Abstract
This study characterized the expression and subcellular localization of the IGF-1R in human corneal epithelial cells. Using a human telomerase-immortalized corneal epithelial cell line, IGF-1R expression and localization was assayed by immunofluorescence and subcellular fractionation followed by western blot. IGF-1R expression was confirmed in primary cultured human corneal epithelial cells. Nuclear localization was assessed under basal and IGF-1 stimulated culture conditions; phosphorylation status of the receptor in response to IGF-1 was demonstrated by western blot. IGF-1R:E-cadherin interactions were detected by immunofluorescence and co-immunoprecipitation of whole cell lysates. The results of this study demonstrated that IGF-1R localized predominantly to the nucleus and in a perinuclear cap pattern which co-localized with the Golgi complex in proliferating corneal epithelial cells. There was no difference in nuclear localization between primary or telomerized cell lines. Subcellular fractionation confirmed IGF-1Rα- and β-subunit localization in soluble and chromatin-bound nuclear fractions. Neither growth factor withdrawal nor IGF-1 stimulation altered nuclear IGF-1R. At points of cell-cell contact, IGF-1R co-localized with E-cadherin; co-immunoprecipitation assays confirmed the presence of an IGF-1R:E-cadherin complex. Importantly, this is the first report to identify IGF-1R in the nucleus and complexed with E-cadherin at points of cell-cell contact in corneal epithelial cells. Nuclear trafficking appeared to be independent of ligand-mediated events at the plasma membrane. The identification of IGF-1R in the nucleus and complexed with E-cadherin suggests novel regulatory functions outside the canonical ligand-induced endocytosis signaling pathway.
Keywords: cornea, epithelium, IGF-1R, E-cadherin
Introduction
The insulin-like growth factor-1 receptor (IGF-1R) is a transmembrane receptor tyrosine kinase with well established regulatory roles in cellular proliferation, differentiation and survival (O'Connor et al., 2000; Valentinis and Baserga, 2001; Vincent and Feldman, 2002; Werner and LeRoith, 1996). Initially translated as a pro-receptor, the IGF-1R is cleaved in the trans-Golgi complex and is later re-assembled into a tetrameric complex consisting of two α-subunits and two β-subunits prior to insertion in the plasma membrane (LeRoith et al., 1995). The α-subunit, which is entirely extracellular, contains a cysteine-rich domain which functions to mediate ligand binding. Both insulin-like growth factor (IGF)-1 and IGF-II have a relatively high affinity for the α-subunit; unlike insulin which has little capacity to activate the IGF-1R (Nakamura et al., 2000; Steele-Perkins et al., 1988). The smaller of the two subunits, the β-subunit spans the plasma membrane and contains an intracellular activation loop with three tyrosine residues responsible for regulating kinase activity, a C-terminal domain which has recently been shown to mediate proteasomal-degradation, and multiple docking sites for cytoplasmic proteins (O'Connor et al., 1997; Sehat et al., 2007).
Trans-phosphorylation of IGF-1R β-subunits occurs following ligand binding, resulting in the recruitment of cytoplasmic docking proteins and downstream effector molecules and subsequent activation of canonical signaling pathways including AKT and/or mitogen activated protein kinase (MAPK) (Adams et al., 2004). Receptor internalization has been shown to be mediated by both clathrin and caveolin-mediated endocytic trafficking (Huo et al., 2003; Salani et al., 2010). Once internalized, the IGF-1R traffics to the recycling endosome where it is recycled and shuttled back to the cell surface for re-stimulation and signal amplification or proceeds to the late endosome where it undergoes lysosomal degradation, thus mediating signal attenuation (Romanelli et al., 2007). More recently, IGF-1 has been shown to induce ligand-dependent nuclear translocation of the IGF-1R following serum starvation (Sehat et al., 2010). While the IGF-1R does not possess a nuclear localization sequence, the exact mechanism by which IGF-1R traffics to the nucleus is unknown.
In addition to localizing to the nucleus, growth factor receptors, including the IGF-1R, have also been reported to complex with E-cadherin. E-cadherin, a transmembrane glycoprotein, mediates cell-cell adhesion via calcium-dependent homotypic binding and has functional roles in many morphogenetic processes (Takeichi, 1990). The loss of E-cadherin intracellular junctions can disrupt normal differentiation programs and is clinically associated with epithelial mesenchymal transition and tumor invasion (Thiery, 2002). Studies investigating the significance of these growth factor receptor:E-cadherin interactions however, indicate both positive and negative regulation of receptor tyrosine kinases by E-cadherin, which are likely cell type and culture condition specific (Pece and Gutkind, 2000; Qian et al., 2004; Takahashi and Suzuki, 1996). The presence and biological significance of a growth factor receptor complexed with E-cadherin in the corneal epithelium has not been previously established.
The IGF-1R was first characterized in primary cultured rabbit corneal epithelial cells using ligand binding assays (Nakamura et al., 2000). Later studies investigated expression in the human corneal epithelium in situ, where the IGF-1R was shown to localize to both the cytosol and plasma membrane; however, a nuclear role for IGF-1R in the cornea has not yet been reported (Rocha et al., 2002). Moreover, relatively little is known about the biological significance of IGF-1R in corneal epithelial development and in maintenance of normal homeostatic renewal. In the current study, we examined the expression and localization of the IGF-1R in a human corneal epithelial cell line and in primary cultured human corneal epithelial cells. Importantly, we have shown that IGF-1R localizes to the nucleus of corneal epithelial cells and is associated with nuclear chromatin. We have further identified the presence of IGF-1R complexed with E-cadherin at points of cell-cell contact in non-differentiated cells. Collectively these findings suggest important new regulatory pathways for IGF-1R in the corneal epithelium.
Materials & Methods
Cell culture
Human telomerized corneal epithelial cells (hTCEpi) were initially isolated and thereafter routinely maintained in serum-free keratinocyte growth media (KGM-2, Lonza, Walkersville, MD) containing 0.15 mM calcium and supplemented with 0.4% bovine pituitary extract, 0.1% human epidermal growth factor, 0.1% insulin, 0.1% hydrocortisone, 0.1% transferrin, 0.1% epinephrine and 0.1% gentamicin sulfate amphotericin B as previously described (Robertson et al., 2005). Cells were subcultured on T75 tissue culture flasks (Falcon Labware; BD Biosciences, Bedford, MA), incubated at 37°C in 5% CO2. and passaged every 5 to 7 days. For basal conditions and prior to IGF-1 stimulation, cells were incubated for the indicated amount of time in serum-free keratinocyte basal media containing 0.15 mM calcium without supplements. To investigate ligand-activation of the IGF-1R, cells were stimulated with 100 ng/ml recombinant human IGF-1 (BioVision, Mountain View, CA). For primary cultures (HCEC), human eye bank corneas (Tissue Transplant Services, UT Southwestern Medical Center, Dallas, TX) were digested in dispase (Invitrogen, Carlsbad, CA) overnight at 4°C. Intact epithelial cell sheets were carefully removed and underwent a second digestion in dispase for 2 hours at 37°C. Individual cells were separated by gentle pipetting and seeded onto plastic tissue culture dishes coated with Type 4 collagen (Biocoat, BD Biosciences, San Jose, CA). Cells were cultured in CnT20 cell culture media enriched for progenitor cell culture (Zen Bio, Research Triangle Park, NC).
