Abstract
Hypoxia stimulates tumor angiogenesis by inducing the expression of angiogenic molecules. The negative regulators of this process, however, are not well understood. Here we report that hypoxia induced the expression of insulin-like growth factor binding protein-6 (IGFBP-6), a tumor repressor, in human and rodent vascular endothelial cells (VECs) via a HIF-mediated mechanism. Addition of human IGFBP-6 to cultured human VECs inhibited angiogenesis in vitro. An IGFBP-6 mutant with at least 10,000-fold lower binding affinity for IGFs was an equally potent inhibitor of angiogenesis, suggesting that this action of IGFBP-6 is IGF-independent. The functional relationship between IGFBP-6 and VEGF, a major hypoxia-inducible angiogenic molecule, was examined. While VEGF alone increased angiogenesis in vitro, co-incubation with IGFBP-6 abolished VEGF-stimulated angiogenesis. The in vivo role of IGFBP-6 in angiogenesis was tested in flk1:GFP zebrafish embryos, which exhibit green fluorescence protein in developing vascular endothelium, permitting visualization of developing blood vessels. Injection of human IGFBP-6 mRNA reduced the number of embryonic inter-segmental blood vessels by ∼40%. This anti-angiogenic activity is conserved in zebrafish because expression of zebrafish IGFBP-6b had similar effects. To determine the anti-angiogenic effect of IGFBP-6 in a tumor model, human Rh30 rhabdomyosarcoma cells stably transfected with IGFBP-6 were inoculated into athymic BALB/c nude mice. Vessel density was 52% lower in IGFBP-6-transfected xenografts than in vector control xenografts. These results suggest that the expression of IGFBP-6 in VECs is up-regulated by hypoxia and IGFBP-6 inhibits angiogenesis in vitro and in vivo.
Introduction
Hypoxia is widely recognized as an important driving force for tumor angiogenesis. As tumors grow, the original vasculature becomes insufficient to supply oxygen to the growing tumor cells, and local hypoxia develops. Hypoxia stimulates new blood vessel formation via the induction of angiogenic factors. This neovascularization is critical for tumor progression as it provides tumor cells with oxygen, nutrients, and growth factors 1, 2, 3. A key player in the hypoxia response is hypoxia-inducible factor-1 (HIF-1), which is a heterodimeric transcription factor composed of HIF-1α and HIF-1β. While HIF-1β (also known as ARNT) is constitutively expressed, HIF-1α is sensitive to O2 tension. When O2 levels are high, HIF-1α is hydroxylated by prolyl hydroxylase domain-containing proteins (PHDs), then ubiquitinated and targeted for proteosomal degradation. In hypoxic conditions, HIF-1α hydroxylation is inhibited, and it enters the nucleus where it forms a dimer with HIF-1β. The dimerized HIF-1 complex binds to hypoxia response elements (HREs) of its target genes and promotes their transcription. HREs are present in many angiogenic genes including vascular endothelial growth factor (VEGF), a major angiogenic gene, and its receptor, Flk1 4, 5.
Recent studies suggest that there are also negative feedback mechanisms that inhibit angiogenesis during prolonged hypoxia 6. An et al. 7 reported that expression of RGC32, which inhibits VEGF- and hypoxia-induced angiogenesis, is up-regulated by hypoxia. Likewise, hypoxia induces the expression of GRS5, which inhibits VEGF-induced angiogenesis through p38 MAPK, via HIF-1 activation 8. Compared to our relatively comprehensive understanding of the induction of angiogenic genes by hypoxia/HIF-1, however, the negative regulators in hypoxia-induced tumor angiogenesis are not well understood.
Insulin-like growth factor binding proteins (IGFBP) 1-6 bind IGFs with high affinity in extracellular fluids and regulate IGF actions at target tissues 9. Some IGFBPs also possess distinct biological actions that are independent of IGFs 9. IGFBP-6 is unique among the six IGFBPs because of its 50-fold greater affinity for IGF-II, making it a relatively specific inhibitor of IGF-II actions 10. Recent studies suggest that IGFBP-6 is a tumor suppressor that inhibits the growth of a number of IGF-II-dependent tumors 10. In addition to its ability to bind and sequester IGF-II, recent in vitro studies suggest that IGFBP-6 also has IGF-independent actions.11
An early report showed that hypoxia increased IGFBP-6 mRNA levels in bovine endothelial cells 12, but expression of IGFBP-6 and its relationship to hypoxia have not been studied in human endothelial cells. More importantly, the role of IGFBP-6 in normal and tumor angiogenesis, if any, is unknown. The objective of the present study was therefore to test the hypothesis that hypoxia induces IGFBP-6 gene expression in vascular tissues, and that this induction of IGFBP-6 negatively regulates hypoxia-induced tumor angiogenesis.
Materials and Methods
Experimental animals
Wild type and flk1:GFP transgenic zebrafish (Danio rerio) were kept at 28°C under a 14:10-h light-dark photoperiod and fed twice daily. Embryos were obtained from natural breeding and were kept at 28.5°C in embryo-rearing solution supplemented with 0.003% (weight/volume) 2-phenylthiourea (Sigma, St. Louis, MO)13. All experiments with zebrafish were conducted at Ocean University of China with approved protocols. Female athymic BALB/c nude mice were obtained from the Animal Resource Centre, Western Australia. Xenografting studies were performed at the University of Melbourne in accordance with institutional guidelines of the University of Melbourne and Austin and Repatriation Medical Centre Animal Ethics and Biosafety Committees.
Cell culture and hypoxia treatment
Human umbilical vein endothelial cells (HUVECs) and rat brain vessel endothelial cells were obtained from ATCC and maintained in 45% a-MEM (Gibco) supplemented with 45% F-10 (Gibco), 10% fetal bovine serum (FBS), 100U/ml penicillin, and 100μg/ml streptomycin. Normoxic cells (20% O2) were maintained in a humidified-air atmosphere incubator containing 95% air/5% CO2 at 37°C. Hypoxic cells (1% O2) were maintained in a controlled atmosphere chamber at 37°C. Rh30 human rhabdomyosarcoma cells were stably transfected with an empty vector or human IGFBP-6 expression plasmids and cultured using published protocols14.
Western immunoblotting
Nuclear extracts from cultured HUVECs were prepared following previously published procedure18. Western immunoblotting analysis was carried out as reported17 using a polyclonal anti HIF-1α antibody (Novus Biologicals LLC., Littleton, CO) at a dilution of 1:500. The second antibody used was a goat anti-rabbit antibody (Kang Chen Bio-tech, Shanghai, China) at a dilution of 1:2000.
