Abstract
N-Acyl-l-homoserine lactones (AHLs) are a major class of quorum sensing signals used by Gram-negative bacteria to regulate gene expression in a population-dependent manner, thereby enabling group behavior. Enzymes capable of generating and catabolizing AHL signals are of significant interest for the study of microbial ecology and quorum-sensing pathways, for understanding the systems that bacteria have evolved to interact with small molecule signals, and for their possible use in therapeutic and industrial applications. The recent structural and functional studies reviewed here provide detailed insight into the chemistry and enzymology of bacterial communication.
The social lives of bacteria
Humans are certainly not the first to use information warfare. Nature is rife with examples of communication for cooperation and for subterfuge, with the growing field of sociomicrobiology (see Glossary) representing possibly the most reductionistic extreme [1]. In one example, various Gram-negative bacteria produce cell permeable N-acylated-l-homoserine lactones (AHLs) at a low basal rate. If these signals are allowed to accumulate, they bind cognate transcriptional regulators and act as autoinducing signals. Because the concentration of autoinducers often mirrors the local population density, they act as a sort of census to regulate gene expression in a population-dependent manner. A number of strategies to interfere with these signaling pathways have been discovered, including AHL-degrading enzymes with activities that work as a type of censorship to block interbacterial communication. The study of these processes enhances our understanding of microbial ecology and suggests novel therapeutic strategies. Towards these ends, recent advances in the study of AHL generation and decay have enabled a detailed understanding of the enzymology that underlies the social lives of bacteria.
Bacterial census: AHL synthesis
Although bacteria have evolved many chemically diverse communication systems (Box 1), in the present review we consider only AHLs, which are produced by Proteobacteria. The two protein components of the AHL signaling system within the producing organism are the inducer protein (I) and the receptor protein (R). Inducer proteins are the synthases responsible for the formation of AHLs; they are designated LuxI-type AHL synthases, after the LuxI protein from Vibrio fischeri. The number of AHLs that have been characterized as quorum-sensing molecules in bacteria greatly exceeds the number of the corresponding synthases that have been studied in detail. Nevertheless, sequence homology among AHL synthases suggests that they are structurally similar and probably follow similar chemical mechanisms. Early studies of the archetypal AHL synthase LuxI from V. fischeri established that the enzyme's substrates are S-adenosylmethionine (SAM) and an acylated acyl carrier protein (ACP) [2]. Two distinct chemical reactions are required to form the AHL: acyl transfer from the ACP to the amino group of SAM, and lactonization of SAM with concomitant expulsion of S-methylthioadenosine (MTA) (Figure 1). The usual metabolic function of SAM is as a methyl group donor, so its role as the source of the amino acid in AHL synthesis is unusual.
Figure 1.
Chemical reaction catalyzed by LuxI. All known LuxI-type AHL synthases utilize SAM as a substrate; product diversity comes from the different acyl-ACPs that are used as the second substrate. Abbreviations: AHL, acyl homoserine lactone; SAM, S-adenosyl methionine; ACP, acyl carrier protein; MTA, methylthioadenosine.
The structures of three AHL synthases have been determined by X-ray crystallography: EsaI from Pantoea stewartii, which catalyzes the formation of 3-oxohexanoyl homoserine lactone [3]; LasI from Pseudomonas aeruginosa, whose product is 3-oxo-dodecanoyl homoserine lactone [4]; and TofI from Burkholderia glumae, which catalyzes formation of octanoyl homoserine lactone (C8-HSL) [5]. The synthases exhibit an α-β-α fold that is similar to that observed in GCN5-related histone acetyltransferases (GNATs), although no sequence similarity between AHL synthases and GNATs is evident. The recently determined structure of TofI is a major advance, because it provides the first view of ligands bound to an AHL synthase. Previous predictions about substrate binding modes, which were based on the structural similarity between AHL synthases and GNATs (reviewed in [5]), are largely borne out by the TofI structure in which the product MTA and the inhibitor J8-C8 (which is a structural analog of the product C8-HSL) are bound (Figure 2). The binding pocket for the acyl chain of substrate and product forms a deep cavity such that the hydrophobic acyl chain is completely removed from the aqueous solvent. MTA is bound in a cleft, but remains partially exposed to the solvent. There is a stacking interaction between the ribose ring of MTA and Trp33, a residue that is conserved in AHL synthases; functional analysis reveals that substitution of Trp33 with non-aromatic residues abrogates TofI activity [5]. In the absence of ligands, residues 32–40 are disordered, but they form part of an ordered loop when Trp33 interacts with MTA (and presumably SAM).
Figure 2.
Structure of AHL synthase TofI from Burkholderia glumae (Protein Data Bank code: 3p2h). The inhibitor J8-C8, whose structure is given in the inset, is bound in the center of the protein with the C8 acyl chain extending into a hydrophobic pocket. Methylthioadenosine (MTA) is bound in the pocket at the top of the protein. Residues 32–40 are shown in red; this portion of the protein is disordered in the absence of ligands, but becomes stabilized through interactions with MTA.
