Abstract
Laminin α1 (Lama1), which is a subunit of laminin-1 (laminin-111), a heterotrimeric ECM protein, is essential for embryonic development and promotes neurite outgrowth in culture. Because the deletion of Lama1 causes lethality at early embryonic stages in mice, the in vivo role of Lama1 in neural development and functions has not yet been possible to determine. In this study, we generated conditional Lama1 knockout (Lama1CKO) mice in the epiblast lineage using Sox2-Cre mice. These Lama1CKO mice survived, but displayed behavioral disorders and impaired formation of the cerebellum. Deficiency of Lama1 in the pial basement membrane of the meninges resulted in defects in the conformation of the meninges. During cerebellar development, Lama1 deficiency also caused a decrease in the proliferation and migration of granule cell precursors, disorganization of Bergmann glial fibers and endfeet, and a transient reduction in the activity of Akt. A marked reduction in numbers of dendritic processes in Purkinje cells was observed in Lama1CKO mice. Together, these results indicate that Lama1 is required for cerebellar development and functions.
Keywords: Laminin α1 chain, meninges, and cerebellum
1. Introduction
Two principal neuronal cell types, Purkinje and granule cells, are essential for the normal development and functioning of the cerebellum. Purkinje cells, which develop first, migrate along the radial glial system from the germinative zone. In contrast, granule neurons migrate from the proliferative zone in the external granule layer (EGL) to the internal granule layer (IGL), guided by Bergmann glial processes (Hatten, 1999; Herrup and Kuemerle, 1997; Rakic and Sidman, 1973).
Extracellular matrix (ECM) proteins and their receptors closely participate in cerebellar development. For example, vitronectin, is expressed in differentiated granule neurons and acts to decrease sonic hedgehog-induced proliferation of granule cell precursors (GCPs) and to promote neural differentiation (Pons et al., 2001). In mice, CNS-specific knockout of integrin β1, one of the major receptors of ECM proteins, and of its downstream molecules, integrin-linked kinase (ILK) and focal adhesion kinase (FAK), causes defects in the formation of folia by decreasing the proliferation of GCPs and causes abnormalities in the formation of Bergmann glia and Purkinje cells (Blaess et al., 2004; Graus-Porta et al., 2001; Mills et al., 2006; Watanabe et al., 2008). Other ECM protein receptors, α- and β-dystroglycan, regulate the migration of cerebellar granule neurons (Qu and Smith, 2004; Satz et al., 2010).
Laminins comprise a family of heteromeric ECM proteins consisting of α, β, and γ chains (Aumailley et al., 2005; Miner and Yurchenco, 2004). Laminins are required for basement membrane assembly (Li et al., 2003; Li et al., 2005), and they regulate cellular behavior through interactions with cell surface receptors, including integrins, syndecans, and α-dystroglycan (Gullberg and Ekblom, 1995; Miner and Yurchenco, 2004). Laminin α1 (Lama1) is the first laminin to be expressed during mouse embryogenesis (Miner et al., 2004; Smyth et al., 1999) and in vitro studies have demonstrated it to have a number of biological activities, including promotion of cell adhesion, migration, neurite outgrowth, angiogenesis, and tumor metastasis (Ekblom et al., 2003; Ichikawa et al., 2009; Kleinman et al., 1990). Mouse embryos that are deficient in Lama1 lack Reichert’s membrane and die by embryonic day 7 (E7) (Alpy et al., 2005; Miner et al., 2004). Mutant mice expressing a truncated Lama1 lack the C-terminal LG4 and LG5 subdomains and die before E6.5, despite the presence of both the embryonic basement membrane and Reichert’s membrane (Scheele et al., 2005). A missense mutation and conditional knockout of the Lama1 gene in mice disrupt retinal vascular development and inner limiting membrane formation (Edwards et al., 2010).
Although Lama1 is present in the meninges and in larger vessels in the late developmental stages of the CNS (Andrae et al., 2004), the in vivo role of Lama1 in the CNS is unknown. In this report, we created conditional null mice using epiblast-specific Sox2-Cre and found that Lama1 was essential for the proliferation and migration of GCPs in the cerebellum. Lama1 was also required for formation of Bergmann glial processes and for the localization and dendritic formation of Purkinje cells.
2. Results
2.1. Generation of conventional and conditional Lama1-knockout mice
To generate conventional (Lama1KO) and conditional (Lama1CKO) Lama1 null mice, two ES clones were isolated by transfecting the targeting vector, which contained the PGK-neor-PGK-tk cassette flanked by two loxP sequences in intron 15 and a third loxP sequence in intron 17 of the Lama1 allele (supplementary material Fig. S1A). These cells were transiently transfected with a CMV-Cre expression plasmid, and ES clones containing either the Lama1-deleted allele or floxed allele were obtained. The ES clones from each were injected into blastocysts to generate either Lama1KO or floxed-Lama1 mice. Homozygous Lama1KO (Lama1del/del) mice died by embryonic day 7 (E7) because of the absence of Reichert’s membrane (data not shown), similar to Lama1 null mice described in a previous report (Alpy et al., 2005; Miner et al., 2004). Lama1 mRNA and protein were absent in homozygous Lama1KO mice (data not shown). Southern blotting and genomic PCR confirmed the genotype of Lama1KO mice (supplementary material Fig. S1B). The floxed-Lama1 (Lama1flox/flox) mice were fertile and phenotypically normal. To identify the role of Lama1 in tissue development and functions at later stages, we created Lama1CKO (Lama1flox/del;Sox2cre/+) mice by crossing floxed-Lama1 mice with Sox2-Cre transgenic mice. The Sox2 gene is expressed in the inner cell mass, epiblast, and extraembryonic ectoderm prior to gastrulation, and in the prospective neural plate and chorion at the onset of gastrulation. All epiblast cells appear to have undergone a recombination event of Sox2-Cre by E6.5 (Hayashi et al., 2002). Lama1CKO mice underwent complete gestation and survived postnatally. Both genders of Lama1CKO mice showed normal sexual development and fertility. A genomic PCR analysis demonstrated that the floxed segment of the floxed-Lama1 allele was almost completely deleted in the tail of Lama1CKO mice (supplementary material Fig. S1C), and that this deleted allele was detected in almost all adult tissues (supplementary material Fig. S1D). Subsequent RT-PCR analysis revealed the absence of Lama1 mRNA in the cerebellum of Lama1CKO mice (supplementary material Fig. S1E).