RT-PCR and Quantitative real-time RT-PCR (qRT-PCR)
Total RNA was isolated from cultured cells using Trio Reagent (Nitrogen, Carlsbad, CA). RNA (1 μg) was reverse transcribed using M-MLV (Protégé, Madison, WI) and oligo(dT)18 primer (Sangon, Shanghai, China). RT-PCR was carried out using premix Taq polymerase (Takara, Dalian, China) in a MyCycler Thermal Cycler (Bio-Rad, Hercules, CA). RT-PCR products were fractionated by 1.5% agarose gel electrophoresis, stained with ethidium bromide, and photographed using a Chemidoc XRS Gel Documentation System (Bio-Rad, Hercules, CA).
qRT-PCR was carried out in an iCycler iQ Multicolor real-time PCR detection system (Bio-Rad Laboratories, Hercules, CA) using SYBR Green PCR Master Mix (Applied Biosystems, Foster City, CA). Primers used were as follows: β-actin: 5′- CCAGATCATGTTTGAGACC - 3′ and 5′- GCCAGGTCCAGACGCAGG - 3′; human IGFBP-6: 5′- CTGCCTCAACTTCATTCTC -3′ and 5′- AGTCTTCTTGCTCCTCCTTA -3′. Serial dilutions of plasmid DNA ranging from 108 to 103 molecules/μl for β-actin and 107 to 102 molecules/μl for human IGFBP-6 were used to generate standard curves. After 3 min incubation at 95°C, amplification was performed as follows: 95°C, 10 s, 60°C, 30 s, for 40 cycles. Each assay for an unknown sample was performed in duplicate along with the standard DNA and negative control. The number of molecules of a particular gene transcript was calculated based on the standard curve and normalized to β-actin mRNA levels.
In vitro capillary-like tube formation assay
The effect of IGFBP-6 on angiogenesis was examined using an in vitro tube formation assay as reported previously 15. Briefly, aliquots (50 μL) of Matrigel were plated into individual wells of 96 well tissue culture plates and allowed to polymerize at 37°C. After incubation in serum-free medium for 12 h, HUVEC cells were trypsinized and re-suspended in culture medium containing 1% FBS. HUVEC cells (1×104) were plated into each well with or without recombinant human IGFBP-611, human VEGF 165 (R&D System, Minneapolis, MN), and/or human pigment epithelium-derived factor (PEDF) (ProSpec-Tany Techno Gene Ltd., East Brunswick, NJ). After 12 h incubation, a picture was taken to cover the entire surface area of each well using a phase-contrast microscope. The number of total nodes and tube length were measured. The total node number in the control group (BD Matrigel) is 40-56/well. The results are expressed as a percentage of the control group.
Plasmid construction, capped mRNA synthesis, and microinjection
To study the effects of IGFBP-6 on angiogenesis in zebrafish embryos, a DNA fragment containing the open reading frame (ORF) of human IGFBP-6 (with the stop codon deleted) was amplified by RT-PCR (forward primer 5′- TTGGATCCGCCACCATGACCCCCC -3′ and reverse primer 5′ –TGCGAATTCCGCC GCTACTCCCA -3′). The amplified PCR product was digested with EcoR I and BamH I and subcloned into the pCS2-eGFP vector 16 to create pCS2-hIGFBP-6:eGFP. The construct was verified by DNA sequencing. The construction of zebrafish IGFBP-6b expression plasmids was reported recently 17. Capped mRNA was synthesized using a mMESSAGE mMACHINE kit (Ambion, Inc., Austin, TX).
Microinjection and in vivo angiogenesis assay in zebrafish
To visualize and quantify the effect of IGFBP-6 on angiogenesis in zebrafish embryos, capped mRNA was microinjected into flk1:GFP transgenic zebrafish embryos19 as reported previously18. After injection, embryos were placed in embryo rearing solution and kept at 28.5°C. Live embryos were imaged at 24 and 30 hpf. GFP mRNA and phenol red injected embryos were used as controls.
In vivo tumor angiogenesis experiments
Xenografting experiments were performed as described previously 14. Briefly, empty vector or human IGFBP-6 transfected Rh30 cells (1 × 107 per mouse) were injected subcutaneously into 5- 6 week old female athymic BALB/c nude mice. Five control and 4 IGFBP-6-overexpressing mice were studied. Animal care, feeding, maintenance, and surgery have been reported previously 14. After 27 d, mice were sacrificed. Xenografts were excised and fixed in formalin. Paraffin sections (4 μm) were cut and stained with hematoxylin and eosin. Blood vessels, as defined by erythrocyte-containing luminal structures, were counted in 6-10 fields at 100× magnification in each tumor.
Statistics
Data are shown as mean ± standard error (SE). Differences among groups were analyzed by one-way ANOVA followed by Fisher's LSD post-hoc test or by unpaired t-test (SPSS, Chicago, IL).
Results
Hypoxia up-regulates IGFBP-6 expression in vascular endothelial cells through HIF-1
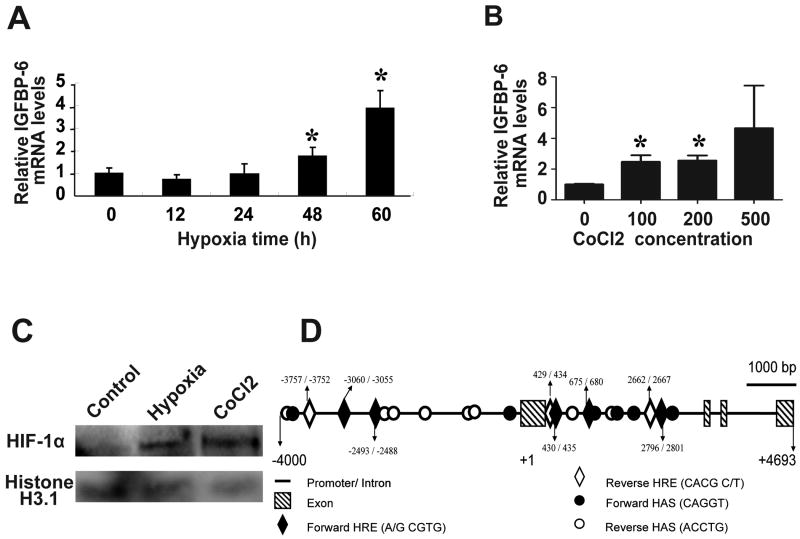
To determine the possible effect of hypoxia on IGFBP-6 expression, human umbilical vascular endothelial cells (HUVECs) were subjected to hypoxia (1% O2) treatment for up to 60 h. RNA was isolated and IGFBP-6 mRNA levels were determined by qRT-PCR. As shown in Fig. 1A, hypoxia treatment resulted in a significant increase in IGFBP-6 mRNA levels in HUVECs after 48 and 60 h (Fig. 1A). Next, HUVEC cells were treated with various concentrations of the hypoxia mimetic CoCl2. CoCl2 can activate hypoxia-dependent pathways under normal oxygen levels by stabilizing HIF-1α and is often used as a hypoxia mimetic. CoCl2 significantly increased IGFBP-6 mRNA levels at 100 and 200 μM (Fig. 1B). Similar induction of IGFBP-6 by hypoxia and CoCl2 was also observed by RT-PCR in HUVECs and rat brain vessel endothelial cells (Supplemental Fig. S1). Further analyses showed that hypoxia (12 h) and CoCl2 (6 h)-treatment markedly increased HIF-1α levels in HUVECs (Fig. 1C) and increased the expression of PDK1 (Supplemental Fig. S1), a well known HIF-1 target gene1-3. Sequence analysis of the human IGFBP-6 promoter suggested that there are 8 canonical HREs located in the promoter region of human IGFBP-6 gene: 5 in the forward orientation and 3 in the reverse orientation (Fig. 1D). These results suggest that hypoxia induces the expression of IGFBP-6 in human and rodent VECs, likely via a HIF-1-mediated mechanism.