Antibodies
The following antibodies were used: a rabbit polyclonal anti-IGF-1Rβ (Cell Signaling, Danvers, MA), a rabbit polyclonal anti-IGF-1Rβ (Santa Cruz Biotechnology, Santa Cruz, CA), a mouse monoclonal anti-IGF-1Rα (Abcam, Cambridge, MA), a mouse monoclonal anti-E-cadherin (Sino Biological, Beijing, China), a rabbit monoclonal anti-tyrosine 1135 IGF-1Rβ (Cell signaling, Danvers, MA), a mouse monoclonal anti-GM130 (BD Transduction Laboratories, Franklin Lakes, NJ), a mouse monoclonal anti-calnexin (Millipore, Temecula, CA), a rabbit polyclonal anti-HDAC2 (Sigma, St. Louis, MO), a rabbit polyclonal anti-GADPH (Santa Cruz Biotechnology, Santa Cruz, CA), a rabbit polyclonal anti-SP1 (Millipore, Temecula, CA), a rabbit monoclonal anti-histone H3 (Cell Signaling, Danvers, MA), a pan-cytokeratin clone AE3 (Biogenesis, Kingston, NH), a rabbit polyclonal anti-E-cadherin (Cell signaling, Danvers, MA), a mouse monoclonal anti-β-actin (Sigma, St. Louis, MO), and a rabbit IgG (Santa Cruz Biotechnology, Santa Cruz, CA). A commercially available blocking peptide was used to confirm specificity of the IGF-1Rβ polyclonal antibody used for immunofluorescence (Cell Signaling, Danvers, MA).
Immunofluorescence
For immunofluorescent studies, cells were seeded onto glass coverslips and allowed to adhere overnight. Cells were fixed in 4% paraformaldehyde (Electron Microscopy Sciences, Fort Washington, PA) in phosphate buffered saline (PBS) and permeabilized in 0.2% Triton X-100. Cells were blocked using 0.5% bovine serum albumin (Sigma, St. Louis, MO) and incubated in primary antibody overnight. Samples were subsequently washed in PBS and stained with an Alexa Fluor 488 or 555 secondary antibody (Cell Signaling, Danvers, MA) for one hour at room temperature. Nuclei were counterstained with DRAQ5 (Cell Signaling, Danvers, MA). F-actin was labeled using alexa-fluor 543 phalloidin (Invitrogen, Carlsbad, CA). All samples were mounted on slides using Prolong gold antifade reagent (Invitrogen, Carlsbad, CA) and imaged on a Leica SP2 laser scanning confocal microscope (Leica Microsystems, Heidelberg, Germany) using a 63× water objective. All images were sequentially scanned to avoid spectral crosstalk between channels.
Subcellular fractionation
Cellular fractions were separated using a protein subcellular fractionation kit (Thermo Fisher, Rockford, IL) according to manufacturer instructions. Briefly, cells were harvested using trypsin-EDTA and subsequently washed with chilled PBS. Proteins were extracted from individual fractions using respective buffers, followed by centrifugation. Protein concentration was determined using a Bradford assay (BioRad, Hercules, CA). 50 µg of protein was loaded per lane for each fraction. Individual fractions were electrophoresed through a 4–15% precast linear gradient polyacrylamide gel (BioRad, Hercules, CA) and immunblotted as described below. Controls for cross-contamination of individual fractions included antibodies against GADPH (cytosolic extract), GM-130 (membrane extract), SP1 (soluble nuclear extract), histone H3 (chromatin-bound nuclear extract), HDAC2 (total nuclear extract) and pan-cytokeratin clone AE3 (cytoskeletal extract). For analysis of changes in cytosolic and nuclear fractions under different experimental conditions, proteins were further normalized using GAPDH and HDAC2, respectively.
SDS PAGE and Immunoblot
For collection of whole cell lysates, cells were lysed directly in the 100 mm culture dish using 0.4 ml lysis buffer. All cells were lysed in radioimmunoprecipitation buffer (RIPA) buffer containing a protease inhibitor cocktail tablet (Complete-Mini, Roche Diagnostics, Indianapolis, IN) and a protease and phophatase single-use inhibitor cocktail, (Thermo Fisher, Rockford, IL) on ice for 10 minutes. Lysates were snap frozen in liquid nitrogen, vortexed and briefly centrifuged to remove the precipitate. All lysates were boiled for 5 minutes in 4× sample buffer pH 6.8 containing 0.25 M tris, 8% lauryl sulfate, 40% glycerol, 20% mercaptoethanol and 0.04% bromophenol blue, resolved on a 4–15% precast linear gradient polyacrylamide gel (Bio-rad, Hercules, CA) and subsequently transferred to a polyvinyl difluoride (PVDF) membrane. Membranes were blocked in 1–5% non-fat milk for 1 hour at room temperature and blotted using one of the following antibodies: IGF-1Rβ, IGF-1Rα and β-actin (Sigma-Aldrich, St. Louis, MO) overnight at 4°C. Following a 1 hour incubation with an anti-mouse secondary antibody (Amersham Biosciences, Piscataway, NJ), protein was visualized using ECL Plus Detection Reagents (Amersham Biosciences, Piscataway, NJ) and imaged on a Typhoon Variable Mode Imager. For western blots of whole cell lysates, β-actin was used as a loading control. For subcellular fractionation experiments, protein was normalized as described above.
Immunoprecipitation
For immunoprecipitation assays, whole cell lysates were immunoprecipitated with IGF-1Rβ (Cell Signaling, Danvers, MA), IGF-1Rβ (Santa Cruz Biotechnology, Santa Cruz, CA) or a rabbit IgG control. 11 µl of IGF-1Rβ or 4 ul IgG control were incubated with 4 mg of whole cell lysates with continuous rocking overnight at 4°C. 70 µl of immobilized protein A plus (Thermo Fisher, Rockford, IL) was then added with continuous rocking for 2 hours at room temp. Following centrifugation, the pellet was washed with immunoprecipitation buffer (Thermo Fisher, Rockford, IL). 4× sample buffer was added to the resulting pellet and boiled for 5 minutes. Immunoprecipitated lysates were then electrophoresed and immunoblotted as described above.
