Abstract
Intercellular interactions in the cell microenvironment play a critical role in determining cell fate, but the effects of these interactions on pathways governing human embryonic stem cell (hESC) behavior have not been fully elucidated. We and others have previously reported that 3-D culture of hESCs affects cell fates, including self-renewal and differentiation to a variety of lineages. Here we have used a microwell culture system that produces 3-D colonies of uniform size and shape to provide insight into the effect of modulating cell-cell contact on canonical Wnt/β-catenin signaling in hESCs. Canonical Wnt signaling has been implicated in both self-renewal and differentiation of hESCs, and competition for β-catenin between the Wnt pathway and cadherin-mediated cell-cell interactions impacts various developmental processes, including the epithelial-mesenchymal transition. Our results showed that hESCs cultured in 3-D microwells exhibited higher E-cadherin expression than cells on 2-D substrates. The increase in E-cadherin expression in microwells was accompanied by a downregulation of Wnt signaling, as evidenced by the lack of nuclear β-catenin and downregulation of Wnt target genes. Despite this reduction in Wnt signaling in microwell cultures, embryoid bodies (EBs) formed from hESCs cultured in microwells exhibited higher levels of Wnt signaling than EBs from hESCs cultured on 2-D substrates. Furthermore, the Wnt-positive cells within EBs showed upregulation of genes associated with cardiogenesis. These results demonstrate that modulation of intercellular interactions impacts Wnt/β-catenin signaling in hESCs.
Keywords: Stem cell, micropatterning, cell signaling, cell adhesion
1. Introduction
Due to their unique capacity for unlimited self-renewal and the potential to differentiate into all adult cell types [1, 2], human embryonic stem cells (hESCs) hold tremendous promise for a variety of cell-based applications, including drug and toxicity testing and tissue engineering. However, much is still unknown about the mechanisms that control self-renewal and differentiation of these cells. Stem cell fate decisions are affected by a variety of microenvironmental cues, including but not limited to soluble factors, the extracellular matrix, and intercellular interactions [3, 4].
Various materials-based systems have been developed to study the effects of modulating cell-cell contact and colony morphology on ESC behavior. A forced aggregation method based on centrifugation of single-cell suspensions of hESCs into V-bottom 96 or 384-well plates generated uniformly sized EBs in a high throughput manner, and this approach was utilized to determine that differences in EB size affect efficiency of cardiogenesis [5, 6]. Another approach used to control EB size involved 2-D patterning of hESCs on Matrigel using microcontact printing to study how colony size, and ultimately EB size, affects commitment to different differentiation trajectories [7]. 3-D cell patterning strategies utilizing microwell culture platforms have also been used to study intercellular interactions in ESCs [8, 9]. A microwell array engineered from PEG hydrogels to control aggregate size of ESCs has been used to study the differential commitment of mESC-derived EBs to endothelial or cardiac lineages depending on EB size [8, 10]. We have previously demonstrated that growth of hESCs in microwells surrounded by a protein-resistant self-assembled monolayer (SAM) promotes either long-term self-renewal [9] or subsequent embryoid body (EB)-mediated differentiation to cardiomyocytes [11]. Elucidating the developmental pathways through which colony morphology regulates human pluripotent stem cell self-renewal and differentiation will facilitate design of culture systems to better regulate these diverse cell fates.
In this study we employed a 3-D microwell system and 2-D culture to determine how cadherin-mediated cell-cell interactions modulate canonical Wnt signaling in hESCs. In epithelial cells such as hESCs, β-catenin provides a physical linkage between E-cadherin and the actin cytoskeleton in adherens junctions [12]. β-catenin also acts as a transcription co-factor of the canonical Wnt signaling pathway [13]. Canonical Wnt binding to its cell surface receptor inhibits phosphorylation and degradation of β-catenin in the cytosol, permitting β-catenin translocation to the nucleus where it complexes with TCF or LEF to induce transcription of canonical Wnt-responsive genes. Competition for β-catenin between adherens junctions and the canonical Wnt pathway can affect various aspects of vertebrate development, including the epithelial-mesenchymal transition (EMT) [14–16], mesoderm patterning [17], and neural crest development [18]. Canonical Wnt signaling is involved in many cell differentiation processes [19]. Studies of vertebrate development in vivo [20, 21] and in vitro studies in ESCs [22–25] have revealed a stage-specific role for the canonical Wnt/β-catenin pathway in cardiogenesis; canonical Wnt activation is necessary for early mesoderm induction but inhibition is later required for cardiac specification from these mesodermal progenitors. Additionally, canonical Wnt/β-catenin signaling has been implicated in regulating self-renewal of hESCs, though there is some debate as to the nature of its role. Activation of canonical Wnt signaling by treatment of hESCs with the GSK3-inhibitor BIO was able to maintain pluripotency over short time scales (4–5 days) in the absence of feeder-conditioned media [26]. However, a later study showed that canonical Wnt signaling was insufficient to maintain self-renewal upon subculture of hESCs and instead a new model was proposed in which canonical Wnt signaling promotes proliferation of hESCs, which can accelerate either self-renewal or differentiation depending on the context of other signaling cues [27]. A follow-up study confirmed that activation of canonical Wnt signaling in undifferentiated hESCs promotes proliferation but not necessarily self-renewal [28]. However, another recent study demonstrated that canonical Wnt receptor Frizzled-7 is necessary for hESC self-renewal [29]. While canonical Wnt signaling clearly plays a role in hESC developmental events, we lack a complete understanding of how this pathway regulates cell self-renewal and differentiation.
In this study, we hypothesized that the differences in cell-cell interactions in 2-D culture and 3-D microwells affect signaling through the canonical Wnt pathway by altering localization of β-catenin. To test this hypothesis, we compared Wnt/β-catenin signaling in undifferentiated hESCs cultured in 2-D and 3-D morphologies, and in EBs formed from these cultures.
2. Materials and Methods
2.1 Microwell fabrication
Microwells were prepared as previously described [9]. Briefly, soft lithography was used to pattern the wells in polyurethane using PDMS stamps. E-beam evaporation was then used to coat the areas outside of the wells with a thin layer of gold. Finally, a tri(ethylene glycol)-terminated alkanethiol self-assembled monolayer (EG3) was assembled on the gold surface.
2.2 hESC culture and embryoid body differentiation
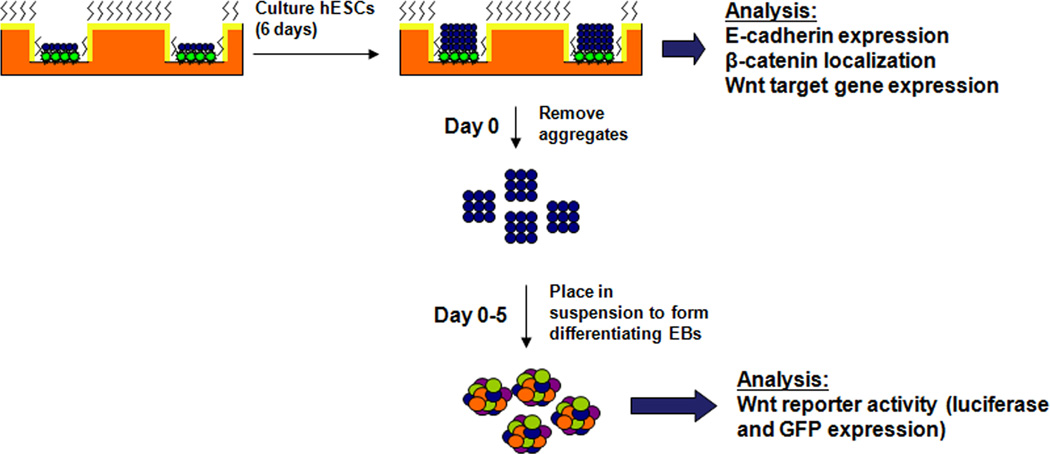
The culture and differentiation processes are illustrated in Fig. 1. H1, H7 and H9 hESCs (passages 25–50) were cultured on tissue culture polystyrene (TCPS) 6-well plates or in microwells. Both substrates were coated with growth factor reduced Matrigel (BD Bioscience, Medford, MA) for 1 hour at 37°C. Unconditioned medium (UM/F−) composed of DMEM/F12 (Invitrogen) containing 20% Knockout Serum Replacer (Invitrogen), 1× MEM nonessential amino acids (Invitrogen), 1 mL L-glutamine (Sigma), and 0.1 mM β-mercaptoethanol (Sigma) was conditioned on irradiated mouse embryonic fibroblasts for 24 hours and supplemented with 4 ng/mL bFGF, resulting in the culture medium CM/F+. Microwells were seeded with cells as previously described [9]. For differentiation studies, hESCs were cultured for 6 days in CM/F+ supplemented with 2 µM BIO (Sigma). To initiate differentiation, colonies were detached from the Matrigel matrix at day 6 of culture using 1 mg/mL dispase (Invitrogen) and placed in suspension UM/F− in Corning 3741 low attachment plates. Following 24 hours in UM/F−, the EBs were maintained in suspension for 4 more days in EB20 medium: DMEM/F12 (Invitrogen) containing 20% fetal bovine serum (Invitrogen), 1× MEM nonessential amino acids (Invitrogen), 1mL L-glutamine (Sigma), and 0.1mM β-mercaptoethanol (Sigma).
