Abstract
We examined the effect of Mdm2 on regulation of the ApoCIII promoter and its cross-talk with p53 and nuclear receptor SHP. Overexpression of Mdm2 markedly enhanced ApoCIII promoter activity by HNF4α. A direct association of Mdm2 protein with the HNF4α protein was observed by co-immunoprecipitation. Ectopic expression of p53 decreased HNF4α activation of the ApoCIII promoter and antagonized the effect of Mdm2. Co-expression of SHP further strengthened p53 inhibition and abolished Mdm2 activation of the ApoCIII promoter. Mdm2 inhibited p53-mediated enrichment of HNF4α to the ApoCIII promoter while simultaneously reducing p53 binding and increasing recruitment of SHP to the ApoCIII promoter. The results from this study implicate a potentially important function of Mdm2 in regulation of lipoprotein metabolism.
Keywords: nuclear receptor, promoter activity, tumor suppressor, oncogene, lipoprotein
1. Introduction
Plasma levels of lipoproteins that contain apolipoprotein III (ApoCIII) predict coronary heart disease (CHD) and are associated with the development of metabolic syndrome [1]. ApoCIII causes hypertriglyceridemia by inhibiting the clearance of TG-rich lipoproteins. ApoCIII gene expression is regulated by several pathways. Insulin reduces ApoCIII gene transcription via the insulin-response element in the ApoCIII promoter [2]. ApoCIII expression is also down-regulated by nuclear receptor peroxisome proliferator-activated receptor alpha (PPARα) [3] and up-regulated by hepatocyte nuclear factor 4 alpha (HNF4α) [4]. Nuclear receptor small heterodimer partner (SHP) functions as a transcriptional repressor of the promoter activity of its target genes [5]. SHP [6] and tumor suppressor p53 [7] independently repress the transactivation of HNF4α by interacting with HNF4α.
Mdm2 is an oncogene that interacts with p53 and promotes the ubiquitination and proteasome-mediated degradation of p53 [8]. Although the function of p53 has been linked to atherosclerosis [9], it remains elusive whether Mdm2 plays a role in lipoprotein metabolism. This study identified Mdm2 as a potent activator of the ApoCIII promoter through HNF4α. The results implicate a likely regulatory function of Mdm2 in lipoprotein metabolic pathways.
2. Materials and Methods
2.1. Plasmids, cell culture, and transfection
Plasmids were obtained from Drs. Frances Sladek (ApocIII Luc) [4], Xiongbin Lu (HA-Mdm2) [10], and Gerhart Ryffel (Myc-HNF4α) [11]. Other plasmids were from our laboratory [6] or purchased from Addgene. M2 antibody, anti-FLAG M2 Magnetic Beads and anti-HA agarose were purchased from Sigma. Mouse monoclonal antibody against Myc-tag was purchased from Cell Signaling. Hela cells were cultured in Dulbecco’s modified Eagle’s medium (DMEM) with 10% fetal bovine serum. Transfection was performed by Lipofectamine 2000 according to the manufacturer’s instructions.
2.2. Co-immunoprecipitation and ChIP assays
Cells were transfected with the indicated plasmids using Lipofectamine 2000 (Invitrogen), lysed in 500 μl lysis buffer and immunoprecipitated with anti-Myc, anti-Flag, anti-GFP, or anti-HA antibodies for 4 hr at 4°C. The beads were washed four times with the lysis buffer. ChIP assays were performed as previously described [12].
2.3. Statistical analysis
Data are expressed as mean ± SEM. Statistical analyses were carried out using Student’s unpaired t test; p < 0.01 was considered statistically significant.
3. Results and Discussion
3.1. Mdm2 enhances HNF4α transactivation of the ApoCIII promoter
Expression of Mdm2 alone did not activate the ApoCIII promoter (not shown). However, ectopic expression of Mdm2 markedly enhanced ApoCIII promoter activation by HNF4α in a dose-dependently fashion (Fig. 1A). Co-IP revealed a physical association of Mdm2 with the HNF4α protein (Fig. 1B), suggesting that Mdm2 interacts with HNF4α to enhance its transactivation.