A wide variety of AHLs with different acyl chains are found in nature. Chain lengths from C4 to C18 have been observed; a ketone is frequently found at C3, which is sometimes reduced to a hydroxyl group; and in some cases the acyl chain is branched or unsaturated [6]. AHL synthases typically exhibit strict, but not absolute, substrate specificity. For example, the best substrate for RhlI, an AHL synthase from P. aeruginosa, is butyryl-ACP, but it can catalyze the slow formation of N-hexanoylhomoserine lactone [7]. The structures of EsaI and TofI suggest that substrate specificity is determined by the size of the tunnel in which the acyl chain of acyl-ACP binds. Substitution of two residues of an AHL synthase from Erwinia carotovora to increase the volume of the acyl chain tunnel altered the specificity so that it produced N-(3-oxooctanoyl)-l-homoserine lactone rather than its normal product N-(3-oxohexanoyl)-l-homoserine lactone [8]. However, the binding pocket for acyl-ACP in LasI is open-ended, so no such clear structural basis for the specificity of LasI has been observed. Another potential source of specificity for the reaction in vivo that has not been explored carefully is the ACP itself. The P. aeruginosa genome encodes three ACPs, and in vitro kinetic studies indicate that only two are good substrates for RhlI [9]. Given the myriad proteins with which ACPs interact [10], it could be that those organisms that express more than one ACP distribute the chore of acylation specificity determination between holo-ACP synthases and the AHL synthases for which the acyl-ACPs are substrates.
One of the most surprising findings in recent work was the discovery that some bacteria produce p-coumaroyl-homoserine lactone when they are grown in the presence of p-coumarate [11]. Because p-coumarate is not a bacterial metabolite, but is a component of lignin in plants, the implication of this finding is that the bacteria rely on a plant host to supply the sidechain needed to form the signaling molecule. This observation extends the interspecies cross-talk that AHLs can mediate to the synthesis of the AHLs themselves. Although not yet demonstrated, bacteria presumably express the enzymes necessary to convert p-coumarate to p-coumaroyl-ACP. A strain of Bradyrhizobium sp. has been shown to produce cinnamoyl-homoserine lactone [12], so N-aroyl-homoserine lactones might be more common than has been recognized to date.
An early question about the mechanism of AHL synthases was whether the acyl group was transferred from acyl-ACP to the enzyme to form a covalent intermediate in the course of the reaction, following a ping-pong mechanism. This hypothesis was initially supported by observations of the RhlI reaction when the concentrations of butyryl-ACP and SAM were varied [7]. However, product inhibition studies ruled out a ping-pong mechanism and established that SAM was the first substrate to bind to RhlI, and MTA was the last product to dissociate. In contrast, studies of TofI established that the inhibitor J8-C8, which binds to the site that the acyl group of acyl-ACP must occupy, can bind to the enzyme in the absence of SAM, so some uncertainty remains about the order of substrate binding in AHL synthases. Consideration of the relative locations of the ligand binding sites revealed in the TofI structure suggests that acyl-ACP and SAM must bind from opposite faces of the enzyme in order to be consistent with the order of substrate binding observed in the RhlI reaction; otherwise, SAM binding would block acyl-ACP from reaching its binding site.
Interest in developing compounds that disrupt quorum-sensing systems into pharmacological agents provides urgency to the task of defining the chemical mechanism of AHL synthases (Box 2). Structural characterization of the proteins provides one route to designing inhibitors, but characterization of the chemical mechanism can provide insights into the nature of the intermediates that form during the catalytic cycle and the transition states for the chemical transformations. This important information can be leveraged to design potent, specific inhibitors. Currently, the general features of the chemical transformations that occur in the AHL synthase reaction are clear, but many details remain to be defined.
One of the interesting features of the AHL synthase reaction is that two distinct chemical reactions are catalyzed, acylation and lactonization. A priori, neither reaction would seem to be a chemical prerequisite for the other, so either reaction could occur first. However, N-butyryl-SAM was detected as an intermediate in rapid-mixing chemical quench experiments, demonstrating that acylation precedes lactonization [13].
Lactonization occurs via nucleophilic attack of the carboxylate oxygen on the methylene carbon adjacent to the positively-charged sulfur of SAM. Alternative mechanisms in which S-methylthioadenosine is eliminated prior to lactone formation in the RhlI reaction can be discounted based on the fact that no solvent protons are incorporated into the product, and the predicted intermediate, N-butyrylvinylglycine, is not turned over by the enzyme [9]. The mechanistic possibilities for lactonization span a continuum from a purely SN2 mechanism, in which the carboxylate oxygen adds to carbon concomitant with expulsion of MTA, to an SN1 mechanism, in which MTA departs first, leaving an electropositive carbon to which the carboxylate oxygen adds. Computational studies suggest that the latter is a better description of the reaction, which proceeds with considerable electropositive character at the methylene carbon at the transition state [9].