Results
IGF-1R expression and localization
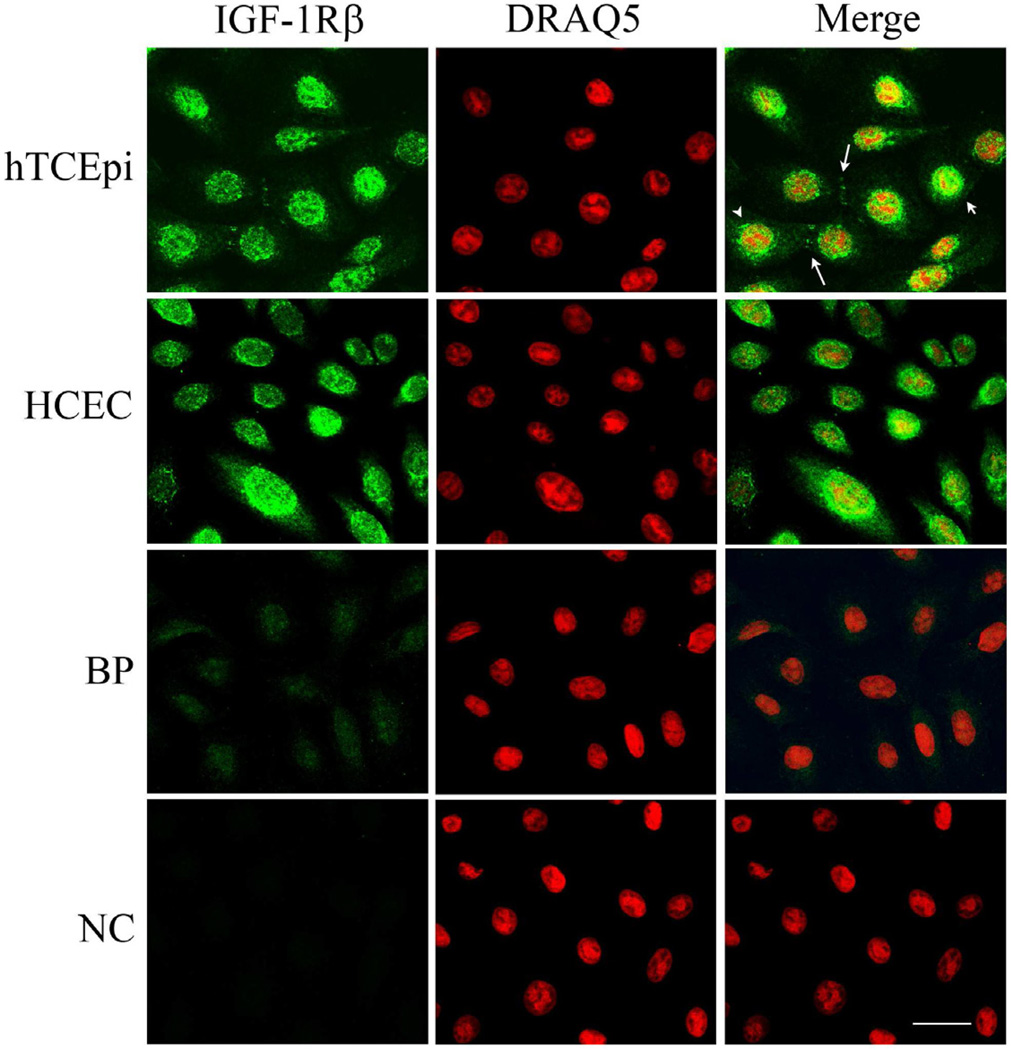
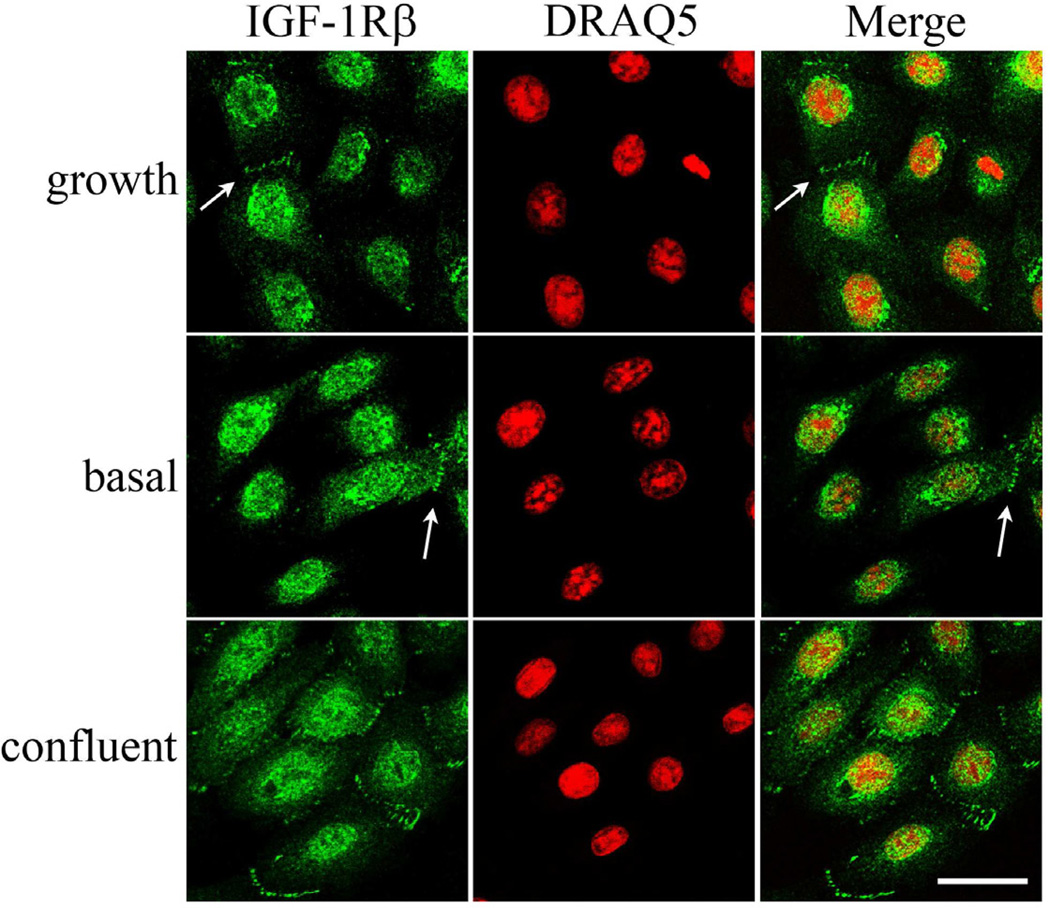
Double-labeling experiments with antibodies directed against the β-subunit of the IGF-1R and a nuclear counterstain demonstrated the intracellular presence of IGF-1R in the nucleus of hTCEpi cells under normal growth conditions (Figure 1). The distribution of the nuclear IGF-1R pattern was variable, with some cells staining robustly for IGF-1R in the nucleus, while other cells exhibited weak nuclear labeling and strong perinuclear staining. The localization pattern of IGF-1R was confirmed in HCEC (Figure 1). These staining patterns were ubiquitous amongst all cells examined for both hTCEpi and HCEC; no cells were detected that were negative for IGF-1R. IGF-1R also was occasionally identified at specific areas of cell-cell contact (Figure 1, large arrow). No plasma membrane labeling was detected with either antibody. A blocking peptide confirmed antibody specificity; no staining was evident in the negative control (Figure 1).
Figure 1.
Localization of IGF-1Rβ in corneal epithelial cells. IGF-1Rβ was detected in hTCEpi cells (first row) and HCEC cells (second row) using alexa fluor 488 (green, first column); nuclei were counterstained with DRAQ5 (red, second column); merged images (third column). Nuclear (small arrow) and juxtanuclear staining (arrow head) was noted in a variable distribution among cells. IGF-1Rβ was also detected at points of cell-cell contact (large arrow). A blocking peptide (BP, third row) was used to confirm antibody specificity. Negative control (NC, bottom row), primary antibody omitted. Scale: 22.5 µm.
