Abstract
It is well established that 4-Hydroxynonenal (HNE) plays a major role in oxidative stress-induced signaling and the toxicity of oxidants. Surprisingly our recent studies also demonstrate that low levels of HNE generated during oxidative stress promote cell survival mechanisms and proliferation. Since the expression and secretion of VEGF is known to be affected by Oxidative stress, during present studies, we have examined dose dependent effect of HNE on VEGF expression and secretion in a model of Retinal Pigment Epithelial (RPE) cells in culture. Results of these studies showed that while inclusion of 0.1μM HNE in the medium caused increased secretion of VEGF, its secretion and expression was significantly suppressed in the presence of >5 μM HNE in the media. These concentration dependent hormetic effects of HNE on VEGF secretion could be blocked by the over expression of GSTA4-4 indicating that these effects were specifically attributed to HNE and regulated by GSTA4-4. VEGF secreted in to the media showed angiogenic properties as indicated by increased migration and tube formation of HUVEC in matrigel when grown in media from RPE cells treated with 1 μM HNE. The corresponding media from GSTA4-4 over expressing RPE cells had no effect on migration and tube formation of HUVEC in matrigel. These results are consistent with earlier studies showing that at low concentrations, HNE promotes proliferative mechanisms and suggest that HNE induces VEGF secretion from RPE cells that acts in a paracrine fashion to induce angiogenic signaling mechanism in the endothelial cells. These findings may suggest a role of HNE and GSTA4-4 in oxidative stress induced proliferative retinopathies.
Keywords: Lipid peroxidation, 4-Hydroxy-t-2-nonenal, Glutathione S-transferasesA4-4, Vascular Endothelial Growth Factor, Vascular Endothelial Growth Factor Receptor, Retinal pigment epithelium cells
1. Introduction
4-hydroxynoninal (HNE) is the predominant end-product of lipid peroxidation (LPO) that contributes to cytotoxicity of oxidants via electrophilic attack on DNA and proteins [1,2]. It contributes to toxicity by inducing pro-apoptotic signaling through multiple pathways and also by necrosis. We and others have established that HNE is involved in regulation of gene expression and cell cycle signaling in a concentration dependent manner, and that its concentration in cells is regulated through a coordinated action of GSTA4-4, that catalyzes its conjugation to GSH and RLIP76, that transports GS-HNE conjugate out of cells. GstA4 knock-out mice having impaired HNE metabolism and increased HNE levels in tissues are more sensitive to the toxicity of oxidant chemicals/oxidative stress suggesting the role of HNE in the mechanisms of toxicity of oxidant xenobiotics and a protective role of GSTA4-4 against oxidative stress. However, recent studies have shown that unless subjected to oxidative stress, GstA4 knock-out mice have a normal phenotype, and surprisingly show a noticeable increase in their life span [3]. Other intriguing aspects of HNE-induced signaling observed in our studies and also reported by other investigators are its concentration dependent effects causing apoptosis at high concentrations, but promoting proliferation at low concentrations. These contrasting concentration dependent effects of HNE are observed in most of the cell types studied so far. The physiological significance of the contrasting hormetic effects of HNE on signaling and the mechanisms responsible for a surprisingly higher life span of GstA4 knock-out mice are not understood and need to be investigated.
Our recent studies show that besides being toxic HNE also induces defense mechanisms against oxidative stress to prevent its own toxic effects and protect the neighboring cells from a run-away apoptosis . These studies have shown that HNE induces defense mechanisms such as transcriptional activation of heat shock factors (HSFs), induction of HSP70, induction of anti-oxidant enzymes, and the activation of Daxx mediated anti-apoptotic mechanisms. More importantly, these studies suggest that oxidative stress (UV radiation or H2O2)-induced activation of these defense mechanisms requires HNE. Together, these findings suggest a requirement of HNE for the activation of defense mechanisms against oxidative stress for cell survival. VEGF is a homo-dimeric protein of about 34–45 kDa and it is implicated in angiogenesis in cancers and also in retinal microenvironment [4]. In vaso-proliferative disorders, including ARMD and diabetic retinopathy [5] the retinal pigment epithelial layer has been suggested to be the source of VEGF and it has been shown that oxidative stress causing agents and HNE can induce VEGF secretion from RPE cells. Thus, during present studies we have systematically investigated the dose dependent effect of HNE, an inevitable consequence of oxidative stress, on the expression of VEGF and VEGFR to address the hypothesis that at low concentration HNE activates various survival mechanisms. We have also evaluated the possible physiological consequences of HNE induced secretion of VEGF secreted from RPE cells shows angiogenic effects in an in vitro model. Here we show, for the first time that HNE exerts a hormetic (concentration dependent opposite effect) on the secretion of VEGF, i.e. at low levels HNE causes increased secretion of VEGF from RPE cells but at higher concentration, it inhibits VEGF secretion.