The roles played by individual amino acid residues in the AHL synthase reaction are unclear, although mutagenesis studies of conserved and random residues have identified critical residues in RhlI [14, 15]. The pH-dependence of kinetic parameters in the RhlI reaction show that the ionization states of two residues are important for catalysis and binding [9]. The acylation reaction is expected to require catalysis by acidic and basic enzyme sidechains, but structural studies of GNAT superfamily acetyltransferases have demonstrated that this can be accomplished in different ways. More detailed interpretation of the pH kinetics would be greatly aided by structures of AHL synthases with bound substrates or products.
Bacterial censorship: AHL degradation
Whenever an organism evolves a competitive advantage, it is almost an inevitable corollary that competing organisms will develop interfering strategies. Such appears to be the case with quorum sensing, as several different enzymes capable of disrupting AHL-based quorum sensing have been discovered. The majority of these “quorum-quenching” enzymes can be categorized into two distinct groups: AHL lactonases, which catalyze hydrolytic ring opening of the lactone to form an N-acyl-homoserine product, and AHL acylases, which catalyze hydrolytic cleavage of the amide bond to form homoserine lactone and free fatty acid. Although the role of these enzymes in their native environments is not always clear [16], their quorum quenching abilities and their utility as biochemical tools, as well as in potential industrial and therapeutic applications, are quite promising.
AHL lactonases
AHL lactonases can be further divided into three categories that show homology to different protein superfamilies: amidohydrolases, paraoxonases and metallo-β-lactamases [17–19]. We will focus on examples of AHL lactonases in the metallo-β-lactamase superfamily, which are arguably the most active based on available kcat/KM values, and the best defined in terms of catalytic mechanism.
The first quorum-quenching enzyme discovered was the autoinducer inactivator A (AiiA) from the Gram-positive bacterium Bacillus thruingiensis, later found to be widespread among Bacillus isolates [19, 20]. AiiA was originally identified as member of the metallo-β-lactamase superfamily through the recognition of the conserved metal-binding motif HXHXDH. Although a few initial reports questioned the necessity of metal ions for catalysis, AiiA contains a binuclear zinc ion cluster at its active site that is essential for both proper folding and catalytic activity, as shown by metal analysis, extended X-ray absorption fine structure (EXAFS), NMR and a series of X-ray crystal structures [21, 22]. The direct participation of the metal ions in catalysis was first demonstrated by comparison of hydrolysis kinetics for AHLs and sulfur-substituted analogs by a series of different AiiA metalloforms [23]. These measurements revealed a substantial effect on the relative kcat values with respect to the identity of the metal ion substitutions, reflecting a kinetically significant interaction that occurs between the ring's leaving group heteroatom and the metal ion center during substrate turnover.
The determination of product-bound structures shed light on the position of the substrate at the active site and its interactions with the metal center and other nearby residues, leading to the proposal of the following mechanism (Figure 3) [24, 25]: the AHL ring would bind at the active site, placing its less hindered face toward the metal center, with its carbonyl carbon interacting with one of the zinc atoms (zinc-1, which is ligated by three histidines) and the leaving group oxygen placed over the other zinc atom (zinc-2, which is ligated by a histidine and two aspartates). The hydroxide ion that bridges both metal ions attacks the lactone's carbonyl carbon, leading to formation of a tetrahedral adduct that is stabilized by zinc-1 and the phenol side chain of the neighboring Tyr194. This tyrosine is not conserved in the metallo-β-lactamase superfamily, but is conserved in all of the AHL lactonases identified to date. The expected structure of the tetrahedral adduct parallels that of an observed phosphate ligand found at the active site of the related lactonase AiiB [26]. The collapse of the tetrahedral adduct leads to expulsion of the oxygen leaving-group, which is stabilized, possibly as the anion, by zinc-2. The Asp108 residue, originally ligated to zinc-2 and the bridging hydroxide in the resting enzyme, takes an alternative conformation in which zinc-2 is released and the side chain is repositioned to shuttle a proton from the newly formed carboxyl group to the leaving group. This proton transfer results in formation of the ring-opened N-acylhomoserine product, which can now form a bidentate bridge between the metal ions through its carboxylate, resulting in the structure observed by X-ray crystallography (Figure 4). Product release, followed by regeneration of the starting enzyme, completes the catalytic cycle. Stable isotope incorporation studies support this addition-elimination mechanism [21]. One early mechanistic proposal placed the lactone ring in the opposite orientation, with the carbonyl ligating instead to zinc-2 [27]; however, this proposal is inconsistent with reaction modeling and the product-bound structures, and probably reflects the non-productive binding mode of the inhibitor complex from which the proposal was derived. The AHL lactonase mechanism shares many similarities with that of the more distantly related metallo-β-lactamase enzymes, notably in the use of zinc-2 to stabilize the leaving group [28]. These mechanistic similarities further support its inclusion in this diverse enzyme superfamily.
Figure 3.