Fig.1.
Hypoxia up-regulates IGFBP-6 gene expression in cultured human umbilical vascular endothelial cells (HUVECs) through an HIF-1-mediated mechanism. A) Hypoxia treatment increases IGFBP-6 mRNA levels. Cells were cultured under hypoxia (1% O2) for the indicated time. RNA was isolated and IGFBP-6 mRNA levels were determined by real-time quantitative PCR and normalized for β-actin mRNA levels. Values are shown as mean ± SE (n=4). *, P<0.05 vs. 0 h. B) CoCl2, a chemical HIF-1 inducer, increases IGFBP-6 mRNA expression levels. HUVECs were treated with the indicated concentrations of CoCl2 for 5 h. Results are mean ± SE (n=3). *, P<0.05 vs. control. C) Hypoxia and CoCl2 treatment increases nuclear HIF-1α levels. HUVECs were subjected to hypoxia (0.1%) for 12 h or 500 μM CoCl2 for 6 h. The nuclear protein was prepared and analyzed by Western blot using the indicated antibodies. D) Human IGFBP-6 gene has 8 canonical HRE and 14 putative HAS sites in the promoter/enhancer region.
IGFBP-6 inhibits angiogenesis through an IGF-independent mechanism in vitro
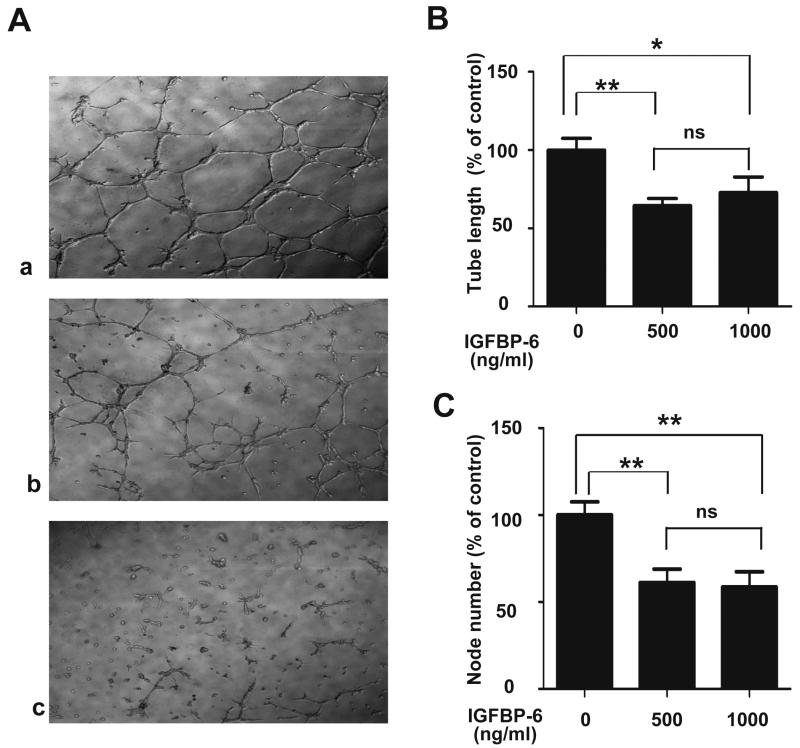
We next examined the effect of IGFBP-6 on angiogenesis using an in vitro capillary-like tube formation assay. VECs can form blood vessel networks when grown on Matrigel 15. As shown in Fig. 2A, when grown on BD Matrigel Matrix Phenol Red Free, HUVEC cells formed highly organized capillary-like networks. Addition of IGFBP-6 resulted in fewer and partially formed capillary-like structures (Fig. 2A). To quantify the IGFBP-6 effect, mean tube length and node number were determined. As shown in Fig. 2B, IGFBP-6 significantly decreased tube length at concentrations of 500 ng/ml (61 ± 8% of control, P<0.01) and 1000 ng/ml (64 ± 5% of control, P<0.05). Likewise, IGFBP-6 significantly decreased node number compared with the control group (Fig. 2C, P < 0.01).
Fig. 2.
IGFBP-6 inhibits angiogenesis in vitro. A) Tube formation assay was performed using HUVECs in the absence (panel a) or presence of IGFBP-6 (panel b: 500 ng/ml, and panel c: 1000 ng/ml) on culture dishes coated with BD Matrigel. Representative micrographs are shown. The effect of IGFBP-6 was quantified by measuring the average tube length (B) and node number (C). Results are expressed as a percentage of control (mean ± SE, n = 9. *, P < 0.05; **, P < 0.01 vs. control).
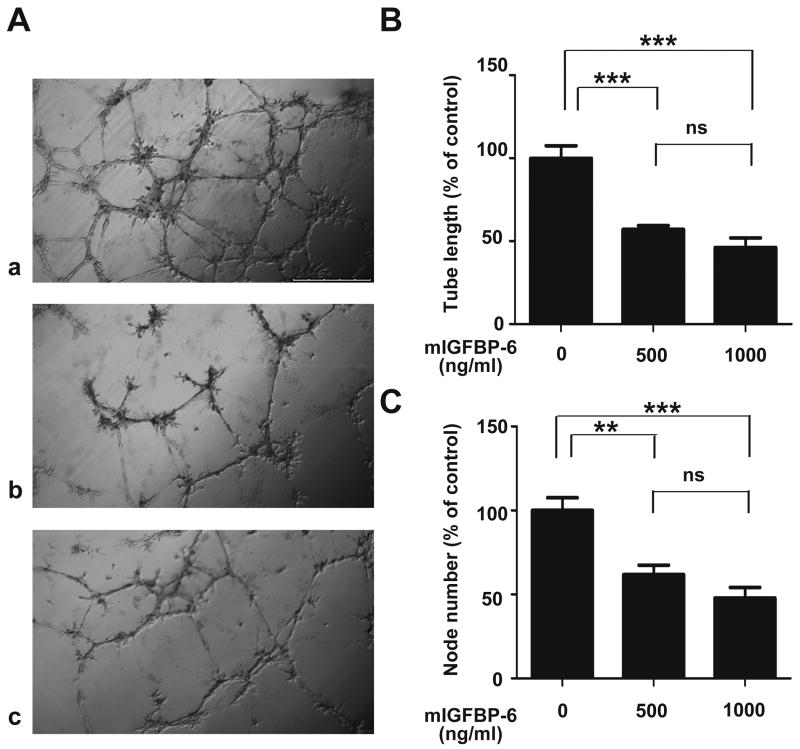
To determine whether the inhibitory effects of IGFBP-6 on angiogenesis are IGF-independent, we tested the activity of mIGFBP-6, an IGFBP-6 mutant that has at least 10,000-fold lower binding affinity for IGF-II than native IGFBP-6 11. As shown in Fig. 3, this non-IGF binding mutant IGFBP-6 had essentially the same inhibitory activity as native IGFBP-6. The mean tube length in the presence of 500 ng/ml mIGFBP-6 was 57 ± 2% of the control level (P<0.001) and the node number was 62 ± 6% of control (P<0.01). Similar results were obtained when 1000 ng/ml mIGFBP-6 was added (Fig. 3). These in vitro results suggest that IGFBP-6 inhibits angiogenesis through an IGF-independent mechanism.
