Abstract
Tumors expressing the chemokine receptor CXCR4 have been reported to be more aggressive and to produce more metastatic seeding in specific organs, such as the bone marrow. However, evaluation of tumors for CXCR4 expression requires testing of ex vivo biopsy samples, and is not routinely done in cancer management. In prior work to address this issue, we and others have developed tracers for positron emission tomography (PET) that targeted CXCR4, but in addition to binding to CXCR4 these tracers also bound to red blood cells (and to other unrelated targets) in vivo. Here we report two new tracers based on the CXCR4 peptide antagonist 4F-benzoyl-TN14003 (T140) that bind to CXCR4, but not to undesired targets. These tracers, NOTA-NFB and DOTA-NFB, show slight reductions in both 1) binding affinities for CXCR4 and 2) inhibition of CXCL12 induced migration, compared to T140, in vitro. Both NOTA-NFB and DOTA-NFB specifically accumulate in CXCR4-positive, but not CXCR4-negative, tumor xenografts in mice and allow clear visualization of CXCR4 expression by PET. Evaluation of NOTA-NFB and DOTA-NFB for their potential to mobilize immune cells and progenitor cells from the bone marrow to the peripheral blood revealed slightly reduced, but still comparable, results to the parent molecule T140. The tracers reported here may allow the evaluation of CXCR4 expression in primary tumors and metastatic nodules, and enable better informed, more personalized treatment for patients with cancer.
Keywords: T140, CXCR4 imaging, PET, copper-64
1. Introduction
Chemokine receptors (CKRs) are a subfamily of seven-transmembrane domain, G-protein coupled receptors, that upon binding to their chemokine ligands, guide the migration of cells within an organism [1]. More than 40 chemokines and 19 chemokine receptors have been identified in humans [2–5]. Among CKRs, CXCR4 is unusual in the breadth of its biological activities beyond leukocyte recruitment, which include roles in the development of the hematopoietic, cardiovascular, and nervous systems during embryogenesis [6]. The natural human ligand of CXCR4 is the chemokine CXCL12/SDF-1, and both ligand and receptor have been highly conserved during evolution [7–8].
One of the physiological roles of CXCR4 is in directing cells to organ sites that express high levels of CXCL12, suggesting that this interaction plays a key role in the chemotaxis, homing, and retention of hematopoietic cells during homeostasis. Of particular interest, CXCR4-CXCL12 plays a role in stem cell homing to and retention in the bone marrow [9]. Previous studies have shown that mobilization of stem cells from the bone marrow by treatment with granulocyte colony-stimulating factor (G-CSF) involves degradation of CXCL12 in the bone marrow, promoting cell egress [10]. Moreover, specific disruption of the interaction between CXCR4 and CXCL12 in the bone marrow, by use of antagonists, acts additively with G-CSF treatment to improve stem cell mobilization [1, 11–13].
In addition to its extensive physiological roles, CXCR4 has also been found to be expressed by various human cancers including breast, prostate, lung, colon and multiple myeloma [14–21], and has been suggested to be involved in the process of cancer cell metastasis [22–23]. CXCR4 expression can be induced by a number of transcription factors, including hypoxia inducible factor-1 (HIF-1), NF-κB and oncoproteins PAX3-FKHR and RET/PTC, which have been shown to be expressed by cancers [14, 24]. There is evidence, too, that high levels of CXCR4 expression in cancers correlate with poor prognosis, and with resistance to chemotherapy. In the case of cancers of hematopoietic origin, this is thought to occur in part due to enhanced interactions between cancers and bone marrow stroma [20–21].
Collectively, the data on CXCR4 in cancer suggest that this receptor increases tumor cell survival and/or growth and/or metastasis, making it a potentially attractive therapeutic target [14, 24–25]. Currently, CXCR4 expression in a tumor can only be evaluated using excised tissue specimens, which limits data to the site of biopsy and to a single point in time. The development of CXCR4-specific positron emission tomography (PET) tracers would enable non-invasive evaluation of CXCR4 expression in whole tumors and, thereby, could be expected to promote personalized treatment for patients with cancer.
4-F-benzoyl-TN14003 (T140) is a high affinity CXCR4 peptide antagonist consisting of 14 amino acids, one disulfide bridge between Cys4 and Cys13, an amidated C terminus, and a 4-fluorobenzoyl group at the N-terminus (Fig. 1). Administration of T140 to mice induces in vivo mobilization of stem cells from the bone marrow, which is additive in effect with G-CSF treatment [12]. We have previously labeled T140 with 18F and found that it binds to CXCR4 in tumors, but this was masked in vivo because it also bound to red blood cells (RBCs) [26]. Moreover, the radiolabeling of T140 with 18F was laborious and time consuming, limiting its practicality in clinical settings.
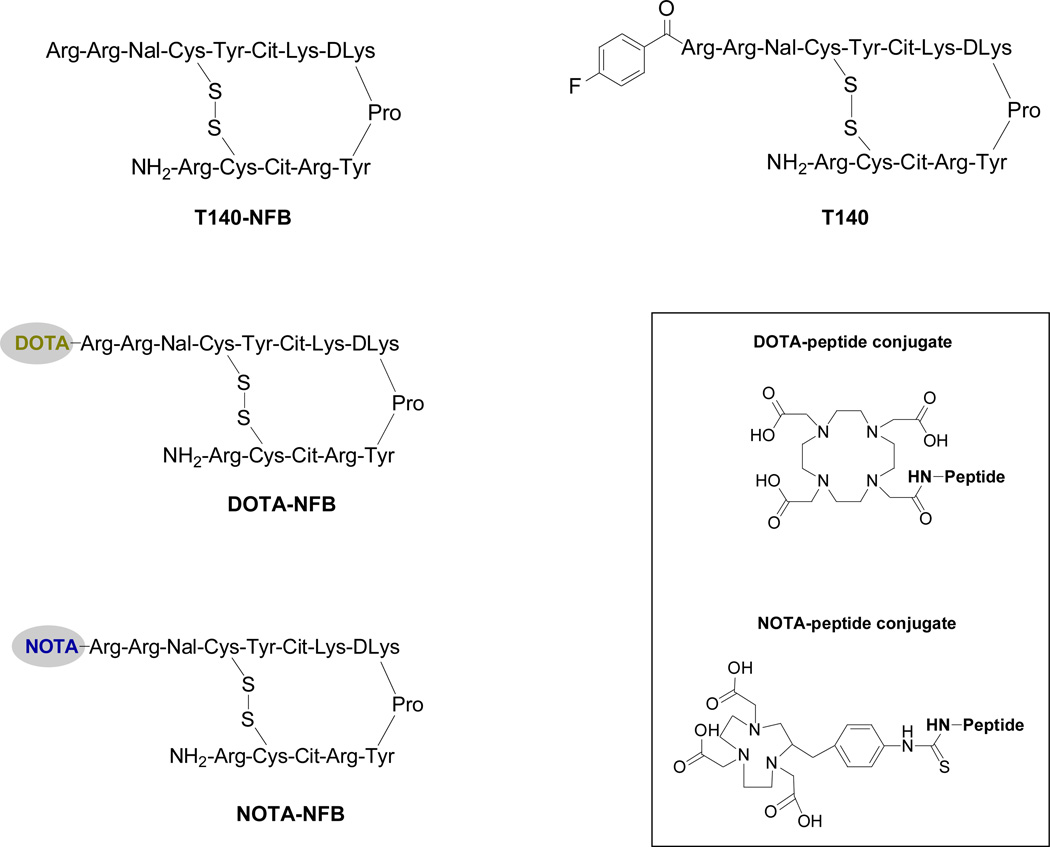
Figure 1.