Chemical mechanism of AHL lactonase. A dinuclear zinc ion cluster at the active site uses both zinc ions (Zn1 and Zn2) to catalyze the ring-opening hydrolysis of AHL substrates with diverse N-acyl substitutions.
Figure 4.
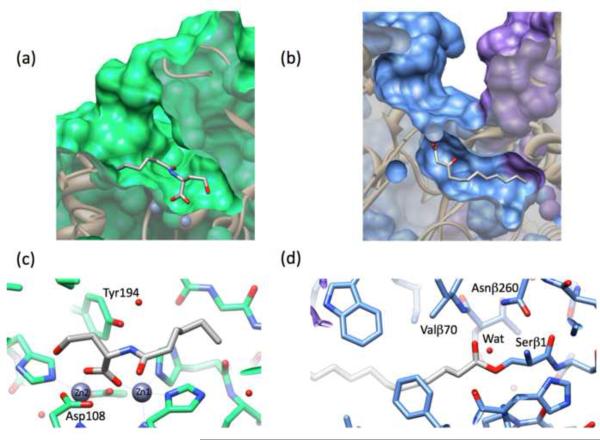
Structures of AHL degrading enzymes. Substrate binding and hydrolysis occurs by very different mechanisms in AHL lactonase as compared to AHL acylase. (a) A cut-away view of the AHL lactonase AiiA from Bacillus thuriengiensis in complex with the bound product N-hexanoyl-l-homoserine (Protein Data Bank code: 3DHB) shows that the lactone ring, now opened, binds deep within the protein, with the N-acyl chain making weaker non-selective interactions along the surface of a wide cavity. (b) Conversely, a cut-away view of the AHL acylase PvdQ from Pseudomonas aeruginosa in complex with the bound product 3-oxo-dodecanoate shows that the N-acyl chain binds deep within a hydrophobic cavity, leaving the lactone ring, not seen here in the product complex, to make less selective interactions with the surface of a wide cavity. The β-chain is shown in blue and the α-chain in purple (Protein Data Bank code: 2wyc). To illustrate mechanistic differences, close-up views of reaction intermediates are shown in panels (c,d). (c) The active site of AiiA (in green) makes specific interactions between each zinc ion and the product's carboxylate (in grey) (Protein Data Bank code: 3DHB). (d) The active-site serine β1 of PvdQ (in blue) makes a covalent ester with the AHL substrate (in grey), allowing visualization of a trapped reaction intermediate (Protein Data Bank code: 2wyb). Abbreviation: Wat, a water molecule.
The substrate preference of AiiA is primarily determined by selective recognition of the chiral (S)-homoserine lactone moiety, around which the enzyme is observed to clamp down upon ligand binding [24]. The N-acyl chain of the substrate does not seem to bind tightly, but rather binds in a relatively unconstrained manner by making multivariate interactions with a wide hydrophobic groove along the surface of the protein (Figure 4). These interactions are reflected in the strict selectivity of AiiA for the chiral lactone moiety and its broad substrate tolerance for the acyl chain, which allows facile hydrolysis of acyl chains that are 4 – 12 carbons long (or possibly longer) and tolerance for 3-oxo substituents [21, 29]. In fact, the recently identified N-aroyl-HSL quorum sensing signals are two of the best substrates for AiiA, reflecting the diversity of N-substitutions that are effectively processed [30].
AHL acylases
The second category of quorum-quenching enzymes, AHL acylases, are members of the N-terminal nucleophile (NTN) hydrolase superfamily. Other family members include penicillin G acylase, penicillin V acylase, glutaryl-7-aminocephalosporanic acid acylase and aceulin acylase; each catalyzes an amide hydrolysis reaction. The most fully characterized AHL acylase is PvdQ from P. aeruginosa [31]. Much like other NTN hydrolases, PvdQ is produced as an inactive precursor protein containing a signal peptide that directs export into the periplasmic space. Export is followed by an intermolecular cleavage to remove the signal peptide. The resulting protein undergoes two autoprocessing events in which a short linker sequence is excised from the middle of the protein, leaving an N-terminal peptide (α-peptide) and a C-terminal peptide (β-peptide). The α- and β-peptides associate non-covalently to form the mature heterodimeric enzyme. Autoprocessing is essential for enzyme activation because the newly formed N-terminal amine of Ser1 on the β-peptide (Serβ1) plays an integral role in catalysis.
NTN hydrolases have been described as having a “single-amino-acid catalytic centre,” but a number of PvdQ residues other than Serβ1 are suspected to facilitate turnover, as highlighted in a recently proposed mechanism (Figure 5) [32, 33]: The AHL substrate binds close to Serβ1, which uses its N-terminal amine as a general base to deprotonate, through an intervening water molecule, the sidechain hydroxyl. The deprotonated sidechain of Serβ1 attacks the carbonyl carbon of the AHL's amide. Formation of the tetrahedral adduct, stabilized by the backbone amide of Valββ70 and the Asnββ260 sidechain, is followed by collapse and expulsion of the homoserine lactone moiety, with protonation of the leaving amine by a bound water molecule. This forms a covalent ester intermediate with the sidechain of Serβ1 (Figure 5), of which subsequent hydrolysis can lead to formation and release of the fatty acid product and reformation of the resting enzyme. Most of the steps in this proposed mechanism are based on analogy to the related NTN hydrolases, and several mechanistic details specific to PvdQ are not firmly established. However, the proposed mechanism is consistent with the recent PvdQ structures reported for product-bound complexes as well as the structure of the covalent ester intermediate that was trapped at acidic pH values [33].