2. Materials and Methods
2.1 Material
HNE was purchased from Cayman Chemical (Ann Arbor, MI). Bradford reagent, bisacrylamide, and SDS for SDS PAGE were obtained from Bio-Rad (Hercules, CA). Western blot stripping buffer was obtained from Pierce Co. (Rockford, IL). EGM-2 bullet kit medium was purchased from Lonza (Walkersville, MD). The cell culture medium DMEM, Lipofectamine 2000 transfection reagent, and fetal bovine serum were purchased from GIBCO (Invitrogen, Carlsbad, CA). All other reagents and chemicals including DMSO, G418 (geneticin), and 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT), etc. were purchased from Sigma-Aldrich (St. Louis, MO).
2.2 Cell lines
The simian virus SV40-transformed human fetal male RPE 28 cells (Coriell Institute, Camden, NJ) that exhibit epithelioid morphology and retain physiological functions characteristic of the primary human RPE cells were cultured in standard medium containing 10% fetal bovine serum and antibiotics in a humidified incubator at 37°C in 5% CO2 atmosphere as described before [6]. The HUVEC (Lonza, Walkersville, MD) cells were grown in EGM-2 bullet kit. All studies were conducted by using cells of passages 10–20 for RPE and 2–6 for HUVEC. The cells were trypsinized and passaged every 3–4 days.
2.3 Cell viability assay
The cytotoxicity of HNE to RPE cells was measured by the 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyl-2H-tetrazolium Bromide (MTT) assay as described before [7] with minor modifications. Briefly, 2 × 104 cells in 190 μl of medium were seeded in 96-well microtiter plates and allowed to attach for 24 h. Next day, HNE in 10 μL PBS was added to achieve the desired concentration. After 12 h incubation, 10 μl of a stock solution of MTT (5 mg/ml in PBS) was added to each well and the plates were incubated for additional 4 h at 37°C, centrifuged, and the medium was decanted. Cells were subsequently dissolved in 100 μl DMSO with gentle shaking for 2 h at room temperature, followed by measuring absorbance at 562 nm in a micro plate reader (El x 808 BioTek Instruments, Inc). A dose-response curve was plotted and the concentration of HNE causing a 50% reduction in formazan crystal formation (IC50) was determined.
2.4 LDH assay
To measure lactate dehydrogenase (LDH) released from the RPE cells, a commercially available cytotoxicity detection kit was used as briefly described below: Cells (2 × 104 in 190 μl of medium) were seeded in 96-well microtiter plates and allowed to attach for 24 h. The next day, 10 μl of PBS containing the desired concentration of HNE was added. After 12 h incubation, total culture medium was collected and centrifuged to remove contaminating cells and cellular debris. The volume of media was then measured. For assay, 100 μl of each sample was transferred to a 96 well microtiter plate, 100 μl of LDH reagent mixture was added to each well and incubated for up to 30 min at room temperature. After incubation 50 μl stop solution was added and the absorbance of samples was measured at 490 nm.
2.5 Transient transfection with hGSTA4
Transient transfection of RPE cells was performed with hGSTA4 as described by us before [6,13]. Briefly, RPE cells at a density of 5 × 105 cells per 100 mm Petri dish were plated and the dishes having >70% confluent cells were used for the transfection with 24 μg of either empty pTarget-T vector (VT) or the pTarget vector containing the open reading frame (ORF) of the restored Kozak hGSTA4 sequence (hGSTA4-Tr). Lipofectamine 2000 reagent (Invitrogen, Carlsbad, CA) was used for transfection as per the manufacturer s instructions.
2.6 Western Blot Analysis
The cells with or without specified treatment(s), were pelleted, washed thrice with PBS, re-suspended in RIPA lysis buffer (50 mM Tris-HCl, pH 7.5; 1% NP-40; 150 mM NaCl; 1 mg/ml aprotinin; 1 mg/ml leupeptin; 0.5 mM phenylmethylsulfonyl fluoride; 1 mM Na3VO4; 1 mM NaF) at 4°C for 30 min, and lysed by sonication. Cell debris was removed by centrifugation at 14,000 g for 30 min at 4°C to obtain clear extracts. Western blot analyses were performed with the extracts containing 25–75 μg protein. Protein was determined by the method of Bradford [8] throughout these studies.