Fig. 3.
The anti-angiogenic action of IGFBP-6 is IGF-independent. A) Tube formation assay was performed using HUVECs in the absence (panel a) or presence of mIGFBP-6, an IGFBP-6 mutant with impaired IGF binding (panel b: 500 ng/ml, and panel c: 1000 ng/ml). The effect was quantified by measuring the average tube length (B) and node number (C). Results, are expressed as a percentage of control (mean ± SE, n = 6. *, P<0.05; **, P<0.01; ***, P<0.0001 vs. control. ns, not significantly different).
IGFBP-6 inhibits VEGF-stimulated angiogenesis in vitro
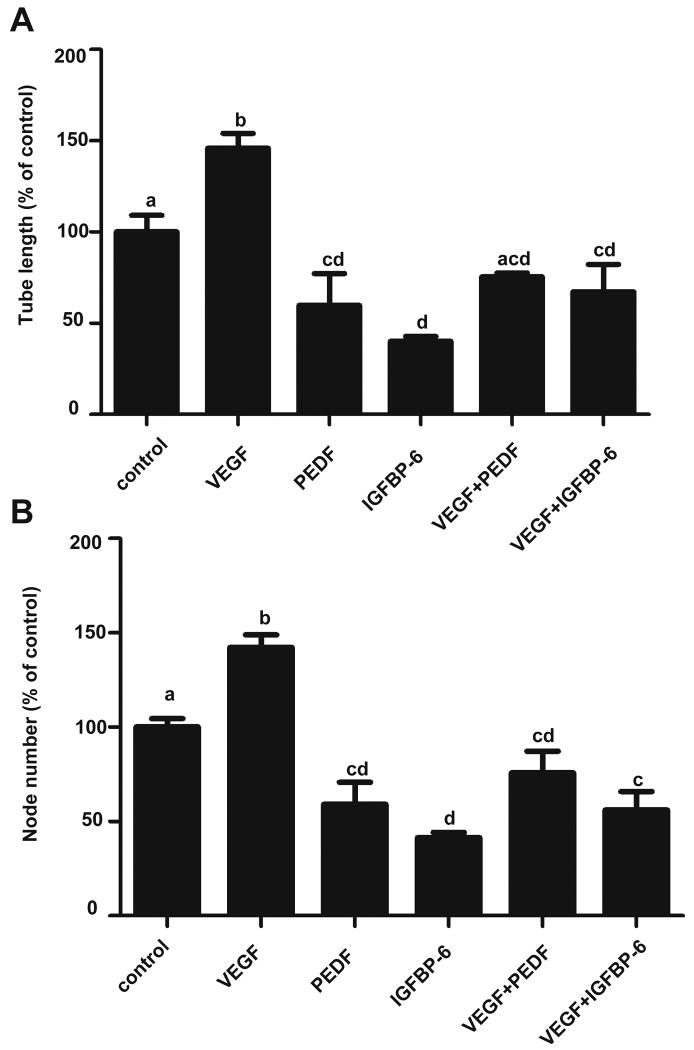
We next investigated the functional relationship between IGFBP-6 and VEGF. As mentioned earlier, hypoxia promotes angiogenesis by inducing angiogenesis-promoting molecules such as VEGF. Human pigment epithelium-derived factor (PEDF), which inhibits angiogenesis was used as a control. As shown in Fig. 4, addition of VEGF165 resulted in a significant increase in the average tube length and the node number. As expected, PEDF significantly decreased in both parameters (Fig. 4). PEDF reduced VEGF-induced angiogenesis to or below the basal levels (Fig. 4). Addition of IGFBP-6 with VEGF165 also abolished VEGF-stimulated angiogenesis. In fact, addition of IGFBP-6 with VEGF165 significantly reduced the average tube length and node number below control values (Fig. 4). These results suggest that IGFBP-6 inhibits basal as well as VEGF-stimulated angiogenesis in vitro.
Fig. 4.
IGFBP-6 inhibits both basal and VEGF-stimulated angiogenesis. Tube formation assay was performed using HUVECs in the presence of VEGF (25 ng/ml), PEDF (916 ng/ml), IGFBP-6 (1000 ng/ml), VEGF+PEDF, and VEGF+IGFBP-6. The average tube length (A) and node number (B) were determined and are expressed as a percentage of the control group (mean ± SE, n=3). Groups labeled with different letters are statistically different from each other at P < 0.01.
