Abstract
Purpose
The aim of this study was to elucidate the effect of a bovine hydroxyapatite/collagen (BHC) block in one-wall intrabony periodontal defects in dogs.
Methods
A one-wall intrabony periodontal defect (4 mm wide and 5 mm deep) was prepared bilaterally at the mesial side of the mandibular fourth premolar in five beagle dogs. After thorough root planing, block-type BHC (4×5×5 mm) was placed on one side. The contralateral defect area did not receive any material as a sham-surgery control. Histological analysis of the sites was performed after an 8-week healing period.
Results
Two of five samples in the experimental group healed well without dissipation of the graft materials, and histological analysis revealed excellent regeneration of the periodontal tissues. However, most of the grafted materials had been displaced in the other three samples, leaving only a small portion of the graft. The measured parameters exhibited large standard deviations, and the mean values did not differ significantly between the experimental and sham-surgery control sides.
Conclusions
The application of BHC alone-without a barrier membrane-to wide, one-wall intrabony periodontal defects yielded inconsistent results regarding both periodontal regeneration and substantivity of the graft materials. Thus, the use of a barrier membrane for noncontained-type defects is recommended to improve the stability of the grafted material, and to condense it.
Keywords: Collagen, Guided tissue regeneration, Histology
INTRODUCTION
The concept of guided tissue regeneration (GTR) for periodontal disease has been extended to guided bone regeneration for implant surgery. Both treatment modalities commonly require a barrier membrane to prevent epithelial downgrowth and to provide provisional space for regeneration [1]. Indeed, a barrier membrane has been considered essential for the achievement of successful results.
These membranes can be categorized based on their bioresorbability as either nonresorbable or resorbable. Nonresorbable membranes, such as expanded polytetrafluoroethylene, may suit the requirements for the ideal barrier membrane with regard to both mechanical strength and cell occlusiveness [2,3]. However, the frequent prevalence of wound dehiscence associated with their use can produce an adverse outcome [4]. The necessity of a second operation to remove the membrane is another drawback of the nonresorbable-type membrane. In contrast, resorbable membranes, such as the double-layered porcine type I collagen membrane, exhibit relatively good handling properties and can be resorbed over a reasonable period, negating the need for an additional surgical intervention [5]. However, these membranes are vulnerable to collapse due to their low mechanical stability. It is thus recommended that they be reinforced by combining them with bone graft material [6].
Since the use of membranes increases the cost and extends the operation time, bone grafting without a barrier membrane has been attempted as an alternative treatment modality. Tight filling of the bone substitute particles themselves at the intrabony defect reportedly protects the epithelial cells from migration without the need for a barrier membrane [7,8], and it seems that bone grafting alone can act as both a membrane and a scaffold if the grafted materials are well localized and maintained within the defect. However, a meta-analysis found that the use of a membrane in the treatment of intrabony defects might enhance clinical parameters compared to a bone graft alone [9,10].
Several preclinical studies found no distinctive differences between the aforementioned treatment modalities (i.e., bone graft alone versus a combination of barrier membrane and bone graft) [6,11,12]. It appears to be difficult to localize a particulated bone graft within the defect without the aid of a barrier membrane [13], especially when there are few bony walls surrounding the osseous defect, which is a critical factor in the estimation of regenerative potential. Favorable results would be expected even following flap debridement alone for contained defects, such as extraction sockets, and three-wall defects, where blood clots can be stably maintained. Conversely, noncontained defects such as two- or one-wall defects have fewer sources of progenitor cells compared to contained defects, and stabilization of blood clots is difficult to achieve by itself.
In order to achieve ease of handling without the need to apply a membrane, bovine hydroxyapatite (BH) particles incorporated with a porcine type-I collagen matrix have been developed. This material can be stabilized without dissipation, like a hard sponge, which allows it to be adapted and condensed into irregular defects. BH has been used widely for the correction of periodontal osseous defects and augmentation of resorbed alveolar ridges around dental implants [5,8,14-19], and the similarity of its porosity and microstructure to human bone provide an excellent osteoconductive environment within osseous defects. Bovine hydroxyapatite/collagen (BHC) has combined the advantages of BH and a collagen matrix, and it can be softened to produce favorable manageability when soaked. Several preclinical and clinical studies have indicated that a combination of BHC grafting and GTR enhances the periodontal regeneration in periodontal intrabony defects [19-22]. BHC is expected to maintain the dimensions of the defect space during the healing phase, even in the noncontained type, such as a one-wall intrabony defect. If the stability of the grafted material within the defect is guaranteed, this may remove the need for a barrier membrane.