Figure 5.
Chemical mechanism of the AHL acylase PvdQ. An N-terminal serine residue (Serβ1), unmasked by an autoprocessing reaction, serves as a nucleophile for the formation of a covalent intermediate (which can be visualized using X-ray crystallography, as shown in Figure 4). Subsequent hydrolysis of the intermediate completes the catalytic cleavage of the amide bond in AHL substrates.
Substrate recognition by PvdQ sharply contrasts with that of AHL lactonase. As described above, AHL lactonase clamps down over the substrate's lactone ring and only makes weak non-selective interactions with the N-acyl chain. Conversely, PvdQ binds the N-acyl chain of the substrate and does not have any obvious binding site for the lactone ring (Figure 4). The deep hydrophobic pocket of PvdQ, in which the substrate's N-acyl chains binds, makes extensive and specific hydrophobic interactions that enforce a more strict selectivity for acyl chains that are ≥ 8 carbons long (Figure 4) [31]. In contrast, the lactone ring is not observed in the current PvdQ structures, but is predicted to be located within a large solvent-filled cleft of the heterodimer [33]. There are no obvious residues placed for selective interaction with the homoserine lactone moiety, predicting a broad selectivity for this part of the substrate that is also often observed with other related NTN hydrolases. This prediction is also consistent with a proposed alternative non-AHL substrate of PvdQ that would carry a much larger moiety linked to a fatty acid chain [34].
Concluding remarks
The recent structural determinations of enzymes capable of AHL synthesis and degradation have allowed a more detailed understanding of the basis of their catalytic mechanisms and substrate selectivity. These studies provide a foundation for understanding the native functions of the enzymes, for choosing the proper enzyme as a tool to understand quorum sensing, and for more directed engineering efforts to optimize their functions. The homology of these enzymes to others in their respective superfamilies, most notably to β-lactam processing enzymes, highlights intriguing parallels between how bacteria interact with γ-lactones and β-lactams. Given that cell-to-cell signaling has been proposed as the primary function of many natural products that are used clinically as antibiotics [35], the homology between antibiotic-inactivating enzymes and quorum-regulating enzymes suggests a possible common “etymology” for these elements of bacterial communication.
Box 1. Chemical diversity of quorum-sensing signals.
N-Acyl-l-homoserine lactones represent just one of the “dialects” used for quorum-sensing. Bacteria use a chemically-diverse range of signaling molecules for communication, each having different biosynthetic pathways, chemical properties, binding proteins, and degradation mechanisms. A sampling of this diversity is represented in Figure I by the chemical structures of N-(3-oxo-hexanoyl)-l-homoserine lactone (3-oxo-C6-HSL) from Photobacterium fischeri [36], p-coumaroyl-l-HSL (pCHSL) from Rhodopseudomonas palustris [37], diffusible signal factor (DSF) from Xanthomonas campestris [38], cholera autoinducer-1 (CAI-1) from Vibrio cholerae [39], 2-heptyl-3-hydroxy-4-quinolone (pseudomonas quinolone signal or PQS) from P. aeruginosa [40], autoinducer-2 (AI-2) from Vibrio harveyi [41], and AgrD1 thiolactone (autoinducer peptide I, or AIP-I) from Staphylococcus aureus [42].
Figure I(Box 1).
Selected chemical structures of interbacterial signaling molecules. Abbreviations: see text.
Box 2. Quorum-sensing systems as drug targets.
Antibiotics currently in use are either bactericidal (bacteria-killing) or bacteriostatic (growth inhibitory). As resistance to these drugs continues to rise, new strategies for treating bacterial infections are being sought. One approach that has attracted interest is to target the components of bacterial physiology that are required for infectious activity, but not for viability. Since quorum-sensing systems regulate expression of virulence factors, among other things, inhibition of quorum-sensing should attenuate virulence. The observation that the alga Delisea pulchra produces halogenated furanones (i.e. α,β-unsaturated five-membered lactones) that interfere with AHL-mediated signaling [43] stimulated the search for other compounds that inhibit quorum-sensing [44, 45].
Most of the compounds that have been identified to date target the transcription factor to which the signal molecule binds, rather than inhibit the synthase responsible for its synthesis. Investigation of LuxR expressed in E. coli revealed that furanones increased the rate of LuxR turnover, although furanone binding to LuxR was not demonstrated [46].