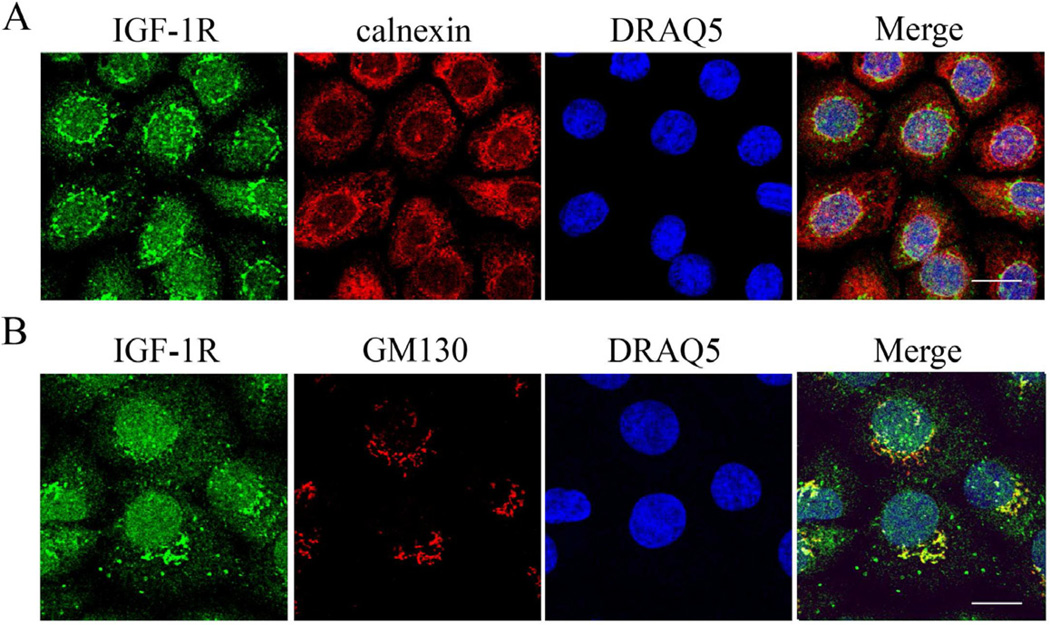
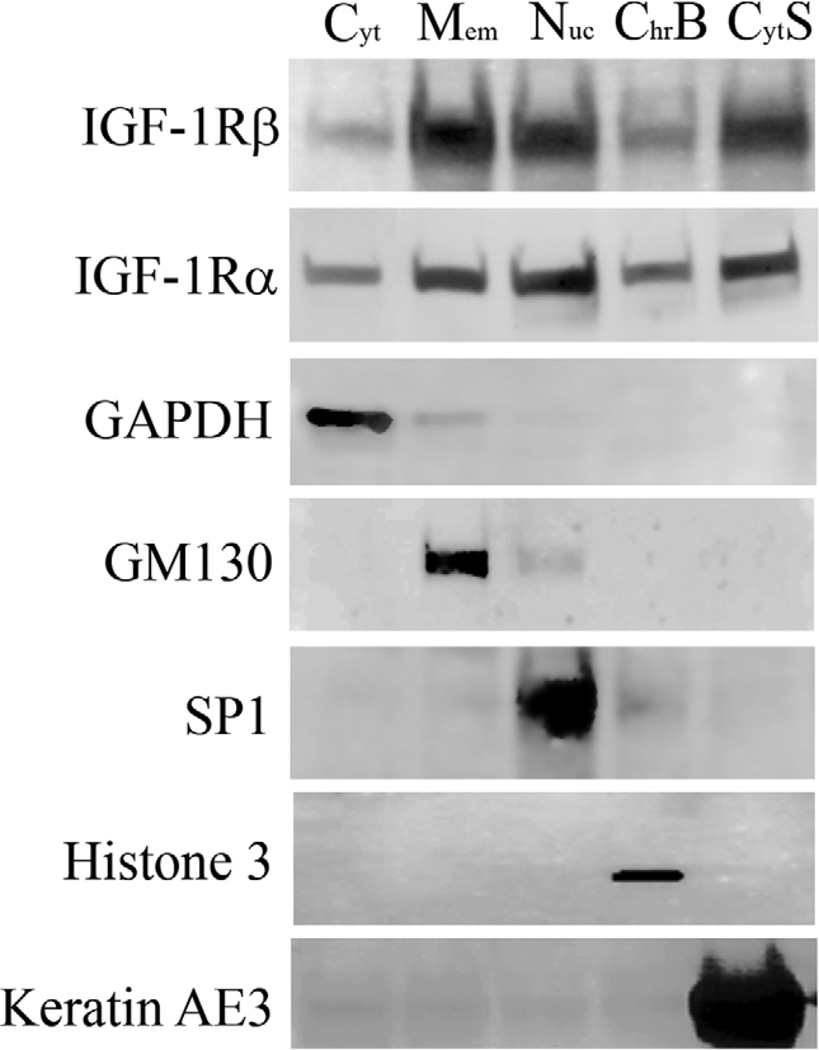
Triple-labeling of the IGF-1Rβ subunit with either the endoplasmic reticulum marker calnexin or the Golgi matrix protein GM130 and DRAQ5 demonstrated robust co-localization of the IGF-1Rβ outside the nucleus with the Golgi complex (Figure 2B). Specifically, the IGF-1Rβ was distributed in a perinuclear cap pattern which was consistent throughout all the cells with extranuclear IGF-1R in both cell lines. No co-localization was detected with the endoplasmic reticulum. Subcellular fractionation confirmed the nuclear localization of the receptor, with both α- and β-subunits present in both the soluble nucleus and chromatin-bound lysates (Figure 3). Based upon equal protein loading for individual fractions, low levels of IGF-1R were detected in the cytosolic fraction, with higher levels detected in the soluble nuclear fraction and membrane portion. Antibodies specific for each cellular compartment confirmed enrichment of specific fractions of interest.
Figure 2.
Juxtanuclear localization of IGF-1Rβ. (A) Triple-labeling of hTCEpi cells with IGF-1Rβ (green), ER-protein calnexin (red), and nuclear counter-staining with DRAQ5 (blue). Scale: 13.35 µm. (B) Triple-labeling of hTCEpi cells with IGF-1Rβ (green), the Golgi matrix protein GM130 (red), and nuclear counter-staining with DRAQ5 (blue). Scale: 12.4 µm. Outside the nucleus, IGF-1Rβ co-localized exclusively with the Golgi matrix protein GM130 in a perinuclear cap pattern. No co-localization was noted with the ER. No staining was noted in the negative control which omitted the primary antibodies (not shown).
Figure 3.
Subcellular fractionation of hTCEpi cells. Immunoblotting confirmed the presence of IGF-1Rα- and β-subunits throughout all cellular compartments including nuclear (soluble and chromatin bound) fractions. Fractionation experiments were performed under normal growth conditions, with media supplemented with growth factors. Subcellular fraction controls include GAPDH (cytoplasm), GM130 (membrane), SP1 (soluble nucleus), histone 3 (chromatin bound), and keratin AE3 (cytoskeleton). For each fraction, 50 µg of protein was loaded per lane. Blots are representative of 3 repeated experiments.