Schematic structure of T140 derivatives.
To address both issues we have developed several derivatives of T140 that add a chelator to the peptide to make it amenable to labeling with 64Cu in high radiochemical yield. Two of these derivatives (64Cu-DOTA-NFB and 64Cu-NOTA-NFB, Fig. 1) were found to bind specifically to CXCR4, without binding to RBC. 64Cu-DOTA-NFB and 64Cu-NOTA-NFB were then evaluated for their potential for in vivo imaging of CXCR4 in tumor-bearing mice. The results shown here demonstrate the usefulness of PET for evaluating a drug’s binding profile in vivo and in helping to guide the elimination of “off-target” interactions during drug development.
2. Materials and Methods
2.1. General
1,4,7,10-Tetraazacyclododecane-1,4,7,10-tetraacetic acid mono (N-hydroxysuccinimide ester) (DOTA-NHS-ester) and S-2-(4-isothiocyanatobenzyl)-1,4,7-triazacyclononane-1,4,7-triacetic acid (p-SCN-Bn-NOTA) were purchased from Macrocyclics (Dallas, TX). All other solvents and chemicals were purchased from Sigma-Aldrich (St. Louis, MO). 64Cu was produced at the NIH by the irradiation of a thin layer of 64Ni (Isoflex, USA) electroplated on a solid gold internal target plate of the CS-30 cyclotron utilizing the nuclear reaction 64Ni(p,n)64Cu and separated from the target material as 64CuCl2 by anion chromatography as previously described [27].
C18 cartridges (Waters Corporation, Milford, MA) were each activated with both 5 mL of EtOH and 10 mL of water. Radio-TLC was performed on an AR-2000 Bioscan scanner (Washington, DC), using silica gel plates (LK6DF, 60 Å, 200 mm, Whatman) and 1% ethylenediaminetetraacetic acid (EDTA), 5% ammonium-acetate in water : methanol (1:1) as a developing solvent. T140 with free amino terminus (T140-NFB, Fig. 1) and lysine protected with N-[1-(4,4-dimethyl-2,6-dioxocyclohex-1-ylidene)ethyl] (Dde) were purchased from C.S. Bio Co. (Menlo Park, CA). Radioactive samples were measured on a gamma counter (1480 Wizard 3, Perkin–Elmer, Boston, MA).
High-performance liquid chromatography (HPLC) was performed on a system with a variable wavelength detector operating at 280 nm and with a radioactivity detector containing a NaI crystal. Two systems were used:
Reversed-phase (RP) HPLC system using a Higgins preparative C18 column (5 µm, 20 × 250 mm). The flow was set at 12 mL/min using a gradient system, starting from 95% of solvent A (0.1% TFA in water) and 5% of solvent B (0.1% TFA in acetonitrile (ACN)) and changing to 35% solvent A and 65% solvent B at 35 min.
Analytical RP-HPLC using a Vydac C4 (214TP5415, 5 µm, 4.6 × 150 mm) column, and a gradient system starting from 100% of solvent A and 0% of solvent B and changing to 70% solvent A and 30% solvent B at 30 min, with a flow of 1.5 mL/min.
2.2. Synthesis of DOTA-NFB and NOTA-NFB
The conjugation procedure with the two chelators was done similarly to the known procedures [28]. DOTA-NHS-ester with T140-NFB peptide was conducted by dissolving the peptide (2–3 mg, 0.84–1.26 µmol) in 0.2–0.3 mL dimethylformamide (DMF). Then, 1.2 eq of DOTA-NHS ester in 0.1 mL of DMF were added, followed by the addition of 5 eq of diisopropylethylamine. The reaction was mixed at 4 °C overnight. Thereafter, hydrolysis of Dde, was done by adding 2% (V/V) hydrazine. The mixture was then incubated for 10 min at 25 °C. Conjugation of p-SCN-Bn-NOTA with T140-NFB was conducted similarly to the above procedure, except that p-SCN-Bn-NOTA was dissolved in dimethylsulfoxide (DMSO). Purification of the conjugated peptides was conducted on HPLC using system 1. The retention times were as follows: DOTA-NFB, 13.9 min and NOTA-NFB, 15.2 min The desired peptide conjugates were collected and the solvent was removed by lyophilization.
The purity of the desired peptide was analyzed by analytical HPLC using system 2. The retention times of DOTA-NFB and NOTA-NFB were 13.51 and 13.72 min respectively, and the chemical purities were greater than 95%. Mass spectrometry analysis employed a Waters LC-MS system (Waters, Milford, MA). LC–MS confirmed the molecular mass of the conjugated peptides--DOTA-NFB: observed 1212.58 [(M+H+)/2], calculated: 2423.82 (C106H167N37O25S2); NOTA-NFB: observed 1244.59 [(M+H+)/2], calculated: 2487.93 (C110H167N37O24S3).
2.3. 64Cu-radiolabeling
64Cu-chloride was converted to 64Cu-acetate by adding 0.5 mL of 0.4 M ammonium acetate (NH4OAc) solution (pH = 5.5) to 20 µL 64Cu-chloride. 64Cu-acetate solution (0.2 mL; 10–12 mCi, 370–444 MBq) was added into a solution of conjugated peptide (30 µg) in 0.4 M NH4OAc (pH = 5.5). The reaction was stirred for 20 min at 40 °C. Complexation of 64Cu and the conjugated peptide was monitored by radio-TLC (Rf [64Cu-DOTA-NFB, 64Cu-NOTA-NFB] = 0.4, Rf [64Cu-free] = 0.9). For both conjugated peptides, radio-TLC showed incorporation of greater than 97%.
The reaction vial was then diluted with 10 mL of water and loaded onto an activated C18 Sep-Pak cartridge. The cartridge was washed with water (10 mL), and the desired labeled peptide was eluted with 1 mM HCl in ethanol (1 mL) into a glass test tube. The ethanol was evaporated for 5 min under a stream of argon at 40 °C and then reformulated with saline.
The overall radiochemical yield for both peptides was 91 ± 3% (not decay-corrected, n = 5), calculated from the start of synthesis to the reformulation of the labeled peptide with saline. 64Cu-DOTA-NFB and 64Cu-NOTA-NFB were achieved with a total radiosynthesis time of 50–55 min and a specific activity of 0.33 – 0.40 mCi/µg (12.21 – 14.8 MBq/µg).
Quality control (QC) analysis was performed on HPLC using system 2. The retention times were: 64Cu-DOTA-NFB 13.60 min and 64Cu-NOTA-NFB 13.80 min, and the radiochemical purity was greater than 99%.
2.4. Cell culture
Chinese hamster ovarian (CHO) cells and CHO cells that were stably transfected with CXCR4 (CHO-CXCR4, expressing 6.8 × 105 receptors per cell) were a kind gift from Dr. David McDermott (NIAID, NIH, Bethesda, MD) [26]. CHO cells were grown in F-12K medium (ATCC). Jurkat cells (ATCC) were grown in RPMI. Both cell culture media were supplemented with 10% fetal bovine serum, 1 mM sodium pyruvate, 2 mM L-glutamine and non-essential amino acids (GIBCO) at 37 °C under an atmosphere containing 5% CO2.
