Abstract
The purpose of writing this review is to showcase the Molecular Imaging and Contrast Agent Database (MICAD; www.micad.nlm.nih.gov) to students, researchers and clinical investigators interested in the different aspects of molecular imaging. This database provides freely accessible, current, online scientific information regarding molecular imaging (MI) probes and contrast agents (CA) used for positron emission tomography, single-photon emission computed tomography, magnetic resonance imaging, x-ray/computed tomography, optical imaging and ultrasound imaging. Detailed information on >1000 agents in MICAD is provided in a chapter format and can be accessed through PubMed. Lists containing >4250 unique MI probes and CAs published in peer-reviewed journals and agents approved by the United States Food and Drug Administration (FDA) as well as a CSV file summarizing all chapters in the database can be downloaded from the MICAD homepage. Users can search for agents in MICAD on the basis of imaging modality, source of signal/contrast, agent or target category, preclinical or clinical studies, and text words. Chapters in MICAD describe the chemical characteristics (structures linked to PubChem), the in vitro and in vivo activities and other relevant information regarding an imaging agent. All references in the chapters have links to PubMed. A Supplemental Information Section in each chapter is available to share unpublished information regarding an agent. A Guest Author Program is available to facilitate rapid expansion of the database. Members of the imaging community registered with MICAD periodically receive an e-mail announcement (eAnnouncement) that lists new chapters uploaded to the database. Users of MICAD are encouraged to provide feedback, comments or suggestions for further improvement of the database by writing to the editors at: micad@nlm.nih.gov
Keywords: Molecular imaging probes, Contrast agents, Database, positron emission tomography (PET), single-photon emission computed tomography (SPECT), magnetic resonance imaging (MRI), x-ray/computed tomography (x-ray/CT), optical imaging (OI), ultrasound imaging
INTRODUCTION
The intent of this review is to introduce the Molecular Imaging and Contrast Agent Database (MICAD; www.micad.nih.gov) to students, researchers and clinical investigators who are interested in studying the different disease processes with molecular imaging techniques or to those who are involved in the development of diagnostic agents or drugs that can be used to treat or diagnose diseases such as cancer, cardiovascular diseases etc. The database is a freely accessible online source of scientific information regarding molecular imaging (MI) probes and contrast agents (CAs) that have in vivo data published in peer reviewed journals [1, 2]. The primary mission of MICAD is to promote research, development, application and translation of molecular imaging research and techniques to the biological sciences and drug discovery for the rapid advancement of clinical sciences to detect, diagnose, treat and monitor various malignancies or pathological conditions in humans [3]. In addition, information available in the database can help avoid duplication of efforts and may catalyze and promote collaboration(s) between academic institutions and the pharmaceutical industry to perform research and develop new MI probes and CAs. Any MI probe or CA published during the last ~4 decades that is in pre-clinical development, under evaluation in clinical trials, or commercially available for use in the clinic can be considered for inclusion in MICAD. It is pertinent to mention that MICAD is not involved in the research, discovery, development, synthesis, application, or distribution of any MI probes or CAs. The database provides only information regarding MI probes and CAs that are used as tracers to detect and track the activity or level of potentially useful or known biomarkers (e.g., receptors) in diseased as compared to control or normal tissues. Information in the database also pertains to MI probes that are used to observe and understand the different molecular events or processes that occur in the different biological systems and are involved in the initiation and development of diseases such as cancer, cardiovascular diseases etc. If the information obtained from the pre-clinical studies (using mice, rats, non-human primates, etc.) is useful it can possibly be used to develop a novel MI probe or CA that will have clinical application. Conventional clinical methods may require surgical intervention or multiple laboratory investigations of the blood, urine, or biopsied tissues to detect, diagnose, stage and develop a treatment for a disease. In comparison, the use of MI probes is minimally invasive and can help healthcare providers stage,and monitor the progression or treatment of a disease in the patient with the same imaging techniques that were initially used to detect and diagnose the condition [3]. It is believed that molecular imaging will become a powerful tool and a synergistic component of biomedical research to develop therapies for the advancement of individualized medicine [4, 5].
In 2002 the United States National Institutes of Health (NIH) created a Common Fund for Medical Research (previously known as the NIH Roadmap; www.nihroadmap.nih.gov) for the rapid progress of scientific areas that could assist in the efficient translation of research into clinical applications. The three critical areas of research identified for development to achieve this goal were new pathways to discovery, research teams of the future, and re-engineering clinical research [6, 7]. One of the themes for the new pathways to discovery was the Molecular Libraries and Imaging (MLI) program which was designed to address important issues pertaining to the identification of molecules that can be used to diagnose patient disease and to develop targeted drugs to treat various ailments. The primary goal of the MI program is to enhance the awareness of known efforts and to promote the discovery and application of novel reagents in the imaging sciences and medicine, particularly to detect and diagnose cancers and to monitor the disease after initiation of the treatment. The three main components of MLI are: high-specificity/high-sensitivity molecular imaging probes, MICAD, and the Imaging Probe Development Center [8]. In 2004, a panel composed of NIH and extramural experts in molecular imaging established the framework to implement MICAD as a part of the National Center of Biotechnology Information (NCBI) PubMed database. The MICAD website is a source of free, concise and up-to-date information regarding MI probes and CAs in an easy-to-read textual format that is accessible through the PubMed website (www.pubmed.gov). A team of staff scientists (curators or editors) is dedicated to maintain the database with guidance from the trans-NIH panel through collaboration between the NCBI at the National Library of Medicine (NLM) and the Cancer Imaging Program of the National Cancer Institute of the NIH. Since its launch in September 2005 the number of chapters in MICAD has grown from 45 to >1000 (at the time of writing this article) and 15 – 20 new chapters are added to the database every month. The number of monthly visits to the MICAD website has increased >30-fold since inception of the program and the database is a popular source of information among the imaging community at the national and international level.