2.7 Wound healing assay
In vitro wound-healing assay was performed using previously described method [9]. Briefly, HUVECs (8×104) were grown to confluence on 12-well tissue culture plates for 48 h, and then starved in serum-free maintenance medium for 4 h. A “wound” was made by scraping the middle of the cell monolayer with a sterile 10 μL micropipette tip. Floating cells were removed by extensive washing with PBS and the conditioned media obtained from RPE cell cultures with or without HNE treatment was added to each of the wells. Cells were stained with crystal violet and photographed using a phase-contrast microscope at 12 h after wounding.
2.8 Estimation of VEGF
RPE cells (2×104) grown in 96-well plates were incubated with serum-free medium containing varying concentrations (0–20 μM) of HNE. At the end of 12 h incubation, supernatants were collected and secreted VEGF was measured using an enzyme-linked immunosorbent assay (ELISA) according to manufacturer s (R&D Systems, Inc, Minneapolis, MN) recommendations and absorbance was measured using a microtiter plate reader (Fischer Scientific, PA) with a test wavelength of 450 nm and a reference wavelength set at 550 nm.
2.9 Tube-formation Assay
Growth factor-reduced (GFR) Matrigel (BD Biosciences, New Bedford, MA) was added to wells of a cold 96-well plate (45 μl/well), and then incubated at 37°C for 1 h to allow gelling. Overnight starved HUVEC cells were seeded onto the Matrigel. Medium collected from HNE-treated and untreated RPE conditioned medium was then placed on these cells. Cells were observed directly and number of tubes and length of the tubes were measured using Image J software under dark field illumination on an inverted light microscope at low power. Photographs were also taken with a digital camera under the same conditions.
2.10 Statistical Analysis
Statistical analyses were performed by using Student s t test. P value < 0.05 is considered as statistically significant. Representatives of p-value in figures include “*”<0.05. Analysis of Variance (ANOVA) was used for the analysis of VEGF secretion on exposure to HNE.
3. RESULTS
3.1 HNE causes toxicity to RPE cells
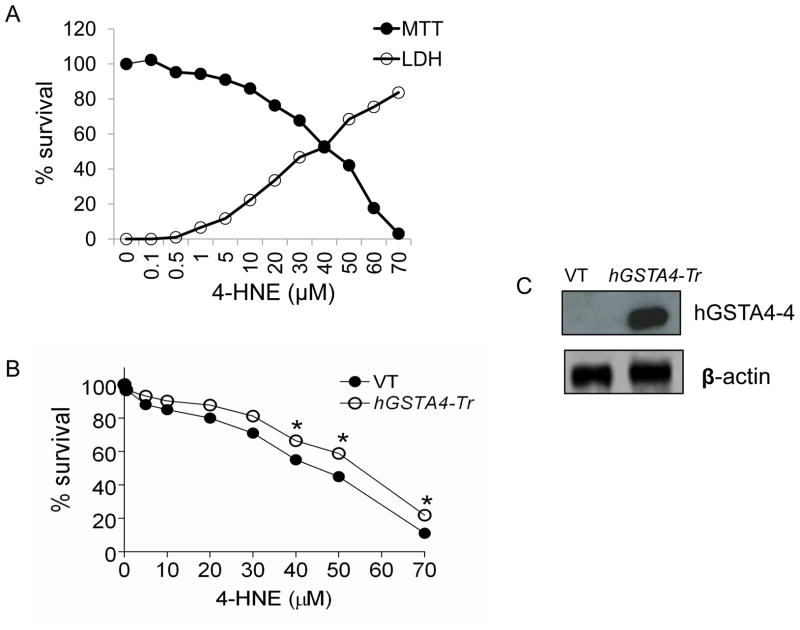
HNE cytotoxicity to cells is well known [10–14]. To assess the effect of HNE accumulation on RPE cells in the retinal microenvironment as a result of oxidative stress, we examined the cytotoxicity of HNE to RPE cells. These studies were particularly conducted to assess HNE toxicity at physiologically relevant, low concentrations. HNE treatment decreased the viability of RPE cells in a dose-dependent manner. At physiologically relevant concentrations (below 5 μM), the toxicity of HNE was only minimal but it increased remarkably at higher concentrations. As shown in Figure 1A, the IC50 of HNE was found to be 45 μM ± 4.2 that was similar in range to the value previously reported [6,15] by us for these cells. The results of LDH leakage from the cells we consistent to those of MTT assay indicating only minimal HNE toxicity at physiologically relevant concentrations. The protective role of hGSTA4-4, against HNE cytotoxicity to RPE cells was evaluated by comparing the effect of HNE on empty vector-transfected (VT), and hGSTA4-transfected (GSTA4-Tr) cells using the MTT assay. The over expression of hGSTA4-4 in transiently transfected cells was confirmed by Western blot analysis where a robust expression of hGSTA4-4 was seen. Cells over expressing hGSTA4-4 were significantly protected against HNE toxicity (Fig. 1B) as illustrated by a shift in HNE IC50 value (IC50 values for the vector, and hGSTA4 transfected cells; 45 μM ± 4.2 μM, and 59 ± 3.7 μM, respectively).