2.5. Transwell migration assay
Migration medium (600 µL, RPMI supplemented with 1% fetal bovine serum) containing CXCL12 (PeproTech, Rocky Hill, NJ) at a concentration of 100 ng/mL, was placed into the lower chamber of a Costar 24-well Transwell with 5 µm pore size (Corning Corp, Corning, NY). Some wells also contained T140, DOTA-NFB or NOTA-NFB at various concentrations. Jurkat cells (105 in 100 µL migration medium) were placed in the upper chamber. Cells were collected from the lower chamber after 3 h of migration at 37 °C, and counted by flow cytometry (LSR-II, Becton Dickinson) using counting beads (Spherotech). Percent specific migration was calculated as 100 × ((the number of cells migrating with chemokine present) − (the number of cells migrating without chemokine present)) / (the total number of input cells)) [29].
2.6. CXCR4 receptor binding assay
CHO-CXCR4 and CHO cells were trypsinized and resuspended in binding buffer (PBS containing 50 mM HEPES, 1 mM CaCl2, 5 mM MgCl2, 0.5% (w/v) BSA and 0.3 mM NaN3). Incubation was conducted in a 96-well filter plate (Millipore) with a total volume of 200 L containing 105 cells, 0.02 µCi (0.74 kBq) 125I-CXCL12 (Perkin-Elmer) and 0–1000 nM of DOTA-NFB or NOTA-NFB for 1 h on a shaker at room temperature. After incubation, cells were washed four times with binding buffer. Dried filter membranes were punched out, and cell bound radioactivity was measured using a gamma counter. Binding results were calculated using Prism (GraphPad, La Jolla, CA).
2.7. Mouse studies
Athymic nude mice and C57BL/6 mice were purchased from Taconic (Germantown, NY) and housed under pathogen free conditions. All animal studies were conducted in accordance with the principles and procedures outlined in the National Institutes of Health (NIH) Guide for the Care and Use of Animals, and under protocols approved by the NIH Clinical Center Animal Care and Use Committee (CC/ACUC). Tumor xenograft model: Mice were injected with 106 CXCR4-positive or CXCR4-negative CHO tumor cells subcutaneously (s.c.) on the shoulders. The tumors were allowed to develop for 2 weeks prior to imaging or biodistribution studies. Mobilization studies: Mice were injected s.c. with different amounts of CXCR4 antagonist in a volume of 200 µL. Blood was collected into heparin-containing tubes by heart puncture after anesthesia, and stained as described in the flow cytometry section, below.
2.8. Biodistribution
Fifty µCi (1.85 MBq) of 64Cu-DOTA-NFB or 64Cu-NOTA-NFB in a volume of 100 µL saline were injected through the tail veins of tumor-bearing mice. For blocking experiments, 50 µg of unlabeled DOTA-NFB or NOTA-NFB were co-injected with the labeled peptide 64Cu-DOTA-NFB or 64Cu-NOTA-NFB, respectively. At 4 h post injection, blood was drawn from the heart, under anesthesia, and the mice were then sacrificed. Spleen, liver, muscle, kidneys, intestine and tumors were removed and weighed. All organs were assayed for radioactivity using a gamma counter. The results were calculated as percent injected dose per gram tissue (%ID/g). Each group contained 5–6 mice.
2.9. PET studies
Tumor-bearing mice were anesthetized using isoflurane/O2 (1.5–2% v/v) and injected with 100 µCi (3.7 MBq) of 64Cu-DOTA-NFB or 64Cu-NOTA-NFB, in a volume of 150 µL PBS. PET scans were performed using an Inveon DPET scanner (Siemens Medical Solutions) at 1, 2, 4 and 24 h post-injection. For blocking experiments, 100 µCi (3.7 MBq) of 64Cu-DOTA-NFB or 64Cu-NOTA-NFB were co-injected with 50 µg of unlabeled DOTA-NFB or NOTA-NFB, respectively. Each group contained 5–6 mice. The images were reconstructed by a three-dimensional ordered subsets expectation maximization (3D-OSEM) algorithm, with no correction for attenuation or scatter. Image analysis was done using ASI Pro VM™ software. The %ID/g for a tissue was determined by drawing regions of interest (ROIs) surrounding the entire organ on the coronal images. The radioactivity contained in the ROI divided by the dose administered to the animal gave the %ID and the volume of the ROI was converted to mass assuming a density of 1 for all tissues.
2.10. Flow cytometry
Flow data were acquired using an LSR-II cytometer (Becton Dickinson) and analyzed using FlowJo (Tree star). For counting cells, counting beads (Spherotech) were added to each sample.
CXCR4 expression by cells and tumors
Tumors were dissociated by incubating small pieces of the tumor in DMEM (Gibco) containing Liberase TM (Roche) and DNase (Sigma) for 20 min at 37° C and then passed through a 70 µm filter (Falcon). Cells were blocked in HBSS buffer supplemented with 2% rat serum and 2% mouse serum (Jackson ImmunoResearch labs) on ice for 10 min, and thereafter cells were stained with PE-conjugated anti-human CXCR4 (R&D). Cells from tumors were also stained using FITC-labeled anti-mouse MHC-I H-2Dk (Becton Dickinson).
White cell mobilization studies
Blood (100 µL) was treated with RBC lysis buffer (ACK lysing buffer, Quality Biological), washed and blocked in HBSS buffer supplemented with 2% rat serum and 2% mouse serum (Jackson ImmunoResearch labs) on ice for 10 min, and thereafter cells were stained with FITC anti-mouse CD45.2, Alexa700 anti-mouse CD3, APC-eFluor 780 CD45R (B220), PerCP-Cy5.5 anti-mouse CD11b, APC anti-mouse Gr-1, and eFluor 450 anti-mouse F4/80 (eBioscience). Cells were gated for size to exclude dead cells and to select the following phenotypes: white blood cells (WBC) were gated on CD45.2; T cells were gated on CD3+B220−; B cells were gated on B220+CD3−; total macrophages were gated on CD11b+Gr-1−; and mature macrophages were gated on F4/80+ after gating for total macrophages.
2.11. Colony assay
Blood (150 µL) from treated and from control mice was added to separate 3 mL volumes of MethoCult GF (StemCell) according to the manufacture’s instructions. Colonies were counted 14 days after plating using an inverted microscope (Zeiss).
2.12. Statistical analysis
Results were expressed as mean and standard error (SE). Two-tailed unpaired Student’s t tests were used to determine differences between groups. P values <0.05 were considered statistically significant.
3. Results
3.1. Chemistry and radiochemistry
Conjugation of DOTA-NHS ester and p-SCN-Bn-NOTA was done on the amino terminus of T140 peptide. The peptide used for the conjugation did not contain the fluoro-benzoyl group (NFB) at the amino terminus and had N-[1-(4,4-dimethyl-2,6-dioxocyclohex-1-ylidene)ethyl] (Dde) protecting groups on the lysine residues in order to achieve conjugation exclusively on the N-terminus (Fig. 1). The conjugated peptides (DOTA-NFB and NOTA-NFB) were obtained in reasonable chemical yield of 50–62% after HPLC purification, and were analyzed by both analytical HPLC and mass spectroscopy to confirm the purity and identity of the products.
64Cu complexation into the desired labeled peptide was very efficient. Following incubation at 40 °C for 20 min, almost no free 64Cu was detected by radio-TLC and no HPLC purification was required. The overall radiochemical yield was 91 ± 3% (not decay-corrected, n = 5), calculated from the start of synthesis to the reformulation of the labeled peptide with saline. The labeled peptides had a specific activity of 0.33 – 0.40 mCi/µg (12.21 – 14.8 MBq/µg) and a radiochemical purity greater than 99%.