2.2. Lama1CKO mice display abnormal behaviors
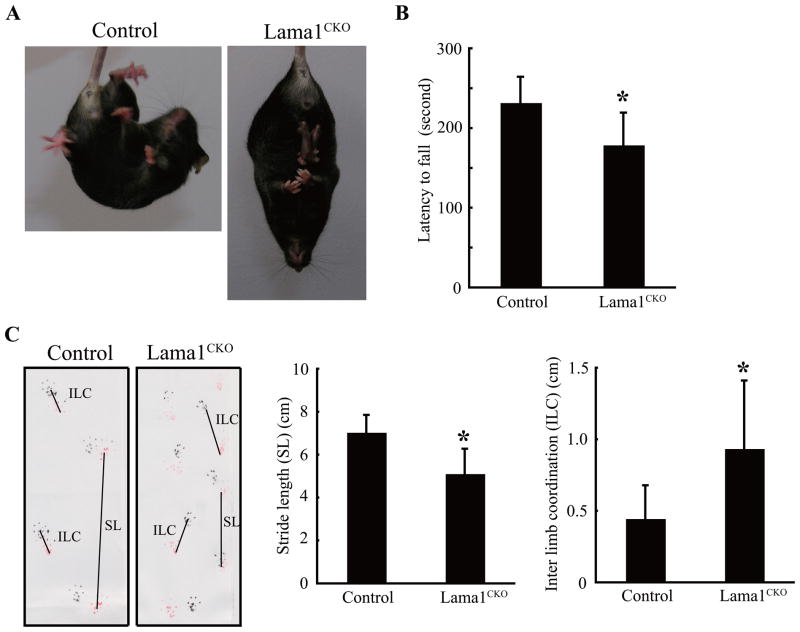
Three behavioral tests were conducted to determine whether Lama1CKO mice have abnormalities in behavior: the tail suspension test, the rotarod test, and footprint analysis. In the tail suspension test, Lama1CKO mice showed hugging behavior whereas control Lama1flox/del mice showed normal escape-oriented movements: running forward and backwards, body torsions with attempts to catch the suspended body (Fig. 1A). In the rotarod test, the movement time of Lama1CKO mice was slower than that of control mice, indicating that Lama1CKO mice have motor deficits (Fig. 1B). In the footprint test, Lama1CKO mice showed a significant reduction in stride length and an increase in interlimb coordination when compared with control mice, indicating the occurrence of locomotion disorder in Lama1CKO mice (Fig. 1C). These behavioral abnormalities suggest the occurrence of defects in neuronal functions, which result in the disequilibrium and lack of coordination seen in Lama1CKO mice.
Fig 1.
Impaired behavior of Lama1CKO mice. (A) Tail suspension test of adult mice. Lama1CKO mice exhibit hugging behavior. (B) RotaRod performance at 8–15 weeks of age. Lama1CKO mice show a marked decrease in latency to fall as compared with control Lama1flox/del mice. The bar graph shows the mean and SD of time to fall (n=10) (*, P < 0.001; two-sided t-test) (C) Representative footprints of control and Lama1CKO mice. Lama1CKO mice showed a significant reduction in stride length (SL) and increase in interlimb coordination (ILC) compared with control Lama1flox/del mice. The graphs show the mean and SD of length. (n=5) (*, P < 0.001; two-sided t-test).
2.3. Reduced size and structural defects in the cerebellum of Lama1CKO mice
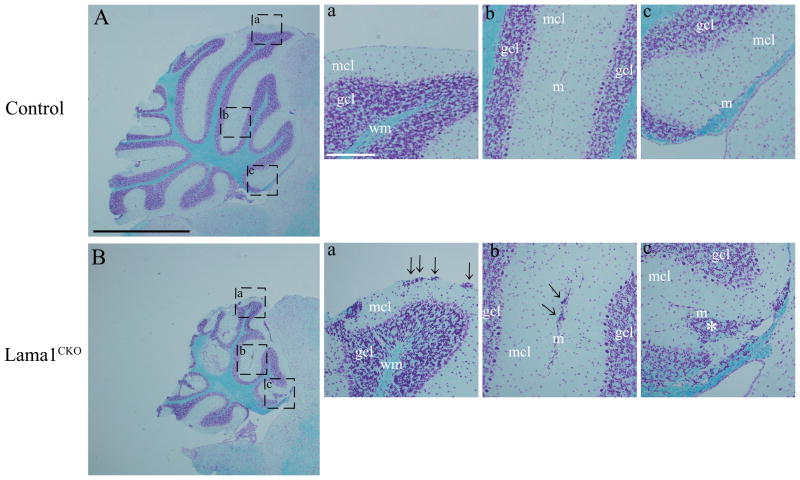
For further investigation of the neurological dysfunction, we analyzed histological sections of the brains of adult Lama1CKO mice. The cerebellum was reduced in size and the superior colliculus was appreciably exposed to the surface of the brain compared to the situation in control Lama1flox/del mice (supplementary material Fig. S2). To analyze the morphological changes in the cerebellum in more detail, we examined sagittal sections stained with Luxol Fast Blue (Fig. 2A and B). In the cerebellum of Lama1CKO mice, the depth of folia decreased and aggregation of granule cells appeared at the cerebellum surface, along the line of fusion of adjacent cerebellar folia under the meninges (arrows in Fig. 2Ba and b). The meninges, which form the fissures of folia, were not in the correct location in Lama1CKO mice (asterisk in Fig. 2Bc). These results suggest that the behavioral dysfunctions seen in Lama1CKO mice are due at least in part to cerebellar defects.
Fig. 2.
Cerebellar defects in Lama1CKO mice. Sagittal sections of adult control (A) and Lama1CKO mice at 8 weeks age (B) were stained with Luxol Fast Blue. The cerebellum of Lama1CKO mice is smaller than that of control mice, and the formation of some folia is disrupted. (a–c) High magnification view of areas indicated. In Lama1CKO mice, the aggregation of granule cells in the molecular layer was observed under meninges of the cerebellar surface and fusion lines of folia (arrows). The meninges, which form the fissure of the folia, were completely lacking (asterisk). mcl, molecular cell layer; gcl, granule cell layer; wm, white matter; m, meninges. Black scale bars: 1.0 mm, White scale bars: 100 μm.