In mouse models of P. aeruginosa infections, furanones increase bacterial clearance and decrease tissue damage [47, 48]. Interestingly, halogenated furanones were also found to inhibit Staphylococcus epidermidis biofilm formation, even though this process is coordinated by a different signal, autoinducer-2 (see Box 1), not AHL [49]. Despite these promising observations, the road to clinical use of quorum-sensing inhibitors is not without obstacles. The stability and toxicity of halogenated furanones may make them unsuitable for use in humans. Furthermore, compounds that bind to the receptor proteins are not guaranteed to be inhibitors of quorum-sensing, but may instead act as activators [50].
Acknowledgements
Work in the authors' laboratories was supported in part by a grant from the Robert A. Welch foundation (F-1572 to WF) and a grant from the NIH (GM59653 to PAT).
Glossary
- Autoinducer
a signaling molecule that is produced by a microorganism, accumulates in the growth medium and leads to induction of a subset of genes found in the same organism. These signals are used in quorum sensing to sense local cell density.
- Holo-ACP synthase
an acyl-carrier protein synthase bearing a serine residue that has been post-translationally modified to carry a 4'-phosphopantetheinyl substituent. This modification introduces a thiol-containing flexible “arm” that covalently binds the acyl chain (linked as a thioester) that is used as a substrate for AHL synthesis.
- Lactonization
formation of a cyclic ester. In the reaction catalyzed by AHL synthases, this process results in formation of the α-amino-γ-butyrolactone ring.
- Metalloforms
variants of a metalloprotein in which the identity or stoichiometry of the metal content differs. These variants can be found endogenously or can be reconstituted in an experimental system.
- Nucleophilic attack
donation of an electron pair from a donor atom to an acceptor electrophile to form a covalent chemical bond.
- Ping-pong mechanism
a double-displacement bireactant mechanism. The binding of the first substrate is followed by release of a product and (usually) formation of a covalent enzyme intermediate before the second substrate is bound and processed to complete the reaction.
- Sociomicrobiology
the study of group behavior in microorganisms. Group behavior is often mediated by communication systems that rely on the production and detection of small diffusible signaling molecules such as AHLs.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Parsek MR, Greenberg EP. Sociomicrobiology: the connections between quorum sensing and biofilms. Trends Microbiol. 2005;13:27–33. doi: 10.1016/j.tim.2004.11.007. [DOI] [PubMed] [Google Scholar]
- 2.Schaefer AL, et al. Generation of cell-to-cell signals in quorum sensing: Acyl homoserine lactone synthase activity of a purified Vibrio fischeri LuxI protein. Proc. Nat. Acad. Sci. USA. 1996;93:9505–9509. doi: 10.1073/pnas.93.18.9505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Watson WT, et al. Structural basis and specificity of acyl-homoserine lactone signal production in bacterial quorum sensing. Molecular Cell. 2002;9:685–694. doi: 10.1016/s1097-2765(02)00480-x. [DOI] [PubMed] [Google Scholar]
- 4.Gould TA, et al. Structure of the Pseudomonas aeruginosa acylhomoserinelactone synthase LasI. Mol. Microbiol. 2004;53:1135–1146. doi: 10.1111/j.1365-2958.2004.04211.x. [DOI] [PubMed] [Google Scholar]
- 5.Chung J, et al. Small-molecule inhibitor binding to an N-acyl-homoserine lactone synthase. Proc. Natl. Acad. Sci. U.S.A. 2011;96:4360–4365. doi: 10.1073/pnas.1103165108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Thiel V, et al. New structural variants of homoserine lactones in bacteria. ChemBioChem. 2009;10:1861–1868. doi: 10.1002/cbic.200900126. [DOI] [PubMed] [Google Scholar]
- 7.Parsek MR, et al. Acyl homoserine-lactone quorum-sensing signal generation. Proc. Natl. Acad. Sci. U.S.A. 1999;96:4360–4365. doi: 10.1073/pnas.96.8.4360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brader G, et al. Altering substrate chain length specificity of an acylhomoserine lactone synthase in bacterial communication. J. Biol. Chem. 2005;280:10403–10409. doi: 10.1074/jbc.M408603200. [DOI] [PubMed] [Google Scholar]
- 9.Raychaudhuri A, et al. Chemical mechanism and substrate specificity of RhlI, and acylhomoserine lactone synthase from P. aeruginosa. Biochemistry. 2005;44:2974–2981. doi: 10.1021/bi048005m. [DOI] [PubMed] [Google Scholar]