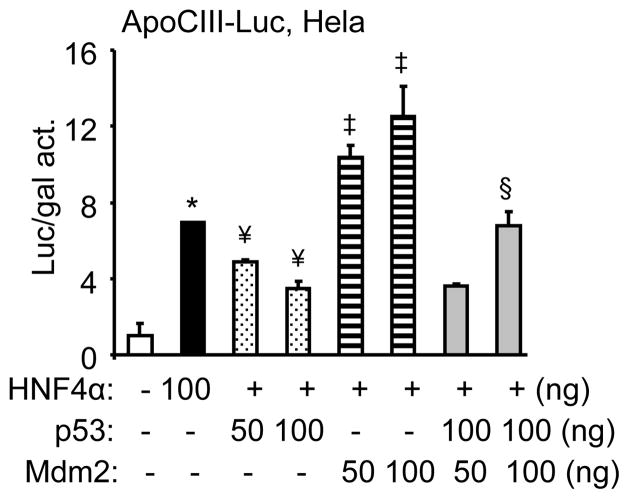
Fig. 1. Mdm2 activates the ApoCIII promoter through HNF4α.
(A) Transient transfection assays to determine the effect of Mdm2 on the ApoCIII promoter activity by HNF4α. Luciferase (luc) activity (act) was determined and normalized by β-gal activity. Data are expressed as means ± SEM of triplicate assays. The experiments were repeated three times with similar results and one representative result is shown. *p<0.01 vs. control (−) pcDNA3 group; ¥p<0.01 vs. HNF4α alone. (B) Immunoprecipitation and Western blots to determine the interaction between Mdm2 and HNF4α proteins.
3.2. p53 antagonizes Mdm2 activation of the ApoCIII promoter
Overexpression of p53 dose-dependently decreased HNF4α transactivation of the ApoCIII promoter (Fig. 2), which was in agreement with an early report showing that p53 represses HNF4α expression [13]. When p53 and Mdm2 were co-expressed, the activation of Mdm2 was significantly diminished by p53, suggesting that p53 antagonizes the effect of Mdm2.
Fig. 2. Mdm2 activation of the ApoCIII promoter is antagonized by p53.
Transient transfection assays to determine the effect of p53 on Mdm2-mediated ApoCIII promoter activity. The experiments were performed as in Fig. 1. *p<0.01 vs. control (−) pcDNA3 group; ¥ ‡p<0.01 vs. HNF4α alone; §p<0.01 vs. p53 or Mdm2 alone.
3.3. SHP strengthens p53 inhibition and abolishes Mdm2 activation of the ApoCIII promoter
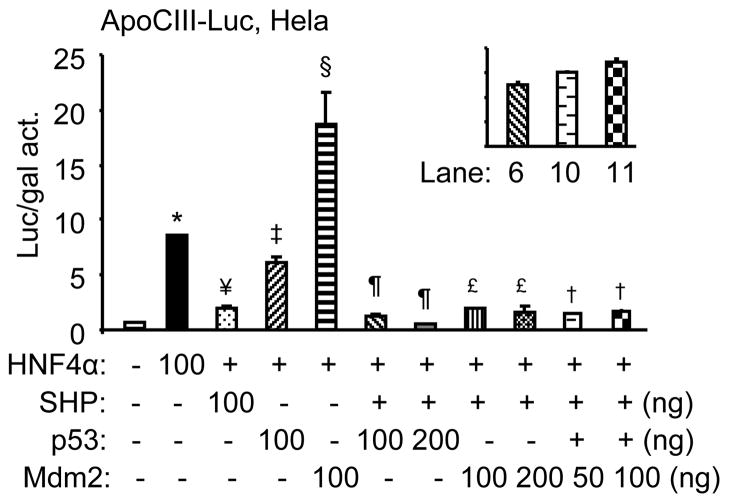
Consistent with our previous results [6], expression of SHP alone significantly inhibited ApoCIII promoter activity by HNF4α (Fig. 3). When SHP and p53 were co-transfected in cells, the inhibitory effect of p53 on ApoCIII promoter activity was strengthened by SHP. Because SHP and p53 both interact with HNF4α and inhibit its activity [6; 13], and SHP does not affect the transcriptional activity of p53 on its reporters [14] (not shown), the further reduced HNF4α transactivation in the co-presence of SHP and p53 compared with SHP or p53 alone may represent an additive inhibitory effect. Conversely, when SHP and Mdm2 were co-expressed in cells, the activation of Mdm2 was completely abolished by SHP. The results suggest that the inhibition of SHP on HNF4α is stronger than the activation of Mdm2.
Fig. 3. Mdm2 activation of the ApoCIII promoter is abolished by SHP.
Transient transfection assays to determine the effect of SHP on Mdm2-mediated ApoCIII promoter activity in the absence or presence of p53. The experiments were performed as in Fig. 1. *p<0.01 vs. control (−) pcDNA3 group; ¥, ‡, §p<0.01 vs. HNF4α alone; ¶p<0.01 vs. p53 or SHP alone; £, †p<0.01 vs. p53 or Mdm2 alone.