The aim of this study was thus to elucidate the effect of BHC grafting without a barrier membrane in one-wall periodontal intrabony defects in dogs.
MATERIALS AND METHODS
Animals
Five male beagle dogs, 15 months old and weighing about 10 to 15 kg, were used. The animals had intact dentition and a healthy periodontium. Animal selection, management, preparation, and surgical protocols followed routine procedures approved by the Animal Care and Use Committee, Yonsei Medical Center, Seoul, Korea.
Surgical procedures
The basic surgical protocol and defect site preparation-with the exception of the application of the experimental biomaterial-followed routine procedures established in previous studies [23]. Briefly, local infiltration anesthesia under general anesthesia was performed for the duration of the surgical procedure, which was performed under sterile conditions in an operating room. Following extraction of the mandibular third premolars, 8 weeks of healing were allowed. One-wall intrabony defects (4 mm wide and 5 mm deep) were prepared bilaterally (one on each side) using a high-speed fissure bur and a bone chisel at the mesial side of the mandibular fourth premolar. Following thorough planing of the root surface exposed within the defect, a reference notch was ditched at the mostapical-junction of the root adjacent to the margin of the defect. Block-type BHC (4×5×5 mm) was placed on one side (Fig. 1). The contralateral defect area did not receive any material, and was treated as a sham-surgery control. The animals were randomly assigned to the groups. Flaps were positioned coronally and sutured (Monosyn 4.0 Glyconate Monofilament, B. Braun Melsungen AG, Tuttlingen, Germany). The sutures were removed after 10 days, and a soft diet was provided throughout the study period.
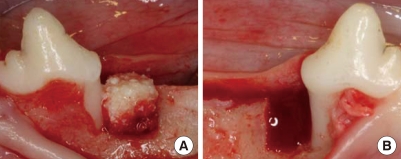
Figure 1.
Clinical photograph showing the bovine hydroxyapatite/collagen block graft site (A) and the sham-surgery control site (B) in one-wall, intrabony, periodontal defects prepared at the mesial side of the mandibular fourth premolar.
Sacrifice and histological processing
After a further 12-week healing period, the dogs were sacrificed by anesthesia drug overdose. Block sections including segments with the defects were obtained and fixed in 10% neutral buffered formalin for 10 days. After rinsing in sterile water, the sections were decalcified in 5% formic acid, dehydrated in a graded series of ethanol solutions, and embedded in paraffin. Step-serial, 5-µm-thick sections were cut in a mesial-distal vertical plane at intervals of approximately 80 µm. The mostcentral-sections of each defect site were selected based on the width of the root canal, and were stained with hematoxylin and eosin and subsequently used for the histological and histometric analyses.
Histological analysis
Overall healing patterns, including the presence of inflammation, soft tissue and bone healing, and attachment gains were observed under a light microscope (BX50, Olympus, Tokyo, Japan). After capturing the microscope images, histometric analysis was performed using a PC-based image analysis system (Image-Pro Plus, Media Cybernetics, Silver Spring, MD, USA). The measurements were made using a methodology established in previous studies [23]. The following parameters were measured:
Defect height (DH): distance from the cementoenamel junction (CEJ) to the apical extension of the reference notch.
Long junctional epithelial distance (LJE): distance from the CEJ to the apical end of the junctional epithelium.
Connective tissue attachment (CTA): distance from the end of the junctional epithelium to the coronal extension of the new cementum.
Cementum regeneration (CR): distance from the apical extension of the reference notch to the coronal extension of the new cementum or a cementum-like substance on the root surface.
Bone regeneration height (BR): distance from the apical extension of the reference notch to the coronal extension of the new bone along the root surface.