What MICAD contains
All chapters in MICAD are indexed, have assigned PubMed identification numbers (PMID) and can be searched on the PubMed database. The database includes, but is not limited to, chapters summarizing data regarding MI probes and CAs developed for positron emission tomography (PET), single-photon emission computed tomography (SPECT), magnetic resonance imaging (MRI), x-ray/computed tomography (x-ray/CT), ultrasound imaging, optical imaging (OI), planar radiography, and planar gamma imaging. New imaging modalities are added to the database as and when studies using the latest techniques are published. All literature references within the chapters have links to the corresponding abstracts that are accessible in the MEDLINE/PubMed databases. Chemical structures displayed in the chapters have links to the chemical substance abstracts in the PubChem database, a component of PubMed. In addition, to keep the imaging community informed of the latest developments regarding an agent the chapters are revised and updated as soon as new information regarding the agent is published. To improve the performance of the database the MICAD website and search engine undergo a periodic review and are redesigned and upgraded accordingly. MICAD is an evolving database that actively seeks feedback and recommendations from the users through various communication channels and platforms, including exhibits setup at various national and international imaging or related scientific meetings and conferences.
The MICAD Home Page
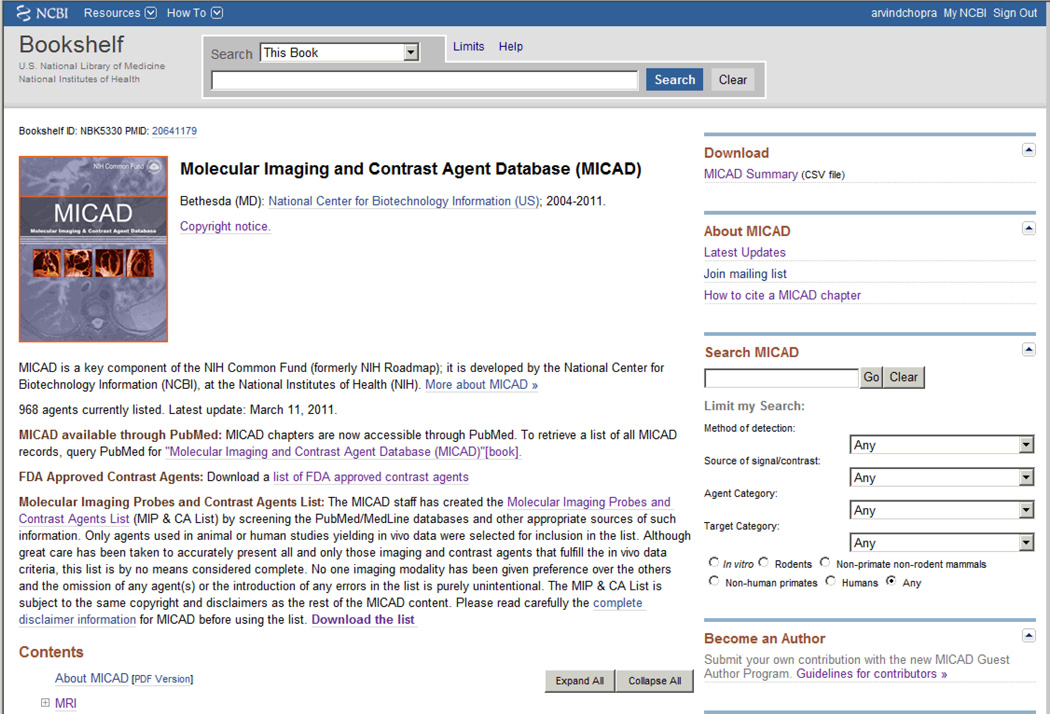
The MICAD program is supported through the NIH Common Fund and in keeping with the current US government policies it is available in the public domain with no copyright protection in the United States. No registration or subscription is required to access the database. A brief description of the background, scope, and mission of the database is available on the home page of MICAD (Fig. 1). The MICAD homepage is a part of the Bookshelf Section of the NCBI website (www.ncbi.nih.gov) and information regarding >1000 MI probes and CAs can be viewed either through PubMed by searching for “Molecular Imaging and Contrast Agent Database (MICAD)”[book] or by using the PMID number 20641179. The MICAD home page is also accessible directly on the web at www.micad.nih.gov. Each week the MICAD web site is updated by the addition of three to five chapters (150 – 250 chapters/year). Imaging agents in the database are searchable using either a single or any combination of six search filters available on the MICAD home page. The searches may be based on text words (e.g. Parkinson’s, single chain antibodies etc.), modality of detection (MRI, OI, PET etc.), source of signal or contrast (Alexa 650, Cy5.5, 123I, 99mTc, gadolinium, microbubbles, nanoparticles (NPs) etc.), agent category (antibodies, compounds, NPs, peptides, proteins etc.) or target category (such as enzymes, receptors, transporters, non-targeted etc.). Search results can be limited to chapters that meet desired criteria (e.g. in vitro studies, rodent or human studies etc.) by using an appropriate option provided below the search filters.
Figure 1.
The MICAD home page.*
* The MICAD home page can be accessed through PubMed by searching for “Molecular Imaging and Contrast Agent Database (MICAD) [Internet]”, by using PMID 20641179 or by accessing the web site address: www.micad.nlm.nih.gov.
Based on the modality used for imaging the largest category of probes described in MICAD is for PET (42%), followed by those for SPECT (31%), OI (12%) and MRI (12%) (Table 1). Multimodal, x-ray/CT and ultrasound probes constitute only about 4% of the total chapters in the database. Among the PET agents ~41% have a 18F label, ~31% are labeled with 11C and ~28% contain 64Cu, 124I, 68Ga and 89Zr as tracers. For SPECT agents 99mTc is the label of choice (~42%) followed by 111In (~29%) and other nuclides such as 123/125/131I and 177Lu have been used to label ~29% of the agents. Most MRI agents contain either Gd (~35%) or iron oxide (~25%) as the source of signal and in the remaining (~40%) the signal is generated by a variety of elements or compounds such as 19F, 17O and supraparamagnetic iron oxide. In ~30% of OI agents the signal is generated by Cy5.5 and rest of the agents (~70%) in this category are labeled with IRDye 800CW, various Alexa fluor dyes, quantum dots or bioluminescent luciferases. The majority of ultrasound agents (~75%) have a microbubble scaffold and all x-ray/CT agents contain non-radioactive iodine as the contrast generator. Most of the imaging agents in MICAD (Table 2) have a platform derived from small molecules or compounds (~42%) or from antibodies or peptides (~20% each) and ~30% have a backbone composed of polypeptides, proteins, nucleic acids, NPs etc.