IGFBP-6 inhibits embryonic angiogenesis in vivo and this action is conserved in zebrafish
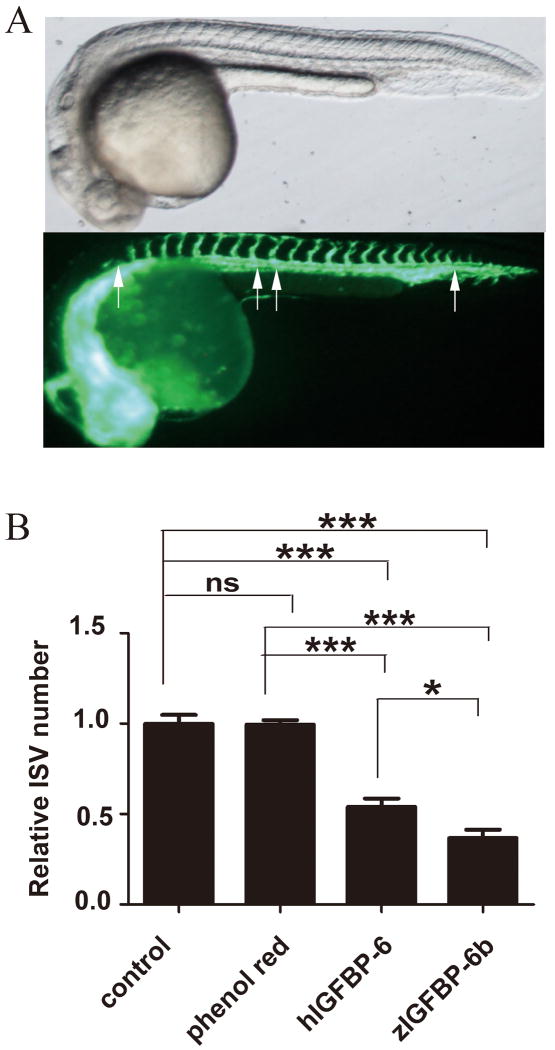
To test the effect of IGFBP-6 in an in vivo setting, capped mRNA encoding human IGFBP-6 was synthesized and injected into flk1:GFP embryos. The flk1:GFP transgenic zebrafish line is widely used for studying vascular development. In this line, GFP expression, driven by the flk1 gene promoter, is exclusively expressed in vascular epithelial cells in the developing vasculature19, thus permitting visualization of the developing blood vessels in vivo (Fig. 5A). Phenol red was injected as control. As shown in Fig. 5A, embryos injected with human IGFBP-6 mRNA had fewer inner segmental vessels (ISV) compared to embryos injected with phenol red or intact embryos (Fig. 5B). As shown in Fig. 5B, the mean relative number of ISVs in the intact control group was 100 ± 5% at 30 hpf. A similar value (100 ± 3%) was obtained in the phenol red injected group. Injection of GFP mRNA also had no effect (not shown). In contrast, human IGFBP-6 significantly decreased relative ISV number to 54 ± 5% of control (P<0.0001). These results, consistent with the in vitro data, suggest that human IGFBP-6 inhibits angiogenesis in this in vivo model.
Fig. 5.
Human and zebrafish IGFBP-6 inhibits blood vessel formation in zebrafish embryos in vivo. A) A 30 hour post fertilization (hpf) flk1:GFP transgenic zebrafish embryo. The upper panel is a bright field view and lower panel is a GFP view. Arrows indicate inner segmental vessels (ISV). B) 1-2 cell stage flk1:GFP transgenic zebrafish embryos were microinjected with phenol red, capped mRNA encoding human IGFBP-6, or zebrafish IGFBP-6 (zIGFBP-6b). The embryos were raised to 30 hpf and the number of inner segmental vessels (ISV) was counted. Results are expressed as a percentage of control (mean ± SE, n=3, *, P<0.05; ***, P<0.001).
To determine whether this anti-angiogenic activity is conserved in zebrafish, we prepared capped mRNA for the zebrafish igfbp-6b gene and injected it into flk1:GFP embryos. As shown in Fig. 5B, zebrafish IGFBP-6b significantly inhibited embryonic vascular development (37±5%, P<0.001). These results suggest that IGFBP-6 is an anti-angiogenic factor in vivo, and this action is conserved between human and zebrafish IGFBP-6.
IGFBP-6 inhibits rhabdomyosarcoma xenograft angiogenesis
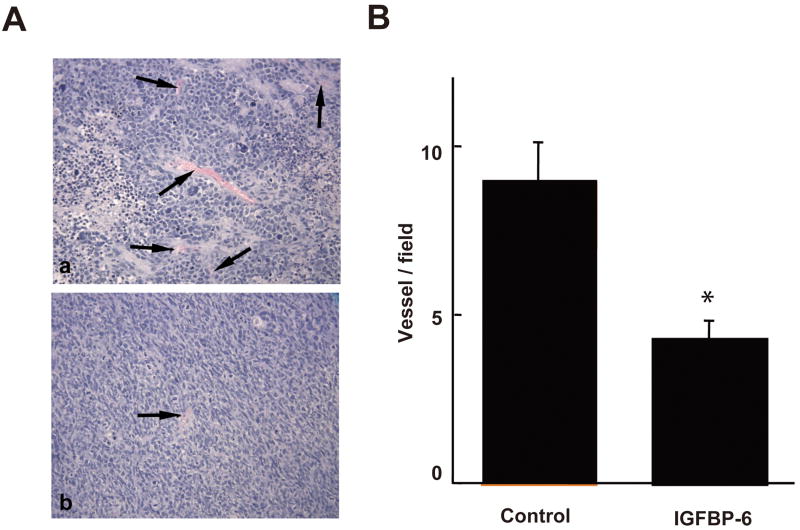
We next investigated the role of IGFBP-6 in tumor angiogenesis by xenografting empty vector- or human IGFBP-6-transfected Rh30 human rhabdomyosacoma cells into athymic BALB/c nude mice. We previously showed that overexpression of human IGFBP-6 in these tumor cells inhibits cell proliferation and promotes apoptosis in vitro and reduces xenograft growth in vivo 14. After 27 d, tumor volume of the IGFBP-6-transfected xenografts was 60% lower than that of vector control xenografts (0.34 ± 0.08 vs. 0.86 ± 0.06 cm2, P < 0.05), confirming our previous results 14. Blood vessel density, as defined by erythrocyte-containing luminal structures per field, was quantified. Vessel density was 52% lower in IGFBP-6-transfected xenografts than in vector control xenografts (4.3 ± 0.5 vs. 8.9 ± 1.2 vessels/field, P<0.05, Fig. 6), suggesting that IGFBP-6 inhibits tumor angiogenesis in this in vivo model.
Fig. 6.
IGFBP-6 inhibits angiogenesis in Rh30 human rhabdomyosarcoma xenografts in vivo. Nude mice were injected with Rh30 cells that had been stably transfected with an IGFBP-6-expressing (n=4) or control (n=5) vector. After 27 d, tumors were excised and fixed. Paraffin sections were cut and stained with hematoxylin and eosin. A) A representative view of xenografts from the control (panel a) and IGFBP-6-overexpressing Rh30 cells (panel b). Some blood vessels, defined as erythrocyte-containing luminal structures, are indicated by arrows. B) Quantitative results. Blood vessels were counted in 6-10 fields at 100× magnification in each tumor. Results are shown as mean ± SE, n = 4-5. * p<0.05.
Discussion
In this study, we have provided several lines of experimental evidence supporting the notion that IGFBP-6 is an hypoxia-inducible gene that plays a negative role in hypoxia-induced tumor angiogenesis. We show that prolonged hypoxia induces IGFBP-6 expression in human and rat VECs, likely via HIF-1 activation. Our in vitro assays, using cultured human HUVECs, and in vivo studies, using flk1:GFP zebrafish embryos, suggest that IGFBP-6 is a potent anti-angiogenic molecule. This action is conserved across species and is IGF-independent. Furthermore, we provide direct evidence for an inhibitory role of IGFBP-6 in tumor angiogenesis in Rh30 human rhabdomyosacoma xenografts.