3.4. Mdm2 alters the enrichment of HNF4α, p53, and SHP to the ApoCIII promoter
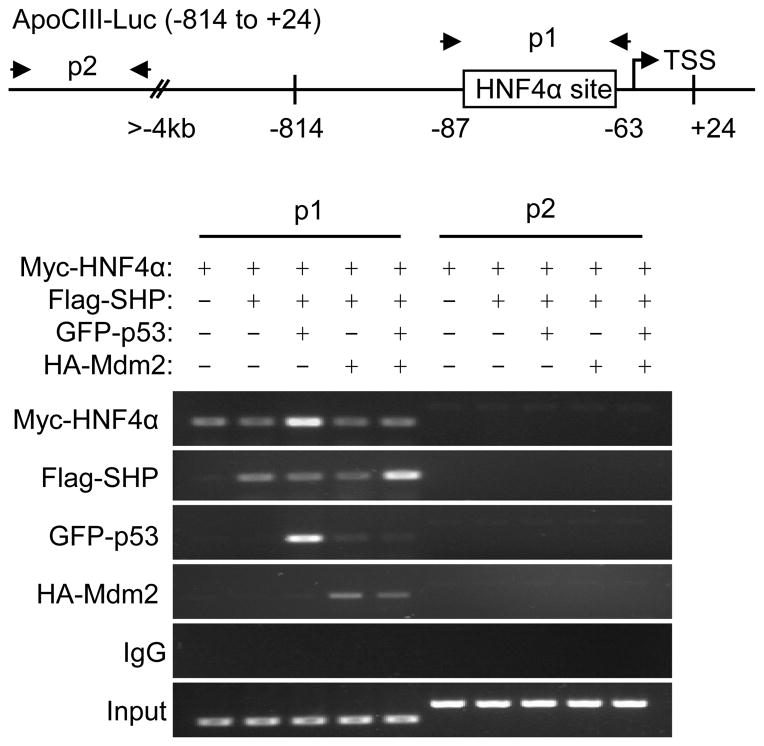
We employed ChIP assays to determine whether the recruitment of HNF4α, p53, or SHP to the ApoCIII promoter was altered by Mdm2. SHP did not affect the physical association of HNF4α with the ApoCIII promoter when co-expressed with HNF4α (Fig. 4, lane 2 vs. 1). p53 did not affect SHP, but markedly increased HNF4α binding to the ApoCIII promoter (lane 3 vs. 1). When Mdm2 was co-expressed with SHP in the absence of p53, the association of SHP and HNF4α to the ApoCIII promoter was not affected by Mdm2 (lane 4 vs. 1&2). However, Mdm2 dramatically reduced the recruitment of p53, as well as p53-dependent association of HNF4α, to the ApoCIII promoter (lane 5 vs. 3). At the same time, SHP association with the ApoCIII promoter was enhanced by Mdm2.
Fig. 4. Mdm2 alters the recruitment of HNF4α, p53, and SHP to the ApoCIII promoter.
Hela cells were co-transfected with Myc-HNF4α, Flag-SHP, GFP-p53, HA-Mdm2 plasmids, and the ApoCIII promoter (−814 to +24); and anti-Myc, anti-Flag, anti-GFP, and anti-HA antibodies were used to co-immunoprecipitate each corresponding protein, respectively. Two sets of primers were designed for ChIP assays. P1 could detect Co-IP of each protein on exogenously overexpressed ApoCIII promoter, whereas p2 was located 4 kb upstream from TSS and thus served as a negative control.
Mdm2 reduces the physical association of p53 with the ApoCIII promoter, which may contribute to its efficient antagonism of p53’s inhibition. At the same time, Mdm2 increases the recruitment of SHP to the ApoCIII promoter, which may further increase the inhibitory effect of SHP and decrease Mdm2’s counteracting effect.
The finding of this study is important because it points to a potential role of Mdm2 in lipoprotein metabolism. Future studies using mouse models are required to further address this question.
Highlights.
Mdm2 enhances HNF4α activation of the ApoCIII promoter via interaction with HNF4α
p53 antagonizes the effect of Mdm2 activation of the ApoCIII promoter
SHP strengthens p53 inhibition but abolishes Mdm2 activation of the ApoCIII promoter
Mdm2 alters the enrichment of HNF4α, p53 and SHP to the ApoCIII promoter
Acknowledgments
We thank Drs. Frances Sladek, Xiongbin Lu, Gerhart Ryffel, and Paul Neilsen for providing the plasmids. Y.Z. is supported by NIH T32CA092347 Multidisciplinary Cancer Research Training Program (MCRTP). This work is in part supported by National Institutes of Health DK080440 to L.W.