Figure 1. Schematic of microwell culture/EB differentiation process.
hESCs were cultured in 3-D microwells or on 2-D substrates for 6 days. At day 6 the cells were analyzed for E-cadherin expression, β-catenin localization, and Wnt target gene expression. For differentiation studies, on day 6 aggregates were enzymatically removed from either 3-D microwells or 2-D substrates and placed in suspension in medium containing serum. During the 5 day suspension period, Wnt reporter activity was assessed via assays for luciferase and GFP expression to quantify differences in Wnt signaling.
2.3. Plasmid construction, lentiviral production and infection of hESCs
The 7 TCF/LEF binding site sequences were obtained by direct PCR of plasmid M50 (Addgene plasmid 12456) and the GreenFire (GF) sequences were obtained by direct PCR of the pGreenFire1-mCMV Plasmid (System Bioscience TR010PA-1). These two sequences were cloned into pSicoR PGK puro (Addgene plasmid 11586) digested by Xba I (NEB) and Xho I (NEB). The constructed Wnt reporter plasmid was named 7TGFP and verified by sequencing. This 7TGFP vector was cotransfected with the helper plasmids psPAX2 and pMD2.G (Addgene plasmids 12260 and 12259) into HEK-293TN cells (System Biosciences) for virus production. Virus-containing medium was collected at 48 and 72 hours after transfection and used for infection of hESCs in the presence of 6µg/mL polybrene (Sigma). Transduced cells were cultured in mTeSR1 on Matrigel for three days and then clonally isolated in mTeSR1 with 1 µg/mL puromycin.
2.4 Immunocytochemistry and Imaging
hESCs cultured in microwells or on Matrigel-coated glass coverslips were fixed in 4% paraformaldehyde (EMS) for 20 min at room temperature. Samples were blocked and permeabilized for 1 hour in blocking buffer, PBS (Invitrogen) containing 5% chick serum (Invitrogen) and 0.2% Triton X-100 (Sigma). Primary antibodies (see Supplemental Table 1 for list) were incubated overnight at 4°C in blocking buffer, and after subsequent washes in PBS cells were incubated in blocking buffer containing chicken anti-mouse Alexa 488 (1:500, Invitrogen) and donkey anti-goat Alexa 555 (1:500, Invitrogen) for 1 hour at room temperature. Following PBS washes, the cell nuclei were labeled with TOPRO-3 iodide (1:500, Invitrogen) for 20 min. Samples were then mounted on coverslips using ProLong Gold anti-fade reagent (Invitrogen) and imaged using a Bio-Rad Radiance 2100 Multiphoton Rainbow microscope (Bio-Rad).
2.5 Flow cytometry and cell sorting
Cells were detached from microwells or Matrigel-coated 6-well TCPS plates using 0.25% trypsin-EDTA (Invitrogen), fixed in 1% paraformaldehyde for 10 min at 37°C, and permeabilized in ice-cold 90% methanol for 30 min. Primary antibodies (see Supplemental Table 1 for list) were incubated overnight in FACS buffer (PBS with 2%FBS and 0.1% Triton X-100). Following 2 PBS washes, cells were incubated with goat anti-rabbit Alexa488 (1:1000, Invitrogen) for 30 min at room temperature (for Oct4 flow only). After 2 PBS washes, samples were analyzed on a FACSCaliber flow cytometer (Becton Dickinson Immunocytometry Systems, BDIS) using CellQuest software. For 7TGP Wnt reporter line studies, EBs were rinsed with PBS and cells were singularized using Accutase (Invitrogen). Cells were then resuspended in FACS buffer for analysis. For cell sorting experiments, singularized Wnt reporter cells were resuspended in HBSS (Invitrogen) containing 2% FBS and separated into GFP-positive and GFP-negative populations using a FACSVantageSE cell sorter (BDIS). Following collection, both cell populations were centrifuged to collect cell pellets for RNA extraction.
2.6 Quantitative polymerase chain reaction (qPCR)
For RNA extraction, cells were dissociated with 0.25% Trypsin-EDTA. Total RNA was extracted using an RNeasy Mini Kit (Qiagen) according to the manufacturer’s instructions. cDNA was generated from 1 µg of RNA using Omniscript reverse transcriptase (Qiagen) and oligo-dT primers (Invitrogen). For 7TGP Wnt reporter lines, an RNeasy Micro Kit (Qiagen) was used for the RNA extraction from cell pellets due to the small number of cells, and 400 ng of RNA was used to generate cDNA. Quantitative PCR (qPCR) was then performed using iQ SYBR Green Supermix (Bio-Rad) and 1 µL cDNA on an iCycler (Bio-Rad). Primer sequences are supplied in Supplemental Table 2. Relative expression was found using the comparative cycle threshold (CT) method using the reference gene glyceraldehyde-3-phosphate dehydrogenase (GAPDH). Fold difference was then calculated as 2−ΔΔCT or −(2ΔΔCT).
2.7 Western blotting
Nuclear and cytoplasmic extracts were isolating using a NE-PER kit (Pierce). Proteins were quantified using a BCA protein assay (Pierce), resolved on a 12% polyacrylamide gel and transferred to a nitrocellulose membrane. After blocking with 5% powdered milk in TBS + 1% Tween-20 for 1 hour at room temperature, membranes were labeled with primary antibodies (see Supplementary Table 1 for list) overnight at 4°C followed by horseradish peroxidase-conjugated secondary antibodies for 1 hour at room temperature. Protein levels were detected via a SuperSignal West Pico Chemiluminescent Substrate (Pierce). Equal protein loading and proper separation of nuclear and cytoplasmic extracts were confirmed via β-actin and Histone2b levels.
2.8 Luciferase Assay
EBs were rinsed in PBS and cells were singularized using Accutase. Then, 100,000 cells/well were placed in 96-well plates. Luciferase expression was quantified using the Bright-Glo Luciferase Assay (Promega) according to the manufacturer’s instructions. Expression was normalized to total cell number using a CellTiter-Glo Luminescent Cell Viability Assay (Promega). Plates were read on an Infinite F500 microplate reader (Tecan).
2.9 Statistics
Data are presented as mean ± standard deviation (SD), and p-values were determined using an unpaired Student’s t-test or one-way ANOVA.
3. Results
3.1 Modulating E-cadherin expression
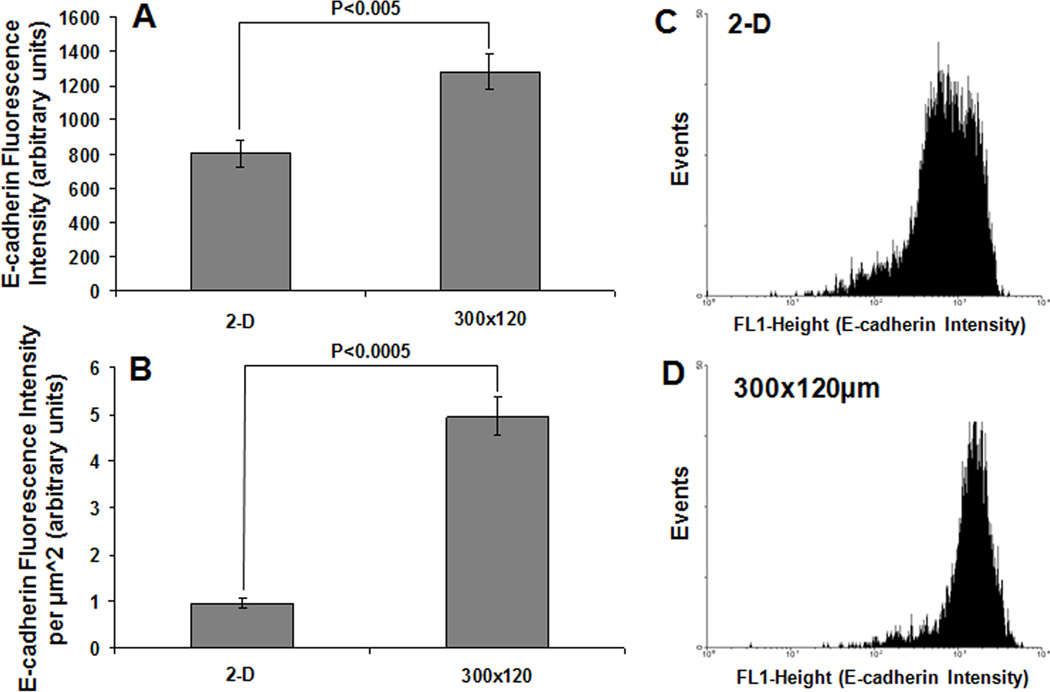
Since a primary difference between 2-D and 3-D culture is the relative amount of cell-cell vs. cell-matrix contact and E-cadherin mediates intercellular chemical and mechanical interactions between epithelial cells in adherens junctions, we compared E-cadherin expression in hESCs cultured in 2-D and 3-D formats. H9 hESCs were cultured for 6 days on 2-D TCPS plates or in 3-D microwells with a 300 µm lateral dimension and 120 µm depth (300×300×120 µm). The cells were singularized and removed from the microwells, and the intensity of E-cadherin expression per cell was quantified via flow cytometry. The fraction of cells expressing Oct4 was also compared to identify effects of colony morphology on differentiation state of the cells (Fig. S1A). Flow cytometry for co-expression of Oct4 and SSEA4 also revealed no statistically significant differences between hESCs cultured in microwells and on 2-D substrates (data not shown). Fig. 2A demonstrates that the average fluorescence intensity of E-cadherin per cell in microwells was approximately 50% greater than E-cadherin expression in cells cultured on 2-D substrates. Representative histograms of E-cadherin expression in cells cultured in 2-D and 3-D are shown in Fig. 2C–D. Additionally, when differences in cell size (Fig. S1B) were taken into consideration by normalizing the average intensity to cell surface area, assuming a spherical shape, the difference in E-cadherin surface density between 2-D and 3-D cultures was even more pronounced, with the 300×300×120 µm microwells exhibiting a 5-fold higher normalized E-cadherin intensity than cells cultured on 2-D substrates (Fig. 2B). Increased E-cadherin expression per cell was also observed in microwell-cultured hESCs as compared to 2-D cultured hESCs with the H7 hESC line (Fig. S2).