- 10.Butland G, et al. Interaction network containing conserved and essential protein complexes in Escherichia coli. Nature. 2005;433:531–537. doi: 10.1038/nature03239. [DOI] [PubMed] [Google Scholar]
- 11.Schaefer AL, et al. A new class of homoserine lactone quorum-sensing signals. Nature. 2008;454:595–600. doi: 10.1038/nature07088. [DOI] [PubMed] [Google Scholar]
- 12.Ahlgren NA, et al. Aryl-homoserine lactone quorum sensing in stem-nodulating photosynthetic bradyrhizobia. Proc. Natl. Acad. Sci. U.S.A. 2011;108:7183–7188. doi: 10.1073/pnas.1103821108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Raychaudhuri A, et al. Reactivity and reaction order in acylhomoserine lactone formation by Pseudomonas aeruginosa RhlI. Biochemistry. 2008;47:2893–2898. doi: 10.1021/bi702009n. [DOI] [PubMed] [Google Scholar]
- 14.Parsek MR, et al. Analysis of random and site-directed mutations in RhII, a Pseudomonas aeruginosa gene encoding an acylhomoserine lactone synthase. Mol Microbiol. 1997;26:301–310. doi: 10.1046/j.1365-2958.1997.5741935.x. [DOI] [PubMed] [Google Scholar]
- 15.Kambam PK, et al. Altering the substrate specificity of RhlI by directed evolution. Chembiochem. 2009;10:553–558. doi: 10.1002/cbic.200800636. [DOI] [PubMed] [Google Scholar]
- 16.Roche DM, et al. Communications blackout? Do N-acylhomoserine-lactone-degrading enzymes have any role in quorum sensing? Microbiology. 2004;150:2023–2028. doi: 10.1099/mic.0.26977-0. [DOI] [PubMed] [Google Scholar]
- 17.Afriat L, et al. The latent promiscuity of newly identified microbial lactonases is linked to a recently diverged phosphotriesterase. Biochemistry. 2006;45:13677–13686. doi: 10.1021/bi061268r. [DOI] [PubMed] [Google Scholar]
- 18.Draganov DI, et al. Human paraoxonases (PON1, PON2, and PON3) are lactonases with overlapping and distinct substrate specificities. J Lipid Res. 2005;46:1239–1247. doi: 10.1194/jlr.M400511-JLR200. [DOI] [PubMed] [Google Scholar]
- 19.Dong YH, et al. AiiA, an enzyme that inactivates the acylhomoserine lactone quorum-sensing signal and attenuates the virulence of Erwinia carotovora. Proc Natl Acad Sci U S A. 2000;97:3526–3531. doi: 10.1073/pnas.060023897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lee SJ, et al. Genes encoding the N-acyl homoserine lactone-degrading enzyme are widespread in many subspecies of Bacillus thuringiensis. Appl Environ Microbiol. 2002;68:3919–3924. doi: 10.1128/AEM.68.8.3919-3924.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Thomas PW, et al. The Quorum-Quenching Lactonase from Bacillus thuringiensis Is a Metalloprotein. Biochemistry. 2005;44:7559–7569. doi: 10.1021/bi050050m. [DOI] [PubMed] [Google Scholar]
- 22.Liu D, et al. Three-dimensional structure of the quorum-quenching N-acyl homoserine lactone hydrolase from Bacillus thuringiensis. Proc Natl Acad Sci U S A. 2005;102:11882–11887. doi: 10.1073/pnas.0505255102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Momb J, et al. The quorum-quenching metallo-gamma-lactonase from Bacillus thuringiensis exhibits a leaving group thio effect. Biochemistry. 2006;45:13385–13393. doi: 10.1021/bi061238o. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu D, et al. Mechanism of the quorum-quenching lactonase (AiiA) from Bacillus thuringiensis. 1. Product-bound structures. Biochemistry. 2008;47:7706–7714. doi: 10.1021/bi800368y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Momb J, et al. Mechanism of the quorum-quenching lactonase (AiiA) from Bacillus thuringiensis. 2. Substrate modeling and active site mutations. Biochemistry. 2008;47:7715–7725. doi: 10.1021/bi8003704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liu D, et al. Structure and specificity of a quorum-quenching lactonase (AiiB) from Agrobacterium tumefaciens. Biochemistry. 2007;46:11789–11799. doi: 10.1021/bi7012849. [DOI] [PubMed] [Google Scholar]
- 27.Kim MH, et al. The molecular structure and catalytic mechanism of a quorum-quenching N-acyl-l-homoserine lactone hydrolase. Proc Natl Acad Sci U S A. 2005;102:17606–17611. doi: 10.1073/pnas.0504996102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Crowder MW, et al. Metallo-beta-lactamases: novel weaponry for antibiotic resistance in bacteria. Acc Chem Res. 2006;39:721–728. doi: 10.1021/ar0400241. [DOI] [PubMed] [Google Scholar]
- 29.Wang LH, et al. Specificity and enzyme kinetics of the quorum-quenching N-Acyl homoserine lactone lactonase (AHL-lactonase) J Biol Chem. 2004;279:13645–13651. doi: 10.1074/jbc.M311194200. [DOI] [PubMed] [Google Scholar]