VECs are critical components of the vasculature and play a crucial role in the maintenance of vascular integrity. These cells are hypoxia-tolerant and can divide under hypoxic conditions. There is a large body of published data suggesting that major components of the IGF signaling pathway are expressed and functional in VECs 20. IGFs are evolutionarily conserved polypeptides that regulate a wide range of cellular processes including mitogenesis, differentiation, migration, and survival 21. These actions are largely mediated through the IGF-I receptor (IGF-IR), a receptor tyrosine kinase. Ligand binding of the IGF-IR activates a number of major signaling pathways, including the Ras -MAPK and the PI3 kinase-Akt pathways. IGF signaling has been implicated in hypoxia-induced tumor angiogenesis 20. IGF-I stimulates HIF-1α expression in endothelial cells via the PI3 kinase/mTOR signaling pathway 22. IGF-I also stimulates VEGF expression and secretion via HIF-1-dependent and -independent mechanisms 23. Furthermore, pharmacological blockade or tissue-specific knockout of the IGF-IR decreased retinal neovascularization 24, 25
Cultured mammalian VECs express IGFBP 2-6 12, 26, 27. IGFBPs synthesized by VECs are involved in transfer of IGFs across the VEC barrier 28, 29. IGFBPs bind IGFs and inhibit or potentiate their actions in the vasculature 20. Han and colleagues have shown that IGFBP-6 mRNA expression was very low in cultured bovine VECs and increased with exposure to hypoxia 12. The mechanism underlying this hypoxia induction, however, was not studied. In this study, we have extended this observation to human and rat VECs, suggesting the up-regulation of IGFBP-6 expression in VECs is conserved across species. Furthermore, we provide evidence that HIF-1 activation is likely involved in hypoxia-induced IGFBP-6 gene expression. Hypoxia or CoCl2 treatments increased nuclear HIF-1α levels and increased expression of established HIF-1 target genes such as PDK1. Sequence analysis of the human IGFBP-6 gene showed that there are 8 canonical HREs located in the promoter region: 5 in the forward orientation and 3 in the reverse orientation. It has been proposed that cooperative interactions between the HRE and an adjacent HIF-1 ancillary sequence (HAS) are critical to confer hypoxia responsiveness 18. In the promoter region of human IGFBP-6, we found 14 putative HAS, 6 in the forward orientation and 8 in the reverse orientation. HIF-1 has also been shown to induce the expression of a related gene, IGFBP-1, and hypoxia increased IGFBP-1 expression in the liver through an HRE located in intron 2 of its gene 30. More recent studies show that the induction of IGFBP-1 gene expression by hypoxia is mediated through HIF-1 both in vitro and in vivo 18. There is a clear temporal difference between IGFBP-1 and IGFBP-6 regulation by hypoxia: hypoxia increases IGFBP-1 gene expression rapidly in liver/hepatocytes, whereas the up-regulation of the IGFBP-6 gene was not observed until 48 h of hypoxia. This timing is interesting as it implies that IGFBP-6 induction by hypoxia may act as a negative feedback mechanism in hypoxia-induced angiogenesis.
A number of mechanisms underlying the inhibitory actions of IGFBP-6 on tumor growth have been suggested. IGFBP-6 has been shown to inhibit tumor cell proliferation and increase apoptosis, most likely by binding to and inhibiting IGF-II actions 10, 11, 14. IGFBP-6 has been recently reported to interact with Ku80, a DNA-end binding protein, in the cytoplasm to regulate cell survival, and this effect may be IGF-dependent 31. IGFBP-6 acts as an effector of the tumor suppressor gene semaphorin 3B in lung cancer cells, although IGF-II partially abrogated its effects 32. A number of recent studies also indicate that IGFBP-6 may suppress tumor growth via IGF-independent actions. IGFBP-6 has a functional nuclear localization signal and is capable of translocation to the nucleus in an IGF-independent manner 33. IGFBP-6 inhibited nasopharyngeal carcinoma cell proliferation, invasion, and metastasis by directly binding to the promoter of the tumor suppressor EGR-1 and increasing its expression; IGF-dependence of this effect was not directly studied 34. In contrast to the above studies, IGFBP-6 has also been shown to promote rhabdomyosarcoma and colon cancer cell migration via p38 MAPK and crosstalk with other MAPKs in an IGF-independent manner 11, 35.
The results of the present study suggest an additional and novel mechanism by which IGFBP-6 inhibits tumor growth. Our in vitro and in vivo data show that IGFBP-6 is a potent angiogenesis inhibitor. This action is IGF-independent and is evolutionarily conserved in human and fish. Ongoing solid tumor growth requires angiogenesis which is driven by relative hypoxia 36. Inhibition of hypoxia-induced angiogenesis by IGFBP-6 may, therefore, contribute to its tumor suppressor properties. There are several plausible mechanisms by which IGFBP-6 inhibits angiogenesis: 1) since IGFBP-6 suppresses VEGF-induced tube formation when added together in cultured HUVECs, IGFBP-6 may inhibit VEGF action by directly binding to VEGF itself and/or affect VEGF receptor-mediated signaling; 2) IGFBP-6 has been shown to activate p38 MAPK in an IGF-independent manner and this is required for its ability to promote rhabdomyosarcoma and colon cancer cell migration35. It is possible that IGFBP-6 inhibits angiogenesis via a similar mechanism. 3) IGFBP-6 has a functional nuclear localization signal and is capable of translocation to the nucleus33. A recent study reports that IGFBP-6 inhibits nasopharyngeal carcinoma cell proliferation, invasion, and metastasis by directly binding to the promoter of the tumor suppressor gene EGR-1 and increasing its expression34. The possible involvement of nuclear IGFBP-6 in the anti-angiogenic action cannot be ruled out. Future studies aimed at elucidating the molecular basis of IGF-independent inhibition of angiogenesis by IGFBP-6 should deepen our understanding of hypoxia-regulated tumor angiogenesis. It should be pointed out that our current model on the hypoxia-induced IGFBP-6 expression in VECs and the anti-angiogenic action of IGFBP-6 is based on results from gain-of-functional experiments, either by adding large amounts of purified IGFBP-6 to cultured human cells in vitro or by overexpressing IGFBP-6 in animal tissues in vivo. Future studies are needed to determine whether the local levels of IGFBP-6 in tumor vasculature are sufficiently high to cause a significant decrease in angiogenesis in vivo. It will also be interesting to test whether knockdown of IGFBP-6 in VECs using short double-stranded RNA (siRNA) will enhance hypoxia-induced angiogenesis and increase tumor growth. Moreover, tissue-specific IGFBP-6 knockout mouse lines will be needed to determine the physiological role of the hypoxia-induced IGFBP-6 expression by VECs in vivo. Understanding the signaling pathways leading to the negative regulation of angiogenesis will likely provide important tools to develop novel therapeutic strategies to hinder deregulated tumor angiogenesis.