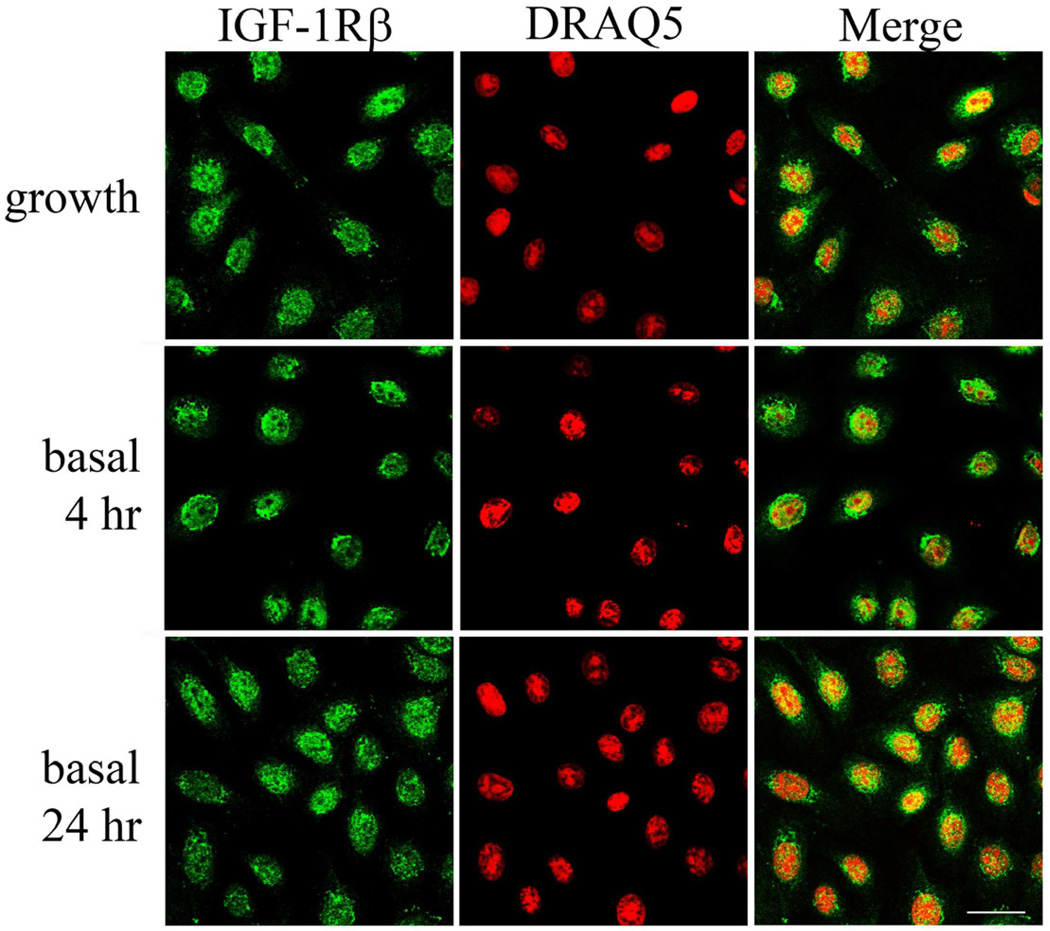
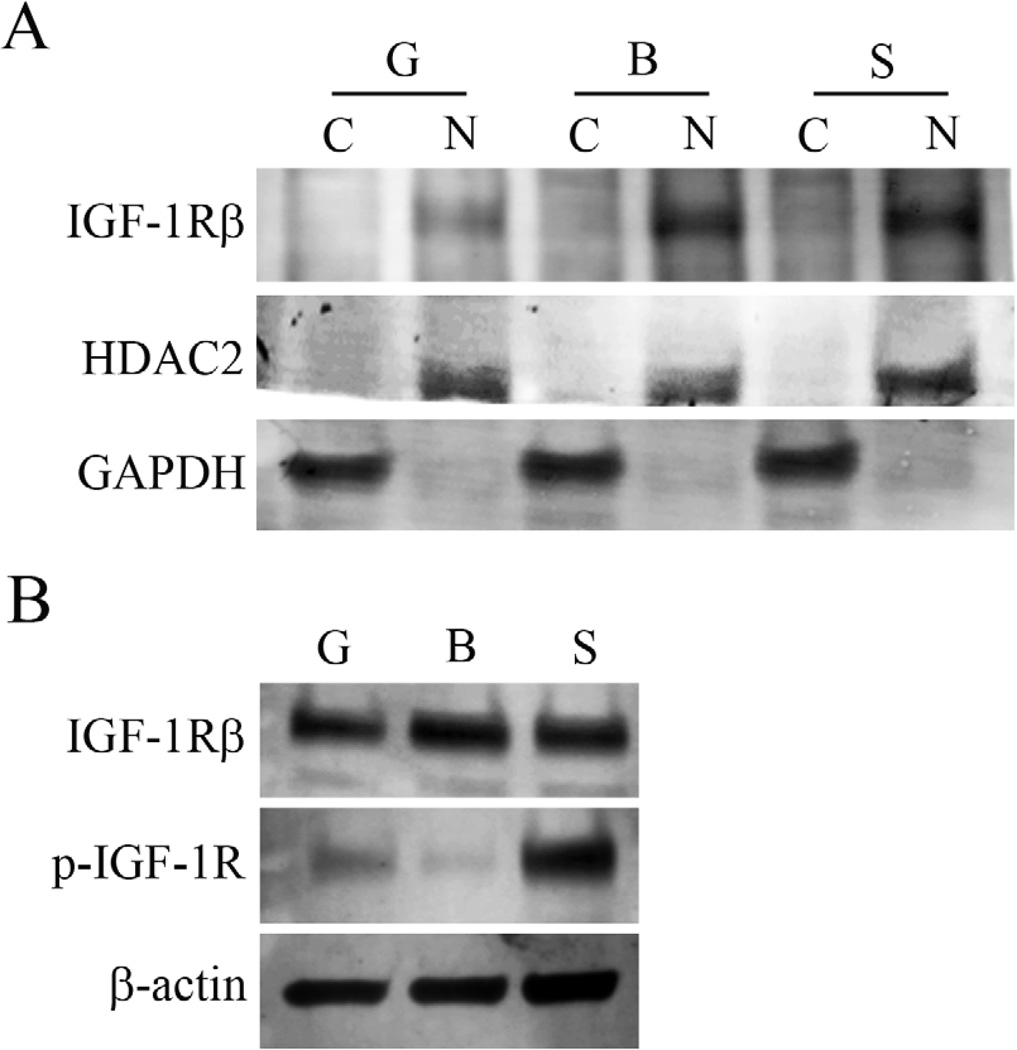
When cultured under basal media without supplements, no change was noted in the localization of IGF-1R after 2, 4, 24 hours (Figure 4, representative images at 4 and 24 hours shown). Subsequent stimulation with IGF-1 failed to alter nuclear localization (data not shown). Similar to immunofluorescence, subcellular fractionation experiments confirmed that nuclear expression of the IGF-1R was unaltered after growth factor withdrawal or following IGF-1 stimulation (Figure 5A). In contrast, whole cell lysates demonstrates robust phosphorylation of the IGF-1R after stimulation with IGF-1 (Figure 5B).
Figure 4.
Nuclear localization and growth factor withdrawal. hTCEpi cells were double-labeled with IGF-1Rβ (green) and DRAQ5 (nuclear counterstain, red) after culture in normal growth media containing supplements (growth) and in basal media for 4 and 24 hours. Culture in basal media in the absence of growth factors failed to alter nuclear localization of the receptor. Scale: 22.5 µm.
Figure 5.
Subcellular distribution of IGF-1Rβ. hTCEpi cells were grown for 24 hours under normal growth or basal media conditions, with or without IGF-1 stimulation. (A) Culture under basal conditions in the absence of growth factors with and without stimulation failed to alter nuclear localization of the IGF-1R. Little to no IGF-1Rβ was detected in cytosolic extracts. (B) Western blotting of whole cell lysates for total and phosphorylated IGF-1R. Phosphorylated IGF-1R was reduced after culture in basal media for 24 hours; subsequent stimulation with IGF-1 resulted in robust receptor phosphorylation. G: growth; B: basal; S: IGF-1 stimulation; C: cytosolic extract; N: nuclear extract. GAPDH and HDAC2 were used to confirm absence of contamination between fractions and as loading controls for cytosolic and nuclear fractions, respectively in (A). β-actin was used as a loading control for whole cell lysates experiments shown in (B). Blots are representative of 3 repeated experiments.