- 30.Momb J, et al. Enzymic disruption of N-aroyl-l-homoserine lactone-based quorum sensing. Chembiochem. 2010;11:1535–1537. doi: 10.1002/cbic.201000191. [DOI] [PubMed] [Google Scholar]
- 31.Huang JJ, et al. Utilization of acyl-homoserine lactone quorum signals for growth by a soil pseudomonad and Pseudomonas aeruginosa PAO1. Appl Environ Microbiol. 2003;69:5941–5949. doi: 10.1128/AEM.69.10.5941-5949.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Duggleby HJ, et al. Penicillin acylase has a single-amino-acid catalytic centre. Nature. 1995;373:264–268. doi: 10.1038/373264a0. [DOI] [PubMed] [Google Scholar]
- 33.Bokhove M, et al. The quorum-quenching N-acyl homoserine lactone acylase PvdQ is an NTN-hydrolase with an unusual substrate-binding pocket. Proc Natl Acad Sci U S A. 2010;107:686–691. doi: 10.1073/pnas.0911839107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nadal Jimenez P, et al. Role of PvdQ in Pseudomonas aeruginosa virulence under iron-limiting conditions. Microbiology. 2010;156:49–59. doi: 10.1099/mic.0.030973-0. [DOI] [PubMed] [Google Scholar]
- 35.Yim G, et al. Antibiotics as signalling molecules. Philos Trans R Soc Lond B Biol Sci. 2007;362:1195–1200. doi: 10.1098/rstb.2007.2044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Eberhard A, et al. Structural identification of autoinducer of Photobacterium fischeri luciferase. Biochemistry. 1981;20:2444–2449. doi: 10.1021/bi00512a013. [DOI] [PubMed] [Google Scholar]
- 37.Schaefer AL, et al. A new class of homoserine lactone quorum-sensing signals. Nature. 2008;454:595–599. doi: 10.1038/nature07088. [DOI] [PubMed] [Google Scholar]
- 38.Deng Y, et al. Listening to a new language: DSF-based quorum sensing in Gram-negative bacteria. Chem Rev. 2011;111:160–173. doi: 10.1021/cr100354f. [DOI] [PubMed] [Google Scholar]
- 39.Wei Y, et al. Mechanism of Vibrio cholerae autoinducer-1 biosynthesis. ACS Chem Biol. 2011;6:356–365. doi: 10.1021/cb1003652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pesci EC, et al. Quinolone signaling in the cell-to-cell communication system of Pseudomonas aeruginosa. Proc Natl Acad Sci U S A. 1999;96:11229–11234. doi: 10.1073/pnas.96.20.11229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chen X, et al. Structural identification of a bacterial quorum-sensing signal containing boron. Nature. 2002;415:545–549. doi: 10.1038/415545a. [DOI] [PubMed] [Google Scholar]
- 42.Mayville P, et al. Structure-activity analysis of synthetic autoinducing thiolactone peptides from Staphylococcus aureus responsible for virulence. Proc Natl Acad Sci U S A. 1999;96:1218–1223. doi: 10.1073/pnas.96.4.1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Manefield M, et al. Halogenated furanones inhibit quorum sensing through accelerated LuxR turnover. Microbiology. 2002;148:1119–1127. doi: 10.1099/00221287-148-4-1119. [DOI] [PubMed] [Google Scholar]
- 44.Geske GD, et al. Small Molecule Inhibitors of Bacterial Quorum Sensing and Biofilm Formation. Journal of the American Chemical Society. 2005;127:12762–12763. doi: 10.1021/ja0530321. [DOI] [PubMed] [Google Scholar]
- 45.Muh U, et al. Novel Pseudomonas aeruginosa Quorum-Sensing Inhibitors Identified in an Ultra-High-Throughput Screen. Antimicrob. Agents Chemother. 2006;50:3674–3679. doi: 10.1128/AAC.00665-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Manefield M, et al. Halogenated furanones inhibit quorum sensing through accelerated LuxR turnover. Microbiology. 2002;148:1119–1127. doi: 10.1099/00221287-148-4-1119. [DOI] [PubMed] [Google Scholar]
- 47.Wu H, et al. Synthetic furanones inhibit quorum-sensing and enhance bacterial clearance in Pseudomonas aeruginosa lung infection in mice. Journal of Antimicrobial Chemotherapy. 2004;53:1054–1061. doi: 10.1093/jac/dkh223. [DOI] [PubMed] [Google Scholar]
- 48.Christensen LD, et al. Impact of Pseudomonas aeruginosa quorum sensing on biofilm persistence in an in vivo intraperitoneal foreign-body infection model. Microbiology. 2007;153:2312–2320. doi: 10.1099/mic.0.2007/006122-0. [DOI] [PubMed] [Google Scholar]
- 49.Lönn-Stensrud J, et al. Furanones, potential agents for preventing Staphylococcus epidermidis biofilm infections? Journal of Antimicrobial Chemotherapy. 2009;63:309–316. doi: 10.1093/jac/dkn501. [DOI] [PubMed] [Google Scholar]
- 50.Smith KM, et al. Library screening for synthetic agonists and antagonists of a Pseudomonas aeruginosa autoinducer. Chem Biol. 2003;10:563–571. doi: 10.1016/s1074-5521(03)00107-8. [DOI] [PubMed] [Google Scholar]