Figure 2. 3-D microwell confinement increases expression of E-cadherin per cell.
Average intensity of E-cadherin fluorescence per cell in 300×300×120 µm microwells and on 2-D substrates at day 6 as determined by flow cytometry (A). Cells in microwells possessed significantly higher E-cadherin expression per cell. The elevated E-cadherin expression in microwells was more dramatic when fluorescence intensity was normalized to cell surface area to take into account differences in cell size (B). Panels (C) and (D) show sample histograms of E-cadherin intensity in populations from 2-D Matrigel-coated tissue culture polystyrene (TCPS) controls and 300×300×120 µm microwells respectively.
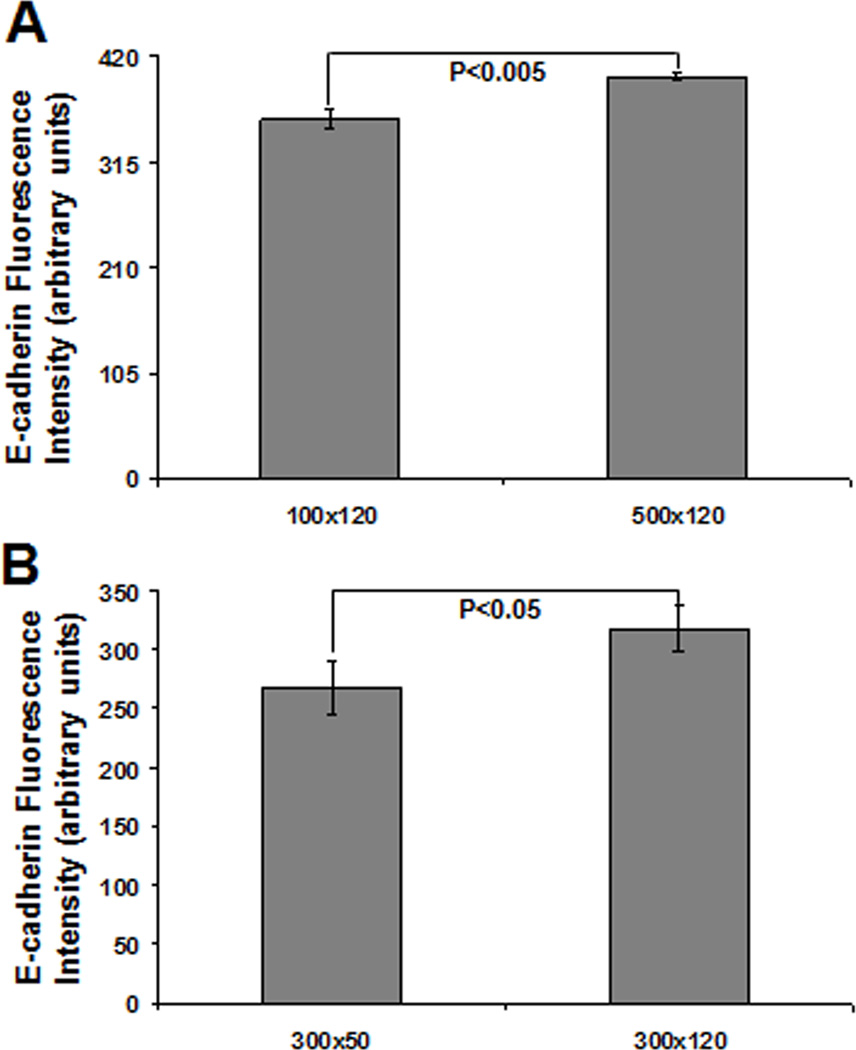
To further assess the effect of cell-cell contact on E-cadherin expression, we examined the extent to which E-cadherin expression changes with microwell dimensions. We found that increasing microwell lateral dimension from 100 to 500 µm at a constant depth of 120 µm led to a small but significant increase in E-cadherin expression per cell (Fig. 3A). Average E-cadherin expression per cell also increased when the lateral dimension was held constant at 300 µm and the depth was increased from 50 µm to 120 µm (Fig. 3B). These results demonstrate that the increased cell-cell interactions in 3-D compared to 2-D cultures of hESCs led to an increase in E-cadherin expression, and that hESCs cultured in larger microwells exhibited greater E-cadherin expression than those cultured in smaller microwells.
Figure 3. E-cadherin expression can be modulated by varying microwell dimensions.
Average intensity of E-cadherin fluorescence per cell in 300×300×50, 100×100×120, 300×300×120, and 500×500×120 µm microwells at day 6 as determined by flow cytometry. Increasing microwell lateral size from 100 to 500 µm (A) or increasing microwell depth from 50 to 120 µm (B) led to increased E-cadherin expression per cell.
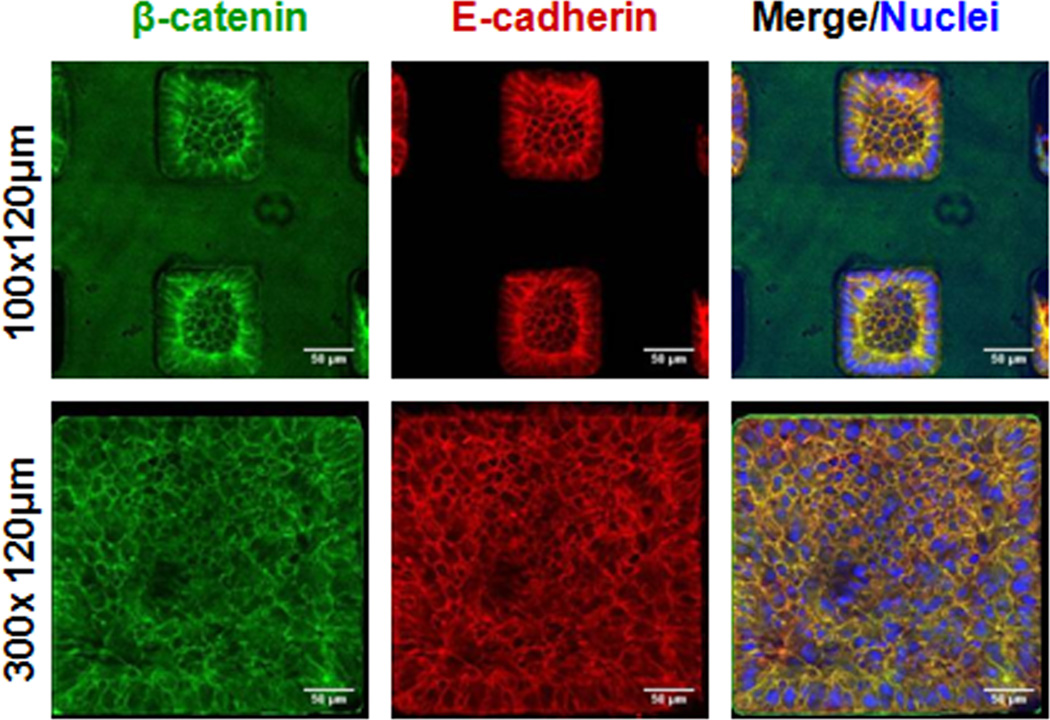
To verify appropriate co-localization of E-cadherin and associated junctional proteins in hESCs cultured in microwells, we performed immunocytochemistry for E-cadherin and β-catenin. Cells were grown in 100×100×120 and 300×300×120 µm microwells for 6 days, and then they were fixed and labeled with antibodies for E-cadherin and β-catenin. Confocal microscopy revealed that β-catenin co-localized with E-cadherin at the cell membrane in both microwell sizes (Fig. 4). The co-localization of β-catenin and E-cadherin was also observed in the H1 line (Fig. S3), and no qualitative differences were observed in extent of E-cadherin localization to the membrane at different depths within the microwell (Fig. S4).