Figure 1. Effect of HNE toxicity in RPE cells.
(A) RPE cells were treated with 0–70 μM of HNE for 12 h and assayed for cytotoxicity by MTT and LDH assay. (B) RPE cells were transiently transfected with empty p-Target vector (VT) and p-Target vector containing the ORF of hGSTA4 sequence (hGSTA4-Tr). VT and hGSTA4-Tr cells were treated with 0–70 μM of HNE for 12 h and assayed for cytotoxicity by MTT assay. The plots show the percent cell survival (mean ± SD, n = 4) at different concentrations of HNE. (C) Over-expression of hGSTA4-4 in transfected cells was confirmed by western blot analysis.
3.2 HNE increases VEGF secretion in RPE cells
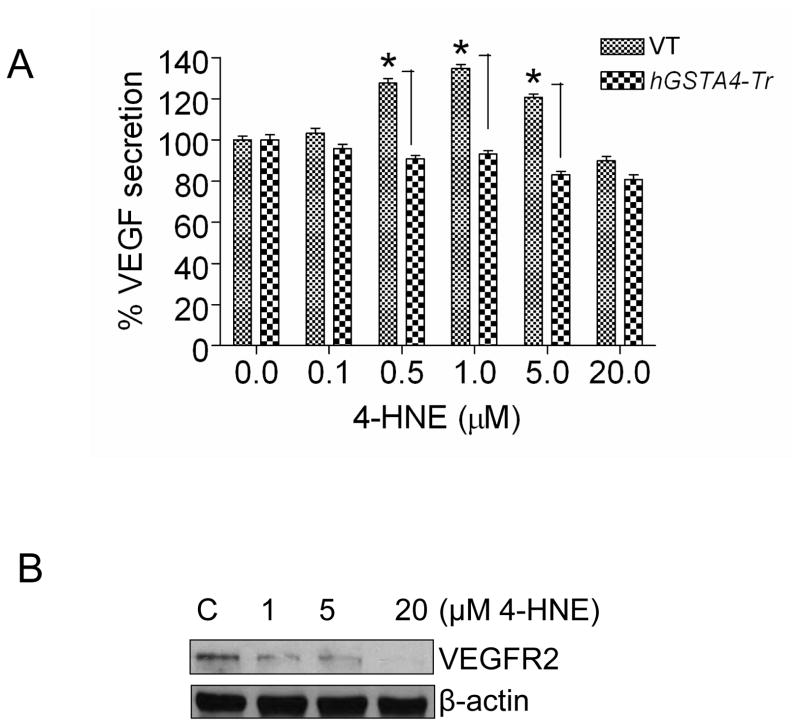
Previous studies from our laboratory have shown that HNE is involved in oxidative stress-induced signaling in RPE cells [6,15]. Since VEGF secretion has been shown to be affected by oxidative stress and also by HNE, we studied dose dependent effect of HNE on the expression and secretion of VEGF in these cells. The results of these experiments presented in Fig. 2A suggested that the inclusion of HNE in the media at concentrations as low as of 0.1 to 1.0 μM caused a remarkable increase in the secretion of VEGF from RPE cells into the media. Optimal secretion of VEGF was observed at 1.0 μM HNE that showed a plateau thereafter up to about 5 μM of HNE and decreased when concentrations were higher than 5 μM. HNE-induced secretion of VEGF was abrogated in hGSTA4 transfected cells indicating HNE as the causative factor for induction of VEGF secretion. The effect of HNE on the expression of VEGF receptor (VEGFR-2) was also studied. The results of these experiments (Fig. 2B) showed that 1 μM HNE which optimally induced VEGF secretion caused a significant suppression of VEGFR-2 expression in RPE cells and at high HNE concentration VEGFR-2 expression was almost completely abrogated.
Figure 2. Effect of HNE on VEGF secretion and VEGFR-2 expression in RPE cells.