3.2. Competitive binding assay with 125I-CXCL12 radioligand
In order to determine whether the CXCR4 binding characteristics of T140 were affected by modification on the N-terminus, we evaluated the affinities of DOTA-NFB and NOTA-NFB to CHO and CHO-CXCR4 cells in a competitive binding assay using 125I-CXCL12. The IC50 of DOTA-NFB to CXCR4 was two-fold lower (68 nM) than NOTA-NFB (138 nM), (Fig. 2A). Both DOTA-NFB and NOTA-NFB had higher IC50 values than reported for T140 itself (2.5 nM) [26]. These results imply that introduction of DOTA or NOTA molecules at the N-terminus reduced binding affinities to CXCR4, but the peptides retained CXCR4 binding that was reasonable for imaging applications.
Figure 2.
(A) Competitive binding assay of DOTA-NFB and NOTA-NFB with 125I-CXCL12 using CHO and CHO-CXCR4 cell lines. Results were done in triplicate and are from two experiments (B) Inhibition of CXCL12-induced migration of Jurkat cells by T140, DOTA-NFB or NOTA-NFB. Results shown are averages of 3 experiments ± SE. (C) Histograms of CXCR4 expression evaluated by flow cytometry showing an isotype control antibody staining of CHO-CXCR4 (red line), CHO (blue line), CHO-CXCR4 (light green line), cells from excised CHO-CXCR4 (black line) and CHO (turquoise line) tumors. Results shown are representative of two experiments.
3.3. Activities of T140 derivatives as CXCR4 antagonists in vitro
We also compared the relative abilities of DOTA-NFB, NOTA-NFB, and T140 to inhibit cell migration toward CXCL12. In this assay we used Jurkat T-cells that express high levels of CXCR4 [30]. We used CXCL12 in the lower wells at 100 ng/mL, which is the lowest concentration that induces maximal migration [30], with or without various concentrations of DOTA-NFB, NOTA-NFB, and T140. All peptides displayed a dose dependant inhibition of cell migration towards CXCL12. As might be expected from the binding affinities, T140 was a better inhibitor at concentrations of 100 ng/well and 1 µg/well, and DOTA-NFB was a slightly better inhibitor than NOTA-NFB (Fig. 2B). None of the peptides inhibited cell migration at a concentration of 10 ng/well (Fig. 2B).
3.4. MicroPET imaging studies
CHO cells and CHO-CXCR4 cells were evaluated for CXCR4 expression by flow cytometry before being injected into mice, and at the time the tumors were excised. Greater than 98% of the CHO-CXCR4 tumor cells retained CXCR4 expression levels in vivo that were similar to the levels before injection (Fig. 2C).
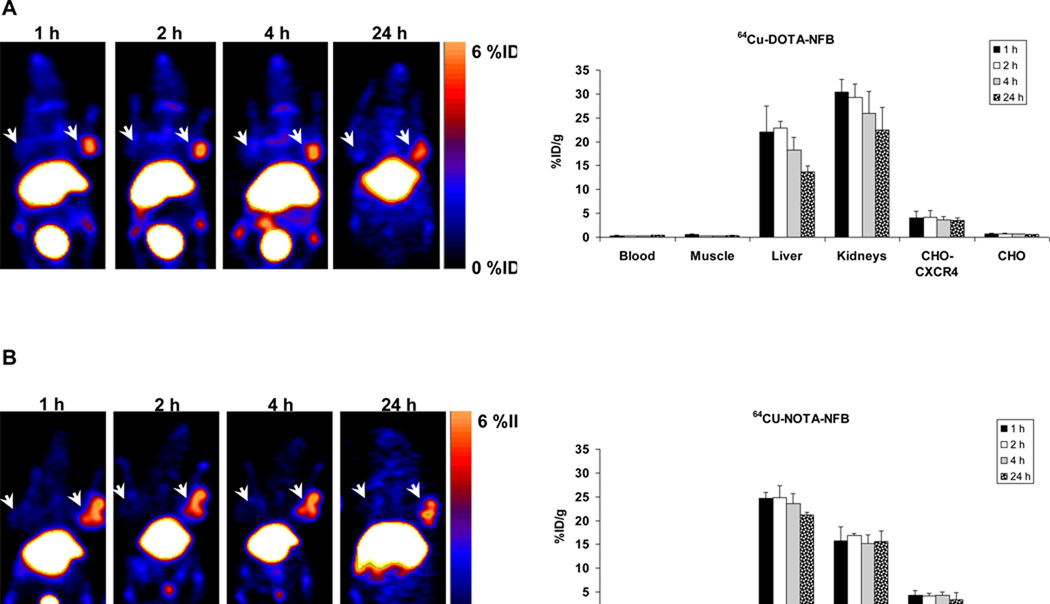
The usage of 64Cu-DOTA-NFB and 64Cu-NOTA-NFB for imaging CXCR4 expression in tumors was evaluated by static microPET scans using mice bearing subcutaneous CHO-CXCR4 and CHO tumors. In the case of both peptide tracers, CXCR4-positive, but not CXCR4-negative, tumors were clearly visualized (Fig. 3). These images showed that, unlike with the parent molecule T140 [26], there was almost no accumulation of the tracer in the blood, and there was low background. Both peptides displayed very low binding to human RBCs both in vitro and in vivo (data not shown). The %ID/g was calculated from PET images for the blood, muscle, liver, kidneys, CHO-CXCR4 tumor and CHO-CXCR4-negative tumor at different time points (Fig. 3). The uptakes in the positive tumors were constant over time with values of 4.09 ± 1.37 %ID/g and 4.34 ± 1.00 %ID/g at 1 h post-injection and 3.58 ± 0.67 %ID/g and 4.38 ± 0.68 %ID/g at 4 h post-injection for 64Cu-DOTA-NFB and 64Cu-NOTA-NFB, respectively. At all time points, accumulation of the peptide tracers was 8–10 times higher in the CXCR4-positive tumors than that in the negative tumors.
Figure 3.
(A) Representative coronal PET images (Left) and uptake calculation (Right) of mice injected with 100 µCi of 64Cu-DOTA-NFB (B) Representative coronal PET images (Left) and uptake calculation (Right) of mice injected with 100 µCi of 64Cu-NOTA-NFB. Arrows indicate CHO-CXCR4 tumor (right shoulder) and CHO tumor (left shoulder). Uptake results are calculated from PET scans and are shown as averages of 5–6 mice ± SE.
With 64Cu-NOTA-NFB the signal-to-background ratio was higher at all time points than with 64Cu-DOTA-NFB (Fig. 3). This was likely because 64Cu-DOTA-NFB had higher uptake in the blood than 64Cu-NOTA-NFB (0.35 ± 0.08 %ID/g for 64Cu-DOTA-NFB and 0.13 ± 0.02 %ID/g for 64Cu-NOTA-NFB at 4 h post-injection).
High uptake of both tracers was evident in the liver and kidneys with little clearance over time (Fig. 3). 64Cu-DOTA-NFB showed 13.63 ± 1.24 %ID/g in the liver and 22.43 ± 4.70 %ID/g in the kidneys at 24 h post-injection (Fig. 3A). 64Cu-NOTA-NFB had higher uptake in the liver (21.17 ± 0.64 %ID/g) and slightly lower accumulation in the kidneys (15.62 ± 2.16 %ID/g) at 24 h post-injection (Fig. 3B).