Figure 4. β-catenin co-localized with E-cadherin in microwells.
Cells in 100×100×120 and 300×300×120 µm microwells were fixed and labeled with antibodies against β-catenin (green) and E-cadherin (red) on day 6. Nuclei were stained with TOPRO-3-iodide (blue). Both microwell sizes showed β-catenin co-localized with E-cadherin at the cell membrane
3.2 β-catenin localization and Wnt pathway downregulation
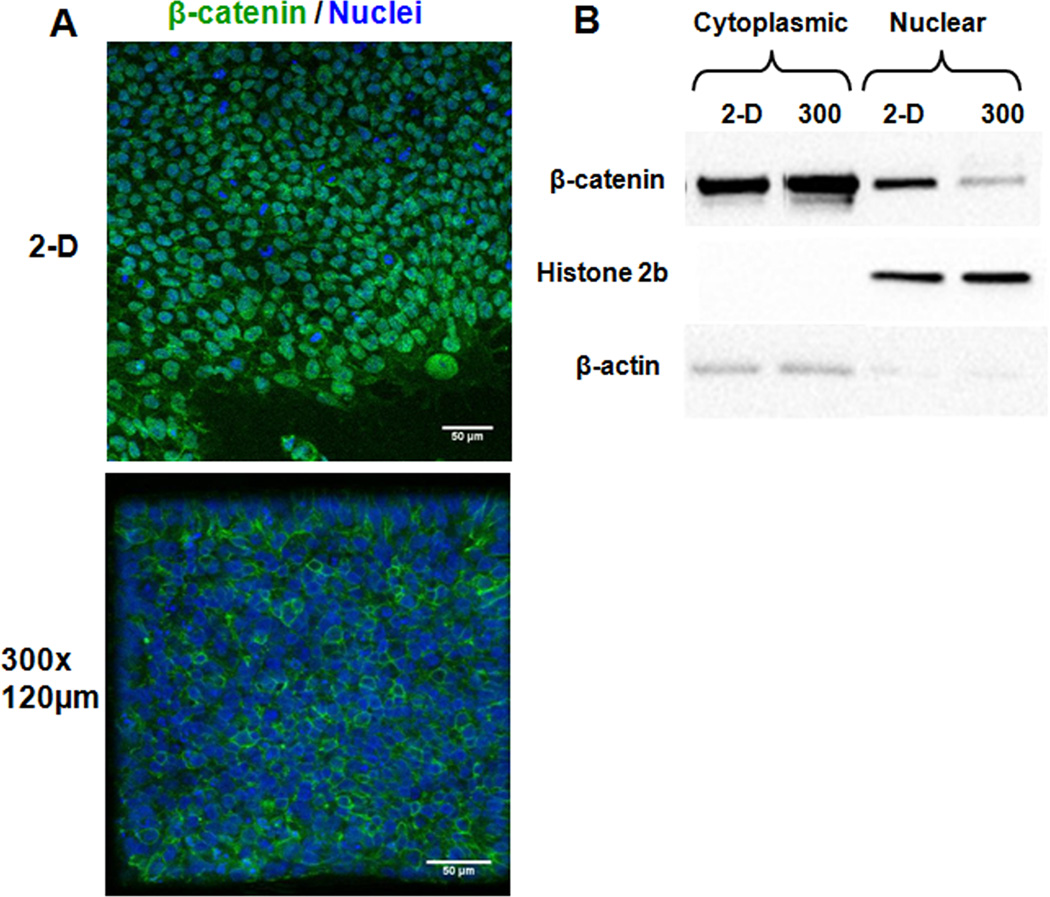
β-catenin localization within the cell is suggestive of its function. Membrane localized β-catenin likely mediates cadherin linkages with the cytoskeleton at adherens junctions while nuclear localized β-catenin interacts with co-factors to initiate transcription of genes regulated by canonical Wnt signaling. Immunocytochemistry for β-catenin revealed that hESCs cultured on 2-D substrates contained both nuclear and membrane-bound β-catenin, while hESCs cultured in 300×300×120 µm microwells contained almost exclusively membrane-bound β-catenin (Fig. 5A). This observation suggests that the increased extent of cell-cell contact and elevated expression of E-cadherin in 3-D culture results in sequestration of β-catenin to the cell membrane. Nuclear and cytoplasmic protein extracts were also isolated from hESCs cultured on 2-D substrates and in 300×300×120 µm microwells, and western blot analysis of these extracts for presence of β-catenin confirmed both nuclear and cytoplasmic β-catenin localization in cells cultured on 2-D substrates but predominant cytoplasmic localization in cells cultured in 3-D microwells (Fig. 5B).
Figure 5. Microwell confinement of undifferentiated hESCs affects β-catenin localization.
Cells in 300×300×120 µm microwells and on 2-D substrates (Matrigel-coated glass coverslips) were labeled with an antibody for β-catenin (green) and a TOPRO-3-iodide nuclear stain (blue) at day 6 (A). Cells in microwells showed an absence of detectable nuclear β-catenin. This localization difference was confirmed via western blot analysis of nuclear and cytoplasmic protein extracts collected at day 6 from 300×300×120 µm microwells and 2-D Matrigel-coated TCPS plates (B).
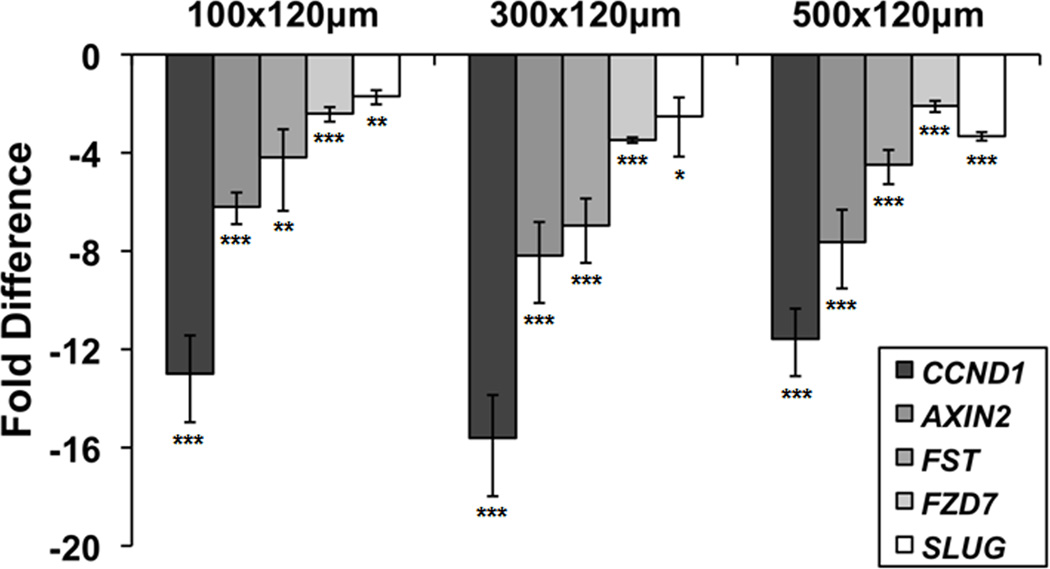
To determine if the reduction in nuclear β-catenin in hESCs cultured in microwells as compared to on 2-D substrates resulted in a downregulation of canonical Wnt signaling, we extracted RNA from hESCs grown for 6 days on 2-D substrates, 100×100×120 µm, 300×300×120 µm, and 500×500×120 µm microwells and quantified expression of 5 Wnt target genes via qPCR [30–33]. Flow cytometry confirmed that at this point in culture, over 98% of hESCs grown in all four conditions expressed Oct4 (Fig. S1). hESCs cultured in all microwell sizes exhibited downregulation of FST, AXIN2, CCND1, FZD7 and SLUG compared to cells grown on 2-D substrates (Fig. 6). However, statistically significant differences between cells harvested from different microwell sizes were not observed. Downregulation of these target genes was also observed in H1 hESCs cultured in 3-D microwells as compared to cells on 2-D substrates (Fig. S5). The qPCR results suggest that the reduction of nuclear β-catenin in microwells compared to 2-D substrates is linked to a downregulation of Wnt signaling in undifferentiated hESCs.
Figure 6. Downregulation of canonical Wnt-responsive genes in microwell-cultured hESCs.
qPCR analysis of Wnt target gene expression in three microwell sizes (100×100×120, 300×300×120, and 500×500×120 µm) at day 6. Fold difference is relative to 2-D control (Matrigel-coated TCPS plates). Wnt downstream genes were downregulated in all microwells as compared to the 2-D control (* indicates P<0.05, ** indicates P<0.005, *** indicates P<0.0005 compared to same-day 2-D control), but there was no statistically significant difference between different microwell sizes.