Table 1.
Chapters in MICAD Based on Modality Used for Imaging.*
| Modality | Chapters in MICAD (%) |
|---|---|
| MRI | 12 |
| Multimodal | 1 |
| Optical | 12 |
| PET | 42 |
| SPECT | 31 |
| Ultrasound | 2 |
| x-ray/CT | 1 |
There are approximately 4200 agents in the MIP & CAList on the MICAD home page. Of these, >1000 are in MICAD
Table 2.
Category of Agents in MICAD.
| Scaffold | Percentage |
|---|---|
| Antibodies | 19 |
| Small molecules/compounds | 42 |
| Peptides | 20 |
| Proteins | 2 |
| Polypeptides | 4 |
| Others* | 13 |
Nanoparticles, nucleic acids, metals, etc.
DOWNLOADING INFORMATION FROM MICAD
Summary of Chapters in MICAD
Under the Download Section of the MICAD home page (Fig. 1) a comma separated values (CSV) file (spreadsheet) which lists and summarizes information regarding all entries in the database is available for download. This list, updated weekly, can be sorted according to the desired parameter (e.g. agent category, target, in vitro studies, non-human primate or human studies etc.) after it is downloaded. From this list it is apparent that almost all MICAD chapters (>95%) have data generated from in vitro and rodent studies. Data derived from non-human primate and human investigations (clinical trials) are available for 15 and 20% of the agents, respectively. Only ~10% of the agents have data obtained from non-primate non-rodent animals (e.g. rabbits, guinea pigs etc.). Of the MI probes and CAs available in MICAD, <3% have been through a complete cycle of investigations i. e. in vitro studies, and investigations in rodents, non-primate non-rodent animals, non-human primates and clinical trials. Because the agents published in MICAD are selected at random, without bias toward a specific MI modality, the list of agents in this CSV file is only a qualitative reflection of the published literature as a whole.
A list of all the MI probes and CAs (the MIP & CA List) published in peer-reviewed journals (currently ~4500 in number) is also available for download from the MICAD homepage (Fig. 1). Updates for this list are available three to four times a year and 100 –150 new agents may be added with each update (~500 agents/year). A PubMed abstract regarding each agent in the list can be viewed by clicking on its PMID. The MIP & CA list also contains information regarding the target and possible imaging applications of each agent. Agents in the list can be sorted or screened on the basis of mode of action, target, modality used to detect the signal etc.
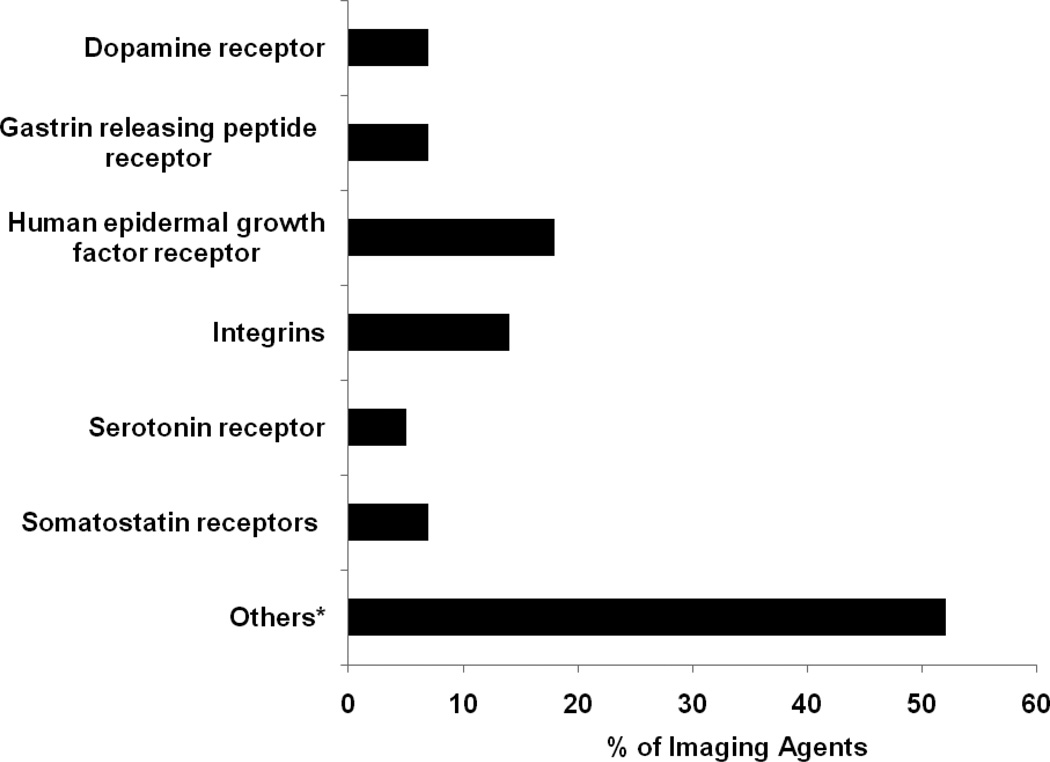
The MIP & CA list reveals that ~30% of MI probes and CAs are targeted toward receptors (Table 3) followed by enzymes (~5%; tyrosine kinase, carbonic anhydrase IX etc.), transporters of serotonin, dopamine, amino acids etc. (~4% each), antigens (CD20, CD33, vascular endothelial growth factor etc.; ~4%) and tissues (liver, brain, heart, bone etc; ~4%). Approximately 16% of the imaging agents are non-targeted and have application in perfusion studies of the heart, brain and the vasculature. The two main classes of receptors targeted by the agents (Figure 2) are the epidermal growth factor receptor (~18%; primarily the HER1, HER2 and HERvIII subtypes) and the integrins (~6.5%; mostly the alpha(v)beta(3) subtype). Approximately 2 to 3% of the probes target the receptors for benzodiazepines, gastrin releasing peptide, dopamine, nicotine-acetylcholine, sigma, serotonin, somatostatin etc., respectively. Other tracers in this category (~50% of the total) target several different receptors such as those for androgens, estrogens, folic acid and vascular endothelial growth factor. No imaging agents have been reported to investigate the receptors for the different types of fibroblast growth factors and only a few agents are available to investigate receptors that are known to modulate the various physiological processes involved in development of the malignant phenotype such as those for cytokines, tumor necrosis factor and the transforming growth factors.