Statistical analysis
The means and standard deviations of the experimental and control data (n=5) were obtained from the measurements taken from the central section of each defect. The Wilcoxon signed-rank test was used to compare the experimental and control data using a statistical software program SPSS ver. 15.0 (SPSS Inc., Chicago, IL, USA), with the level of significance set at 5%.
RESULTS
Clinical findings
All surgical wounds were well closed at stitch removal, and the overall healing period was uneventful. No sites that underwent a surgical procedure exhibited any clinical signs of postoperative complications including infection, wound dehiscence, or abnormal bleeding following the surgery.
Histologic findings and histometric analysis
Two types of healing patterns were observed in the experimental group at 12 weeks postoperatively: favorable and unfavorable.
Favorable-healing group (n=2)
There was a certain amount of residual BH within the defect. New cementum had lined the entire denuded root surface under the apical end of the junctional epithelium (Fig. 2). No direct ankylosis was found. Some areas showing root resorption were covered with new cementum. Numerous blood vessels were observed within the periodontal ligamental space between the newly formed bone and the root surface. Obliquely running Sharpey's fibers were embedded into both sides of new cementum and new bone. The residual BH had a high porosity and was interconnected. Newly formed bone had penetrated the inside of the materials and consolidated. In one of the two specimens exhibiting favorable healing, new bone had filled the defect, reaching the BH (Fig. 3). Cementoblast-like cells had lined a seam of new cementum covering the root. In some areas, a resorption lacuna in which odontoclasts were found was observed on the exposed root surface. Apposition of new cementum on the resorbed root surface was simultaneously observed following migration of the odontoclasts at the resorption lacuna.
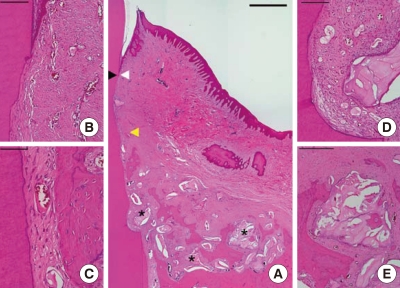
Figure 2.
Histological view of a well-healed sample (#1) in the experimental group (H&E staining). (A) Low magnification. White arrowhead apical end of the junctional epithelium; black arrowhead, coronal end of the new cementum; yellow arrowhead, coronal end of the new bone; star residual graft material (scale bar=1 mm). (B) Higher magnification at the junctional epithelium (scale bar=100 µm). (C) Higher magnification at the middle area of the root surface (scale bar=50 µm). (D) Magnified view at the notch (scale bar=100 µm). (E) Higher magnification of the residual graft material (scale bar=250 µm).
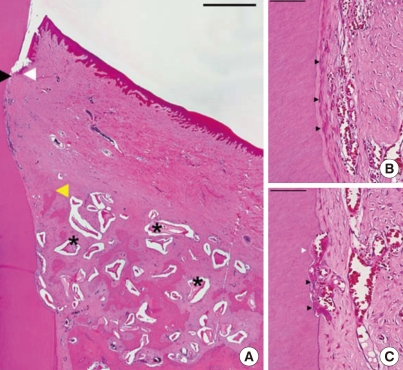
Figure 3.
Histological view of a well-healed sample (#2) in the experimental group (H&E staining). (A) Low magnification. Arrowheads as in Fig. 2 (scale bar=1 mm). (B) Higher magnification at the root surface covered by new cementum and lined with cementoblast-like cells (arrowheads; scale bar=100 µm). (C) Lacunae of root resorption by odontoclasts (black arrowheads) and subsequent apposition of new cementum (white arrowhead; scale bar=50 µm).
Unfavorable-healing group (n=3)
Most of the grafted materials were displaced and only a small portion of BH remained (Fig. 4). Minimal regeneration of periodontal tissues was observed, similar to that seen in the sham-surgery control tissues. A dense fibrous connective tissue had sequestered the displaced graft particles from the neighboring alveolar bone. Degradation of some BH particles by multiple osteoclast-like cells in resorption lacunae was observed.