(A) Effect of HNE on VEGF secretion: RPE cells were transiently transfected with empty p-Target vector (VT) and p-Target vector containing the ORF of hGSTA4 sequence (hGSTA4-Tr). VT and hGSTA4-Tr cells were treated with 0–20 μM of HNE for 12 h and assayed for VEGF secretion by ELISA as mentioned in Method Section. The data represent the mean ± SD (n =3). (B) Effect of 4-HNE on VEGFR-2 expression: RPE cells were treated with 0–20 μM HNE. Cell extracts prepared as described in methods section containing 70 μg protein were subjected to Western blot analysis. Western blot analysis was performed with total cell lysates using indicated antibodies. β-actin was used to control for loading differences.
3.3 VEGF secreted from RPE cells increases migration and wound healing capability of HUVEC cells in vitro
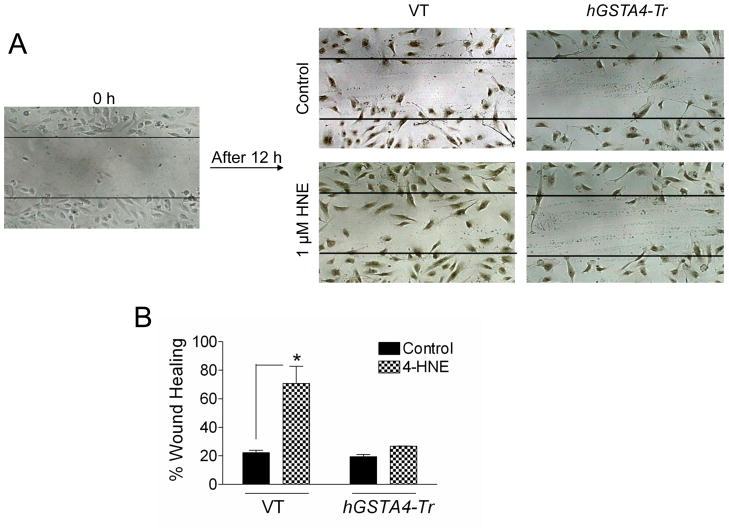
VEGF is implicated as a pro-angiogenic factor that provokes neovascularization in ARMD [16] and RPE has been recognized as an important source for VEGF in this context [5]. Since cell migration is necessary for endothelial cells in neovascularization, we performed an in vitro wound healing migration assay [17] to evaluate the physiological significance of HNE-induced secretion of VEGF in the medium. As described previously for this assay [18], a confluent monolayer of HUVEC cells was scraped across with a pipette tip to create a wounded region. The conditioned medium from RPE cells exposed to 1 μM HNE (that showed maximal secretion of VEGF) or that from control cells without HNE exposure was applied to HUVECs and wound closure or cell migration into the wounding area after 12 h was compared. As shown in Fig. 3, HUVEC cells in the conditioned medium from HNE treated RPE cells showed significantly greater progressive reoccupation of the wounded region as compared to those cells treated with the control RPE conditioned medium without HNE treatment. Similar to the control media, the media from hGSTA4 transfected cells (that did not have increased VEGF secretion Fig. 2A) also did not show any accelerated wound healing in HUVEC cells. Together, these results show that increased secretion of VEGF by low levels of HNE promotes proliferation of endothelial cells in an in vitro model that is consistent with our hypothesis that at low concentrations HNE promotes cell survival mechanisms.
Figure 3. Effect of RPE conditioned medium on HUVEC cells in wound healing assay.
HUVEC cells at confluence were injured by a scratch with a 10 μL pipette tip. Wounded cells were allowed to heal for 12 h in presence of conditioned medium from control VT and hGSTA4-Tr cells and these treated with 0–1 μM of HNE for 12 h. The photograph is representative of three experiments showing similar results. The data in bar graph represents the mean ± SD (n=3).