3.5. Biodistribution
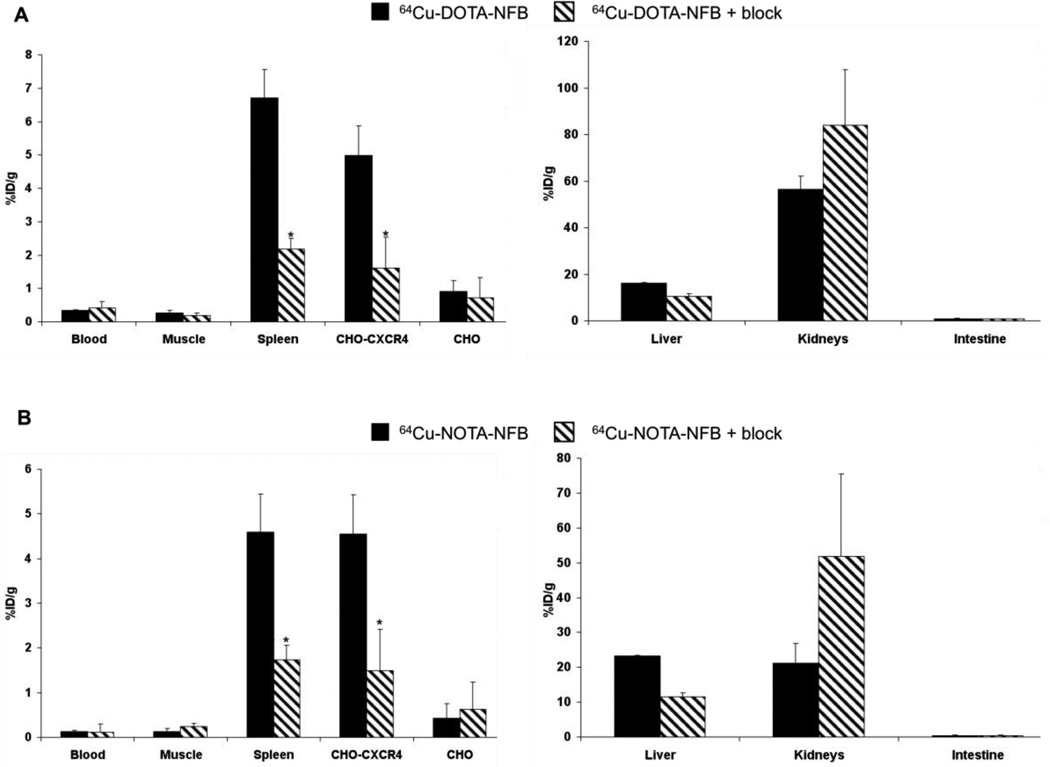
Biodistribution of 64Cu-DOTA-NFB and 64Cu-NOTA-NFB was analyzed by organ dissection with gamma counting in female nude mice that had been inoculated subcutaneously with CHO-CXCR4 and CHO tumors. Data were obtained at 4 h post-injection. Both peptides had uptake in the spleen, which is a CXCR4-expressing organ, (6.70 ± 0.86 %ID/g and 4.59 ± 0.90 %ID/g for 64Cu-DOTA-NFB and 64Cu-NOTA-NFB respectively, Fig. 4) and the CHO-CXCR4 positive tumor (4.98 ± 0.89 %ID/g and 4.55 ± 0.66 %ID/g for 64Cu-DOTA-NFB and 64Cu-NOTA-NFB respectively, Fig. 4). The uptake in these organs was blocked by co-injection of each labeled peptide with 50 µg of unlabeled peptide (Fig. 4). For both peptides, the uptake in the CXCR4-positive tumor was significantly higher than that in the negative tumor (Fig. 4). 64Cu-NOTA-NFB had only one third as much uptake in the blood as compared with 64Cu-DOTA-NFB (0.12 ± 0.01 %ID/g and 0.34 ± 0.02 %ID/g respectively, Fig. 4). 64Cu-DOTA-NFB and 64Cu-NOTA-NFB had high accumulation in metabolic organs, such as liver and kidneys, but none in the intestine (Fig. 4). Surprisingly, co-injection of the labeled peptide with un-labeled peptide resulted in a decrease in the uptake by the liver, although the liver is not known to express high levels of CXCR4 [30]. Blocking led to increases in the kidney, consistent with non-specific accumulation of labeled peptide liberated from specific binding sites in other tissues.
Figure 4.
Biodistribution in female athymic nude mice bearing CHO and CHO-CXCR4 tumors at 4 h post injection of (A) 64Cu-DOTA-NFB (black bars) and co-injection of 64Cu-DOTA-NFB with 50 µg of unlabeled DOTA-NFB for blocking studies (dash bars); and (B) 64Cu-NOTA-NFB (black bars) and co-injection of 64Cu-NOTA-NFB with 50 µg of NOTA-NFB (dash bars). Results shown are averages of 5–6 mice ± SE. *P < 0.01 vs. 64Cu-DOTA-NFB or 64Cu-NOTA-NFB alone.
Since 64Cu-NOTA-NFB had much lower uptake in the blood than 64Cu-DOTA-NFB, it had significantly higher tumor-to-blood (38.88 ± 3.91 vs. 14.50 ± 0.82) and tumor-to-muscle (39.30 ± 2.26 vs. 19.32 ± 2.35) ratios (Table 1). These ratios significantly decreased when the labeled peptide was co-injected with the unlabeled peptide (Table 1), which implies specific binding to CXCR4 in the tumor.
Table 1.
CHO-CXCR4 tumor-to-blood and tumor-to-muscle ratios at 4 h post injection of 64Cu-DOTA-NFB or 64Cu-NOTA-NFB with and without 50 µg of DOTA-NFB or NOTA-NFB, respectively (n = 5 or 6 mice per group).
| Tumor-to- blood |
Tumor-to-blood (block) |
Tumor-to- muscle |
Tumor-to- muscle (block) |
|
|---|---|---|---|---|
| 64Cu-DOTA-NFB | 14.50 ± 0.82 | 3.78 ± 0.22* | 19.32 ± 2.35 | 8.19 ± 2.65* |
| 64Cu-NOTA-NFB | 38.88 ± 3.91 | 12.61 ± 1.90* | 39.30 ± 2.26 | 6.06 ± 1.05* |
P < 0.01 vs. injection of 64Cu-DOTA-NFB and 64Cu-NOTA-NFB alone.