Table 3.
Major Categories of Molecular Imaging and Contrast Agent Targets Based on Published Literature (see MIP & CA List on MICAD Home Page).
| Target Category | Percentage |
|---|---|
| Antigens | 4 |
| Enzymes | 5 |
| Transporters | 4 |
| Receptors | 29 |
| Non-targeted | 16 |
| Organs/tissues | 8 |
| Others* | 34 |
Lipids, infection and inflammation, histones, nucleotides, various receptors with <5 agents, etc.
Figure 2.
Receptors targeted by imaging agents.
* Such as androgen receptors, benzodiazepine receptors, cannabinoid receptors, folate receptors, somatostatin receptors etc. Each constitutes between 1% and 4% of the total.
List of MI probes and CAs Approved by the FDA
A list of ~110 MI probes and CAs approved by the FDA is available on the MICAD home page (Figure 1). This list provides the drug name, active ingredient(s), date of approval, marketing status (i. e. prescription or non-prescription), disease indication(s) for which an agent is approved and a web link to the drug product insert. Among these, 48 agents (~44%) have been approved for x-ray/CT investigations, 43 (~39%) for SPECT and gamma planar imaging, 12 (~11%) for MRI, 3 (~3%) for PET, two (~2%) for OI and two (~2%) for ultrasound applications. Any warnings issued by the FDA regarding the use of an MI probe or a CA are also available on the MICAD home page (Fig. 1). Currently, an FDA warning regarding the use of gadolinium based contrast agents in individuals with certain medical conditions is posted in this section and a web link details the clinical safety of these agents. Warnings issued by the FDA regarding MI probes and CAs are updated as they are announced.
Latest Updates
New chapters are added to the database each week and chapters published within the previous 60 days can be viewed by clicking on the “Latest Updates” link in the “About MICAD” section on the database home page. Each chapter listed in the update can be accessed for reading by clicking on the title; each chapter is also available for download as a PDF. Every month users who have registered with the database receive a list of the new chapters published in MICAD via an email newsletter (eAnnouncement). Agents in the eAnnouncement are presented as a list of abbreviated names that are categorized according to the modality used for imaging and each name is linked to the chapter in MICAD. Individuals interested in receiving the eAnnouncement can register with MICAD through the “Join mailing list” link or by contacting the MICAD editors to request to be added to the mailing list. PubMed links in MICAD chapters are active links so that the search results returned are up-to-date. Therefore, the chapters can be used in publications and can be cited as references. Because the chapters in MICAD are available only as an online publication, the citation must match a specific format to reflect its availability on the MICAD website as follows:
{Author name when applicable}.{Chemical name. Abbreviated name}. In: Molecular Imaging and Contrast Agent Database (MICAD) [database online]. Bethesda (MD): National Library of Medicine (US), NCBI; 2004-{current year}. Available from: http://micad.nih.gov.
Instructions and examples for citing MICAD chapters are available through the “How to cite a MICAD chapter” web link on the home page.
Guest Author Program
A preliminary search of literature on the PubMed database for “imaging and contrast agents” yields >30,000 publications. Among these only about 4500 publications are focused on unique MI probes and CAs (see below) that have in vivo data published in peer-reviewed journals around the world. Earlier the MI probes and CAs were not systematically cataloged in a comprehensive and cumulative manner and information regarding these agents was not easily available to the research community. The development of MICAD is an attempt to collect easily accessible information regarding each MI probes and CAs at a single location for use by the imaging community. Ideally all MI and CAs published in peer-reviewed literature would be included in MICAD, but this is a monumental task as the list is constantly growing because a large number of new imaging agents (and novel techniques/modalities) are published every month and added to the MIP & CA list. Therefore, to promote the rapid expansion of the database and to foster participation by the imaging community in this undertaking, the MICAD editors implemented a program in 2007 to accept chapters from guest authors for publication in the database [1, 2]. Publishing in MICAD has several advantages over print publications because the chapters are available to the readers rapidly, access to the database is free and without subscription, circulation of the articles is worldwide through the internet and information can be printed from the database as necessary. In addition, the chapters are updated periodically and the PubMed links are active. Clicking on the PubMed links in the chapters yields the latest information published in peer-reviewed journals regarding the agents.
Chapters contributed by members of the imaging community include single- author efforts as well as collaborative efforts between members of the same institute or department(s) or from different institutions. Members of the imaging community who are interested in contributing a chapter are encouraged to contact the MICAD team (at micad@nlm.nih.gov) for information regarding the inclusion criteria, the chapter template, the chapter style guide, etc. The MICAD editors also encourage individuals to suggest agents for inclusion in the database. The MICAD editors provide guest authors with all necessary editorial guidance and support, including drawing of the chemical structures for display in the chapter. The editors may also invite individuals working in the imaging field to contribute chapters on interesting MI probes and CAs for quick publication in the database. As of March 2011 ~55 chapters (~6.0% of total) had been contributed to MICAD by guest authors from around the world and more contributions are expected as the database becomes popular among the imaging community. The MICAD editors review all chapters, including those written by guest authors, before publishing online. Information regarding the general policies, criteria for inclusion, content, revision, and style guidelines to write a chapter for MICAD is available on the database home page (Fig. 1).
The “Upcoming MICAD Exhibits” section on the home page provides links to the various scientific meetings and conferences where a MICAD exhibit will be set up. Interested members of the imaging community are welcome to meet with the MICAD editors at the exhibits for a demonstration of the database and to obtain information regarding the database or the Guest Author Program.