3.4 RPE conditioned medium increases tube formation in HUVEC cells in an in vitro angiogenesis assay
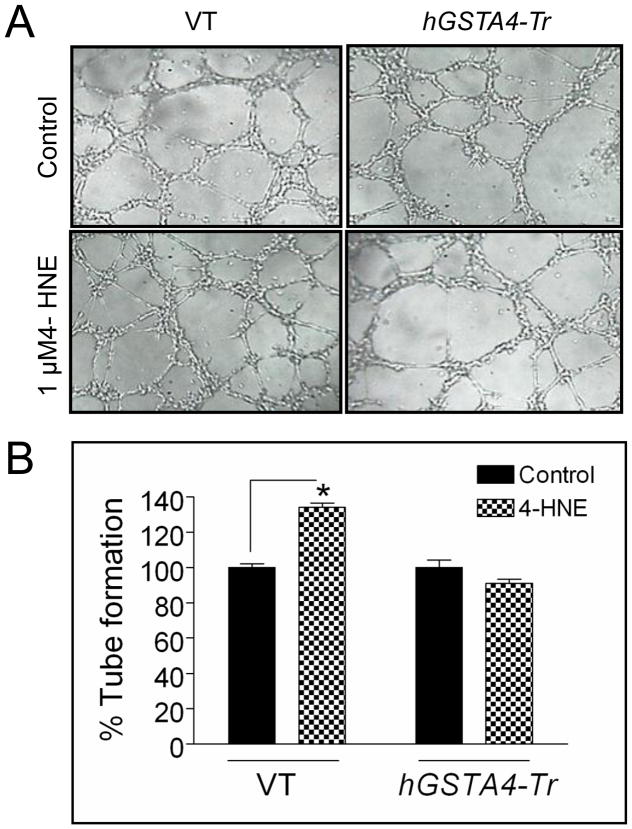
Although angiogenesis is an outcome of multifaceted functionality of several kinds of cells, the tube formation of endothelial cells is a key step in this process [19,20]. To investigate the pro-angiogenic effects of VEGF secreted from HNE-treated RPE cells, we performed in vitro matrigel tube formation assay [20,21]. HUVEC cells, plated on matrigel were grown with and without HNE treated RPE conditioned medium. The length and number of tubes formed in both the cells were measured by Image J software and compared. As shown in Fig. 4, HUVEC cells cultured in media of 1 μM HNE treated RPE cells (with maximal VEGF secretion, Fig. 2) showed increased tube formation as compared to the media of the control cells without HNE treatment. Also, no significant difference in the length and number of tubes formed in HUVEC cells was observed in the presence of conditioned medium from hGSTA4 transfected RPE cells with or without HNE treatment. Together, these results suggest that HNE-induced VEGF secretion contributes to angiogenesis in surrounding epithelial cells that could be inhibited by GSTA4-4.
Figure 4. Effect of RPE conditioned medium on HUVEC cells in tube formation assay.
HUVEC cells were allowed to grow on matrigel for 4 h in presence of conditioned medium from VT and hGSTA4-Tr cells treated with 0–1 μM of HNE for 12 h. The photograph is representative of three experiments showing similar results. The data in bar graph represents the mean ± SD (n=3).
4. Discussion
In present communication we demonstrate that exposure of RPE cells to HNE increases secretion of VEGF from these cells in a concentration dependent manner. Similar to the reported hormetic effect of HNE on EGFR [6], HNE at low levels promotes VEGF secretion in cells and inhibits this secretion at high levels. In a series of experiments we also demonstrate the physiological consequences of the secretion of VEGF by low levels of HNE that may correspond to low levels of oxidative stress. These experiments in which the effect of VEGF secreted in the media of HNE-treated RPE cells was examined on migration and angiogenesis of HUVEC cells were conducted to simulate the effect of HNE-induced secretion of VEGF on retinal micro-environment. The results of these experiments clearly showed that HNE-induced secretion of VEGF promoted increased migration and angiogenesis in HUVEC cells. Furthermore, these effects of HNE were suppressed in GSTA4 transfected cells indicating that the secretion of VEGF and the consequent physiological effects could be specifically attributed to HNE. Increased proliferation of endothelial cells is associated with the excess angiogenesis in the retina. The secreted VEGF exerts its paracrine effect on the surrounding endothelial cells resulting in the proliferation of endothelial cells in the retinal micro-environment that may contribute to the pathologic processes for retinal neo-vascularization. HNE-induced signal to suppress VEGFR-2 expression in RPE cells observed in present studies may be to block the autocrine effects of secreted VEGF on RPE cells. Physiologically relevant concentrations of HNE have been shown to induce EGFR pathway involved in the proliferation of RPE cells [6]. Here we show that at these concentrations, HNE is also associated with the increase in VEGF secretion and proliferation of surrounding endothelial cells. Together, these findings suggest that at physiologically relevant low concentrations HNE seems to promote protective mechanisms but its chronically increased levels due to persistent oxidative stress it may contribute to proliferative retinopathy in the long run.
Our previous studies have shown that oxidative stress-induced signaling in various cell types is mediated via HNE [12,22]. We have previously shown that even a non-toxic mild transient oxidative stress such as UVA exposure to 5 min leads to HNE formation. Moreover, the cells pre-exposed to 5min UVA shows a significant resistance to oxidative stress induced cell death. Also, it is documented that low levels of HNE promote induction of defense mechanisms such as activation of HSF1, Nrf2, Daxx and EGFR. Thus the results of present studies taken together with these previous studies suggest a novel role of HNE in stress induced signaling. In this model, low levels of HNE generated in cells during mild oxidative stress would act as a sensor of oxidative stress to initiate a multitude of signals for promoting mechanisms for defense against oxidative stress. This would be consistent with the observed resistance of mild stress preconditioned cells [23,24], and the activation of HSF1 [25], Nrf2 [26], Daxx [27], EGFR [6,22] and the secretion of VEGF by low levels of HNE observed here. Thus initial low level of HNE formed during oxidative stress seem to minimize the toxicity of initial oxidative insult by increasing the threshold of stress only beyond which cytotoxicity would be manifested. This model would also explain studies showing activation of HSF1 [25], Nrf2 [26], Daxx [27], and p53 [28] in the tissues of GSTA4 null mice [15] and increased life span of these mice [3].