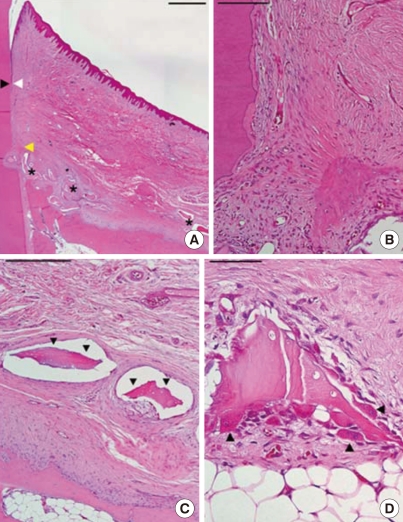
Figure 4.
Histological view of a poorly healed sample in the experimental group (H&E staining). (A) Low magnification. Arrowheads as in Fig. 2 (scale bar=1 mm). (B) Higher magnification at the notch area; minimal new bone was formed (scale bar=100 mm). (C) Fibrous encapsulated graft material (arrowheads; scale bar=250 µm). (D) Degradation of the residual graft material. Arrowheads, osteoclast-like cells (scale bar=50 µm).
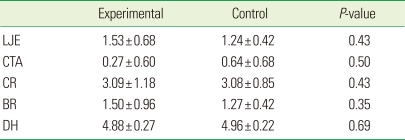
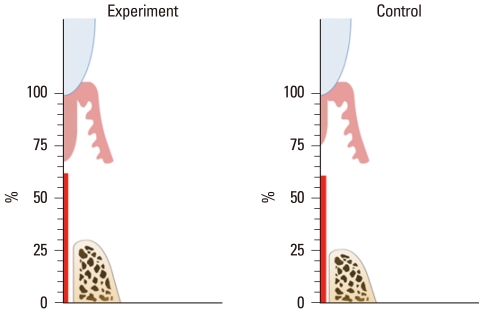
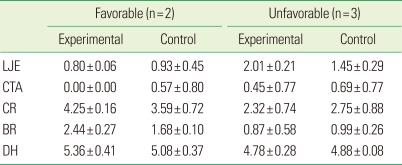
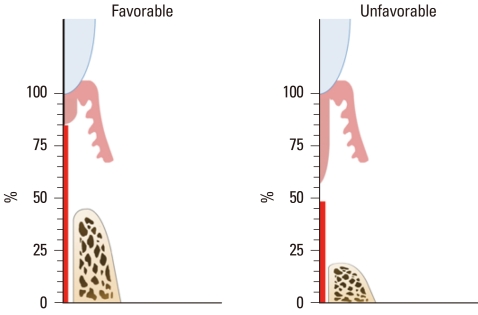
The histometric results of DH, LJE, CTA, CR, and BR are given in Table 1 and Fig. 5. The measured parameters exhibited large standard deviations, but the mean values did not differ significantly between the experimental and sham-surgery control data. Table 2 and Fig. 6 demonstrate the differences in outcomes according to the healing pattern within the same experimental group. In sites showing a favorable-healing pattern, more bone (2.4±0.3 mm, mean±standard deviation) and cementum formation (4.3±0.2 mm), and less extension of the junctional epithelium (0.8±0.1 mm) were observed compared with the unfavorable-healing group (0.9±0.6, 2.3±0.7, and 2.0±0.2 mm, respectively).
Table 1.
Histometric analysis of the measured parameters in the experimental (application of bovine hydroxyapatite/collagen matrix) and sham-surgery control groups (n=5; mean±standard values; mm).
LJE, long junctional epithelium; CTA, connective tissue attachment; CR, cementum regeneration; BR, bone regeneration; DH, defect height.
Figure 5.
Graph illustrating the mean percentage values of the measured parameters.
Table 2.
Comparison of the measured values within the experimental and control groups according to healing pattern (mean±standard deviation; mm).
LJE, long junctional epithelium; CTA, connective tissue attachment; CR, cementum regeneration; BR, bone regeneration; DH, defect height.
Figure 6.
Comparison of the mean percentage values of the measured parameters within the experimental group according to healing pattern (favorable or unfavorable).