3.3 Wnt pathway activity in embryoid bodies (EBs)
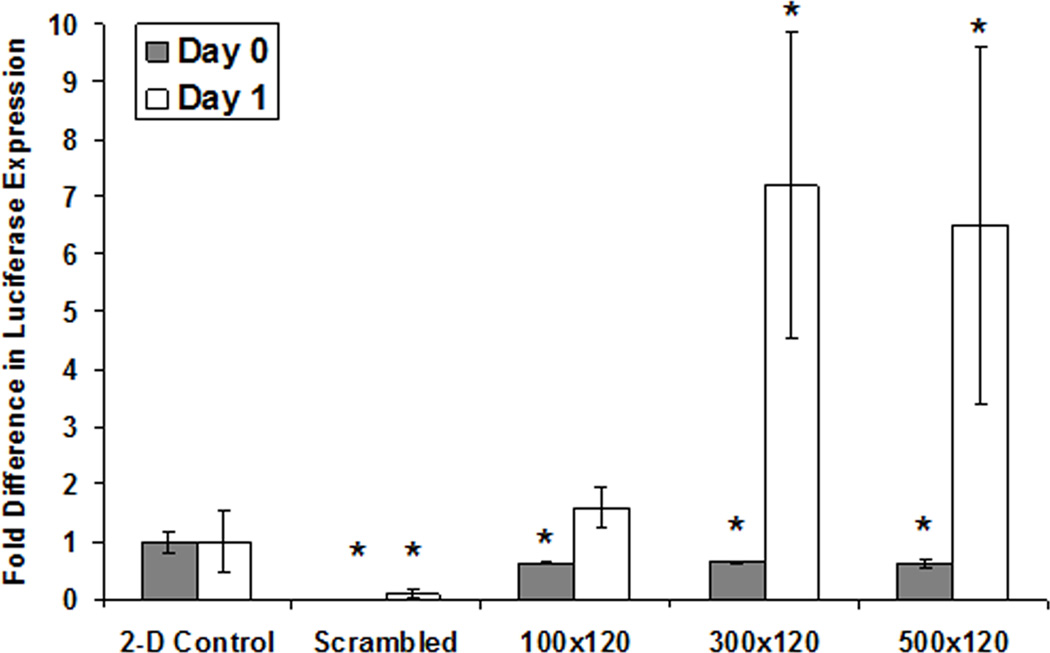
Since microwell-cultured hESCs have been shown to generate EBs with enhanced cardiogenesis when compared to EBs from hESCs cultured on 2-D substrates [11] and early activation of the Wnt pathway has been associated with promoting cardiac differentiation of ESCs [22–25], we next evaluated canonical Wnt signaling in EBs formed from hESCs cultured in microwells and on 2-D substrates. To study Wnt pathway activity in differentiating EBs, we generated a dual reporter line, H9-7TGFP, based on a previously published construct [34] which exhibits both GFP and Luciferase expression when β-catenin/TCF-mediated transcription is active (Fig. S6). Following 6 days of culture, hESC aggregates were removed from microwells or 6-well TCPS plates and placed in suspension for 1 day in UM/F- followed by 4 days in EB20 medium (Fig. 1). Luciferase assays performed at days 0 and 1 of EB formation demonstrated that hESCs in the 100×100×120, 300×300×120 and 500×500×120 µm microwell sizes initially exhibited lower Wnt activity, with fold differences in luciferase expression of 0.63 ± 0.01, 0.64 ± 0.02, and 0.61± 0.07 respectively, relative to the 2-D control (Fig. 7). However, following just one day of differentiation in suspension, EBs generated from microwells exhibited higher Wnt activity than the 2-D controls, with fold differences in luciferase expression of 1.59 ± 0.36, 7.19 ± 2.67, and 6.50 ± 3.10 relative to the 2-D control (Fig. 7).
Figure 7. EBs from microwells exhibited higher Wnt activity following 1 day in suspension.
H9-7TGFP cells were cultured for 6 days in microwells or on 2-D Matrigel-coated TCPS plates. On day 6, aggregates were removed from the substrates and placed in suspension to form EBs. The EBs were collected at day 0 and day 1 and a luciferase assay was performed. Cells cultured in microwells exhibited less canonical Wnt signaling activity at Day 0, but after 24 hours in suspension the EBs from hESCs cultured in all three microwell sizes showed more luciferase expression than EBs from cells cultured on 2-D Matrigel-coated TCPS. Cells that were transfected using a scrambled sequence did not exhibit detectable luciferase activity (* indicates P<0.05 compared to same-day 2-D control).
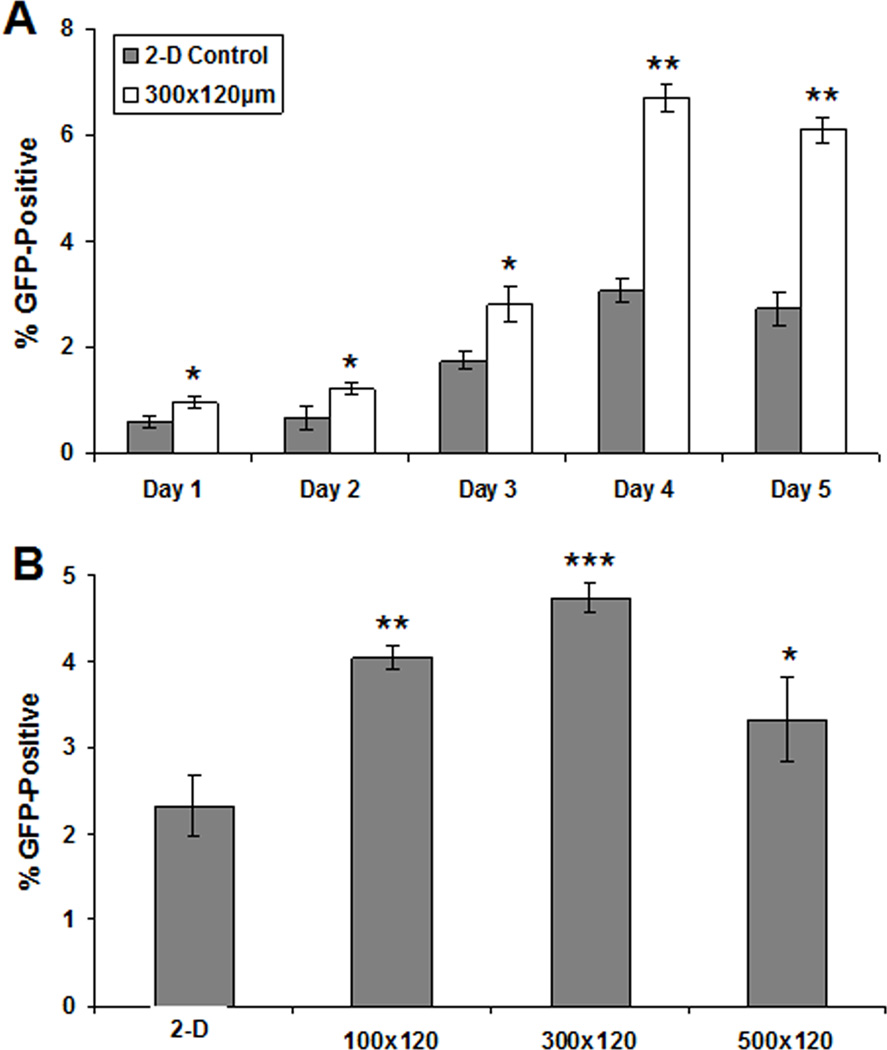
The dynamics of canonical Wnt/β-catenin signaling are critical for cardiogenesis. While early activation is necessary for mesoderm formation, the pathway must then be inhibited to allow for cardiac specification [22, 24]. Since microwell culture has been shown to affect cardiac specification in EBs [10, 11], we evaluated Wnt activity as a function of time during the EB differentiation process. Accordingly, we performed a timecourse of GFP expression in EBs generated from hESCs cultured on 2-D substrates and in 300×300×120 µm microwells using the H9-7TGFP line (Fig. 8A). Flow cytometry assays to evaluate the percentage of GFP-positive cells showed that the microwell-derived EBs contained a higher fraction of GFP-positive cells at each day of suspension culture, and that GFP expression in both 2-D and microwell-derived EBs peaked at day 4. Microwell size also affected the percentage of GFP positive, canonical Wnt-active cells present in EBs. A flow cytometry assay performed at day 4 demonstrated that EBs derived from hESCs cultured in 100×100×120, 300×300×120, and 500×500×120 µm microwells all contained a larger percentage of GFP-positive cells than EBs from 2-D controls, though the 300×300×120 µm microwells produced the highest percentage of GFP-positive cells (Fig. 8B), which is notable since this has previously been shown to be the optimal microwell size for cardiogenesis [11]. This trend was also observed in the H7-7TGFP reporter line (Fig. S7).
Figure 8. Microwell EBs showed earlier onset and higher levels of Wnt activity.