2.4. Defects in the arrangement and dendritic processes in Purkinje cells
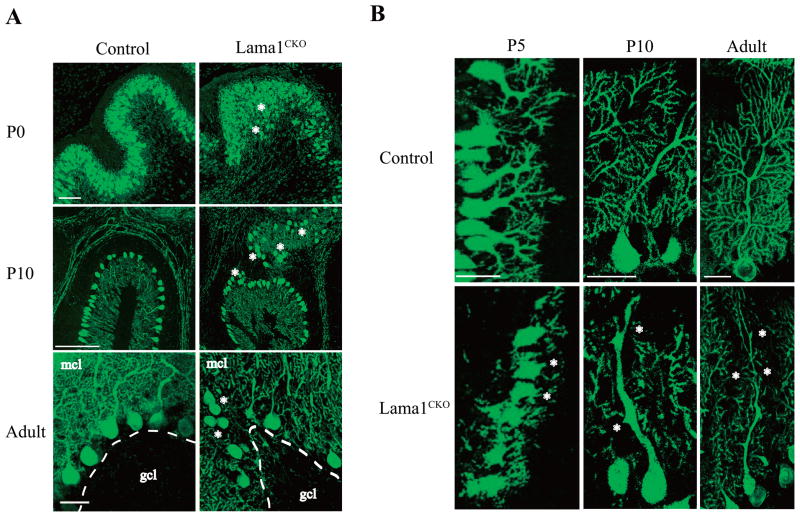
Purkinje cells, which are the main neuronal cells in the cerebellum, originate in the ventricular zone and migrate to the cortex along the radial glial fiber. After this migration, Purkinje cells form a cell layer and extend axons and dendrites (Goldowitz and Hamre, 1998). During postnatal development, Purkinje cell dendrites form synaptic contacts with parallel and climbing fibers in the molecular cell layer (Sotelo, 2004). Because of the remarkable abnormality in the cerebellar formation observed in Lama1CKO mice, we examined the layer formation and neurite extension of Purkinje cells by staining 50 μm thick sections of the cerebellum with an antibody to calbindin, a marker of Purkinje cells (Fig. 3). In Lama1CKO mice at P0, P10, and adult stages, the layer of Purkinje cells was distorted, and the localization of some Purkinje cells was away from the cell layer (Fig. 3A, asterisks). The formation of the dendritic tree of Purkinje cells in the folia of Lama1CKO mice at P5, P10, and adult stages was reduced compared to that of control Purkinje cell dendrites (Fig. 3B, asterisks).
Fig. 3.
Abnormal arrangement and loss of processes in Purkinje cells in Lama1CKO mice. Brains from P0, P5, P10, and adult (8 weeks old) mice were cut into sections of 50 μm using a vibratome. The sections were stained with anti-calbindin D28K. Images were obtained every 2 μm at a thickness of 30 μm using a confocal laser microscope and staked using LSM510 software. (A) In the lobules of Lama1CKO mice, the arrangement of the Purkinje cell layer between the molecular layer (mcl) and granule cell layer (gcl) was disordered at P0, P10 and adult (asterisks). Scale bars: 50 μm. (B) The branching of dendritic processes of Purkinje cells decreased remarkably in Lama1CKO compared to control Lama1flox/del mice at P5, P10 and adult (asterisks). Scale bars: 50 μm.
2.5. The expression of Lama1 and other ECM molecules in the meningeal basement membrane
Lama1 is expressed in the meningeal basement membrane at the surface of the brain, from the late embryonic stage to adulthood (supplementary material Fig. S3). Previous reports also indicated that other laminin α chains, including α2 (Lama2), α4 (Lama4), and α5 (Lama5), are expressed in the meningeal basement membrane (Andrae et al., 2004; Blaess et al., 2004). Because other ECM molecules may compensate for the deletion of Lama1 in Lama1CKO mice, we examined the expression and localization of several ECM molecules in in normal looking cerebellar areas of P5 Lama1CKO mice by immunostaining. In Lama1CKO mice, no expression of Lama1 was detected, consistent with the RT-PCR and immunostaining results (supplementary material Fig. S1E and Fig. 4A), but Lama2 and Lama5 were present in the meningeal basement membrane of Lama1CKO mice (supplementary material Fig. S4A and C). The expression level of Lama2 was slightly increased, whereas that of Lama5 was decreased in the mutant mice. Lama4 was present in the blood vessel basement membrane and its expression level did not change between Lama1CKO and control Lama1flox/del mice (supplementary material Fig. S4B). Nidogen-1, another ECM molecule, was expressed in both the meningeal and blood vessel basement membrane of Lama1CKO mice, and its expression level was the same as that in control mice (supplementary material Fig. S4B). Integrin β1, a main receptor of laminin α chains, was present in the endothelial cells of Lama1CKO mice, and no significant differences were noted between mutant and control mice (supplementary material Fig. S4C). These results indicate that the deletion of Lama1 did not substantially affect the expression of other laminin α chains, nidogen-1, or integrin β1.
Fig. 4.