DISCUSSION
The box-type one-wall intrabony defect model in dogs is a well-established model and has been used to evaluate the effect of particular biomaterials on periodontal regeneration [24,25]. Stavropoulos and Wikesjö [26] evaluated the long-term effect of the GTR procedure by using BHC covered by a collagen membrane in one-wall intrabony defects of two different dimensions (4×4 mm and 6×6 mm) in dogs [26]. Following an 18-month healing period, periodontal regeneration almost the same as the original level was observed, irrespective of the different defect dimensions. However, it is unclear whether the combination of BHC and a collagen membrane would contribute to the best result, since sham-surgery control, BHC-alone, and collagen-membrane-alone groups were not included in the study design. The present study included a sham-surgery control at the contralateral site, and only BHC material was applied to determine the genuine effect of the bone substitute without a covering membrane.
Grafting of BHC in one-wall intrabony periodontal defects yielded inconsistent results regarding the extent of localization and substantivity of the grafted material within the defects. The stability and localization of the grafted material during the initial healing period are critical to correct bone formation. Two of the five experimental samples exhibited almost complete regeneration of new cementum and moderate new bone formation, while the graft materials were maintained and integrated within the defect. However, localization failed in three samples, and the remaining materials could barely be seen in histologic slides. Araújo et al. [27] found that most of the collagen matrix was resorbed, disappearing within 1-2 weeks following grafting. Therefore, early loss of the collagen matrix might be attributable to instability of the loosely embedded particles in the defects, although it is unknown when the grafts were displaced during the healing period. Buccolingually open, one-wall defects could provoke loss of the graft.
Due to individual variance in healing potential, the animals, showing the localization of biomaterials and favorable results in the experimental site (Favorable-healing group), showed favorable periodontal regeneration in the control site as well (Table 2). These animals might rapidly form a firm provisional matrix prior to breaking down the collagen matrix, and thus maintain osteoconductivity. On the other hand, the other three animals (unfavorable-healing group) showed minimal periodontal regeneration and displaced biomaterials in both experimental and control sites. The mean values from histometric measurements of the experimental group were similar to those of the sham-surgery controls, with large standard deviations resulting in no statistically significant differences between the groups, although this could have been due to the small samples.
The proportion of graft material remaining was substantially lower than what was originally applied at surgery, even allowing for the heterogeneity of the healing response. Dissipation or exfoliation of the graft particles from the defect site may be the main reason for this. BHC is composed of 90% BH and 10% porcine type I collagen in weight. Since the BHC was not condensed, but just placed at the defects, the space occupied by the collagen matrix may have resulted in a low density of BH particles. It is known that BH is contained in almost all nonresorbable bone substitutes, and occupies its space in the defect while embedded with the newly formed bone [28,29]. Nevertheless, the possibility of biodegradation by osteoclasts has been reported [14,27,30,31]. In the present study, osteoclast-like multinucleated giant cells were observed surrounding some BH particles. The lack of remaining materials might be influenced by osteoclastic resorption.
The dimensions of the BHC meant that it just fitted the one-wall intrabony defect without a gap. The density of the graft material can influence wound healing. The presence of the condensed graft materials themselves at the coronal entry of the defect can act as a barrier that can block epithelial downgrowth. Conversely, some studies have demonstrated that high-density nonresorbable bone substitute may obstruct the formation of a provisional matrix, thus slowing the overall healing and maturation [32,33]. In this context, the graft material should be placed loosely inside the defect and should be packed at the periphery facing the soft tissue. Since defects such as extraction sockets and three-wall intrabony defects have narrow and small defect entrances, the density of the graft material can be easily controlled. Class II furcation defects also open on one side, surrounding the root surface and alveolar bone. Taheri et al. [8] compared clinical outcomes according to the presence or absence of a barrier membrane, after treating mandibular class II furcation defects with BH grafting. Both treatment modalities resulted in favorable attachment gain and bone fill without any statistically significant differences between the two groups. Therefore, a barrier membrane might be redundant in the case of a contained defect, and the single use of BHC would be more favorable for contained-type defects such as extraction sockets and three-wall intrabony defects. On the other hand, it is difficult to control the density of the graft material in defects with a wide or open entrance. Loosely placed particles that are not contained by a membrane can disperse and are thus unable to act as a barrier.