IGF-1R:E-cadherin complex
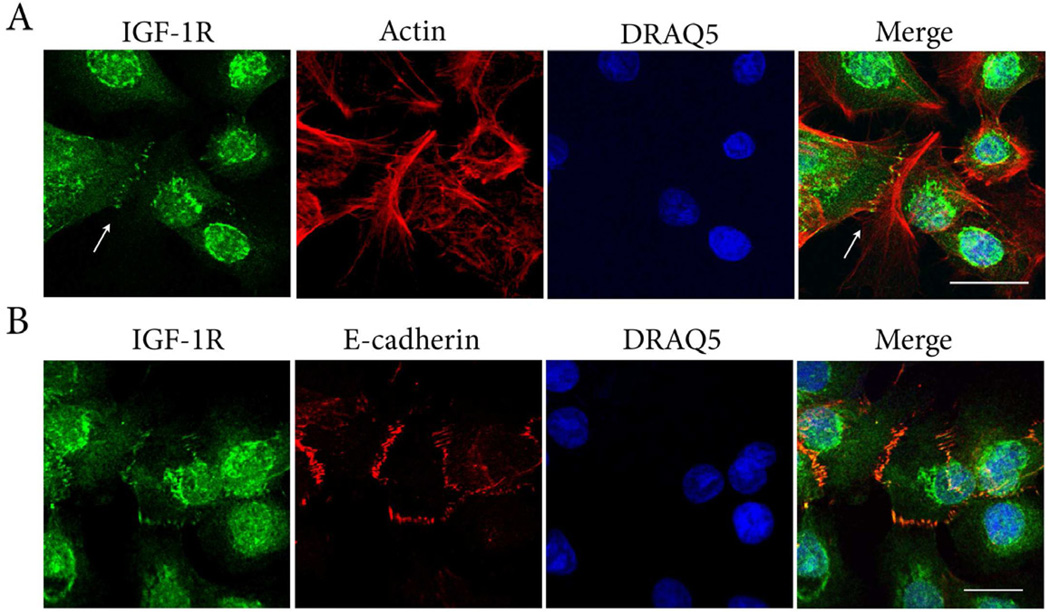
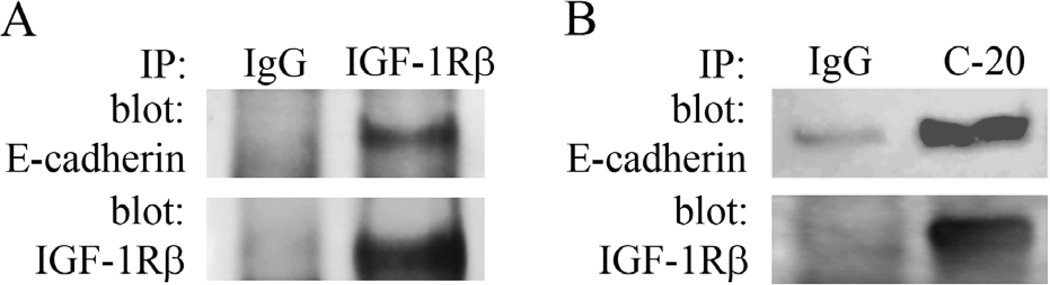
The localization of the IGF-1R at points of cell to cell contact was similarly investigated under growth and basal conditions. IGF-1R was detected at cell points of cell contact under both conditions, and was increased in areas of confluence (Figure 6). Triple-labeling of hTCEpi cells for IGF-1Rβ, phalloidin and DRAQ5 supported the presence of the IGF-1R localized to the ends of the actin cytoskeleton at sites of cell-cell contact (Figure 7A). Triple-labeling of hTCEpi cells for the IGF-1Rβ with E-cadherin and DRAQ5 revealed co-localization between the two proteins (Figure 7B). No fluorescence labeling was detected when both primary antibodies were omitted (data not shown). Immunoprecipitation of hTCEpi cell lysates with 2 different antibodies directed against IGF-1Rβ followed by immunoblotting for E-cadherin confirmed the presence of an IGF-1R:E-cadherin complex at cell-cell contact points (Figure 8). No corresponding band for E-cadherin was noted in the IgG control.
Figure 6.
IGF-1R localization at points of cell-cell contact. hTCEpi cells were double-labeled with IGF-1Rβ (green) and DRAQ5 (nuclear counterstain, red) after culture in normal growth medium and in basal media for 24 hours. The presence of IGF-1R at cell-cell contacts were seen under both growth and growth factor depleted conditions (arrows). At higher densities, as cells spread out and made contact, there was an increase in the frequency of IGF-1R localizing between cells. Scale: 28.6 µm
Figure 7.
IGF-1R:E-cadherin interactions. (A) Triple-labeling of hTCEpi cells with IGF-1Rβ (green), alexa fluor 543-phalloidin (red), and DRAQ5 stained nuclei (blue). Counterstaining with phalloidin demonstrated the presence of the IGF-1R localized to actin junctions at points of cell-cell contact (arrows). Scale: 28.73 µm. (B) Triple-labeling with IGF-1R (green), E-cadherin (red) and DRAQ5 stained nuclei (blue). IGF-1R at areas of cell-cell contact co-localized with E-cadherin (yellow). Scale: 17.12 µm.
Figure 8.
IGF-1R:E-cadherin complex. Co-immunoprecipitation of hTCEpi whole cell lysates using two separate IGF-1R antibodies confirmed an association between IGF-1Rβ and E-cadherin. (A) IGF-1Rβ was immunoprecipitated using a rabbit polyclonal antibody (Cell Signaling). (B) IGF-1Rβ clone C-20 was immunoprecipitated using a rabbit polyclonal antibody (Santa Cruz). Blotting for IGF-1Rβ was used to confirm successful immunoprecipitation. An irrelevant IgG was used as a control.
Discussion
This is the first report localizing the IGF-1R to the nucleus and in association with the E-cadherin complex in non-differentiated corneal epithelial cells. Importantly, we identified the simultaneous expression of the 135 kDa α- and 95 kDa β-subunits within the nuclear compartment which indicates the presence of the full receptor as opposed to the aberrant intracellular trafficking of the β-subunit which has been previously reported to occur during oncogenic transformation or the nuclear accumulation of a proteolytic cleavage fragment following cleavage by γ-secretase (McElroy et al., 2007; Natalishvili et al., 2009). The localization of growth factor receptors to the nucleus has been previously reported (Carpenter, 2003). Among these include the insulin receptor, EGFR and the fibroblast growth factor receptor; of which, the latter two have been shown to undergo both ligand-induced and ligand-independent nuclear translocation (Johnston et al., 1995; Lin et al., 2001; Seol and Kim, 2003).
In agreement with our findings, two recent reports have identified IGF-1R in the nucleus of tumor cells and embryonic kidney cells; however, both studies suggest a ligand dependent role for IGF-1 in mediating IGF-1R nuclear trafficking (Aleksic et al., 2010). In contrast to this prior work, we were unable to detect an increase in nuclear IGF-1R upon ligand stimulation despite evidence of receptor activation at the plasma membrane, suggesting the presence of ligand-independent de novo trafficking. A key difference between this and prior studies however, involves the use of serum in culture media. In those earlier reported studies, as demonstrated by immunofluorescence, serum starvation depleted IGF-1R from the nucleus. While our cells our routinely cultured in serum-free media, we were unable to detect a change in localization in the presence or absence of growth factor when cultured up to 24 hours and nuclear localization appeared unchanged following IGF-1 stimulation.
Non-nuclear expression was detected in a perinuclear cap pattern that exclusively co-localized with the Golgi complex. The pattern of non-nuclear and nuclear expression appeared to be highly variable in our cell line such that in cells with strong perinuclear staining, there was weaker nuclear expression compared to cells with strong nuclear expression that lacked perinuclear localization. Since the Golgi complex functions to direct intracellular trafficking in both an antero- and retrograde manner, the flux in protein localization between the two compartments may reflect retrograde transport from the Golgi to the ER and nucleus (Wang et al., 2010). Due to the highly asynchronous nature of the cell cycle status in these cells, we postulate that the change in the distribution between Golgi and nucleus is cell cycle dependent and would suggest a role for nuclear IGF-1R in mediating proliferative events (Vincent and Feldman, 2002).