3.6. Mobilization studies
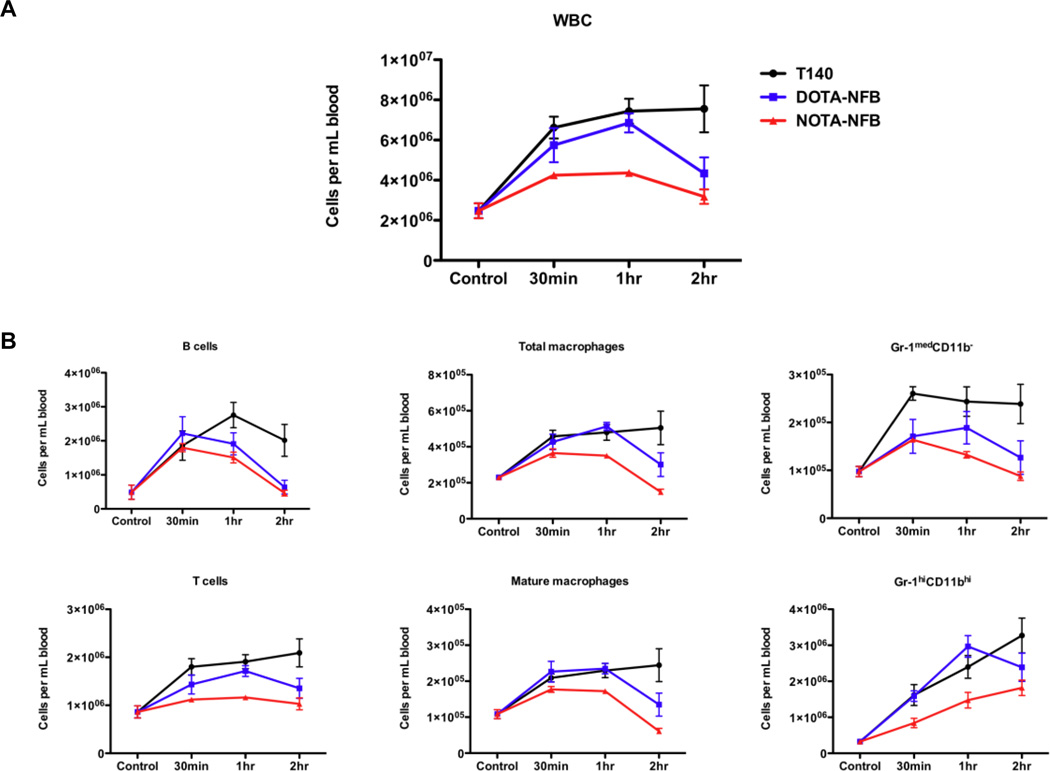
To study the relative abilities of the two new CXCR4 antagonists and T140 to mobilize various types of white blood cells (WBCs) from the bone marrow, T140, DOTA-NFB and NOTA-NFB were injected s.c. in separate, normal mice at a dose of 50 µg per mouse. WBCs were counted in the peripheral blood of these mice after 30 min, 1 and 2 h, and compared to counts in untreated mice.
Both T140 and DOTA-NFB induced a 4-fold increase in WBCs in the blood, while NOTA-NFB had a more subtle effect of about a 2-fold increase (Fig. 5A). When mice were treated with T140 the high WBC numbers in the blood remained relatively stable after 2 h, but for mice injected with DOTA-NFB, the increase in their WBC numbers dropped substantially by 2 h. The profiles of mobilization were generally the same for each type of WBC (with the exception of granulocytes) (Fig. 5B). Overall, NOTA-NFB produced a smaller mobilization effect than the two other peptides for all cell types. DOTA-NFB had a similar profile to T140 up to 1 h post injection, with a decrease of cell numbers at 2 h post injection (Fig. 5B). Mobilization studies done with low amounts of peptides (1 and 5 µg per mouse s.c.) had almost no effect on the number of any type of WBC in the blood (data not shown).
Figure 5.
Mobilization studies in mice. (A) WBCs were counted in the blood of control mice or mice injected with T140, DOTA-NFB or NOTA-NFB by gating on CD45.2 positive cells. (B) Similar patterns of various cell types were observed in the peripheral blood of treated and untreated mice. Gating was done as described in Materials and Methods. Results are representative of two experiments, with at least 3 mice in each group.
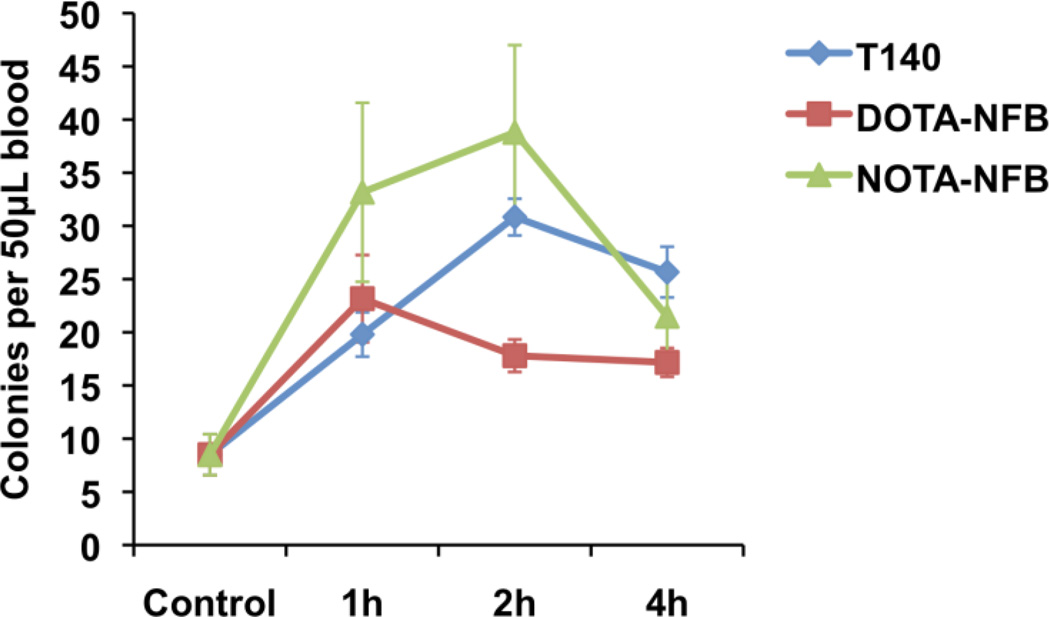
Currently, the only FDA-approved CXCR4 antagonist, Mozobil, is indicated for augmenting stem cell mobilization. Our results with T140 derivatives on WBC mobilization to the peripheral blood suggest that our peptides might also mobilize progenitor cells from the bone marrow to the blood. To test this, we compared T140, DOTA-NFB, and NOTA-NFB using a mouse progenitor colony assay. Injection of 50 µg of each peptide induced mobilization of progenitor cells to the blood (Fig. 6). Interestingly, after T140 injection the number of progenitors in the blood increased steadily and remained substantially elevated between 2 and 4 h post injection, but with NOTA-NFB injection the peak of progenitors cells in the blood was somewhat earlier (between 1 and 2 h postinjection) and decreased sharply between 2 h and 4 h. Both T140 and NOTA-NFB induced mobilization of similar numbers of progenitors to the blood. Unlike the results with mobilization of WBC to the blood, DOTA-NFB displayed lower progenitor cell mobilization than either T140 or NOTA-NFB, showing approximately half the number of progenitor cells mobilized by T140 or NOTA-NFB (Fig. 6).
Figure 6.
Colony assay in mice. Blood from mice treated with T140 derivatives or untreated (control) was collected and incubated for 14 days as described in Materials and Methods. Each group contained at least 3 mice, and the assay was done in duplicate for each mouse.
3. Discussion
Expression of chemokine receptor CXCR4 has been shown by others to occur in a wide range of cancers, and to correlate with aggressive biological behaviors--including increased cell proliferation and metastasis--and to correlate with decreased patient survival [14]. Although CXCR4 might be a useful indicator of prognosis and tumor behavior and a potential target for therapy, the ability to analyze CXCR4 expression by tumors is currently limited to ex vivo evaluation of biopsy samples. The use of CXCR4-specific PET tracers would allow for detection of CXCR4 non-invasively, offering the promise of better informed and customized therapeutic decisions. Sequential PET assessments during the course of illness will be especially valuable in assessing experimental therapies targeting CXCR4.