The present study used the mesial side of the mandibular fourth premolars as a model for a one-wall intrabony defect in dogs. Other studies found enhanced periodontal regeneration compared to the control when using the mesial side of the first molars to prepare periodontal intrabony defects [26,34]. There are large differences between the molars and the premolar with regard to the area and width of the alveolar bone surrounding the defect. A major source of regenerative precursor cells is the neighboring bony wall, including the buccal and lingual plates adjacent to the exposed root surface and the bony base of the defect [25]. Therefore, the first molar area-with its larger surface of bony wall-should possess more favorable regenerative potential than the fourth premolar area.
Application of the BHC alone without a barrier membrane at wide, one-wall, intrabony, periodontal defects yielded inconsistent results regarding both periodontal regeneration and the substantivity of the graft materials. For noncontained-type defects, the stability of the grafted material can be maintained by applying condensed grafted material together with a barrier membrane.
ACKNOWLEDGEMENTS
This study was supported by a grant of the Korea Healthcare Technology R&D Project, Ministry for Health, Welfare & Family Affairs, Republic of Korea (A100158 and A101578).
Footnotes
No potential conflict of interest relevant to this article was reported.
References
- 1.Gottlow J, Nyman S, Karring T, Lindhe J. New attachment formation as the result of controlled tissue regeneration. J Clin Periodontol. 1984;11:494–503. doi: 10.1111/j.1600-051x.1984.tb00901.x. [DOI] [PubMed] [Google Scholar]
- 2.Haney JM, Nilvéus RE, McMillan PJ, Wikesjö UM. Periodontal repair in dogs: expanded polytetrafluoroethylene barrier membranes support wound stabilization and enhance bone regeneration. J Periodontol. 1993;64:883–890. doi: 10.1902/jop.1993.64.9.883. [DOI] [PubMed] [Google Scholar]
- 3.Sigurdsson TJ, Hardwick R, Bogle GC, Wikesjö UM. Periodontal repair in dogs: space provision by reinforced ePTFE membranes enhances bone and cementum regeneration in large supraalveolar defects. J Periodontol. 1994;65:350–356. doi: 10.1902/jop.1994.65.4.350. [DOI] [PubMed] [Google Scholar]
- 4.Murphy KG. Postoperative healing complications associated with Gore-Tex Periodontal Material. Part I. Incidence and characterization. Int J Periodontics Restorative Dent. 1995;15:363–375. [PubMed] [Google Scholar]
- 5.Houser BE, Mellonig JT, Brunsvold MA, Cochran DL, Meffert RM, Alder ME. Clinical evaluation of anorganic bovine bone xenograft with a bioabsorbable collagen barrier in the treatment of molar furcation defects. Int J Periodontics Restorative Dent. 2001;21:161–169. [PubMed] [Google Scholar]
- 6.Paolantonio M. Combined periodontal regenerative technique in human intrabony defects by collagen membranes and anorganic bovine bone. A controlled clinical study. J Periodontol. 2002;73:158–166. doi: 10.1902/jop.2002.73.2.158. [DOI] [PubMed] [Google Scholar]
- 7.Misch CE, Dietsh F. Bone-grafting materials in implant dentistry. Implant Dent. 1993;2:158–167. doi: 10.1097/00008505-199309000-00003. [DOI] [PubMed] [Google Scholar]
- 8.Taheri M, Molla R, Radvar M, Sohrabi K, Najafi MH. An evaluation of bovine derived xenograft with and without a bioabsorbable collagen membrane in the treatment of mandibular Class II furcation defects. Aust Dent J. 2009;54:220–227. doi: 10.1111/j.1834-7819.2009.01122.x. [DOI] [PubMed] [Google Scholar]