Flow cytometry for percentage GFP-positive cells using the H9-7TGFP Wnt reporter line. EBs from hESCs cultured on 2-D Matrigel-coated TCPS controls and in 300×300×120 µm microwells were analyzed at days 1–5 of suspension culture (A). Microwell-derived EBs contained a higher percentage of GFP-positive cells at each time point, and GFP expression peaked at Day 4. Comparison of percentage of GFP-positive cells at Day 4 in EBs from hESCs cultured on 2-D Matrigel-coated TCPS and hESCs cultured in 3 microwell sizes (100×100×120, 300×300×120, and 500×500×120 µm) (B) revealed that all microwell-derived EBs contained more GFP-positive cells than control EBs, with EBs from the 300×300×120 µm size exhibiting the highest percentage (ANOVA test, P<0.0005) of GFP-positive cells (* indicates P<0.05, ** indicates P<0.005, *** indicates P<0.0005 compared to same-day 2-D control).
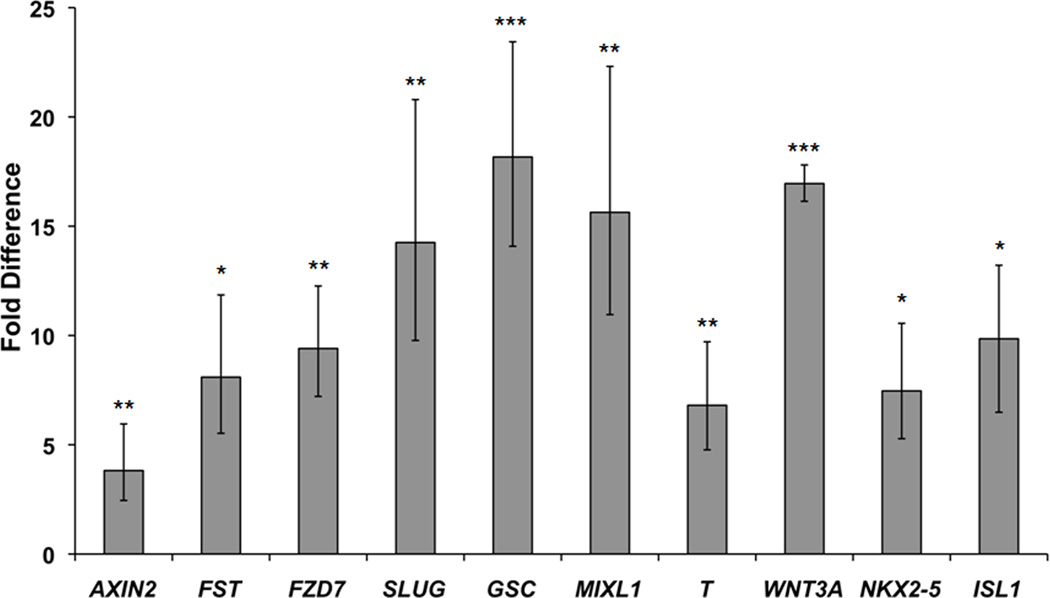
Once we determined that there was significantly more canonical Wnt signaling activity in EBs derived from microwell-cultured hESCs than in EBs from hESCs cultured on 2-D substrates, we sought to establish a link between Wnt activation in EBs and early stages of cardiogenesis. To do so we used fluorescence activated cell sorting (FACS) to isolate the GFP-positive and GFP-negative cell populations in day 3 EBs generated from microwell-cultured hESCs and compared expression of genes related to cardiogenesis in each population. qPCR results demonstrated that canonical Wnt-responsive genes AXIN2, FST, FZD7 and SLUG, primitive streak markers GSC and MIXL1, mesoderm marker T, WNT3A ligand, cardiac transcription factor NKX2-5 and cardiac progenitor marker ISL1 were all upregulated in the GFP-positive population compared to the GFP-negative population (Fig. 9), indicating that Wnt activation is linked to cell differentiation fate. Taken together, these data suggest that the enhanced cardiogenesis observed EBs generated from microwell-cultured hESCs is linked to an upregulation of Wnt signaling at early timepoints during differentiation.
Figure 9. Wnt-active cells in EBs exhibit upregulation of genes related to primitive streak formation and cardiogenesis.
qPCR analysis of GFP-positive and GFP-negative cell populations harvested via FACS at day 3 of suspension culture in EBs generated from 300×300×120 µm microwells. Fold difference is compared to the GFP-negative population. GFP-positive fractions contained higher expression of Wnt target genes AXIN2, FST, FZD7, and SLUG, primitive streak markers GSC and MIXL1, mesoderm marker T, WNT3A, cardiac transcription factor NKX2-5, and cardiac progenitor marker ISL1 (* indicates P<0.05, ** indicates P<0.005, *** indicates P<0.0005 compared to the GFP-negative population).
Discussion
While much progress has been made in identifying small molecules and extracellular matrix components that regulate hESC fates, less is known about the effects of intercellular interactions on hESC self-renewal and differentiation. In this study, we utilized a 3-D microwell array system to elucidate the roles of colony morphology and intercellular interactions in modulating canonical Wnt/β-catenin signaling in hESCs, focusing first on the undifferentiated state. We hypothesized that the increased cell-cell contact in 3-D microwells would increase E-cadherin expression as compared to 2-D substrates, thus sequestering more β-catenin at the adherens junctions and resulting in less β-catenin available to translocate to the nucleus and activate canonical Wnt signaling. This hypothesis was confirmed by flow cytometry assays demonstrating higher E-cadherin expression per cell in microwell-cultured hESCs than in hESCs cultured on 2-D substrates. Consequently, all hESCs cultured in microwells also demonstrated a reduction in nuclear β-catenin and a downregulation in expression of canonical Wnt pathway target genes as opposed to 2-D control hESCs. Additionally, increasing the relative ratio of cell-cell to cell-matrix contact correlated to an increase in E-cadherin expression, indicating that modulation cell-cell interactions using the microwell system was effectively modulating E-cadherin expression. Given the conflicting reports in the literature regarding the role of canonical Wnt/β-catenin signaling in hESC self-renewal [26–29], it is unclear if the promotion of long-term self-renewal previously reported in this microwell system [9] is linked to the downregulation of canonical Wnt signaling described in this study, but it is a possible connection that warrants further study, especially given that the recent development of robust, fully defined culture systems for hESC expansion will enable isolation and study of specific pathways without background signals from conditioned media or feeder cells [35–40]. It has been well-established that the interplay between E-cadherin-mediated signaling and the canonical Wnt/β-catenin pathway affects various stages of vertebrate development [14–17]. Thus, given that the data demonstrating 3-D microwell culture can modulate E-cadherin expression and canonical Wnt/β-catenin signaling in hESCs, it is likely that 3-D culture has the capacity to regulate developmentally-relevant pathways, including those related to the early steps in cardiogenesis.
Previous work with this microwell system demonstrated that all microwell-generated EBs formed more cardiomyocytes than EBs from 2-D controls, while the intermediate size, 300×300×120 µm, was optimal for cardiogenesis [11]. Though hESCs cultured in microwells and on 2-D substrates expressed Oct4 prior to EB formation and the colonies were treated identically during the differentiation process, the EBs from these two systems experienced different trajectories upon differentiation. Thus, the differences in cell-cell interactions experienced by the cells in the undifferentiated state had a substantial effect on signaling pathways that later affected cell fate upon exposure to differentiation cues. Since canonical Wnt activation is necessary for early mesoderm induction [22–24], we used the microwell system to compare the level of canonical Wnt signaling activity in EBs generated from microwell culture to EBs from 2-D substrates. Our results demonstrated that EBs from microwells exhibited higher levels of canonical Wnt signaling than EBs generated from 2-D controls, and there is a link between canonical Wnt activity and expression of genes related to primitive streak formation and cardiogenesis.
Various studies have established a phenomenological link between EB size and differentiation trajectory [7, 10, 11, 41]. An approach to control EB size using microcontact printing to generate uniform colonies of hESCs demonstrated that colony and EB size influenced the ratio of endoderm-biased vs. neural-biased cells and ultimately impacted the efficiency of cardiac induction [7]. A rotary orbital suspension culture method has also been used to generate uniformly sized EBs from mESCs through modulation of the rotational speed, with different size populations showing differential expression of markers related to cardiogenesis [41]. In one of the first attempts to uncover the molecular pathways underlying the effects of modulating intercellular interactions on differentiation of ESCs, another microwell system was used to demonstrate that in mouse ESCs, the enhanced cardiogenesis seen in large EBs (450 µm) as compared to enhanced endothelial differentiation in small EBs (150 µm) was driven by differential expression of non-canonical, β-catenin-independent, Wnt ligands, with Wnt11 being linked to the cardiac fate of the larger EBs [10]. Given the body of literature showing that interactions between cadherin-mediated signaling and the canonical Wnt pathway affect early events in development, including the epithelial-mesenchymal transition and mesoderm formation and patterning, as well as the data presented in this study demonstrating that EBs generated from microwells exhibit higher levels of canonical Wnt/β-catenin signaling during early stages of differentiation, it is possible that Wnt pathway regulation may play a role in these other observations. It is also likely that other juxtacrine or paracrine pathways also play a role in the effects of modulating intercellular interactions on lineage specification, and these pathways warrant further study in the 3-D culture systems described above.