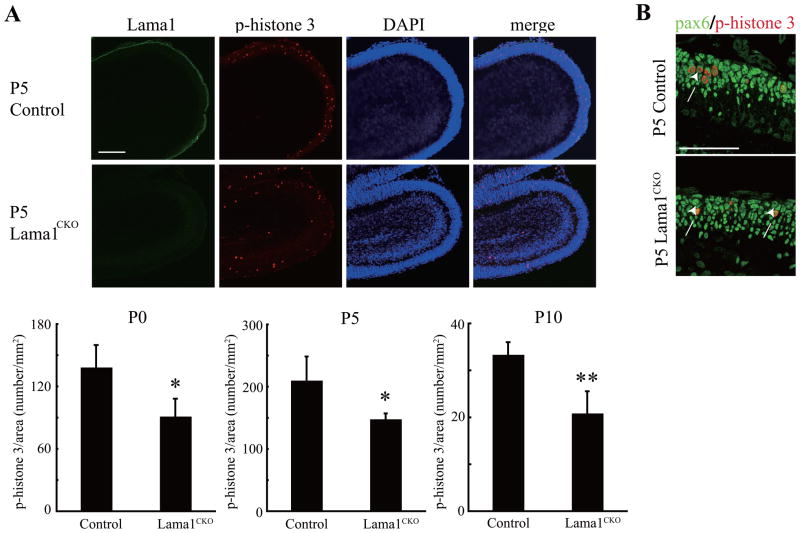
Reduction in granule cell precursor (GCP) proliferation in Lama1CKO mice. (A) Sagittal cerebellar sections at postnatal day 5 (P5) were analyzed by staining with phospho-histone 3 (p-H3). Proliferation of GCPs in Lama1CKO mice was markedly decreased. Fifteen 20 μm thick sections were successively prepared from the midline of the cerebellum. The number of p-H3-positive GCPs was counted and the area of the cerebellum was measured by Image J software. The bar graph shows the mean and SD of the number of p-H3- positive cells per area (n=5) (*, P < 0.02, **, P < 0.005; two-sided t-test). Scale bars: 100 μm. (B) Sagittal cerebellar sections at P5 were analyzed by staining with Pax6 and phospho-histone 3. Phospho-histone 3-positive cells were also positive for Pax6 (arrows). Scale bars: 50 μm
2.6. Lama1 is critical for proliferation of granule cell precursors
The reduced cerebellar size observed in Lama1CKO mice might possibly result from a reduction in proliferation of granule cell precursors (GCPs) and decreased migration of proliferated GCPs. We therefore measured the number of proliferating cells in cerebellar sections from P0, P5, and P10 mice during the growth phase of the cerebellar cortex (Fig. 4A, upper panel, immunostaining of P5 cerebellum; bottom panel, quantitative data from P0, P5, and P10 cerebellums). Proliferating cells within the external granular layer (EGL) were identified by staining with phospho-histone 3 (Ser10), a marker for the M-phase of the cell cycle. We confirmed that phospho-histone 3-positive cells were GCPs by double staining with antibody to Pax6, a marker of GCPs (Fig. 4B). The number of proliferating GCPs in Lama1CKO mice was significantly reduced during cerebellar development compared to that in control Lama1flox/del mice (Fig. 4). In addition, TUNEL assays in the cerebellum at P0, P5, P10, P21, and adulthood revealed no differences in cell death between Lama1CKO and control mice (data not shown).
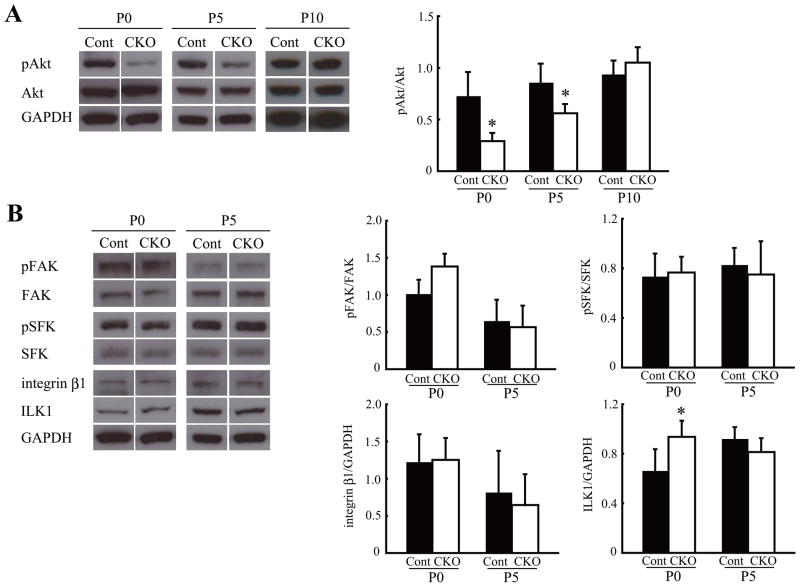
We next examined signaling molecules involved in cerebellar development. Akt is a downstream molecule of PI3-kinase and functions in both the survival and proliferation of progenitor cells in the brain (Barnabe-Heider and Miller, 2003; Groszer et al., 2001). The activation of Akt in P0 and P5 Lama1CKO mice was significantly reduced compared to control. This reduction persisted during P0–P5, but the decrease was repaired at P10 (Fig. 5A). The expression levels of integrin β1 did not change (Fig. 5B). Integrin-linked kinase (ILK1) was increased at P0, but this increase disappeared at P5 (Fig. 5B). We also found no significant differences in the expression and phosphorylation levels of focal adhesion kinase (FAK) and Src family kinase (SFK) in the cerebellum of Lama1CKO and control mice (Fig. 5B). These results suggest that Lama1 is essential for the proliferation of GCPs via activation of the Akt signaling pathway.
Fig. 5.
Reduced proliferation signaling in Lama1CKO mice. Cerebellum of control (Cont) and Lama1CKO mice (CKO) was analyzed by immunoblotting. (A) The phosphorylated Akt level was significantly decreased at P0 and P5 in Lama1CKO mice, compared with control mice, but this reduction was not observed at P10. (B) The expression of ILK1 was increased at P0 in Lama1CKO mice. The activation or expression of other integrin β related molecules was unchanged. The bar graphs show the mean and SD, which was calculated for each signaling density (n=5). (*, P < 0.05; two-sided t-test).