- 9.Trombelli L, Heitz-Mayfield LJ, Needleman I, Moles D, Scabbia A. A systematic review of graft materials and biological agents for periodontal intraosseous defects. J Clin Periodontol. 2002;29(Suppl 3):117–135. doi: 10.1034/j.1600-051x.29.s3.7.x. [DOI] [PubMed] [Google Scholar]
- 10.Reynolds MA, Aichelmann-Reidy ME, Branch-Mays GL, Gunsolley JC. The efficacy of bone replacement grafts in the treatment of periodontal osseous defects. A systematic review. Ann Periodontol. 2003;8:227–265. doi: 10.1902/annals.2003.8.1.227. [DOI] [PubMed] [Google Scholar]
- 11.Blumenthal N, Steinberg J. The use of collagen membrane barriers in conjunction with combined demineralized bone-collagen gel implants in human infrabony defects. J Periodontol. 1990;61:319–327. doi: 10.1902/jop.1990.61.6.319. [DOI] [PubMed] [Google Scholar]
- 12.Nygaard-Østby P, Bakke V, Nesdal O, Nilssen HK, Susin C, Wikesjö UM. Periodontal healing following reconstructive surgery: effect of guided tissue regeneration using a bioresorbable barrier device when combined with autogenous bone grafting. A randomized controlled clinical trial. J Clin Periodontol. 2008;35:37–43. doi: 10.1111/j.1600-051X.2007.01160.x. [DOI] [PubMed] [Google Scholar]
- 13.Parashis A, Andronikaki-Faldami A, Tsiklakis K. Comparison of 2 regenerative procedures--guided tissue regeneration and demineralized freeze-dried bone allograft--in the treatment of intrabony defects: a clinical and radiographic study. J Periodontol. 1998;69:751–758. doi: 10.1902/jop.1998.69.7.751. [DOI] [PubMed] [Google Scholar]
- 14.Berglundh T, Lindhe J. Healing around implants placed in bone defects treated with Bio-Oss. An experimental study in the dog. Clin Oral Implants Res. 1997;8:117–124. doi: 10.1034/j.1600-0501.1997.080206.x. [DOI] [PubMed] [Google Scholar]
- 15.Camelo M, Nevins ML, Schenk RK, Simion M, Rasperini G, Lynch SE, et al. Clinical, radiographic, and histologic evaluation of human periodontal defects treated with Bio-Oss and Bio-Gide. Int J Periodontics Restorative Dent. 1998;18:321–331. [PubMed] [Google Scholar]
- 16.Richardson CR, Mellonig JT, Brunsvold MA, McDonnell HT, Cochran DL. Clinical evaluation of Bio-Oss: a bovine-derived xenograft for the treatment of periodontal osseous defects in humans. J Clin Periodontol. 1999;26:421–428. doi: 10.1034/j.1600-051x.1999.260702.x. [DOI] [PubMed] [Google Scholar]
- 17.Mellonig JT. Human histologic evaluation of a bovine-derived bone xenograft in the treatment of periodontal osseous defects. Int J Periodontics Restorative Dent. 2000;20:19–29. [PubMed] [Google Scholar]
- 18.Yamada S, Shima N, Kitamura H, Sugito H. Effect of porous xenographic bone graft with collagen barrier membrane on periodontal regeneration. Int J Periodontics Restorative Dent. 2002;22:389–397. [PubMed] [Google Scholar]
- 19.Sculean A, Stavropoulos A, Windisch P, Keglevich T, Karring T, Gera I. Healing of human intrabony defects following regenerative periodontal therapy with a bovine-derived xenograft and guided tissue regeneration. Clin Oral Investig. 2004;8:70–74. doi: 10.1007/s00784-004-0254-7. [DOI] [PubMed] [Google Scholar]
- 20.Clergeau LP, Danan M, Clergeau-Guérithault S, Brion M. Healing response to anorganic bone implantation in periodontal intrabony defects in dogs. Part I. Bone regeneration. A microradiographic study. J Periodontol. 1996;67:140–149. doi: 10.1902/jop.1996.67.2.140. [DOI] [PubMed] [Google Scholar]
- 21.Nevins ML, Camelo M, Lynch SE, Schenk RK, Nevins M. Evaluation of periodontal regeneration following grafting intrabony defects with bio-oss collagen: a human histologic report. Int J Periodontics Restorative Dent. 2003;23:9–17. [PubMed] [Google Scholar]