CHAPTER CONTENTS
Each chapter in MICAD has a unique PMID assigned to it so that it can be easily accessed through the PubMed database, via search filters on the MICAD home page or by using appropriate key words on Google or similar search engines. Only literature published in peer-reviewed journals that is indexed in the public domain such as the PubMed and MEDLINE databases is compiled to form appropriate sections of a chapter. Scientific meeting or conference abstracts are not included for publication in MICAD because the abstracts generally lack sufficient information regarding the synthesis, methods used in the study and the imaging characteristics of an agent. If an agent is commercially available, the US government–managed patent database (www.uspto.gov) may be searched to obtain information that is not available in published literature (e.g., synthesis or the final formulation of a compound or biomolecule). To obtain information about the current diagnostic or therapeutic indication(s) for which an agent is approved by the FDA the package (product) insert of a clinical agent may also be reviewed on line (www.fda.gov or www.dailymed.nlm.nih.gov web sites). Information regarding the ongoing clinical evaluation of a MI probes and CAs is available from the www.clinicaltrials.gov web site. However, this site gives only a brief overview of the clinical studies, including those involving MI probes and CAs, and results obtained from an investigation are usually not posted on this web site. Information in MICAD regarding MI probes and CAs that have been evaluated in humans is obtained only from peer-reviewed clinical studies published in scientific journals. Because MICAD does not promote any commercial or only-for-research MI probes, CAs or related products all agents in the database are mentioned strictly by generic names.
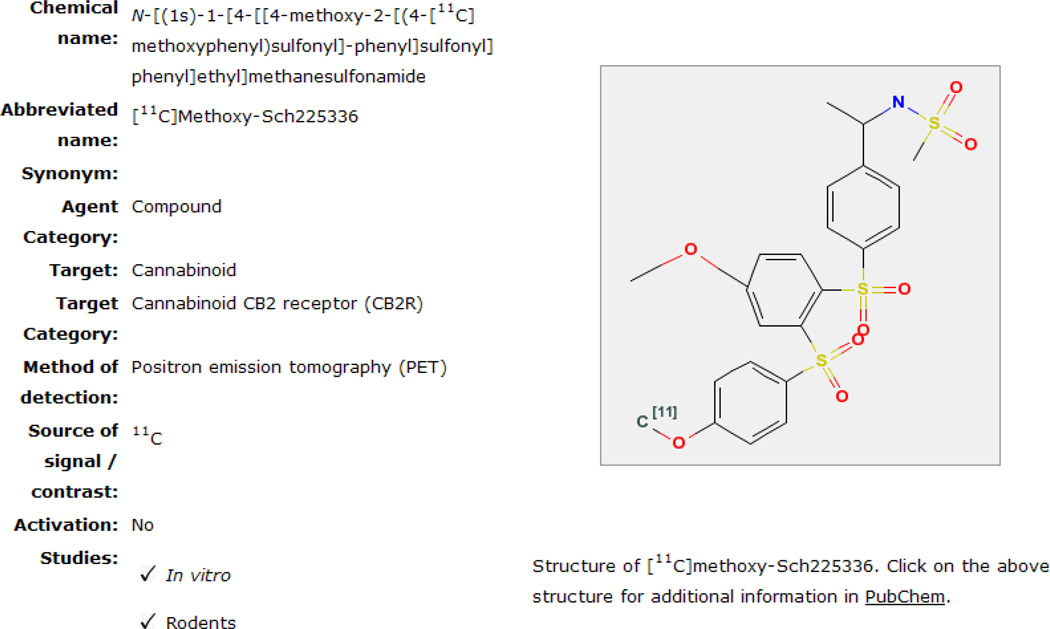
Chapters in MICAD are available as an online textbook format developed at the National Library of Medicine for use through the Entrez database which is a part of the “Bookshelf” infrastructure developed by the NCBI to publish several online books, including MICAD [1, 9]. Agents published in MICAD are either novel or derivatives/modifications of already existing MI probes and CAs. Each entry in MICAD is listed as a chapter and published literature that is consulted to write the chapter is referenced at the end of each chapter. Cited references are numbered in the order in which they appear in the text and each reference is linked to PubMed for easy access to its abstract. Publications used to write a chapter in MICAD should report sufficient details regarding the agent’s chemical or biological synthesis and must contain data obtained from animal or human studies. Information essentially expected in a publication that can be used to write a chapter for MICAD has been discussed earlier by Chopra et al. [2]. Once published online, the MICAD editors from time-to-time update the information when new information or application(s) of the agent is published. Each chapter has a “Created” and an “Updated” date located just below the chapter title to inform the reader of how current is the information. At the start of a chapter the characteristics of the MI probe or CA are summarized in a table (Summary Table) just below the title (Fig. 3). In the summary table the chemical name, its abbreviations and synonyms are presented. The table includes other important characteristics regarding an agent such as the category of an agent (compound, antibody, peptide or nucleotide etc.), target of the agent (epidermal growth factor receptor, amino acid transporter, non-targeted etc.), target category (receptor, antigen etc.), mode of detection (SPECT, PET, MRI etc.), source of signal or contrast (111In, 99mTc, I, Gd etc.) and whether in vivo activation is necessary to generate a signal from the imaging agent. The Summary Table also indicates the type of studies performed with the agent, such as in vitro studies and investigations in animals (rodent, non-primate non-rodent mammals and non-human primates) and/or in humans. The chemical structure of the MI probe or CA, if available, it is displayed next to the Summary Table (Fig. 3) and is linked to the PubChem database where the chemical formula and properties, molecular weight, bioassays etc. of the agent may be available. For MI probes and CAs that have a protein or nucleotide backbone, a web link to the protein and nucleotide sequences, if available through PubMed is provided because the chemical structure for such molecules is rather large and cannot be displayed. Each chapter is divided into several informative sections: Background, Synthesis, In Vitro Studies, Animal Studies, and Human Studies (the final two sections reflect in vivo studies) that are discussed below.
Figure 3.
MICAD chapter summary table and chemical structure.