This ability of IGF-1R to undergo nuclear trafficking to mediate proliferation is consistent with other studies that have shown that within the nucleus, growth factor receptors directly and indirectly regulate expression of genes involved in mediating proliferation and cell growth. Transcriptional targets identified include cyclin D1, cyclooxygenase 2, basic fibroblast growth factor, and c-Jun (Lin et al., 2001; Peng et al., 2001; Reilly and Maher, 2001; Wang et al., 2004). The potential for protein:DNA interactions is well supported by the presence of the IGF-1R in the chromatin-bound insoluble lysate and suggests a role for the IGF-1R in gene regulation. This finding is further supported by earlier reports in other cell lines demonstrating binding of the IGF-1R to genomic DNA with a regulatory role as a transcriptional enhancer (Sehat et al., 2010).
The second key finding in this study is the association of the IGF-1R with E-cadherin in cultured corneal epithelial cells. This finding is supported by the co-localization of the IGF-1R with E-cadherin and co-immunoprecipitation from hTCEpi whole cell lysates. As E-cadherin has well known roles in mediating cellular adhesion and tissue development, the ability of growth factor receptors to bind and disrupt E-cadherin has been implicated in epithelial mesenchymal transitions and tumor invasion (Jamora and Fuchs, 2002; Mauro et al., 2002; Thiery, 2002; Van Roy and Berx, 2008). In human colonic mucosa, IGF-1R has been found complexed with E-cadherin and alpha-v integrin; subsequent stimulation by IGF-1 in this model induces cellular migration (Canonici et al., 2008). In contrast, the interaction of IGF-1R with E-cadherin has been reported to negatively regulate IGF-1 signaling in confluent Madin Darby canine kidney cells (Qian et al., 2004). Similarly, an EGFR:E-cadherin complex has also been reported to positively and negatively regulate EGFR activity in a bi-directional manner influencing both cellular adhesion and homeostatic pathways (Andl and Rustgi, 2005; Pece and Gutkind, 2000; Perrais et al., 2007; Qian et al., 2004). The ability of E-cadherin to influence IGF-1R and EGFR signaling may be due to relative expression levels between the key players dictated in part by cell confluency and calcium-induced junction formation. Further studies are necessary to investigate these regulatory pathways (Andl and Rustgi, 2005).
Collectively, the findings in this study reveal the novel localization of the IGF-1R to the nucleus and E-cadherin complex in human corneal epithelial cells. The biological significance of the IGF-1R to these distinct subcellular compartments within the corneal epithelium is currently unknown; however, intracellular trafficking of receptor tyrosine kinases to specific subcellular compartments may regulate diverse signaling pathways as reported in other cell systems. Thus, investigations into the potential function(s) of the IGF-1R outside of the canonical ligand-mediated signaling pathway are of high significance in furthering our understanding of the role of the IGF-1R in the regulation of corneal epithelial homeostatic events.
IGF-1R localizes to the nucleus in proliferating corneal epithelial cells
Nuclear localization of IGF-1R appeared independent of ligand
IGF-1R complexes with E-cadherin at points of cell-cell contact
Acknowledgments
Supported in Part by NIH Grant R01 EY018219 (DMR), Core Grant EY020799, OneSight Research Foundation, Dallas, Texas (DMR), and a Career Development Award (DMR) and an unrestricted grant from Research to Prevent Blindness, Inc., New York, New York.
Abbreviations
- EGFR
epidermal growth factor receptor
- ER
endoplasmic reticulum
- GAPDH
glyceraldehyde-3-phosphate dehydrogenase
- GM130
Golgi matrix protein
- hTCEpi
human telomerized corneal epithelial cells
- IGF-I
insulin-like growth factor-I
- IGF-II
insulin-like growth factor-II
- IGF-1R
insulin-like growth factor receptor
- kDa
kilodalton
- MAPK
mitogen activated protein kinase
- PBS
phosphate buffered saline
- SP1
specificity protein 1
- SUMO
small ubiquitin-like modifier
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
CR: The authors have no conflict of interest to disclose.
References
- Adams TE, McKern NM, Ward CW. Signalling by the type I insulin-like growth factor receptor: interplay with the epidermal growth factor receptor Growth Factors. 2004;22:89–95. doi: 10.1080/08977190410001700998. [DOI] [PubMed] [Google Scholar]
- Aleksic T, Chitnis MM, Perestenko OV, Gao S, Thomas PH, Turner GD, Protheroe AS, Howarth M, Macaulay VM. Type I insulin-like growth factor receptor translocates to the nucleus of human tumor cells. Cancer Res. 2010;70:6412–6419. doi: 10.1158/0008-5472.CAN-10-0052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andl CD, Rustgi AK. No one-way street: cross-talk between E-cadherin and receptor tyrosine kinase (RTK) signaling. Cancer Biol Ther. 2005;4:28–31. doi: 10.4161/cbt.4.1.1431. [DOI] [PubMed] [Google Scholar]
- Canonici A, Steelant W, Rigot V, Khomitch-Baud A, Boutaghou-Cherid H, Bruyneel E, Van Roy F, Garrouste F, Pommier G, Andre F. Insulin-like growth factor-1 receptor, E-cadherin and alpha v integrin form a dynamic complex under the control of alpha catenin. Int J Cancer. 2008;22:572–582. doi: 10.1002/ijc.23164. [DOI] [PubMed] [Google Scholar]
- Carpenter G. Nuclear localization and possible functions of receptor tyrosine kinases. Current Opinion Cell Biol. 2003;15:143–148. doi: 10.1016/s0955-0674(03)00015-2. [DOI] [PubMed] [Google Scholar]
- Huo H, Guo X, Hong S, Jiang M, Liu X, Liao K. Lipid rafts/caveolae are essential for insulin-like growth factor-1 receptor signaling during 3T3-L1 preadipocyte differentiation induction. J Biol Chem. 2003;278:11561–11569. doi: 10.1074/jbc.M211785200. [DOI] [PubMed] [Google Scholar]
- Jamora C, Fuchs E. Intercellular adhersion, signaling and the cytoskeleton. Nat Cell Biol. 2002;4:101–108. doi: 10.1038/ncb0402-e101. [DOI] [PubMed] [Google Scholar]
- Johnston CL, Cox HC, Gomm JJ, Coombes RC. Fibroblast growth factor receptors (FGFRs) localize in different cellular compartments. A splice variant of FGFR-3 localizes to the nucleus. J Biol Chem. 1995;270:30643–30650. doi: 10.1074/jbc.270.51.30643. [DOI] [PubMed] [Google Scholar]
- LeRoith D, Werner H, Beitner-Johnson D, Robertson CT. Molecular and cellular aspects of the insulin-like growth factor I receptor. Endocr Rev. 1995;16:143–163. doi: 10.1210/edrv-16-2-143. [DOI] [PubMed] [Google Scholar]
- Lin SY, Makino W, Xia W, Matin A, Wen Y, Kwong KY, Bourguignon L, Hung MC. Nuclear localization of EGF receptor and its potential new role as a transcription factor. Nat Cell Biol. 2001;3:802–808. doi: 10.1038/ncb0901-802. [DOI] [PubMed] [Google Scholar]