- 22.Zitzmann NU, Rateitschak-Plüss E, Marinello CP. Treatment of angular bone defects with a composite bone grafting material in combination with a collagen membrane. J Periodontol. 2003;74:687–694. doi: 10.1902/jop.2003.74.5.687. [DOI] [PubMed] [Google Scholar]
- 23.Kim TG, Wikesjö UM, Cho KS, Chai JK, Pippig SD, Siedler M, et al. Periodontal wound healing/regeneration following implantation of recombinant human growth/differentiation factor-5 (rhGDF-5) in an absorbable collagen sponge carrier into one-wall intrabony defects in dogs: a dose-range study. J Clin Periodontol. 2009;36:589–597. doi: 10.1111/j.1600-051X.2009.01420.x. [DOI] [PubMed] [Google Scholar]
- 24.Kim CS, Choi SH, Chai JK, Cho KS, Moon IS, Wikesjö UM, et al. Periodontal repair in surgically created intrabony defects in dogs: influence of the number of bone walls on healing response. J Periodontol. 2004;75:229–235. doi: 10.1902/jop.2004.75.2.229. [DOI] [PubMed] [Google Scholar]
- 25.Kim CS, Um YJ, Chai JK, Cho KS, Moon IS, Choi SH, et al. A canine model for histometric evaluation of periodontal regeneration. Periodontol 2000. 2011;56:209–226. doi: 10.1111/j.1600-0757.2010.00372.x. [DOI] [PubMed] [Google Scholar]
- 26.Stavropoulos A, Wikesjö UM. Influence of defect dimensions on periodontal wound healing/regeneration in intrabony defects following implantation of a bovine bone biomaterial and provisions for guided tissue regeneration: an experimental study in the dog. J Clin Periodontol. 2010;37:534–543. doi: 10.1111/j.1600-051X.2010.01566.x. [DOI] [PubMed] [Google Scholar]
- 27.Araújo MG, Liljenberg B, Lindhe J. Dynamics of Bio-Oss Collagen incorporation in fresh extraction wounds: an experimental study in the dog. Clin Oral Implants Res. 2010;21:55–64. doi: 10.1111/j.1600-0501.2009.01854.x. [DOI] [PubMed] [Google Scholar]
- 28.McAllister BS, Haghighat K. Bone augmentation techniques. J Periodontol. 2007;78:377–396. doi: 10.1902/jop.2007.060048. [DOI] [PubMed] [Google Scholar]
- 29.Esposito M, Piattelli M, Pistilli R, Pellegrino G, Felice P. Sinus lift with guided bone regeneration or anorganic bovine bone: 1-year post-loading results of a pilot randomised clinical trial. Eur J Oral Implantol. 2010;3:297–305. [PubMed] [Google Scholar]
- 30.Hämmerle CH, Chiantella GC, Karring T, Lang NP. The effect of a deproteinized bovine bone mineral on bone regeneration around titanium dental implants. Clin Oral Implants Res. 1998;9:151–162. doi: 10.1034/j.1600-0501.1998.090302.x. [DOI] [PubMed] [Google Scholar]
- 31.Cardaropoli G, Araújo M, Hayacibara R, Sukekava F, Lindhe J. Healing of extraction sockets and surgically produced - augmented and non-augmented - defects in the alveolar ridge. An experimental study in the dog. J Clin Periodontol. 2005;32:435–440. doi: 10.1111/j.1600-051X.2005.00692.x. [DOI] [PubMed] [Google Scholar]
- 32.Stavropoulos A, Kostopoulos L, Nyengaard JR, Karring T. Deproteinized bovine bone (Bio-Oss) and bioactive glass (Biogran) arrest bone formation when used as an adjunct to guided tissue regeneration (GTR): an experimental study in the rat. J Clin Periodontol. 2003;30:636–643. doi: 10.1034/j.1600-051x.2003.00093.x. [DOI] [PubMed] [Google Scholar]
- 33.Araújo M, Linder E, Lindhe J. Effect of a xenograft on early bone formation in extraction sockets: an experimental study in dog. Clin Oral Implants Res. 2009;20:1–6. doi: 10.1111/j.1600-0501.2008.01606.x. [DOI] [PubMed] [Google Scholar]
- 34.Sakata J, Abe H, Ohazama A, Okubo K, Nagashima C, Suzuki M, et al. Effects of combined treatment with porous bovine inorganic bone grafts and bilayer porcine collagen membrane on refractory one-wall intrabony defects. Int J Periodontics Restorative Dent. 2006;26:161–169. [PubMed] [Google Scholar]