Background
This section discusses the rationale to develop the agent, the mechanism of action or activity, the target(s), clinical application (if any) etc. of the probe. Many agents are under development and in the preclinical stage, whereas others have completed the development process and are under evaluation in humans. Some agents are non-targeted because they do not bind to a specific target or exhibit any specific biochemical activity in vivo. Such agents are usually used for diagnostic imaging e.g. Iopamidol which is a non-radioactive iodine-based contrast agent that circulates in the blood pool and non-specifically fills the extracellular fluid spaces. This agent has been approved by the FDA for diagnostic x-ray/CT imaging of the vasculature in humans [10]. Similarly, 1,3-bis-[7-(3-amino-2,4,6-triiodophenyl)-heptanoyl]-2-oleoyl glycerol, another iodine-based contrast agent approved by the FDA, is approved for use only in animals to image the liver or the circulatory system [11, 12]. Recently a “Related Resource Links” section was introduced within the Background section of the chapters. The main purpose of this sub-section is to provide the reader with other relevant information regarding the scaffold, target, imaging properties and applications of the MI probe or CA that are not within the scope of a chapter. Such information can be of much use and interest to imaging scientists for the development of new tracers that can have far superior imaging characteristics compared to the parent compound or macromolecule. An additional benefit is that biology scientists and students in general can access current information regarding the biological properties of a molecule of their interest. In this sub-section, web links are available to related chapters in MICAD, gene information for biological agents (gene identification number in the Genebank, chromosomal location etc.), cellular pathways through which the agent scaffold may mediate its activity (e.g. signal transduction pathway), clinical trials, information from the FDA website and Online Mendelian Inheritance in Man databases etc. The link to all aspects regarding an MI probe or CA from the gene to the protein expression product to clinical trials makes MICAD a unique and single comprehensive source of information on new MI probes and CAs that can be used with the various modalities of molecular imaging.
Synthesis
The chemical or biological processes used to synthesize an imaging probe or CA are described briefly in this section. If available, the yield of each intermediate compound leading to the synthesis and purification of the final product is presented. If a compound is produced using an automated method (e.g., a peptide, polypeptide, and short carbohydrate chain etc.), the equipment, purification column(s) and other procedures used for the synthesis are described briefly in this section. Genetically engineered proteins, monoclonal antibodies (mAbs) or compounds used for imaging purposes are often labeled with nuclides such as 111In, 99mTc, or iodine (123I or 125I) and the methods used to produce and label these biomolecules are detailed in this section. The specific activity, radiochemical purity, and yield of the final labeled product obtained from compounds or biomolecules labeled with radioisotopes such as 18F, 99mTc, 111In, etc. are also given in this section. In addition, the total time required to synthesize and purify probes labeled with radioisotopes that have short half-lives (e.g., 18F, 11C etc.) is reported here. Brief details regarding the chemical procedure used to conjugate a radionuclide to a biomolecule through a chelating agent or a linker may be described in this section as well. In such an agent the number of radionuclide atoms linked to a tracer will depend on the characteristics and number of chelator molecules conjugated to the biochemical. Therefore, investigators should determine and report the number of chelator molecules attached to a biomolecule used as a platform to generate an MI probe or CA. The number of chelator molecules also facilitates determination of the expected molecular weight (theoretical) and specific activity of the purified radiochemical.
If available, the storage conditions and stability of the radioactive or non-radioactive imaging agent (e.g., at different temperatures, in rodent or human serum, etc.) are also provided in this section. The final formulation of an imaging agent, especially for those reconstituted from a freeze-dried kit, may be detailed here as well. Almost all publications have a detailed description or provide relevant references related to the synthesis and labeling of an agent. However, the MICAD editors have noticed that many investigators do not report the specific activity or the stability of a radio-labeled probe [2].
The analytical techniques used to characterize a CA used for MRI, x-ray/CT, OI or ultrasound imaging depends on the chemical nature of the compound or the scaffold used to prepare the agent. In general, MRI agents used in the clinic are non-targeted and have gadolinium (Gd), a paramagnetic metal, as the source of signal, but these agents are linked to nephrotoxicity in some patients, particularly those who have compromised kidney function [13]. As an alternative to Gd-based CAs several MRI agents under development (non-targeted and targeted) have an organic (liposomes) or an inorganic (iron oxide particles) NP platform [14]. A wide size range of NPs (from <100 nm to micron size) has been used for imaging studies under in vivo conditions [15, 16]. Targeted NPs used for MRI usually bear biomolecules such as receptor ligands (peptides, polypeptides, or small proteins) or mAbs on the surface and are directed toward specific organs in the body or tumors that over-express possible diagnostic biomarkers (antigens or receptors). With such an agent, the image quality is affected by the extent of penetration into the target site, the expression level of the target in the tissue, and the binding characteristics and number of biomolecules attached to each NP. Agents used for x-ray/CT imaging are water- soluble small molecules that contain iodine as a source of contrast, and those having an osmolality higher than 800 –900 mosm kg−1 may produce side effects in humans [17]. The chemical composition and the characteristics of a targeting biomolecule attached to the surface of a NP influences the circulation half-life, biodistribution and the quality of MRI or x-ray/CT images generated by it [16, 18, 19]. Although the synthesis and chemical composition of NPs is important information to understand the biochemical and physiological characteristics of the NPs, few investigators provide detailed information regarding the synthesis and composition of these molecules in the publications.
Depending on the nature of the study OI probes may be non-targeted, targeted or activatable as described by Sheth and Mahmood [20]. Targeted OI probes (e.g. those containing a receptor ligand or Ab) may be conjugated to one or more types of fluorescent dyes and are used to detect or quantify the expression of an antigen or a receptor in tissues or on tumor cell surfaces. Chemical linking of the fluorophore to an OI probe can alter the conformational characteristics of the molecule, resulting in partial or complete loss of signal generated by the agent [21]. In general, cells internalize most probes based on Abs or receptor ligands, and the signal generated by these agents can be attenuated because of metabolic degradation or pH changes in the cell compartments. Therefore, analytical characterization of an OI tracer exposed to various stress conditions in vitro can provide a clue as to how suitable the probe will be for in vivo imaging. Approximately 50% of investigators report the number of dye molecules conjugated to an OI agent, but most do not report the stability or analytical profile of the agent after its exposure to an unfavorable environment.