2.7. Lama1 regulates migration of proliferating GCPs
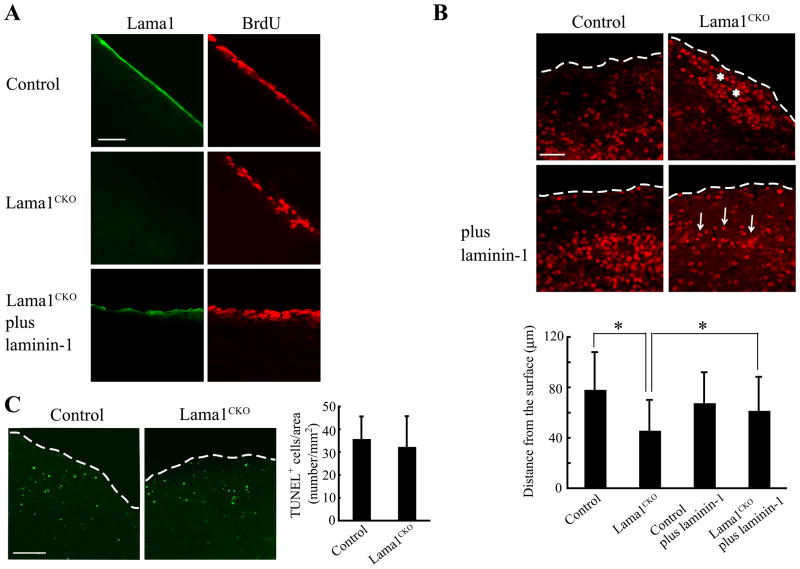
The abnormal cerebellar formation and granule ectopies in Lama1CKO mice may be caused in part by defects in GCP migration. We therefore examined migration of proliferating GCPs in culture using cerebellar slices from Lama1CKO mice, either in the absence or presence of exogenous laminin-1 (Lam-111) (Fig. 6). Sagittal slices of the cerebellum from P10 control and Lama1CKO mice were cultured and labeled with BrdU for 30 min and stained with antibodies for Lama1 and BrdU (Fig. 6A). Lama1 was expressed in the meninges in control cerebellum slices, but was absent from Lama1CKO cerebellum slices. The addition of exogenous laminin-1 in the culture restored Lama1 staining in the meninges of Lama1CKO mice (Fig. 6A). BrdU-positive cells were observed in both control and Lama1CKO mice. After three days of culture, BrdU-positive cells within the external granular layer had moved to the inner layer in control slices, whereas no cell migration was observed in Lama1CKO slices; the BrdU-positive cells remained in the periphery (Fig. 6B). When laminin-1 was added to the culture medium of Lama1CKO cerebellum slices, BrdU-positive cells were again able to migrate to the inner layer (Fig. 6B). Next, we used a TUNEL assay to examine whether cell death affects the migration of GCPs in a culture system. No difference was noted in the number of apoptotic cells in Lama1CKO slices compared to control slices (Fig. 6C). We therefore concluded that the difference in the localization of BrdU-positive cells was due to defects in cell migration. These results suggest that Lama1 is essential for the migration of proliferating GCPs.
Fig. 6.
Reduced migration of proliferating granule cells in Lama1CKO mice. Sagittal slices of the cerebellum of P10 control and Lama1CKO mice were labeled with bromodeoxyuridine (BrdU) and then cultured. After culture, the slices were stained with anti-BrdU antibody and anti-Lama1. (A) At 1h after culture, lama1 was not expressed in the meninges in the slices of Lama1CKO mice, but the expression was recovered by adding 5 μg/ml laminin-1. Scale bars: 20 μm. (B) The migration of BrdU-positive granule cells to the inner layer in Lama1CKO mice decreased after a 72 h culture period (asterisks). However, the decrease could be rescued by adding 5 μg/ml laminin-1 (arrows). The dashed lines show the surface of the cerebellum. Scale bars: 20 μm. The bar graphs show the calculated mean and SD, (n=500 cells). (*, P < 0.001; two-sided t-test). (C) After culturing for 72h, the slices were stained using the TUNEL method. The dashed lines show the surface of the cerebellum. No differences were observed in the number of apoptotic cells in the control or Lama1CKO mice. The bar graphs show the calculated mean and SD. Scale bars: 100 μm.
2.8. Abnormal radial and Bergmann glial fibers in Lama1C K O mice
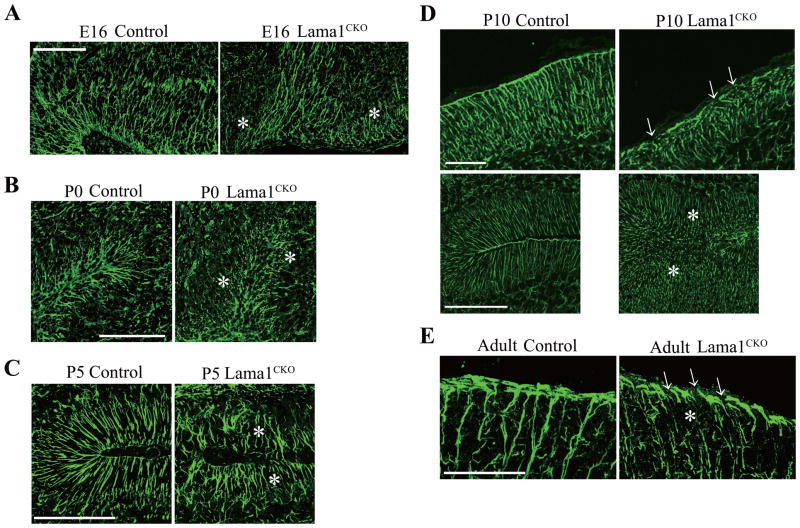
Proliferating GCPs in the external granule layer migrate to the internal granule layer using glial guidance systems (Edmondson and Hatten, 1987; Rakic, 1971; Solecki et al., 2004). In the cerebellum, Bergmann glial cells are first seen in the cortex at the late embryonic period, and they extend their processes to the pial surface. The migration of postmitotic GCPs is guided by surface-mediated interactions with Bergmann glial fibers (Komuro and Rakic, 1998). We therefore investigated the formation of radial glial fibers at E16 and P0, and Bergmann glial fibers at P10 (this is the peak period of postmitotic GCP migration), as well as at P5 and the adult stage, by staining with antibody to radial glial cell marker-2 and glial fibrillary acidic protein (GFAP), a marker for astrocytes (Fig. 7). We found that, in Lama1CKO mice, radial glial fibers and Bergmann glial fibers directed to the meninges were short in length and disoriented (Fig. 7A–E). Bergmann glial endfeet, which run along the meninges, were fragmented and discontinuous compared to those in control mice (Fig. 7D and E). These results suggest that distorted the layer of Purkinje cells and the reduced migration of GCPs in Lama1CKO mice are due to impaired glial guidance and that Lama1 is required for proper glial fiber formation.
Fig. 7.