Footnotes
Competing interests
The authors declare that they have no competing interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kawakami A, Yoshida M. Apolipoprotein CIII links dyslipidemia with atherosclerosis. J Atheroscler Thromb. 2009;16:6–11. doi: 10.5551/jat.e607. [DOI] [PubMed] [Google Scholar]
- 2.Chen M, Breslow JL, Li W, Leff T. Transcriptional regulation of the apoC-III gene by insulin in diabetic mice: correlation with changes in plasma triglyceride levels. J Lipid Res. 1994;35:1918–24. [PubMed] [Google Scholar]
- 3.Staels B, Vu-Dac N, Kosykh VA, Saladin R, Fruchart JC, Dallongeville J, Auwerx J. Fibrates downregulate apolipoprotein C-III expression independent of induction of peroxisomal acyl coenzyme A oxidase. A potential mechanism for the hypolipidemic action of fibrates. J Clin Invest. 1995;95:705–12. doi: 10.1172/JCI117717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hwang-Verslues WW, Sladek FM. Nuclear receptor hepatocyte nuclear factor 4alpha1 competes with oncoprotein c-Myc for control of the p21/WAF1 promoter. Mol Endocrinol. 2008;22:78–90. doi: 10.1210/me.2007-0298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhang Y, Hagedorn CH, Wang L. Role of nuclear receptor SHP in metabolism and cancer. Biochim Biophys Acta. doi: 10.1016/j.bbadis.2010.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhou T, Zhang Y, Macchiarulo A, Yang Z, Cellanetti M, Coto E, Xu P, Pellicciari R, Wang L. Novel polymorphisms of nuclear receptor SHP associated with functional and structural changes. J Biol Chem. 285:24871–81. doi: 10.1074/jbc.M110.133280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Maeda Y, Seidel SD, Wei G, Liu X, Sladek FM. Repression of hepatocyte nuclear factor 4alpha tumor suppressor p53: involvement of the ligand-binding domain and histone deacetylase activity. Mol Endocrinol. 2002;16:402–10. doi: 10.1210/mend.16.2.0769. [DOI] [PubMed] [Google Scholar]
- 8.Haupt Y, Maya R, Kazaz A, Oren M. Mdm2 promotes the rapid degradation of p53. Nature. 1997;387:296–9. doi: 10.1038/387296a0. [DOI] [PubMed] [Google Scholar]
- 9.Guevara NV, Kim HS, Antonova EI, Chan L. The absence of p53 accelerates atherosclerosis by increasing cell proliferation in vivo. Nat Med. 1999;5:335–9. doi: 10.1038/6585. [DOI] [PubMed] [Google Scholar]
- 10.Lu X, Ma O, Nguyen TA, Jones SN, Oren M, Donehower LA. The Wip1 Phosphatase acts as a gatekeeper in the p53-Mdm2 autoregulatory loop. Cancer Cell. 2007;12:342–54. doi: 10.1016/j.ccr.2007.08.033. [DOI] [PubMed] [Google Scholar]
- 11.Lucas B, Grigo K, Erdmann S, Lausen J, Klein-Hitpass L, Ryffel GU. HNF4alpha reduces proliferation of kidney cells and affects genes deregulated in renal cell carcinoma. Oncogene. 2005;24:6418–31. doi: 10.1038/sj.onc.1208794. [DOI] [PubMed] [Google Scholar]
- 12.Zhang Y, Wang L. Nuclear receptor SHP inhibition of Dnmt1 expression via ERRgamma. FEBS Lett. 585:1269–75. doi: 10.1016/j.febslet.2011.03.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Maeda Y, Hwang-Verslues WW, Wei G, Fukazawa T, Durbin ML, Owen LB, Liu X, Sladek FM. Tumour suppressor p53 down-regulates the expression of the human hepatocyte nuclear factor 4alpha (HNF4alpha) gene. Biochem J. 2006;400:303–13. doi: 10.1042/BJ20060614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Neilsen PM, Cheney KM, Li CW, Chen JD, Cawrse JE, Schulz RB, Powell JA, Kumar R, Callen DF. Identification of ANKRD11 as a p53 coactivator. J Cell Sci. 2008;121:3541–52. doi: 10.1242/jcs.026351. [DOI] [PubMed] [Google Scholar]