Supplementary Material
Effects of hypoxia and CoCl2 treatments on IGFBP-6 mRNA levels in cultured human umbilical vascular endothelial cells (A) and rat brain vessel endothelial cells (B). (A) Human umbilical vascular endothelial cells were cultured under normoxia (20%) without (Control) or with CoCl2 at the indicated concentrations for 5h or cultured under hypoxia (1% O2) for the indicated time. Total RNA was isolated and IGFBP-6 mRNA levels were determined by RT-PCR. mRNA levels of PDK, a well-known HIF-1 target gene, were measured as a positive control. β-actin, a housekeeping gene, was used as a loading control. (B) Rat brain vessel endothelial cells were cultured under normoxia (20%) without (Control) or with 1 mM CoCl2 for 5h. IGFBP-6 and β-actin mRNA levels were determined by RT-PCR. Two independent samples from each group are shown.
Acknowledgments
We thank Ms. Lisa Hebda and Catherine Nosal, University of Michigan for proof reading this manuscript. This study was supported by Natural Scientific Foundation of China Grant# 30970357, 30928021, and 30800838, US National Science Foundation Research Grant IOB 0110864, IOS-1051034, NIH 2RO1HL60679, and by grants from the National Health and Medical Research Council of Australia, Cancer Council Victoria and Alfred Research Trust.
References
- 1.Rankin EB, Giaccia AJ. The role of hypoxia-inducible factors in tumorigenesis. Cell Death Differ. 2008;15:678–85. doi: 10.1038/cdd.2008.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fraisl P, Aragones J, Carmeliet P. Inhibition of oxygen sensors as a therapeutic strategy for ischaemic and inflammatory disease. Nat Rev Drug Discov. 2009;8:139–52. doi: 10.1038/nrd2761. [DOI] [PubMed] [Google Scholar]
- 3.Rey S, Semenza GL. Hypoxia-inducible factor-1-dependent mechanisms of vascularization and vascular remodelling. Cardiovasc Res. 2010;86:236–42. doi: 10.1093/cvr/cvq045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Maxwell PH, Wiesener MS, Chang GW, Clifford SC, Vaux EC, Cockman ME, Wykoff CC, Pugh CW, Maher ER, Ratcliffe PJ. The tumour suppressor protein VHL targets hypoxia-inducible factors for oxygen-dependent proteolysis. Nature. 1999;399:271–5. doi: 10.1038/20459. [DOI] [PubMed] [Google Scholar]
- 5.Ohh M, Park CW, Ivan M, Hoffman MA, Kim TY, Huang LE, Pavletich N, Chau V, Kaelin WG. Ubiquitination of hypoxia-inducible factor requires direct binding to the beta-domain of the von Hippel-Lindau protein. Nat Cell Biol. 2000;2:423–7. doi: 10.1038/35017054. [DOI] [PubMed] [Google Scholar]
- 6.Messmer-Blust A, An X, Li J. Hypoxia-regulated angiogenic inhibitors. Trends Cardiovasc Med. 2009;19:252–6. doi: 10.1016/j.tcm.2010.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.An X, Jin Y, Guo H, Foo SY, Cully BL, Wu J, Zeng H, Rosenzweig A, Li J. Response gene to complement 32, a novel hypoxia-regulated angiogenic inhibitor. Circulation. 2009;120:617–27. doi: 10.1161/CIRCULATIONAHA.108.841502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jin Y, An X, Ye Z, Cully B, Wu J, Li J. RGS5, a hypoxia-inducible apoptotic stimulator in endothelial cells. J Biol Chem. 2009;284:23436–43. doi: 10.1074/jbc.M109.032664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Duan C, Xu Q. Roles of insulin-like growth factor (IGF) binding proteins in regulating IGF actions. Gen Comp Endocrinol. 2005;142:44–52. doi: 10.1016/j.ygcen.2004.12.022. [DOI] [PubMed] [Google Scholar]
- 10.Bach LA. IGFBP-6 five years on; not so ‘forgotten’? Growth Horm IGF Res. 2005;15:185–92. doi: 10.1016/j.ghir.2005.04.001. [DOI] [PubMed] [Google Scholar]
- 11.Fu P, Thompson JA, Bach LA. Promotion of cancer cell migration: an insulin-like growth factor (IGF)-independent action of IGF-binding protein-6. J Biol Chem. 2007;282:22298–306. doi: 10.1074/jbc.M703066200. [DOI] [PubMed] [Google Scholar]
- 12.Tucci M, Nygard K, Tanswell BV, Farber HW, Hill DJ, Han VK. Modulation of insulin-like growth factor (IGF) and IGF binding protein biosynthesis by hypoxia in cultured vascular endothelial cells. J Endocrinol. 1998;157:13–24. doi: 10.1677/joe.0.1570013. [DOI] [PubMed] [Google Scholar]
- 13.Westerfield M. The zebrafish Book. University of Oregon Press; 1995. [Google Scholar]
- 14.Gallicchio MA, Kneen M, Hall C, Scott AM, Bach LA. Overexpression of insulin-like growth factor binding protein-6 inhibits rhabdomyosarcoma growth in vivo. Int J Cancer. 2001;94:645–51. doi: 10.1002/ijc.1519. [DOI] [PubMed] [Google Scholar]
- 15.Annabi B, Thibeault S, Lee YT, Bousquet-Gagnon N, Eliopoulos N, Barrette S, Galipeau J, Beliveau R. Matrix metalloproteinase regulation of sphingosine-1-phosphate-induced angiogenic properties of bone marrow stromal cells. Exp Hematol. 2003;31:640–9. doi: 10.1016/s0301-472x(03)00090-0. [DOI] [PubMed] [Google Scholar]
- 16.Li Y, Xiang J, Duan C. Insulin-like growth factor-binding protein-3 plays an important role in regulating pharyngeal skeleton and inner ear formation and differentiation. The Journal of biological chemistry. 2005;280:3613–20. doi: 10.1074/jbc.M411479200. [DOI] [PubMed] [Google Scholar]
- 17.Wang X, Lu L, Li Y, Li M, Chen C, Feng Q, Zhang C, Duan C. Molecular and functional characterization of two distinct IGF binding protein-6 genes in zebrafish. Am J Physiol Regul Integr Comp Physiol. 2009;296:R1348–57. doi: 10.1152/ajpregu.90969.2008. [DOI] [PubMed] [Google Scholar]
- 18.Kajimura S, Aida K, Duan C. Understanding hypoxia-induced gene expression in early development: in vitro and in vivo analysis of hypoxia-inducible factor 1-regulated zebra fish insulin-like growth factor binding protein 1 gene expression. Mol Cell Biol. 2006;26:1142–55. doi: 10.1128/MCB.26.3.1142-1155.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Parker LH, Schmidt M, Jin SW, Gray AM, Beis D, Pham T, Frantz G, Palmieri S, Hillan K, Stainier DY, De Sauvage FJ, Ye W. The endothelial-cell-derived secreted factor Egfl7 regulates vascular tube formation. Nature. 2004;428:754–8. doi: 10.1038/nature02416. [DOI] [PubMed] [Google Scholar]