This microwell system provides a means for isolating and studying the role of intercellular interactions in various hESC processes by providing a 3-D culture platform that constrains the size and shape of growing hESC colonies. Various other 3-D systems have also been reported to support self-renewal of hESCs, including porous polymer scaffolds containing alginate and chitosan [42], encapsulation of cells in alginate [43] or hyaluronic acid hydrogels [44], and culture on microcarriers in stirred suspension reactors [45–47]. These systems may be useful in studying how 3-D culture primes hESCs for differentiation to various lineages. While this study describes the effect of modulating cell-cell interactions on cadherin-mediated signaling via the canonical Wnt/β-catenin pathway, there are other developmentally relevant juxtacrine pathways that are directly regulated by intercellular interactions, such as Notch, TGFα, EGF, and connexin-mediated gap junction signaling [48–55]. The microwell system would facilitate identification of the effects of modulating of intercellular interactions on these pathways and would likely provide additional insight into mechanisms by which hESC colony morphology affects cell fate.
Conclusion
We have utilized a 3-D microwell system to isolate and study the effects of modulating colony morphology and intercellular interactions on canonical Wnt/β-catenin signaling in hESCs and EBs derived from these hESCs. Our results demonstrated that the increase in cell-cell contacts in hESCs cultured in 3-D vs. on 2-D substrates led to higher E-cadherin expression and subsequent downregulation of canonical Wnt signaling in undifferentiated hESCs. However, soon after colonies were detached and placed in suspension to form EB aggregates, microwell-derived EBs showed an early upregulation of Wnt signaling, which is linked to enhanced mesoderm induction and cardiogenesis. The microwell system provides a platform to study the molecular signaling pathways regulated by intercellular interactions and a mechanism to apply these signals to regulate cell fate.
Supplementary Material
Acknowledgements
This study was supported by NIH/NIBIB R01 EB007534 (SPP), NSF EFRI-0735903 (SPP), the UW-Madison Materials Research Science and Engineering Center (MRSEC), and a NSF graduate research fellowship (SMA). The authors would like to thank the WiCell Research Institute for providing cells and reagents, the staff at the University of Wisconsin Comprehensive Cancer Center Flow Cytometry Facility for assistance with flow cytometry and cell sorting, and the W.M. Keck Laboratory for Biological Imaging for support with confocal microscopy.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Thomson JA, Itskovitz-Eldor J, Shapiro SS, Waknitz MA, Swiergiel JJ, Marshall VS, et al. Embryonic stem cell lines derived from human blastocysts. Science. 1998;282(5391):1145–1147. doi: 10.1126/science.282.5391.1145. [DOI] [PubMed] [Google Scholar]
- 2.Odorico JS, Kaufman DS, Thomson JA. Multilineage differentiation from human embryonic stem cell lines. Stem Cells. 2001;19(3):193–204. doi: 10.1634/stemcells.19-3-193. [DOI] [PubMed] [Google Scholar]
- 3.Azarin SM, Palecek SP. Development of scalable culture systems for human embryonic stem cells. Biochem Eng J. 2010;48(3):378. doi: 10.1016/j.bej.2009.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McDevitt TC, Palecek SP. Innovation in the culture and derivation of pluripotent human stem cells. Curr Opin Biotechnol. 2008;19(5):527–533. doi: 10.1016/j.copbio.2008.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Burridge PW, Anderson D, Priddle H, Barbadillo Munoz MD, Chamberlain S, Allegrucci C, et al. Improved human embryonic stem cell embryoid body homogeneity and cardiomyocyte differentiation from a novel v-96 plate aggregation system highlights interline variability. Stem Cells. 2007;25(4):929–938. doi: 10.1634/stemcells.2006-0598. [DOI] [PubMed] [Google Scholar]
- 6.Ungrin MD, Joshi C, Nica A, Bauwens C, Zandstra PW. Reproducible, ultra high-throughput formation of multicellular organization from single cell suspension-derived human embryonic stem cell aggregates. PLoS One. 2008;3(2):e1565. doi: 10.1371/journal.pone.0001565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bauwens CL, Peerani R, Niebruegge S, Woodhouse KA, Kumacheva E, Husain M, et al. Control of human embryonic stem cell colony and aggregate size heterogeneity influences differentiation trajectories. Stem Cells. 2008;26(9):2300–2310. doi: 10.1634/stemcells.2008-0183. [DOI] [PubMed] [Google Scholar]
- 8.Khademhosseini A, Ferreira L, Blumling J, 3rd, Yeh J, Karp JM, Fukuda J, et al. Co-culture of human embryonic stem cells with murine embryonic fibroblasts on microwell-patterned substrates. Biomaterials. 2006;27(36):5968–5977. doi: 10.1016/j.biomaterials.2006.06.035. [DOI] [PubMed] [Google Scholar]
- 9.Mohr JC, de Pablo JJ, Palecek SP. 3-d microwell culture of human embryonic stem cells. Biomaterials. 2006;27(36):6032–6042. doi: 10.1016/j.biomaterials.2006.07.012. [DOI] [PubMed] [Google Scholar]
- 10.Hwang YS, Chung BG, Ortmann D, Hattori N, Moeller HC, Khademhosseini A. Microwell-mediated control of embryoid body size regulates embryonic stem cell fate via differential expression of wnt5a and wnt11. Proc Natl Acad Sci U S A. 2009;106(40):16978–16983. doi: 10.1073/pnas.0905550106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mohr JC, Zhang J, Azarin SM, Soerens AG, de Pablo JJ, Thomson JA, et al. The microwell control of embryoid body size in order to regulate cardiac differentiation of human embryonic stem cells. Biomaterials. 2010;31(7):1885–1893. doi: 10.1016/j.biomaterials.2009.11.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jamora C, Fuchs E. Intercellular adhesion, signalling and the cytoskeleton. Nat Cell Biol. 2002;4(4):E101–E108. doi: 10.1038/ncb0402-e101. [DOI] [PubMed] [Google Scholar]
- 13.Nelson WJ, Nusse R. Convergence of wnt, beta-catenin, and cadherin pathways. Science. 2004;303(5663):1483–1487. doi: 10.1126/science.1094291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Heuberger J, Birchmeier W. Interplay of cadherin-mediated cell adhesion and canonical wnt signaling. Cold Spring Harb Perspect Biol. 2010;2(2):a002915. doi: 10.1101/cshperspect.a002915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Howard S, Deroo T, Fujita Y, Itasaki N. A positive role of cadherin in wnt/beta-catenin signalling during epithelial-mesenchymal transition. PLoS One. 2011;6(8):e23899. doi: 10.1371/journal.pone.0023899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yook JI, Li XY, Ota I, Fearon ER, Weiss SJ. Wnt-dependent regulation of the e-cadherin repressor snail. J Biol Chem. 2005;280(12):11740–11748. doi: 10.1074/jbc.M413878200. [DOI] [PubMed] [Google Scholar]
- 17.Brembeck FH, Schwarz-Romond T, Bakkers J, Wilhelm S, Hammerschmidt M, Birchmeier W. Essential role of bcl9-2 in the switch between beta-catenin's adhesive and transcriptional functions. Genes Dev. 2004;18(18):2225–2230. doi: 10.1101/gad.317604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chalpe AJ, Prasad M, Henke AJ, Paulson AF. Regulation of cadherin expression in the chicken neural crest by the wnt/beta-catenin signaling pathway. Cell Adh Migr. 2010;4(3):431–438. doi: 10.4161/cam.4.3.12138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Clevers H. Wnt/beta-catenin signaling in development and disease. Cell. 2006;127(3):469–480. doi: 10.1016/j.cell.2006.10.018. [DOI] [PubMed] [Google Scholar]
- 20.Marvin MJ, Di Rocco G, Gardiner A, Bush SM, Lassar AB. Inhibition of wnt activity induces heart formation from posterior mesoderm. Genes Dev. 2001;15(3):316–327. doi: 10.1101/gad.855501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schneider VA, Mercola M. Wnt antagonism initiates cardiogenesis in xenopus laevis. Genes Dev. 2001;15(3):304–315. doi: 10.1101/gad.855601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Naito AT, Shiojima I, Akazawa H, Hidaka K, Morisaki T, Kikuchi A, et al. Developmental stage-specific biphasic roles of wnt/beta-catenin signaling in cardiomyogenesis and hematopoiesis. Proc Natl Acad Sci U S A. 2006;103(52):19812–19817. doi: 10.1073/pnas.0605768103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tran TH, Wang X, Browne C, Zhang Y, Schinke M, Izumo S, et al. Wnt3a-induced mesoderm formation and cardiomyogenesis in human embryonic stem cells. Stem Cells. 2009;27(8):1869–1878. doi: 10.1002/stem.95. [DOI] [PubMed] [Google Scholar]