We and others have previously developed CXCR4-specific PET tracers, but imaging results in animal models were suboptimal due to off-target accumulation or poor pharmacokinetics of the labeled compounds and their metabolites [30–34]. Interestingly, when we radiolabeled CXCR4 antagonists AMD3100 and 4F-T140 and evaluated them using PET imaging, we found that both molecules had additional binding sites in vivo, and that they were not as specific as had been reported [26, 30]. 64Cu-AMD3100 had extremely high accumulation in the liver, which express very low levels of CXCR4. Nevertheless, 64Cu-AMD3100 could be competed by unlabeled drug, suggesting specific binding to another target in that organ [30]. 18F-T140 had specific binding to RBCs, which do not express CXCR4, and this binding could be overcome by co-injection of a small amount of unlabeled peptide [26]. Another less favorable aspect of labeling 18F-T140 was that the radiosynthesis route was laborious and had low yield.
Our next step was to try and develop new peptides based on T140 that would retain T140’s high affinity and biological properties, while improving radiolabeling efficiency, and improving the selectivity and specificity for binding to CXCR4 in vivo. We developed two new derivatives of T140, DOTA-NFB and NOTA-NFB, which bind to CXCR4, but not to RBCs, and therefore can be injected in lower mass. The maximum amount of tracer injected in this study was 15 µg/kg. Therapy with T140 as a stem cell mobilization agent in combination with G-CSF is given at a dose of 5 mg/kg, and at even higher doses when administered by itself. Hence, the amount of tracer being injected is much lower than the treatment dose and may not induce pharmacological effect.
The two derivatives were designed to have a chelator rather than a 4-fluoro-benzoyl group (NFB) attached to the N-terminus, so they could be labeled readily with copper-64. Both peptides maintain the inhibitory effect on migration of Jurkat T cells, have reasonable binding affinities to CXCR4, display highly specific accumulation in CXCR4-positive, but not CXCR4-negative, tumors and produce high tumor-to-background ratios. Overall our results suggest that the N-terminus of T140 is required for binding to RBC, but that it also affects to some extent the binding to CXCR4. It is possible that these molecules cause some steric interference with the binding to CXCR4 and that the size of the chelator matters. This issue is important for further improvement of T140 both as a drug and as a tracer, and merits further investigation. It is also of note that, although NOTA-NFB and DOTA-NFB had lower affinity for CXCR4 than T140, all three peptides had similar abilities to mobilize WBCs and progenitor cells.
The downside of copper-64 labeled T140 derivatives, when compared to fluorine-18 labeled T140, is high, long-lasting uptake in the liver and kidneys. A similar phenomenon has also observed when the chelator was coupled to one of the two lysine groups of the peptide [35]. A probable explanation is that metabolites of the labeled peptides, possibly after peptide internalization, accumulate in the liver and kidneys.
NOTA-NFB and DOTA-NFB derivatives of T140 represent a step closer toward the development of an optimal CXCR4 tracer for PET imaging. Both are readily labeled with copper-64, do not show undesired binding to unrelated targets in vivo, and allow visualization of CXCR4 expression in cancers. It is our hope that the use of these tracers in the diagnosis and management of patients with cancer may lead to more effective interventions tailored to an individual’s needs. In addition, we believe that our work suggests that PET and other imaging modalities such as single photon emission computed tomography (SPECT) can be used to advantage during drug development. Firstly, for drugs such as T140, which can be labeled for imaging without altering their structure, PET/SPECT can be used to evaluate drug binding/distribution in the whole organism, and secondly, PET/SPECT can be used in selected cases to test the effects of chemical modifications on optimizing on-target, and minimizing off-target activities.
Acknowledgments
This research was supported in part by the Intramural Research Programs (IRPs) of the National Institute of Biomedical Imaging and Bioengineering (NIBIB) and the National Institute of Allergy and Infectious Diseases (NIAID), National Institutes of Health (NIH), and by the International Cooperative Program of the National Science Foundation of China (NSFC) (81028009).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Burger JA, Peled A. CXCR4 antagonists: targeting the microenvironment in leukemia and other cancers. Leukemia. 2009;23:43–52. doi: 10.1038/leu.2008.299. [DOI] [PubMed] [Google Scholar]
- 2.Scotton CJ, Wilson JL, Scott K, Stamp G, Wilbanks GD, Fricker S, Bridger G, Balkwill FR. Multiple actions of the chemokine CXCL12 on epithelial tumor cells in human ovarian cancer. Cancer Res. 2002;62:5930–5938. [PubMed] [Google Scholar]
- 3.Zimmerman NP, Vongsa RA, Wendt MK, Dwinell MB. Chemokines and chemokine receptors in mucosal homeostasis at the intestinal epithelial barrier in inflammatory bowel disease. Inflamm Bowel Dis. 2008;14:1000–1011. doi: 10.1002/ibd.20480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rollins BJ. Chemokines. Blood. 1997;90:909–928. [PubMed] [Google Scholar]
- 5.Rossi D, Zlotnik A. The biology of chemokines and their receptors. Annu Rev Immunol. 2000;18:217–242. doi: 10.1146/annurev.immunol.18.1.217. [DOI] [PubMed] [Google Scholar]
- 6.Klein RS, Rubin JB. Immune and nervous system CXCL12 and CXCR4: parallel roles in patterning and plasticity. Trends Immunol. 2004;25:306–314. doi: 10.1016/j.it.2004.04.002. [DOI] [PubMed] [Google Scholar]
- 7.Horuk R. Chemokine receptors. Cytokine Growth Factor Rev. 2001;12:313–335. doi: 10.1016/s1359-6101(01)00014-4. [DOI] [PubMed] [Google Scholar]
- 8.Gupta SK, Lysko PG, Pillarisetti K, Ohlstein E, Stadel JM. Chemokine receptors in human endothelial cells. Functional expression of CXCR4 and its transcriptional regulation by inflammatory cytokines. J Biol Chem. 1998;273:4282–4287. doi: 10.1074/jbc.273.7.4282. [DOI] [PubMed] [Google Scholar]
- 9.Lapidot T, Dar A, Kollet O. How do stem cells find their way home? Blood. 2005;106:1901–1910. doi: 10.1182/blood-2005-04-1417. [DOI] [PubMed] [Google Scholar]
- 10.Petit I, Szyper-Kravitz M, Nagler A, Lahav M, Peled A, Habler L, Ponomaryov T, Taichman RS, Arenzana-Seisdedos F, Fujii N, Sandbank J, Zipori D, Lapidot T. G-CSF induces stem cell mobilization by decreasing bone marrow SDF-1 and up-regulating CXCR4. Nat Immunol. 2002;3:687–694. doi: 10.1038/ni813. [DOI] [PubMed] [Google Scholar]
