Abstract
The arachidonate 5-lipoxygenase-activating protein (ALOX5AP) gene has been associated with stroke. The majority of the reported ALOX5AP associations have considered non-radiologically confirmed infarcts as the stroke phenotype. We assessed the association of genetic variants in ALOX5AP with stroke defined by the presence infarcts on brain Magnetic Resonance Imaging (MRI). We studied 202 persons with MRI-defined brain infarcts cases and 487 healthy individuals of Caribbean Hispanic ancestry. Another sample of European ancestry comprised of 1,823 persons with MRI-defined brain infarct and 7,578 controls. Subjects were genotyped for the four SNPs that define ALOX5AP HapA haplotype. No association was found between SNPs and MRI-defined brain infarcts. Our data do not support the hypothesis that variants in ALOX5AP are associated with risk of MRI-defined brain infarcts.
Keywords: MRI-defined brain infarcts, ALOX5AP
1. Introduction
The genome-wide linkage scan published by deCODE (Helgadottir, et al., 2004) implicated SNPs and haplotypes in the ALOX5AP and PDE4D genes in ischemic stroke (IS) in the Icelandic population. Their results identified a haplotype in the ALOX5AP gene, HapA, defined by the SNPs SG13S25 (rs17222814), SG13S114 (rs10507391), SG13S89 (rs4769874) and SG13S32 (rs9551963), which conferred an increased risk of myocardial infarction (haplotype frequency=0.16, RR=1.80) and stroke (haplotype frequency=0.15, RR=1.67). However, replication of these results in other populations has proven difficult (Zee, et al., 2006).
Failure to replicate associations between ALOX5AP variants and stroke could have been due the diagnosis of stroke on medical records only (Quarta, et al., 2009). The sensitivity of self-reported history of clinical stroke has been questioned since it is likely that patients with ambiguous symptoms or silent strokes are underestimated leading to a higher rate of false-negative results. Results using the WHICAP cohort (Reitz, et al., 2009) suggest that when using MRI scans as validation, the sensitivity and specificity of stroke self-report are low validating the use of neuroimaging techniques to confirm a diagnosis of stroke based on the history.
The present study was designed to confirm or refute an association between MRI-defined brain infarcts (MRI infarcts) and SNPs in ALOX5AP using two different cohorts, i) a community-based predominantly Hispanic case-control sample from the Washington Heights Inwood Columbia Aging Project (WHICAP) and ii) querying the published GWAS results from the Cohorts for Heart and Aging Research in Genome Epidemiology (CHARGE) Consortium meta-analysis.
2. Material and Methods
Caribbean Hispanic population consisted of 202 MRI infarct cases and 487 controls. The four SNPs that define the Hap A haplotype: SG13S25 (rs17222814), SG13S114 (rs10507391), SG13S89 (rs4769874) and SG13S32 (rs9551963) were genotyped at the Illumina Genotyping Service Center, San Diego, California. The CHARGE consortium includes 6 large, prospective, community based cohort studies that have genome-wide variation data coupled with extensive data on multiple phenotypes. Clinical evaluation and MR imaging protocol was described elsewhere (Brickman, et al., 2008, Debette, et al., 2010). Allelic test of association for each of the SNPs were carried out as performed by the Helgadottir et al report.
3. Results
WHICAP cohort. Demographic and risk factors case-control differences
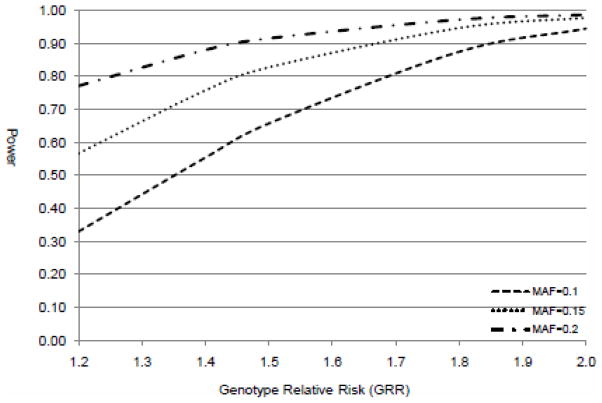
After comparing cases and controls for demographic variables, we found a significantly higher proportion of men than women among MRI stroke cases (p=0.04). The cases differed from controls having a higher frequency of hypertension and myocardial infarction. Only the presence of hypertension and previous myocardial infarction were associated with MRI infarct (Supplemental Table 1). No statistical differences were observed between cases and controls when comparing the distribution of SNP allele frequencies (Supplemental Table 2). Power estimation for the WHICAP cohort (Purcell, et al., 2003) indicated that at significance level of 0.05 and assuming minor allele frequency of 0.15, the study has 85% power to detect odds ratios smaller than the original OR of 1.67 reported by Helgadottir et al.
3.2. - CHARGE cohort
In order to confirm our findings, we also examined the association of the same ALOX5AP SNPs imputed in the 6 CHARGE cohorts as described previously(Debette, et al., 2010) with covert brain infarcts (1 or more MRI infarcts in persons free of clinical strokes). Again, we did not observe any association with MRI infarction (Supplemental Table 3). Power calculations using Quanto program (Gauderman, 2002) revealed that CHARGE study has 99.99% power at 0.05 significance level to detect odds ratio of 1.5 (assuming minor allele frequency of 0.15) given the sample size.