- 24.Ueno S, Weidinger G, Osugi T, Kohn AD, Golob JL, Pabon L, et al. Biphasic role for wnt/beta-catenin signaling in cardiac specification in zebrafish and embryonic stem cells. Proc Natl Acad Sci U S A. 2007;104(23):9685–9690. doi: 10.1073/pnas.0702859104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yang L, Soonpaa MH, Adler ED, Roepke TK, Kattman SJ, Kennedy M, et al. Human cardiovascular progenitor cells develop from a kdr+ embryonic-stem-cell-derived population. Nature. 2008;453(7194):524–528. doi: 10.1038/nature06894. [DOI] [PubMed] [Google Scholar]
- 26.Sato N, Meijer L, Skaltsounis L, Greengard P, Brivanlou AH. Maintenance of pluripotency in human and mouse embryonic stem cells through activation of wnt signaling by a pharmacological gsk-3-specific inhibitor. Nat Med. 2004;10(1):55–63. doi: 10.1038/nm979. [DOI] [PubMed] [Google Scholar]
- 27.Dravid G, Ye Z, Hammond H, Chen G, Pyle A, Donovan P, et al. Defining the role of wnt/beta-catenin signaling in the survival, proliferation, and self-renewal of human embryonic stem cells. Stem Cells. 2005;23(10):1489–1501. doi: 10.1634/stemcells.2005-0034. [DOI] [PubMed] [Google Scholar]
- 28.Cai L, Ye Z, Zhou BY, Mali P, Zhou C, Cheng L. Promoting human embryonic stem cell renewal or differentiation by modulating wnt signal and culture conditions. Cell Res. 2007;17(1):62–72. doi: 10.1038/sj.cr.7310138. [DOI] [PubMed] [Google Scholar]
- 29.Melchior K, Weiss J, Zaehres H, Kim YM, Lutzko C, Roosta N, et al. The wnt receptor fzd7 contributes to self-renewal signaling of human embryonic stem cells. Biol Chem. 2008;389(7):897–903. doi: 10.1515/BC.2008.108. [DOI] [PubMed] [Google Scholar]
- 30.Jho EH, Zhang T, Domon C, Joo CK, Freund JN, Costantini F. Wnt/beta-catenin/tcf signaling induces the transcription of axin2, a negative regulator of the signaling pathway. Mol Cell Biol. 2002;22(4):1172–1183. doi: 10.1128/MCB.22.4.1172-1183.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shtutman M, Zhurinsky J, Simcha I, Albanese C, D'Amico M, Pestell R, et al. The cyclin d1 gene is a target of the beta-catenin/lef-1 pathway. Proc Natl Acad Sci U S A. 1999;96(10):5522–5527. doi: 10.1073/pnas.96.10.5522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vallin J, Thuret R, Giacomello E, Faraldo MM, Thiery JP, Broders F. Cloning and characterization of three xenopus slug promoters reveal direct regulation by lef/beta-catenin signaling. J Biol Chem. 2001;276(32):30350–30358. doi: 10.1074/jbc.M103167200. [DOI] [PubMed] [Google Scholar]
- 33.Willert J, Epping M, Pollack JR, Brown PO, Nusse R. A transcriptional response to wnt protein in human embryonic carcinoma cells. BMC Dev Biol. 2002;2:8. doi: 10.1186/1471-213x-2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fuerer C, Nusse R. Lentiviral vectors to probe and manipulate the wnt signaling pathway. PLoS One. 2010;5(2):e9370. doi: 10.1371/journal.pone.0009370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Braam SR, Zeinstra L, Litjens S, Ward-van Oostwaard D, van den Brink S, van Laake L, et al. Recombinant vitronectin is a functionally defined substrate that supports human embryonic stem cell self-renewal via alphavbeta5 integrin. Stem Cells. 2008;26(9):2257–2265. doi: 10.1634/stemcells.2008-0291. [DOI] [PubMed] [Google Scholar]
- 36.Klim JR, Li L, Wrighton PJ, Piekarczyk MS, Kiessling LL. A defined glycosaminoglycan-binding substratum for human pluripotent stem cells. Nat Methods. 2010;7(12):989–994. doi: 10.1038/nmeth.1532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ludwig TE, Levenstein ME, Jones JM, Berggren WT, Mitchen ER, Frane JL, et al. Derivation of human embryonic stem cells in defined conditions. Nat Biotechnol. 2006;24(2):185–187. doi: 10.1038/nbt1177. [DOI] [PubMed] [Google Scholar]
- 38.Melkoumian Z, Weber JL, Weber DM, Fadeev AG, Zhou Y, Dolley-Sonneville P, et al. Synthetic peptide-acrylate surfaces for long-term self-renewal and cardiomyocyte differentiation of human embryonic stem cells. Nat Biotechnol. 2010;28(6):606–610. doi: 10.1038/nbt.1629. [DOI] [PubMed] [Google Scholar]
- 39.Nagaoka M, Si-Tayeb K, Akaike T, Duncan SA. Culture of human pluripotent stem cells using completely defined conditions on a recombinant e-cadherin substratum. BMC Dev Biol. 2010;10:60. doi: 10.1186/1471-213X-10-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yao S, Chen S, Clark J, Hao E, Beattie GM, Hayek A, et al. Long-term self-renewal and directed differentiation of human embryonic stem cells in chemically defined conditions. Proc Natl Acad Sci U S A. 2006;103(18):6907–6912. doi: 10.1073/pnas.0602280103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sargent CY, Berguig GY, Kinney MA, Hiatt LA, Carpenedo RL, Berson RE, et al. Hydrodynamic modulation of embryonic stem cell differentiation by rotary orbital suspension culture. Biotechnol Bioeng. 2010;105(3):611–626. doi: 10.1002/bit.22578. [DOI] [PubMed] [Google Scholar]
- 42.Li Z, Leung M, Hopper R, Ellenbogen R, Zhang M. Feeder-free self-renewal of human embryonic stem cells in 3d porous natural polymer scaffolds. Biomaterials. 2010;31(3):404–412. doi: 10.1016/j.biomaterials.2009.09.070. [DOI] [PubMed] [Google Scholar]
- 43.Serra M, Correia C, Malpique R, Brito C, Jensen J, Bjorquist P, et al. Microencapsulation technology: A powerful tool for integrating expansion and cryopreservation of human embryonic stem cells. PLoS One. 2011;6(8):e23212. doi: 10.1371/journal.pone.0023212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gerecht S, Burdick JA, Ferreira LS, Townsend SA, Langer R, Vunjak-Novakovic G. Hyaluronic acid hydrogel for controlled self-renewal and differentiation of human embryonic stem cells. Proc Natl Acad Sci U S A. 2007;104(27):11298–11303. doi: 10.1073/pnas.0703723104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lock LT, Tzanakakis ES. Expansion and differentiation of human embryonic stem cells to endoderm progeny in a microcarrier stirred-suspension culture. Tissue Eng Part A. 2009;15(8):2051–2063. doi: 10.1089/ten.tea.2008.0455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Oh SK, Chen AK, Mok Y, Chen X, Lim UM, Chin A, et al. Long-term microcarrier suspension cultures of human embryonic stem cells. Stem Cell Res. 2009;2(3):219–230. doi: 10.1016/j.scr.2009.02.005. [DOI] [PubMed] [Google Scholar]
- 47.Storm MP, Orchard CB, Bone HK, Chaudhuri JB, Welham MJ. Three-dimensional culture systems for the expansion of pluripotent embryonic stem cells. Biotechnol Bioeng. 2010;107(4):683–695. doi: 10.1002/bit.22850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bray SJ. Notch signalling: A simple pathway becomes complex. Nat Rev Mol Cell Biol. 2006;7(9):678–689. doi: 10.1038/nrm2009. [DOI] [PubMed] [Google Scholar]
- 49.Chiba S. Notch signaling in stem cell systems. Stem Cells. 2006;24(11):2437–2447. doi: 10.1634/stemcells.2005-0661. [DOI] [PubMed] [Google Scholar]
- 50.Fox V, Gokhale PJ, Walsh JR, Matin M, Jones M, Andrews PW. Cell-cell signaling through notch regulates human embryonic stem cell proliferation. Stem Cells. 2008;26(3):715–723. doi: 10.1634/stemcells.2007-0368. [DOI] [PubMed] [Google Scholar]
- 51.Levin M. Isolation and community: A review of the role of gap-junctional communication in embryonic patterning. J Membr Biol. 2002;185(3):177–192. doi: 10.1007/s00232-001-0129-7. [DOI] [PubMed] [Google Scholar]
- 52.Nishii K, Kumai M, Shibata Y. Regulation of the epithelial-mesenchymal transformation through gap junction channels in heart development. Trends Cardiovasc Med. 2001;11(6):213–218. doi: 10.1016/s1050-1738(01)00103-7. [DOI] [PubMed] [Google Scholar]
- 53.Singh AB, Harris RC. Autocrine, paracrine and juxtacrine signaling by egfr ligands. Cell Signal. 2005;17(10):1183–1193. doi: 10.1016/j.cellsig.2005.03.026. [DOI] [PubMed] [Google Scholar]
- 54.Watson AJ. The cell biology of blastocyst development. Mol Reprod Dev. 1992;33(4):492–504. doi: 10.1002/mrd.1080330417. [DOI] [PubMed] [Google Scholar]
- 55.Wei CJ, Xu X, Lo CW. Connexins and cell signaling in development and disease. Annu Rev Cell Dev Biol. 2004;20:811–838. doi: 10.1146/annurev.cellbio.19.111301.144309. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.