Aberration of radial and Bergmann glial fibers and endfeet in Lama1CKO mice. Cerebellar sections from E16 (A), P0 (B), P5 (C), P10 (D) and adult (E) mice were stained with anti-radial glial marker-2 or anti-GFAP antibody. In control mice, radial glia and Bergmann glial fibers extended to the meninges in the fissure of folia, whereas in Lama1CKO mice, the glial fibers were discontinuous and fragmented (asterisks). (D) At the surface of the cerebellum, Bergmann glial endfeet are continuous along the meninges in control mice. Some endfeet are disrupted in Lama1CKO mice (D and E, arrows). Scale bars: 100 μm. Scale bars: 200 μm (A and B), 50 μm (C and E), 100 μm (D).
2.9. Disruption of pial basement membranes and abnormal meningeal structure
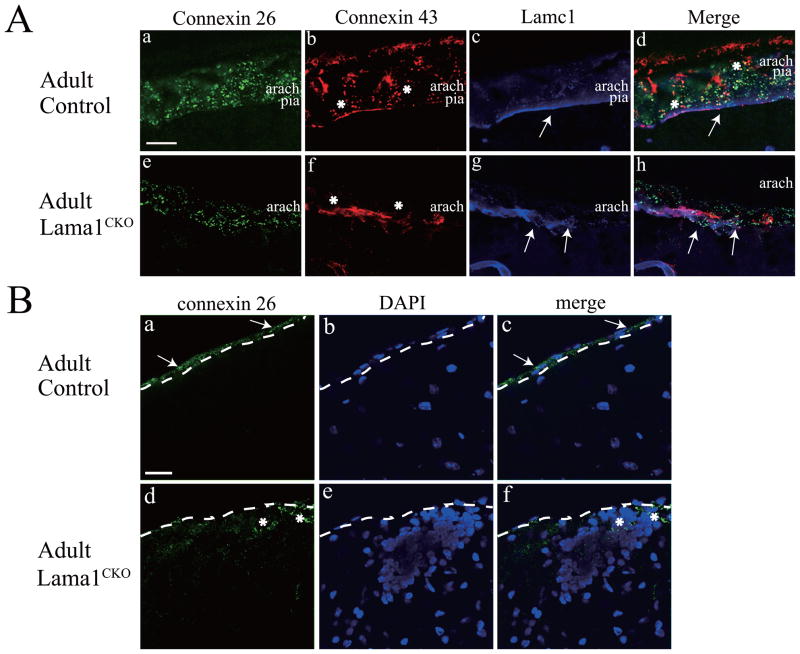
Connexin 26 (Cx26) and 43 (Cx43), gap junction family proteins, are expressed in the cerebellar arachnoid and pia of the meninges (Mercier and Hatton, 2000, 2001; Nagy et al., 2001). To delineate the role of Lama1 in the conformation of the meninges, we examined the localization of Cx26 and Cx43 by immunostaining the meninges in control and Lama1CKO mice. We also examined the pial basement membrane by immunostaining laminin γ1 (Lamc1), which is a subunit of laminin-1 (Lama-111) and some of the other laminins, and a marker of the basement membrane. In control meninges, both Cx26 and Cx43 were expressed in the arachnoid and pia (Fig. 8Aa, b, and d). However Cx26 expression was less prominent in the arachnoid barrier (Fig. 8Ae and h, upper part) than in the arachnoid trabeculae (bottom part). Cx43 expression was nearly absent in the arachnoid of Lama1CKO mice (Fig. 8Af and h). A thin and continuous basement layer at the bottom of the pia was stained by anti-Lamc1 antibody (arrow, Fig. 8Ac and d). The meninges of Lama1CKO mice were thinner and the expression of both Cx26 and Cx43 was reduced (Fig. 8Ae, f, and h). The Lamc1 staining revealed that the pial basement membrane was irregular (Arrows, Fig. 8Ag and h). We also analyzed the area where granule cell ectopies were observed at the cerebellum surface, as shown in Fig. 2Ba (arrows). In the cerebellum of Lama1CKO mice, aggregated granule cells were observed under the meninges by DAPI staining (Fig. 8Be). Some of the ectopically accumulated granule cells near the cerebellum surface were positive for Cx26 staining (Fig. 8Bd, e, and f, asterisks).
Fig. 8.
Abnormal expression of connexin proteins in the meninges of Lama1CKO mice. (A) Sagittal sections of the cerebellar surface of control (a–d) and Lama1CKO (e–h) animals were immunostained with antibodies to connexin 26 (a and c, green), connexin 43 (b and f, red), and Lamc1 (laminin γ1, c and g, blue). Merged images in d and h. Both Cx26 and Cx43 were expressed in the arachnoid and pia of control meninges (a, b, and d). In Lama1CKO mice, Cx26 expression was reduced in the arachnoid barrier (e and h) and Cx43 was almost entirely absent from the arachnoid (f and h, asterisks). Lamc1 was stained in a thin and continuous layer (arrows) of the pial basement membrane in control mice (c and d) but its staining was discontinuous (g and h, arrows) in Lama1CKO mice. Scale bar: 20 μm. (B) Connexin 26 expression in granule ectopies. Sagittal sections of the cerebellar surface of control (a–c) and Lama1CKO (d–f) mice were immunostained with antibodies to connexin 26 (green) with DAPI (blue). Cx26 puncta were present in the pial fibroblastic layer of control mice (a and c, arrows). In Lama1CKO mice, Cx26 were stained in ectopic granule cells near the surface (d and f, asterisks). Aggregated granule cells were observed under the meninges in Lama1CKO mice (e and f). The dashed lines show the borderline between the pial membrane and the surface of the cerebellum. Scale bar: 10 mm.