Highlights.
Low concentration of HNE (0.1–1.0μM) induced secretion of VEGF in RPE cells.
VEGF secreted medium of RPE cells promoted proliferation of endothelial cells.
VEGFR2 expression was attenuated with increasing concentrations of HNE.
These effects of HNE could be blocked by the over expression of GSTA4-4 in cells.
Acknowledgments
**This work was supported in part by NIH Grants EY 04396.
The abbreviations used are
- HNE
4-hydroxy-2-nonenal
- GST
glutathione S-transferase
- LPO
Lipid peroxidation
- VEGF
Vascular Endothelial Growth Factor
- VEGFR
Vascular Endothelial Growth Factor Receptor
- HUVEC
Human Umbilical Vascular Endothelial Cells
- RPE
Retinal Pigment Epithelial Cells
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Chung FL, Chen HJ, Nath RG. Lipid peroxidation as a potential endogenous source for the formation of exocyclic DNA adducts. Carcinogenesis. 1996;17:2105–2111. doi: 10.1093/carcin/17.10.2105. [DOI] [PubMed] [Google Scholar]
- 2.Uchida K, Stadtman ER. Modification of histidine residues in proteins by reaction with 4-hydroxynonenal. Proc Natl Acad Sci USA. 1992;89:4544–4548. doi: 10.1073/pnas.89.10.4544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Singh SP, Niemczyk M, Saini D, et al. Disruption of the mGsta4 gene increases life span of C57BL mice. J Gerontol A Biol Sci Med Sci. 2010;65:14–23. doi: 10.1093/gerona/glp165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yancopoulos GD, Davis S, Gale NW, et al. Vascular-specific growth factors and blood vessel formation. Nature. 2000;407:242–248. doi: 10.1038/35025215. [DOI] [PubMed] [Google Scholar]
- 5.Adamis AP, Shima DT, Yeo KT, et al. Synthesis and secretion of vascular permeability factor/vascular endothelial growth factor by human retinal pigment epithelial cells. Biochem Biophys Res Commun. 1993;193:631–638. doi: 10.1006/bbrc.1993.1671. [DOI] [PubMed] [Google Scholar]
- 6.Vatsyayan R, Chaudhary P, Sharma A, et al. Role of 4-hydroxynonenal in epidermal growth factor receptor-mediated signaling in retinal pigment epithelial cells. Exp Eye Res. 2011;92:147–154. doi: 10.1016/j.exer.2010.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods. 1983;65:55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- 8.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- 9.Xu KP, Yu FS. Cross talk between c-Met and epidermal growth factor receptor during retinal pigment epithelial wound healing. Invest Ophthalmol Vis Sci. 2007;48:2242–2248. doi: 10.1167/iovs.06-0560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Esterbauer H, Schaur RJ, Zollner H. Chemistry and biochemistry of 4-hydroxynonenal, malonaldehyde and related aldehydes. Free Radic Biol Med. 1991;11:81–128. doi: 10.1016/0891-5849(91)90192-6. [DOI] [PubMed] [Google Scholar]
- 11.Awasthi YC, Yang Y, Tiwari NK, et al. Regulation of 4-hydroxynonenal-mediated signaling by glutathione S-transferases. Free Radic Biol Med. 2004;37:607–619. doi: 10.1016/j.freeradbiomed.2004.05.033. [DOI] [PubMed] [Google Scholar]
- 12.Awasthi YC, Sharma R, Sharma A, et al. Self-regulatory role of 4-hydroxynonenal in signaling for stress-induced programmed cell death. Free Radic Biol Med. 2008;45:111–118. doi: 10.1016/j.freeradbiomed.2008.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li J, Sharma R, Patrick B, et al. Regulation of CD95 (Fas) expression and Fas-mediated apoptotic signaling in HLE B-3 cells by 4-hydroxynonenal. Biochemistry. 2006;45:12253–12264. doi: 10.1021/bi060780+. [DOI] [PubMed] [Google Scholar]