- 11.Flomenberg N, Devine SM, Dipersio JF, Liesveld JL, McCarty JM, Rowley SD, Vesole DH, Badel K, Calandra G. The use of AMD3100 plus G-CSF for autologous hematopoietic progenitor cell mobilization is superior to G-CSF alone. Blood. 2005;106:1867–1874. doi: 10.1182/blood-2005-02-0468. [DOI] [PubMed] [Google Scholar]
- 12.Abraham M, Beider K, Wald H, Weiss ID, Zipori D, Galun E, Nagler A, Eizenberg O, Peled A. The CXCR4 antagonist 4F-benzoyl-TN14003 stimulates the recovery of the bone marrow after transplantation. Leukemia. 2009;23:1378–1388. doi: 10.1038/leu.2009.56. [DOI] [PubMed] [Google Scholar]
- 13.Rettig MP, Ansstas G, Dipersio JF. Mobilization of hematopoietic stem and progenitor cells using inhibitors of CXCR4 and VLA-4. Leukemia. 2011 doi: 10.1038/leu.2011.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Furusato B, Mohamed A, Uhlen M, Rhim JS. CXCR4 and cancer. Pathol Int. 60:497–505. doi: 10.1111/j.1440-1827.2010.02548.x. [DOI] [PubMed] [Google Scholar]
- 15.Muller A, Homey B, Soto H, Ge N, Catron D, Buchanan ME, McClanahan T, Murphy E, Yuan W, Wagner SN, Barrera JL, Mohar A, Verastegui E, Zlotnik A. Involvement of chemokine receptors in breast cancer metastasis. Nature. 2001;410:50–56. doi: 10.1038/35065016. [DOI] [PubMed] [Google Scholar]
- 16.Tanaka T, Bai Z, Srinoulprasert Y, Yang BG, Hayasaka H, Miyasaka M. Chemokines in tumor progression and metastasis. Cancer Sci. 2005;96:317–322. doi: 10.1111/j.1349-7006.2005.00059.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Redjal N, Chan JA, Segal RA, Kung AL. CXCR4 inhibition synergizes with cytotoxic chemotherapy in gliomas. Clin Cancer Res. 2006;12:6765–6771. doi: 10.1158/1078-0432.CCR-06-1372. [DOI] [PubMed] [Google Scholar]
- 18.Taichman RS, Cooper C, Keller ET, Pienta KJ, Taichman NS, McCauley LK. Use of the stromal cell-derived factor-1/CXCR4 pathway in prostate cancer metastasis to bone. Cancer Res. 2002;62:1832–1837. [PubMed] [Google Scholar]
- 19.Vicari AP, Caux C. Chemokines in cancer. Cytokine Growth Factor Rev. 2002;13:143–154. doi: 10.1016/s1359-6101(01)00033-8. [DOI] [PubMed] [Google Scholar]
- 20.Azab AK, Runnels JM, Pitsillides C, Moreau AS, Azab F, Leleu X, Jia X, Wright R, Ospina B, Carlson AL, Alt C, Burwick N, Roccaro AM, Ngo HT, Farag M, Melhem MR, Sacco A, Munshi NC, Hideshima T, Rollins BJ, Anderson KC, Kung AL, Lin CP, Ghobrial IM. CXCR4 inhibitor AMD3100 disrupts the interaction of multiple myeloma cells with the bone marrow microenvironment and enhances their sensitivity to therapy. Blood. 2009;113:4341–4351. doi: 10.1182/blood-2008-10-186668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kurtova AV, Tamayo AT, Ford RJ, Burger JA. Mantle cell lymphoma cells express high levels of CXCR4, CXCR5, and VLA-4 (CD49d): importance for interactions with the stromal microenvironment and specific targeting. Blood. 2009;113:4604–4613. doi: 10.1182/blood-2008-10-185827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yoon Y, Liang Z, Zhang X, Choe M, Zhu A, Cho HT, Shin DM, Goodman MM, Chen ZG, Shim H. CXC chemokine receptor-4 antagonist blocks both growth of primary tumor and metastasis of head and neck cancer in xenograft mouse models. Cancer Res. 2007;67:7518–7524. doi: 10.1158/0008-5472.CAN-06-2263. [DOI] [PubMed] [Google Scholar]
- 23.Li JK, Yu L, Shen Y, Zhou LS, Wang YC, Zhang JH. Inhibition of CXCR4 activity with AMD3100 decreases invasion of human colorectal cancer cells in vitro. World J Gastroenterol. 2008;14:2308–2313. doi: 10.3748/wjg.14.2308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Teicher BA, Fricker SP. CXCL12 (SDF-1)/CXCR4 pathway in cancer. Clin Cancer Res. 16:2927–2931. doi: 10.1158/1078-0432.CCR-09-2329. [DOI] [PubMed] [Google Scholar]
- 25.Burger JA, Stewart DJ, Wald O, Peled A. Potential of CXCR4 antagonists for the treatment of metastatic lung cancer. Expert Rev Anticancer Ther. 2011;11:621–630. doi: 10.1586/era.11.11. [DOI] [PubMed] [Google Scholar]
- 26.Jacobson O, Weiss ID, Kiesewetter DO, Farber JM, Chen X. PET of tumor CXCR4 expression with 4-18F-T140. J Nucl Med. 51:1796–1804. doi: 10.2967/jnumed.110.079418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Szajek PP, LP MW, Eckelman WC. Semi-remote production of [64Cu]CuCl2 and preparation of high specific activity [64Cu]Cu-ATSM for PET studies. Radiochim Acta. 2005;93:239. [Google Scholar]
- 28.Liu Z, Yan Y, Chin FT, Wang F, Chen X. Dual integrin and gastrin-releasing peptide receptor targeted tumor imaging using 18F-labeled PEGylated RGD-bombesin heterodimer 18F-FB-PEG3-Glu-RGD-BBN. J Med Chem. 2009;52:425–432. doi: 10.1021/jm801285t. [DOI] [PubMed] [Google Scholar]
- 29.Estes JD, Thacker TC, Hampton DL, Kell SA, Keele BF, Palenske EA, Druey KM, Burton GF. Follicular dendritic cell regulation of CXCR4-mediated germinal center CD4 T cell migration. J Immunol. 2004;173:6169–6178. doi: 10.4049/jimmunol.173.10.6169. [DOI] [PubMed] [Google Scholar]
- 30.Jacobson O, Weiss ID, Szajek L, Farber JM, Kiesewetter DO. 64Cu-AMD3100--a novel imaging agent for targeting chemokine receptor CXCR4. Bioorganic & medicinal chemistry. 2009;17:1486–1493. doi: 10.1016/j.bmc.2009.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Weiss ID, Jacobson O, Kiesewetter DO, Jacobus JP, Szajek LP, Chen X, Farber JM. Positron emission tomography imaging of tumors expressing the human chemokine receptor CXCR4 in mice with the use of 64Cu-AMD3100. Mol Imaging Biol. 2011 Feb 23; doi: 10.1007/s11307-010-0466-y. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nimmagadda S, Pullambhatla M, Pomper MG. Immunoimaging of CXCR4 expression in brain tumor xenografts using SPECT/CT. J Nucl Med. 2009;50:1124–1130. doi: 10.2967/jnumed.108.061325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nimmagadda S, Pullambhatla M, Stone K, Green G, Bhujwalla ZM, Pomper MG. Molecular imaging of CXCR4 receptor expression in human cancer xenografts with [64Cu]AMD3100 positron emission tomography. Cancer Res. 2010;70:3935–3944. doi: 10.1158/0008-5472.CAN-09-4396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Masuda R, Oishi S, Ohno H, Kimura H, Saji H, Fujii N. Concise site-specific synthesis of DTPA-peptide conjugates: Application to imaging probes for the chemokine receptor CXCR4. Bioorg Med Chem. 2011;19:3216–3220. doi: 10.1016/j.bmc.2011.03.059. [DOI] [PubMed] [Google Scholar]
- 35.Jacobson O, Weiss ID, Szajek LP, Niu G, Ma Y, Kiesewetter DO, Farber JM, Chen X. PET imaging of CXCR4 using copper-64 labeled peptide antagonist. Theranostics. 2011;1:251–262. doi: 10.7150/thno/v01p0251. [DOI] [PMC free article] [PubMed] [Google Scholar]