4. Discussion
We found no association between ALOX5AP SNPs and MRI infarcts, suggesting that the SNP associations previously reported might not be risk factors for MRI infarcts. This is the first study that specifically examines the role of ALOX5AP SNPs using MRI defined infarcts in both Hispanic and Caucasian community-based samples. Although the initial reports (Helgadottir, et al., 2004) claimed an association between ALOX5AP and stroke, subsequent independent efforts failed to replicate the initial findings. The lack of replication might be due to small sample sizes or to the use of different populations, phenotype heterogeneity, sampling strategies, genotyping procedures, and/or numbers of loci in the studies. However there are alternative hypothesis. ALOX5AP may explain or be a marker of more severe strokes, since more of the ones studied here are silent strokes; another possibility is that stroke etiologies among our cohorts and previous ones that studied ALOX5AP stroke relationship differ, for example we know that small vessel disease with lacunar infarctions are more often identified in MRI as compared with overt stroke in which cardioembolic or atherosclerotic etiologies are more represented. Our results indicate that ALOX5AP SNPs are not associated with MRI infarcts in the two Europe and North America populations studied. Considering that the two different cohorts were sufficiently powered, we conclude that the ALOX5AP SNPs we studied are not associated with MRI defined brain infarcts.
Supplementary Material
Figure 1.
Power estimation of WHICAP Caribbean Hispanic cohort
Acknowledgments
We acknowledge Dr. DeCarli and the Imaging of Dementia and Aging (IDeA) laboratory for their work in MRI infarct detection under subcontract to P01 AG0027232. We thank all the members of the CHARGE Neurology Working group: Aging Gene-Environment Susceptibility-Reykjavik Study, The Atherosclerosis Risk in Communities Study, The Austrian Stroke Prevention Study, Cardiovascular Health Study, Framingham Heart Study, and Rotterdam Study
Footnotes
Disclosure Statement
The authors disclose no actual or potential conflicts of interest
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Brickman AM, Schupf N, Manly JJ, Luchsinger JA, Andrews H, Tang MX, Reitz C, Small SA, Mayeux R, DeCarli C, Brown TR. Brain morphology in older African Americans, Caribbean Hispanics, and whites from northern Manhattan. Arch Neurol. 2008;65(8):1053–61. doi: 10.1001/archneur.65.8.1053. 65/8/1053 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debette S, Bis JC, Fornage M, Schmidt H, Ikram MA, Sigurdsson S, Heiss G, Struchalin M, Smith AV, van der Lugt A, DeCarli C, Lumley T, Knopman DS, Enzinger C, Eiriksdottir G, Koudstaal PJ, DeStefano AL, Psaty BM, Dufouil C, Catellier DJ, Fazekas F, Aspelund T, Aulchenko YS, Beiser A, Rotter JI, Tzourio C, Shibata DK, Tscherner M, Harris TB, Rivadeneira F, Atwood LD, Rice K, Gottesman RF, van Buchem MA, Uitterlinden AG, Kelly-Hayes M, Cushman M, Zhu Y, Boerwinkle E, Gudnason V, Hofman A, Romero JR, Lopez O, van Duijn CM, Au R, Heckbert SR, Wolf PA, Mosley TH, Seshadri S, Breteler MM, Schmidt R, Launer LJ, Longstreth WT., Jr Genome-wide association studies of MRI-defined brain infarcts: meta-analysis from the CHARGE Consortium. Stroke. 2010;41(2):210–7. doi: 10.1161/STROKEAHA.109.569194. STROKEAHA.109.569194 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gauderman WJ. Sample size requirements for matched case-control studies of gene-environment interaction. Stat Med. 2002;21(1):35–50. doi: 10.1002/sim.973. [pii] [DOI] [PubMed] [Google Scholar]
- Helgadottir A, Manolescu A, Thorleifsson G, Gretarsdottir S, Jonsdottir H, Thorsteinsdottir U, Samani NJ, Gudmundsson G, Grant SF, Thorgeirsson G, Sveinbjornsdottir S, Valdimarsson EM, Matthiasson SE, Johannsson H, Gudmundsdottir O, Gurney ME, Sainz J, Thorhallsdottir M, Andresdottir M, Frigge ML, Topol EJ, Kong A, Gudnason V, Hakonarson H, Gulcher JR, Stefansson K. The gene encoding 5-lipoxygenase activating protein confers risk of myocardial infarction and stroke. Nat Genet. 2004;36(3):233–9. doi: 10.1038/ng1311. ng1311 [pii] [DOI] [PubMed] [Google Scholar]
- Purcell S, Cherny SS, Sham PC. Genetic Power Calculator: design of linkage and association genetic mapping studies of complex traits. Bioinformatics. 2003;19(1):149–50. doi: 10.1093/bioinformatics/19.1.149. [DOI] [PubMed] [Google Scholar]
- Quarta G, Stanzione R, Evangelista A, Zanda B, Di Angelantonio E, Marchitti S, Di Castro S, Di Vavo M, Volpe M, Rubattu S. Phosphodiesterase 4D and 5-lipoxygenase activating protein genes and risk of ischemic stroke in Sardinians. Eur J Hum Genet. 2009;17(11):1448–53. doi: 10.1038/ejhg.2009.71. ejhg200971 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reitz C, Schupf N, Luchsinger JA, Brickman AM, Manly JJ, Andrews H, Tang MX, DeCarli C, Brown TR, Mayeux R. Validity of self-reported stroke in elderly African Americans, Caribbean Hispanics, and Whites. Arch Neurol. 2009;66(7):834–40. doi: 10.1001/archneurol.2009.83. 2009.83 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zee RY, Cheng S, Hegener HH, Erlich HA, Ridker PM. Genetic variants of arachidonate 5-lipoxygenase-activating protein, and risk of incident myocardial infarction and ischemic stroke: a nested case-control approach. Stroke. 2006;37(8):2007–11. doi: 10.1161/01.STR.0000229905.25080.01. 01.STR.0000229905.25080.01 [pii] [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.