In Vitro Studies
This section summarizes the in vitro studies performed with an imaging agent. In general, these studies describe the cellular uptake, target or receptor binding and dissociation properties (with or without competing compounds) of an agent using either cultured mammalian cells or membranes derived from appropriate cells or tissues. Some of these studies provide information regarding the mechanism of action of an agent, the target specificity of the probe, or whether activation of the agent is necessary before it can generate a signal for imaging. In general, most MI probes and CAs do not require activation for imaging, but these studies help investigate the biochemical behavior of an agent under low background conditions. Studies performed to determine the stability and the partition coefficient of small molecule imaging probes are also reported in this section.
Animal Studies
This section is divided into three subsections to provide relevant information about the agent obtained from studies performed in rodents, non-primate non-rodent mammals (such as rabbits, guinea pigs, dogs and pigs), or non-human primates (monkeys and baboons). The rodent subsection presents results obtained from preclinical studies with normal rats or mice, nude mice, or severe combined immunodeficient mice; these animals may or may not bear xenograft tumor implants derived from rodent or human tissue or cell lines. For some studies specific human disease models in rodents or knockout mice are used to characterize the MI probe or CA. Rodents are the most commonly used animals for preliminary investigations, and they are often used to confirm the systemic biodistribution, pharmacokinetics, biochemical characteristics, target specificity and the tumor detection efficiency of radiolabeled or optical imaging agents. A key experiment reported here is the proof of target specificity in the case of targeted probes which can be demonstrated with different experimental approaches (e.g., injecting excess ligand along with the probe, using knockout mice or the target protein, or by comparing the binding of an imaging agent to its target tissue versus the binding in tissue that do not express the target). If suitable results are obtained during preclinical studies in rodents, the molecules may be labeled with therapeutic isotopes (188Re, 90Y, 177Lu, etc.) and evaluated for cancer therapy in other non-rodent non-primate mammals, followed by non-human primates and eventually in humans. Efficacy studies performed in non-human primates such as monkeys and baboons are presented in the Non-Human Primate subsection. Almost 95% of the chapters in MICAD contain information on studies performed in rodents, and ~10% have data from other non-human mammalian species. Although most investigators use 3 animals/group for the in vivo characterization of an imaging agents the use of 5 animals/group is recommended by Eckelman et al. to obtain statistically meaningful results [22].
Human Studies
Information available in this section is obtained only from clinical studies published in scientific journals. The Human Studies section describes results obtained from clinical trials performed with a MI probe or CA to determine the biodistribution, pharmacokinetics, dosimetry, and efficacy of an agent for diagnostic imaging of diseases or from imaging studies performed with radiolabeled therapeutic agents used to treat malignant or metastatic tumors. Some agents are used to detect infections and inflammation, quantify the over-expression of various receptors, or to detect bone growth in humans [23–25]. Investigations to monitor the disease status or the biodistribution of radiolabeled small molecules in patients are also described in this section. Currently ~20% of the chapters in MICAD have information regarding human studies.
Supplemental Information
Any additional information regarding an agent that is available, but not related to the imaging characteristics of the agent (e.g., an alternate method of synthesis, toxicity, pharmacodynamics, metabolic products, etc.) is reported in this section. This information is submitted by members of the imaging community on a voluntary basis and is not reviewed by the MICAD editors for accuracy, reliability, completeness, currency, legality, quality, or appropriateness; the information is presented “as is” in the chapter.
The NIH Support section presents information regarding grants provided by the NIH or affiliated institutions that are used to conduct a part or all of the studies presented in a chapter. The last section of the chapter presents the various references including patents, books, reviews, and research papers consulted to write the chapter. Each reference is linked to PubMed to facilitate access to the abstract of the published paper. If a study is funded with a NIH grant(s), the complete journal article may be accessed free of charge through the PubMed database.
FUTURE DEVELOPMENTS
Recent advances in MI have greatly improved the chances of early detection and the selection of a suitable treatment regimen(s) for various human disorders. This is just the beginning of the developmental process for MI, and an increased use of imaging in the clinic is likely to hasten the advancement of personalized medicine. In addition, the genomic revolution is yielding new information about mutated receptors, new targets for the detection of a variety of pathologies and about orphan nuclear [26] and G-protein-coupled [27] receptors for which a physiological function or association with any disease(s) is not yet clearly defined. As the pathways and the clinical significance of the orphan receptors and their ligands are deciphered new and unique drug targets that can be useful for the early diagnosis and treatment of various ailments will be identified. In this case MI will be an efficient tool to rapidly screen and determine the target specificity of drugs based on different basic platforms (compounds, proteins, mAbs etc.), in pre-clinical investigations and later in clinical trials, well before any one of the drugs can be approved by the FDA for diagnostic and treatment purposes. Consequently, MICAD will play an important role in handling, cataloging and providing large amounts of information pertaining to a multitude of new MI probes and CAs or new modalities or techniques that may have to be developed and utilized to detect the signal(s) generated from these probes. Because MICAD is updated and reviewed periodically, it will provide readily accessible and up-to-date information regarding MI probes and CAs to the imaging community. In this context, for the further improvement of MICAD, users of the database are welcome to suggest changes by writing to the editors at: micad@nlm.nih.gov
ACKNOWLEDGMENTS
We thank Jeremiah McAdams for his assistance with the writing of this review. Funding for the Molecular Imaging and Contrast Agent Database is provided by the Intramural Research Program of the National Institutes of Health.
Footnotes
Conflict of interest disclosure: The authors declare they have no conflict of interest.