- 20.Clemmons DR. Modifying IGF1 activity: an approach to treat endocrine disorders, atherosclerosis and cancer. Nat Rev Drug Discov. 2007;6:821–33. doi: 10.1038/nrd2359. [DOI] [PubMed] [Google Scholar]
- 21.Treins C, Murdaca J, Van Obberghen E, Giorgetti-Peraldi S. AMPK activation inhibits the expression of HIF-1alpha induced by insulin and IGF-1. Biochem Biophys Res Commun. 2006;342:1197–202. doi: 10.1016/j.bbrc.2006.02.088. [DOI] [PubMed] [Google Scholar]
- 22.Treins C, Giorgetti-Peraldi S, Murdaca J, Monthouel-Kartmann MN, Van Obberghen E. Regulation of hypoxia-inducible factor (HIF)-1 activity and expression of HIF hydroxylases in response to insulin-like growth factor I. Mol Endocrinol. 2005;19:1304–17. doi: 10.1210/me.2004-0239. [DOI] [PubMed] [Google Scholar]
- 23.Slomiany MG, Rosenzweig SA. Hypoxia-inducible factor-1-dependent and -independent regulation of insulin-like growth factor-1-stimulated vascular endothelial growth factor secretion. J Pharmacol Exp Ther. 2006;318:666–75. doi: 10.1124/jpet.106.104158. [DOI] [PubMed] [Google Scholar]
- 24.Kondo T, Vicent D, Suzuma K, Yanagisawa M, King GL, Holzenberger M, Kahn CR. Knockout of insulin and IGF-1 receptors on vascular endothelial cells protects against retinal neovascularization. J Clin Invest. 2003;111:1835–42. doi: 10.1172/JCI17455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Smith LE, Shen W, Perruzzi C, Soker S, Kinose F, Xu X, Robinson G, Driver S, Bischoff J, Zhang B, Schaeffer JM, Senger DR. Regulation of vascular endothelial growth factor-dependent retinal neovascularization by insulin-like growth factor-1 receptor. Nat Med. 1999;5:1390–5. doi: 10.1038/70963. [DOI] [PubMed] [Google Scholar]
- 26.Yang YW, Pioli P, Fiorelli G, Brandi ML, Rechler MM. Cyclic adenosine monophosphate stimulates insulin-like growth factor binding protein-4 and its messenger ribonucleic acid in a clonal endothelial cell line. Endocrinology. 1993;133:343–51. doi: 10.1210/endo.133.1.7686482. [DOI] [PubMed] [Google Scholar]
- 27.Moser DR, Lowe WL, Jr, Dake BL, Booth BA, Boes M, Clemmons DR, Bar RS. Endothelial cells express insulin-like growth factor-binding proteins 2 to 6. Mol Endocrinol. 1992;6:1805–14. doi: 10.1210/mend.6.11.1282670. [DOI] [PubMed] [Google Scholar]
- 28.Taylor WR, Nerem RM, Alexander RW. Polarized secretion of IGF-I and IGF-I binding protein activity by cultured aortic endothelial cells. J Cell Physiol. 1993;154:139–42. doi: 10.1002/jcp.1041540117. [DOI] [PubMed] [Google Scholar]
- 29.Gajdusek CM, Luo Z, Mayberg MR. Sequestration and secretion of insulin-like growth factor-I by bovine aortic endothelial cells. J Cell Physiol. 1993;154:192–8. doi: 10.1002/jcp.1041540122. [DOI] [PubMed] [Google Scholar]
- 30.Tazuke SI, Mazure NM, Sugawara J, Carland G, Faessen GH, Suen LF, Irwin JC, Powell DR, Giaccia AJ, Giudice LC. Hypoxia stimulates insulin-like growth factor binding protein 1 (IGFBP-1) gene expression in HepG2 cells: a possible model for IGFBP-1 expression in fetal hypoxia. Proc Natl Acad Sci U S A. 1998;95:10188–93. doi: 10.1073/pnas.95.17.10188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Iosef C, Vilk G, Gkourasas T, Lee KJ, Chen BP, Fu P, Bach LA, Lajoie G, Gupta MB, Li SS, Han VK. Insulin-like growth factor binding protein-6 (IGFBP-6) interacts with DNA-end binding protein Ku80 to regulate cell fate. Cell Signal. 2010;22:1033–43. doi: 10.1016/j.cellsig.2010.02.006. [DOI] [PubMed] [Google Scholar]
- 32.Koyama N, Zhang J, Huqun, Miyazawa H, Tanaka T, Su X, Hagiwara K. Identification of IGFBP-6 as an effector of the tumor suppressor activity of SEMA3B. Oncogene. 2008;27:6581–9. doi: 10.1038/onc.2008.263. [DOI] [PubMed] [Google Scholar]
- 33.Iosef C, Gkourasas T, Jia CY, Li SS, Han VK. A functional nuclear localization signal in insulin-like growth factor binding protein-6 mediates its nuclear import. Endocrinology. 2008;149:1214–26. doi: 10.1210/en.2007-0959. [DOI] [PubMed] [Google Scholar]
- 34.Kuo YS, Tang YB, Lu TY, Wu HC, Lin CT. IGFBP-6 plays a role as an oncosuppressor gene in NPC pathogenesis through regulating EGR-1 expression. J Pathol. 2010;222:299–309. doi: 10.1002/path.2735. [DOI] [PubMed] [Google Scholar]
- 35.Fu P, Liang GJ, Khot SS, Phan R, Bach LA. Cross-talk between MAP kinase pathways is involved in IGF-independent, IGFBP-6-induced Rh30 rhabdomyosarcoma cell migration. J Cell Physiol. 2010;224:636–43. doi: 10.1002/jcp.22156. [DOI] [PubMed] [Google Scholar]
- 36.Helmlinger G, Endo M, Ferrara N, Hlatky L, Jain RK. Formation of endothelial cell networks. Nature. 2000;405:139–41. doi: 10.1038/35012132. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Effects of hypoxia and CoCl2 treatments on IGFBP-6 mRNA levels in cultured human umbilical vascular endothelial cells (A) and rat brain vessel endothelial cells (B). (A) Human umbilical vascular endothelial cells were cultured under normoxia (20%) without (Control) or with CoCl2 at the indicated concentrations for 5h or cultured under hypoxia (1% O2) for the indicated time. Total RNA was isolated and IGFBP-6 mRNA levels were determined by RT-PCR. mRNA levels of PDK, a well-known HIF-1 target gene, were measured as a positive control. β-actin, a housekeeping gene, was used as a loading control. (B) Rat brain vessel endothelial cells were cultured under normoxia (20%) without (Control) or with 1 mM CoCl2 for 5h. IGFBP-6 and β-actin mRNA levels were determined by RT-PCR. Two independent samples from each group are shown.