- Mauro L, Salerno M, Morelli C, Boterberg T, Bracke ME, Surmacz E. Role of the IGF-1 receptor in the regulation of cell-cell adhesion: implications in cancer development and progression. j Cell Physiol. 2002;194:108–116. doi: 10.1002/jcp.10207. [DOI] [PubMed] [Google Scholar]
- McElroy B, Powell JC, McCarthy JV. The insulin-growth factor 1 (IGF-1) receptor is a substrate for γ-secretase-mediated intramembrane proteolysis. Biochem Biophys Res Commun. 2007;358:1136–1141. doi: 10.1016/j.bbrc.2007.05.062. [DOI] [PubMed] [Google Scholar]
- Nakamura M, Chikama T-I, Nishida T. Characterization of insulin-like growth factor-1 receptors in rabbit corneal epithelial cells. Exp Eye Res. 2000;70:199–204. doi: 10.1006/exer.1999.0775. [DOI] [PubMed] [Google Scholar]
- Natalishvili N, Axelson M, Girnita L, Larsson O, Vasilcanu D. Aberrant intracellular IGF-1R β-subunit makes receptor knockout cells (IGF1r−/−) susceptible to oncogenic transformation. Exp Cell Res. 2009;315:1458–1467. doi: 10.1016/j.yexcr.2009.01.008. [DOI] [PubMed] [Google Scholar]
- O'Connor R, Fennelly C, Krause D. Regulation of survival signals from the insulin-like growth factor-I receptor. Biochem Soc Trans. 2000;28:47–51. doi: 10.1042/bst0280047. [DOI] [PubMed] [Google Scholar]
- O'Connor R, Kauffmann-eh A, Liu Y, Lehar S, Evan GI, Baserga R, Blattler WA. Identification of the domains of the insulin-like growth factor I receptor that are required for protection from apoptosis. Mol Cell Biol. 1997;17:427–435. doi: 10.1128/mcb.17.1.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pece S, Gutkind JS. Signaling from E-cadherins to the MAPK pathway by the recruitment and activation of epidermal growth factor recetors upon cell-cell contact formation. J Biol Chem. 2000;275:41227–41233. doi: 10.1074/jbc.M006578200. [DOI] [PubMed] [Google Scholar]
- Peng H, Moffett J, Myers J, Fang X, Stachowiak EK, Maher P, Kratz E, Hines J, Fluharty SJ, Mizukoshi E, Bloom DC, Stachowiak MK. Novel nuclear signaling pathway mediates activation of fibroblast growth factor-2 gene by type 1 and type 2 angiotensin II receptors. Mol Biol Cell. 2001;12:449–462. doi: 10.1091/mbc.12.2.449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrais M, Chen X, Perez-Moreno M, Gumbiner BM. E-cadherin homophilic ligation inhibits cell growth and epidermal growth factor receptor signaling independently of other cell interactions. 2007 doi: 10.1091/mbc.E06-04-0348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian X, Karpova T, Sheppard AM, McNally J, Lowy DR. E-cadherin-mediated adhesion inhibits ligand-dependent activation of diverse receptor tyrosine kinases. EMBO J. 2004;23:1739–1748. doi: 10.1038/sj.emboj.7600136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reilly JF, Maher PA. Importin beta-mediated nuclear import of fibroblast growth factor receptor: role in cell proliferation. J Cell Biol. 2001;152:1307–1312. doi: 10.1083/jcb.152.6.1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson DM, Li L, Fisher S, Pearce VP, Shay JW, Wright WE, Cavanagh HD, Jester JV. Characterization of growth and differentiation in a telomerase-immortalized human corneal epithelial cell line. Invest Ophthal Vis Sci. 2005;46:470–478. doi: 10.1167/iovs.04-0528. [DOI] [PubMed] [Google Scholar]
- Rocha EM, Cunha DA, Carneiro EM, Boschero AC, Saad MJA, Velloso LA. Identification of insulin in the tear film and insulin receptor and IGF-1R on the human ocular surface. Invest Ophthal Vis Sci. 2002;43:963–967. [PubMed] [Google Scholar]
- Romanelli RJ, LeBeau AP, Fulmer CG, Lazzarino DA, Hochberg A, Wood TL. Insulin-like growth factor type-I receptor internalization and recycling mediate the sustained phosphorylation of Akt. J Biol Chem. 2007;282:22513–22524. doi: 10.1074/jbc.M704309200. [DOI] [PubMed] [Google Scholar]
- Salani B, Passalacqua M, Maffioli S, Briatore L, Hamoudane M, Contini P, Cordera R, Maggi D. IGF-1R internalizes with caveolin-1 and PTRF/Cavin in Hacat Cells. PLoS ONE. 2010;5:e14157. doi: 10.1371/journal.pone.0014157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sehat B, Andersson S, Vasilcanu R, Girnita L, Larsson O. Role of ubiquitination in IGF-1 receptor signaling and degradation. PLoS ONE. 2007;2:e340. doi: 10.1371/journal.pone.0000340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sehat B, Tofigh A, Lin Y, Trocme E, Liljedahl U, Lagergren J, Larsson O. SUMOylation mediates the nuclear translocation and signaling of the IGF-1 receptor. Science Signaling. 2010;3:1–11. doi: 10.1126/scisignal.2000628. [DOI] [PubMed] [Google Scholar]
- Seol KC, Kim SJ. Nuclear matrix association of insulin recepetor and IRS-1 by insulin in osteoblast-like UMR-106 cells. Biochem Biophys Res Commun. 2003;306:898–904. doi: 10.1016/s0006-291x(03)01046-5. [DOI] [PubMed] [Google Scholar]
- Steele-Perkins G, Turner J, Edman JC, Hari J, Pierce SB, Stover C, Rutter WJ, Roth RA. Expression and characterization of a functional human insulin-like growth factor receptor. J Biol Chem. 1988;263:11486–11492. [PubMed] [Google Scholar]
- Takahashi K, Suzuki K. Density-dependent inhibition of growth involves prevention of EGF receptor activation by E-cadherin-mediated cell-cell adhesion. Exp Cell Res. 1996;226:214–222. doi: 10.1006/excr.1996.0221. [DOI] [PubMed] [Google Scholar]
- Takeichi M. Cadherins: a molecular family important in selective cell-cell adhesion. Annu Rev Biochem. 1990;59:237–252. doi: 10.1146/annurev.bi.59.070190.001321. [DOI] [PubMed] [Google Scholar]
- Thiery JP. Epithelial-mesenchymal transitions in tumour progression. Nat Rev Cancer. 2002;2:442–454. doi: 10.1038/nrc822. [DOI] [PubMed] [Google Scholar]
- Valentinis B, Baserga R. IGF-1 receptor signaling in transformation and differentiation. Mol Pathol. 2001;54:133–137. doi: 10.1136/mp.54.3.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Roy F, Berx G. The cell-cell adhesion molecular E-cadherin. Cell Mol Life Sci. 2008;65:3756–3788. doi: 10.1007/s00018-008-8281-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincent AM, Feldman EL. Control of cell survival by IGF signaling pathways. Growth Hormone and IGF Res. 2002;12:193–197. doi: 10.1016/s1096-6374(02)00017-5. [DOI] [PubMed] [Google Scholar]
- Wang SC, Lien HC, Xia W, Chen IF, Lo HW, Wang Z, Ali-Seyed M, Lee DF, Bartholomeusz G, Ou-Yang F, Giri DK, Hung MC. Binding at and transactivation of the COX-2 promoter by nuclear tyrosine kinase receptor ErbB-2. Cancer Cell. 2004;6:251–261. doi: 10.1016/j.ccr.2004.07.012. [DOI] [PubMed] [Google Scholar]
- Wang Y-N, Wang H, Yamaguchi H, Lee H-J, Lee H-H, Hung M-C. COPI-mediated retrograde trafficking from the Golgi to the ER regulates EGFR nuclear transport. Biochem Biophys Res Commun. 2010;399:498–504. doi: 10.1016/j.bbrc.2010.07.096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werner H, LeRoith D. The role of the insulin-like growth factor system in human cancer. Adv Cancer Res. 1996;68:183–223. doi: 10.1016/s0065-230x(08)60354-1. [DOI] [PubMed] [Google Scholar]