REFERENCES
- 1.Cheng KT, Menkens A, Bryant S, et al. NIH MICAD initiative and guest author program opportunities. J Nucl Med. 2007;48:19N. [PubMed] [Google Scholar]
- 2.Chopra A, Shan L, Eckelman EC, et al. Important Parameters to Consider for the Characterization of PET and SPECT Imaging Probes. Nuclear Medicine and Biology. 2011 doi: 10.1016/j.nucmedbio.2011.05.011. In Press. [DOI] [PubMed] [Google Scholar]
- 3.Willmann JK, van Bruggen N, Dinkelborg LM, et al. Molecular imaging in drug development. Nat Rev Drug Discov. 2008;7:591–607. doi: 10.1038/nrd2290. [DOI] [PubMed] [Google Scholar]
- 4.Massoud TF, Gambhir SS. Molecular imaging in living subjects: seeing fundamental biological processes in a new light. Genes Dev. 2003;17:545–580. doi: 10.1101/gad.1047403. [DOI] [PubMed] [Google Scholar]
- 5.Piwnica-Worms DR. Introduction to molecular imaging. J Am Coll Radiol. 2004;1:2–3. doi: 10.1016/S1546-1440(03)00024-3. [DOI] [PubMed] [Google Scholar]
- 6.Amidon GL. Molecular pharmaceutics: the NIH Roadmap and the FDA pipeline problem. Mol Pharm. 2004;1:337. doi: 10.1021/mp0400086. [DOI] [PubMed] [Google Scholar]
- 7.Check E. NIH 'roadmap' charts course to tackle big research issues. Nature. 2003;425:438. doi: 10.1038/425438b. [DOI] [PubMed] [Google Scholar]
- 8.Shi ZD, Wu H, Ruddy B, et al. Imaging Probe Development Center: a National Institutes of Health core synthesis resource for imaging probes. J Biomed Opt. 2007;12:051502. doi: 10.1117/1.2778702. [DOI] [PubMed] [Google Scholar]
- 9.Sayers EW, Barrett T, Benson DA, et al. Database resources of the National Center for Biotechnology Information. Nucleic Acids Res. 2011;39:D38–D51. doi: 10.1093/nar/gkq1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Haight AE, Kaste SC, Goloubeva OG, et al. Nephrotoxicity of iopamidol in pediatric, adolescent, and young adult patients who have undergone allogeneic bone marrow transplantation. Radiology. 2003;226:399–404. doi: 10.1148/radiol.2262011471. [DOI] [PubMed] [Google Scholar]
- 11.Weichert JP, Lee FT, Jr, Longino MA, et al. Lipid-based blood-pool CT imaging of the liver. Acad Radiol. 1998;5 Suppl:S16–S19. doi: 10.1016/s1076-6332(98)80047-0. discussion S28–S30. [DOI] [PubMed] [Google Scholar]
- 12.Ford NL, Graham KC, Groom AC, et al. Time-course characterization of the computed tomography contrast enhancement of an iodinated blood-pool contrast agent in mice using a volumetric flat-panel equipped computed tomography scanner. Invest Radiol. 2006;41:384–390. doi: 10.1097/01.rli.0000197981.66537.48. [DOI] [PubMed] [Google Scholar]
- 13.Cheong BY, Muthupillai R. Nephrogenic systemic fibrosis: a concise review for cardiologists. Tex Heart Inst J. 2010;37:508–515. [PMC free article] [PubMed] [Google Scholar]
- 14.Cho EC, Glaus C, Chen J, et al. Inorganic nanoparticle-based contrast agents for molecular imaging. Trends Mol Med. 2010;16:561–573. doi: 10.1016/j.molmed.2010.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Godin B, Driessen WH, Proneth B, et al. An integrated approach for the rational design of nanovectors for biomedical imaging and therapy. Adv Genet. 2010;69:31–64. doi: 10.1016/S0065-2660(10)69009-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Louie A. Multimodality imaging probes: design and challenges. Chem Rev. 2010;110:3146–3195. doi: 10.1021/cr9003538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hasebroock KM, Serkova NJ. Toxicity of MRI and CT contrast agents. Expert Opin Drug Metab Toxicol. 2009;5:403–416. doi: 10.1517/17425250902873796. [DOI] [PubMed] [Google Scholar]
- 18.Hallouard F, Anton N, Choquet P, et al. Iodinated blood pool contrast media for preclinical X-ray imaging applications--a review. Biomaterials. 2010;31:6249–6268. doi: 10.1016/j.biomaterials.2010.04.066. [DOI] [PubMed] [Google Scholar]
- 19.Kozlowska D, Foran P, MacMahon P, et al. Molecular and magnetic resonance imaging: The value of immunoliposomes. Adv Drug Deliv Rev. 2009;61:1402–1411. doi: 10.1016/j.addr.2009.09.003. [DOI] [PubMed] [Google Scholar]
- 20.Sheth RA, Mahmood U. Optical molecular imaging and its emerging role in colorectal cancer. Am J Physiol Gastrointest Liver Physiol. 2010;299:G807–G820. doi: 10.1152/ajpgi.00195.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Longmire MR, Ogawa M, Hama Y, et al. Determination of optimal rhodamine fluorophore for in vivo optical imaging. Bioconjug Chem. 2008;19:1735–1742. doi: 10.1021/bc800140c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Eckelman WC, Kilbourn MR, Joyal JL, et al. Justifying the number of animals for each experiment. Nucl Med Biol. 2007;34:229–232. doi: 10.1016/j.nucmedbio.2007.01.005. [DOI] [PubMed] [Google Scholar]
- 23.Boerman OC, Rennen H, Oyen WJ, et al. Radiopharmaceuticals to image infection and inflammation. Semin Nucl Med. 2001;31:286–295. doi: 10.1053/snuc.2001.26189. [DOI] [PubMed] [Google Scholar]
- 24.Chen JH, Baek HM, Nalcioglu O, et al. Estrogen receptor and breast MR imaging features: a correlation study. J Magn Reson Imaging. 2008;27:825–833. doi: 10.1002/jmri.21330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Roelofs AJ, Thompson K, Gordon S, et al. Molecular mechanisms of action of bisphosphonates: current status. Clin Cancer Res. 2006;12:6222s–6230s. doi: 10.1158/1078-0432.CCR-06-0843. [DOI] [PubMed] [Google Scholar]
- 26.Mukherjee S, Mani S. Orphan nuclear receptors as targets for drug development. Pharm Res. 2010;27:1439–1468. doi: 10.1007/s11095-010-0117-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ozawa A, Lindberg I, Roth B et al. Deorphanization of novel peptides and their receptors. AAPS J. 2010;12:378–384. doi: 10.1208/s12248-010-9198-9. [DOI] [PMC free article] [PubMed] [Google Scholar]