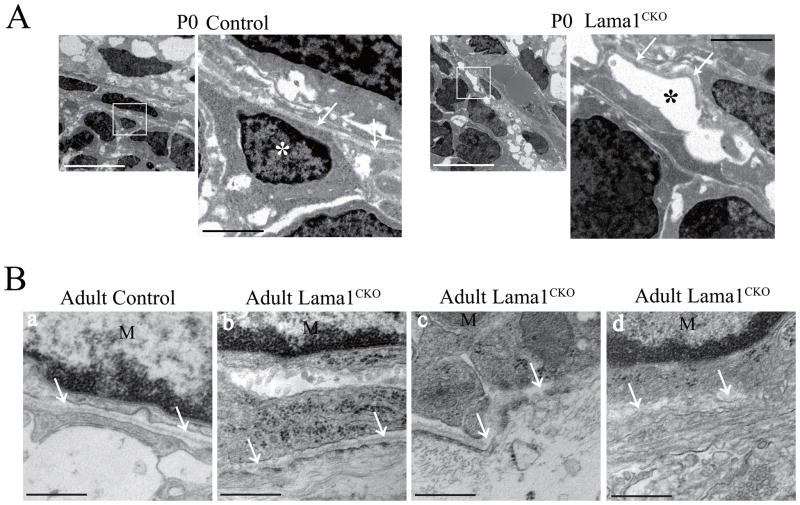
We next used electron microscopy to examine the disruption of the pial basement membrane in Lama1CKO mice at P0 (Fig. 9A) and the adult stage (Fig. 9B). In Lama1CKO mice, the density of glial cells was remarkably low compared with the control and no glial cells were found in the places where they were normally located in control mice (Fig. 9A, asterisk). Moreover, the pial basal lamina in Lama1CKO was curved and discontinuous (Fig. 9A, arrows). Similar results were observed in adult mice (Fig. 9B). These results suggest that Lama1 is required for the formation of the pial basement membrane and the meningeal structure.
Fig. 9.
Disruption of the pial basement membranes in Lama1CKO mice.
(A) Electron micrographs of the cerebellar surface of mice at P0. White boxed areas are enlarged in the right panels. In Lama1CKO mice, the density of glial cells was remarkably low compared with that of the control mice (white and black asterisk). The pial basal lamina of Lama1CKO mice was distorted, curved and discontinuous (arrows). White scale bar: 10 μm, Black scale bar: 2 μm. (B) Electron micrograph of the cerebellar surface of adult mice. Control (a) and Lama1CKO mice (b–d). The pial basal lamina in Lama1CKO mice was often irregular (b, arrows), disorganized (c, arrows) and discontinuous (d, arrows). M: Meningeal cells. Scale bar: 500 nm.
3. Discussion
Lama1 has various biological functions, such as promotion of cell adhesion, migration, neurite outgrowth, angiogenesis, and tumor metastasis (Ekblom et al., 2003; Ichikawa et al., 2009; Kleinman et al., 1990). However, the significance of Lama1 in terms of in vivo cerebellar development and function has been difficult to elucidate because Lama1 knockout mice die in early embryonic stages (Miner et al., 2004). In this report, using conditional knockout mice for Lama1, we demonstrate that Lama1 is important for cerebellar development. Our data show that Lama1 is required for proper conformation of the meninges and for proper formation of the glial fibers.
During cerebellar development, two kinds of glial cells, radial glia and Bergmann glia, function in the migration of Purkinje cells and granule cells, which are different neuron types (Hatten, 1999; Rakic, 1971). During development, the radial glial cells disappear and are replaced by Bergmann glia until birth (Yuasa, 1996; Yuasa et al., 1996). It is conceivable that the observed defects in Bergmann glial fibers cause the aggregated granule cells under the pia and reduced GCP migration in adult Lama1CKO mice. The interaction between GCPs and Bergmann glial fibers is necessary for GCP migration, and several adhesion molecules, such as NCAM, L1, and thrombospondin, contribute to these interactions (Chuong et al., 1987; O’Shea et al., 1990). Because laminin-1 containing Lama1 can interact with the HNK-1 carbohydrates of NCAM and L1 (Hall et al., 1997), Lama1 may be required to organize the contact between GCPs and Bergmann glia under the pia, in concert with these adhesion molecules.
Lama1 also regulates the distribution and dendritic elongation of Purkinje cells. In the mouse cerebellum, Purkinje cell precursors are generated in the primary rhombic lip between the E11 and E13 stages and these precursors migrate to the cortex along the radial glial fibers. Then, during the postnatal 3 weeks, dendrites of the Purkinje cells extend, branch, and form synapses with other neurons. Reelin, a large extracellular protein, promotes migration of Purkinje cells through radial glial guidance and lamination of Purkinje cells (Tissir and Goffinet, 2003). Since Lama1 has epidermal growth factor-like repeats similar to reelin, the abnormal lamination of Purkinje cells in Lama1CKO mice might be due to an impaired radial glial system.
Various endogenous and microenvironmental factors, such as granule cells, growth factors, steroids, thyroid hormone, coricotroprotein-releasing factor, calcium-related molecules, PKCγ, RORα and pleiotrophin-PTP ζ, are involved in dendrite formation and extension in Purkinje cells (Tanaka, 2009). Granule cells contribute to the formation of Purkinje cell dendrites via granule-Purkinje cell interactions, while electrical activity affects dendritic differentiation through calcium signaling. The poor dendritic formation of Purkinje cells in Lama1CKO mice may be due to a reduction in granule cell migration and to abnormal Bergmann glial processes.
Integrin β1, α-dystroglycan, FAK, and ILK, which are receptors and signaling molecules of Lama1, are critical for the proliferation and migration of granule cells and GCPs during cerebellar development (Blaess et al., 2004; Graus-Porta et al., 2001; Mills et al., 2006; Watanabe et al., 2008). Laminin-1 is also reported to modulate sonic hedgehog (SHH) function and to increase proliferation of GCPs (Pons et al., 2001). We showed that Lama1 is essential for the proliferation and migration of GCPs. This Lama1 activity is similar to the activity of the Lama1 receptors, but the defects observed in cerebellar size and motor functions in Lama1CKO mice were considerably more severe compared to those observed in other mutant mice. This may be because Lama1 interacts with both integrin β1 and α-dystroglycan. We found that the expression and activation of integrin β1 signaling were unchanged in Lama1CKO mice. In contrast, the activation of Akt was transiently decreased in the Lama1CKO cerebellum. The PI-3 kinase-Akt pathway is a signaling cascade that involves both integrin β1 and α-dystroglycan, and activation of PI-3 kinase-Akt signaling promotes neural cell proliferation and survival (Barnabe-Heider and Miller, 2003; Groszer et al., 2001). Therefore, the observed decrease in proliferation of GCPs may be due to the reduction in Akt activation at the early postnatal stages. At later stages, Akt phosphorylation levels reached normal levels, similar to those found in WT mice, probably because different external signals induced Akt activation for other cell functions, such as survival. In Lama1CKO mice, the expression levels of Lama4 did not change, but Lama2 expression was slightly increased and Lama5 expression was reduced in the Lama1CKO cerebellum. Double knockout mice for Lama2 and Lama4 showed a reduction in cortical sizes, an increase in apoptotic cells at the ventricular neuroepithelium, and detachment of radial glial cell processes in the cerebral cortex (Radakovits et al., 2009). However, these abnormalities are not observed in single gene knockouts for Lama2 and Lama4 (Radakovits et al., 2009). The meninges have been implicated in the regulation of radial glial survival using a slice culture that lacks the meninges (Radakovits et al., 2009) and in proper morphology of radial glia cells and migration of Cajal-Retzius cells in the cortex(Halfter et al., 2002). Lama1 deficiency resulted in discontinuous pia basement membranes (Fig. 9), although Lama2, Lama5 and nidogen were present in non-disrupted areas of the basement membrane (Supplementary material Fig. S4). These results suggest that Lama1 is required for the formation of the pial basement membrane and the meningeal structure. Thus, Lama1 may serve an important function among laminin α chains expressed in the meninges, as Lama1CKO mice show remarkable phenotypic changes in the cerebellum following a single knockout.