- 14.Chaudhary P, Sharma R, Sharma A, et al. Mechanisms of 4-hydroxy-2-nonenal induced pro- and anti-apoptotic signaling. Biochemistry. 2010;49:6263–6275. doi: 10.1021/bi100517x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sharma A, Sharma R, Chaudhary P, et al. 4-Hydroxynonenal induces p53-mediated apoptosis in retinal pigment epithelial cells. Arch Biochem Biophys. 2008;480:85–94. doi: 10.1016/j.abb.2008.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bressler NM, Bressler SB, Fine SL. Age-related macular degeneration. Surv Ophthalmol. 1988;32:375–413. doi: 10.1016/0039-6257(88)90052-5. [DOI] [PubMed] [Google Scholar]
- 17.Liang CC, Park AY, Guan JL. In vitro scratch assay: a convenient and inexpensive method for analysis of cell migration in vitro. Nat Protoc. 2007;2:329–333. doi: 10.1038/nprot.2007.30. [DOI] [PubMed] [Google Scholar]
- 18.Ohno-Matsui I, Morita J Tombran-Tink, et al. Novel mechanism for age-related macular degeneration:an equilibrium shift between the angiogenesis factors VEGF and PEDF. J Cell Physiol. 2001;189:323–333. doi: 10.1002/jcp.10026. [DOI] [PubMed] [Google Scholar]
- 19.Liekens S, De Clercq E, Neyts J. Angiogenesis: regulators and clinical applications. Biochem Pharmacol. 2001;61:253–270. doi: 10.1016/s0006-2952(00)00529-3. [DOI] [PubMed] [Google Scholar]
- 20.Papetti M, Herman IM. Mechanisms of normal and tumor-derived angiogenesis. Am J Physiol Cell Physiol. 2002;282:C947–C970. doi: 10.1152/ajpcell.00389.2001. [DOI] [PubMed] [Google Scholar]
- 21.Kohn EC, Alessandro R, Spoonster J, et al. Angiogenesis: role of calcium-mediated signal transduction. Proc Natl Acad Sci U S A. 1995;92:1307–1311. doi: 10.1073/pnas.92.5.1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu W, Akhand AA, Kato M, et al. 4-Hydroxynonenal Triggers an Epidermal Growth Factor Receptor-Linked Signal Pathway for Growth Inhibition. J Cell Sci. 1999;112:2409–2417. doi: 10.1242/jcs.112.14.2409. [DOI] [PubMed] [Google Scholar]
- 23.Yang Y, Sharma A, Sharma R, et al. Cells preconditioned with mild, transient UVA irradiation acquire resistance to oxidative stress and UVA induced apoptosis: role of 4-hydroxynonenal in UVA mediated signaling for apoptosis. J Biol Chem. 2003;278:41380–41388. doi: 10.1074/jbc.M305766200. [DOI] [PubMed] [Google Scholar]
- 24.Cheng JZ, Sharma R, Yang Y, et al. Accelerated metabolism and exclusion of 4-hydroxynonenal through induction of RLIP76 and hGST5.8 is an early adaptive response of cells to heat and oxidative stress. J Biol Chem. 2001;276:41213–41223. doi: 10.1074/jbc.M106838200. [DOI] [PubMed] [Google Scholar]
- 25.Jacobs AT, Marnett LJ. Heat shock factor 1 attenuates 4-Hydroxynonenal mediated apoptosis: critical role for heat shock protein 70 induction and stabilization of Bcl-XL. J Biol Chem. 2007;282:33412–33420. doi: 10.1074/jbc.M706799200. [DOI] [PubMed] [Google Scholar]
- 26.Ishii T, Itoh K, Ruiz E, et al. Role of Nrf2 in the regulation of CD36 and stress protein expression in murine macrophages: activation by oxidatively modified LDL and 4-hydroxynonenal. Circ Res. 2004;94:609–616. doi: 10.1161/01.RES.0000119171.44657.45. [DOI] [PubMed] [Google Scholar]
- 27.Khelifi AF, Alcontres MSD, Salomoni P. Daxx is required for stress-induced cell death and JNK activation. Cell Death Differ. 2005;12:724–733. doi: 10.1038/sj.cdd.4401559. [DOI] [PubMed] [Google Scholar]
- 28.Lotem J, Peled-Kamar M, Groner Y, et al. Cellular oxidative stress and the control of apoptosis by wild-type p53, cytotoxic compounds, and cytokines. Proc Natl Acad Sci USA. 1996;93:9166–9171. doi: 10.1073/pnas.93.17.9166. [DOI] [PMC free article] [PubMed] [Google Scholar]