The meninges consist of three membranous layers composed of fibroblasts, extracellular matrix, and a well-organized fluid-containing space. These membranes are the dura mater, arachnoid, and pia mater. These structures serve to protect the central nervous system and to entrap a fluid-filled space that is important for brain function and survival. In the adult brain, gap junctions are abundant in the meninges and can mediate cell-cell communication and cellular morphogenesis, proliferation, and differentiation (Mercier and Hatton, 2001; Nagy et al., 1997). Connexins (Cxs) are implicated in these biological processes through their gap junction and hemichannel activities. Cx43 is expressed in the arachnoid and pia mater of the meninges (Nagy et al., 1999). Glia-specific Cx43 knockout mice display a reduction in size of the cerebellum, granule cell ectopies, and dislamination of Purkinje cells, granule cells, and Bergmann glia (Clark and Barbour, 1997; Muller et al., 1996; Wiencken-Barger et al., 2007). Cx43 is required for cerebellum-dependent motor coordination and motor learning (Tanaka et al., 2008a; Tanaka et al., 2008b). Interestingly, these morphological defects observed in Cx43 knockout mice are similar to those seen in Lama1CKO mice. We found that a deficiency in Lama1 results in the reduction and altered localizations of Cx43 in the cerebellum. Knockout mice for laminin 5 (Lam-332) also show altered expression and localization of Cx43 via laminin-integrin interactions in epithelial cells (Lampe et al., 1998; Isakson et al., 2006). In neuronal cells, laminin has been reported to affect the populations of postnatal neural stem and progenitor cells by altering the expression of connexins (Imbeault et al., 2009). Thus, Lama1 may be required for functional localization of connexins in the cerebellum through a similar mechanism and may have a similar function in cerebellar development.
4. Experimental procedures
4.1. Generation of conventional and conditional Lama1 knockout mice
A mouse genomic clone encoding the 5′ part of Lama1 was isolated from a mouse 129SvJ genomic library by screening with mouse Lama1 cDNA (Sasaki et al., 1988). A DNA fragment containing exons 14 to 18 of Lama1 from the genomic clone was used for gene targeting. The PGKneor-PGKtk cassette from pPNT was flanked with two loxP sequences at each end and inserted into intron 15, and a third loxP sequence was cloned into intron 17 of Lama1. The tk-DTa cassette, which encodes the diphtheria toxin A subunit, was attached to the short arm of the targeting vector for negative selection. The Lama1-loxP construct (supplementary material Fig. S1A) was linearized by ClaI and electroporated into R1 ES cells. The homologous recombinant cells were isolated by drug selection and transiently transfected with the CMV-cre expression plasmid (pBS185, Gibco Life Technology). Two different types of targeted ES clones were isolated, one containing the Lama1-deficient allele and the other containing the floxed-Lama1 allele (supplementary material Fig. S1A).
Two independent clones from each were injected into blastocysts to generate chimeric mice. These chimera were mated with C57BL/6 mice to generate heterozygous null (Lama1del/+) or floxed-Lama1 (Lama1flox/+) mice. Genotypes of the mutant alleles were confirmed by genomic PCR and Southern blotting (supplementary material Fig. S1B and C). These mice were further intercrossed to generate homozygous null (Lama1del/del), heterozygous null/floxed (Lama1del/flox), or floxed-Lama1 (Lama1flox/flox) offspring. Heterozygous Lama1 null mice containing the Sox2-Cre gene (Lama1del/+;Sox2-Crecre/+) were created by crossing with Lama1del/+mice with Sox2-Crecre/+ mice from The Jackson Laboratory, in which the Cre recombinase gene was inserted in the Sox2 locus (Hayashi et al., 2002). Mice with a conditional Lama1-deficiency (Lama1CKO) specifically in epiblasts were created by crossing Lama1del/+;Sox2-Crecre/+) with floxed-Lama1 (Lama1flox/flox) mice. Lama1flox/del mice developed normally and were used as controls.
Cre-mediated recombination in both null and conditional Lama1 alleles was identified by Southern blotting and genomic PCR. DNA was prepared from mice, digested with XbaI, and hybridized with the probe indicated in Figure 1A, the WT Lama1 allele. For PCR, DNA was denatured at 95°C for 15 min and then amplified for 30 cycles (30 sec at 94°C, 60 sec at 60°C, and 90 sec at 72°C) with ExTaq (Takara). The following primer sets were used: wild-type and floxed alleles: E85 (5′-TACTTCAACGTGGTTAGACTTGTGCCTG-3′) and E100 (5′-ATTAGGGCTTGCTATGTCCAGAGAGACAG-3′); null allele, E85 (5′-TACTTCAACGTGGTTAGACTTGTGCCTG-3′) and E114 (5′-TTGTGCCTTTACTAAGCCCTGCTCC-3′); cre allele, E350 (5′-AAAATTTGCCTGCATTACCG-3′) and E360 (5′-ATTCTCCCACCGTCAGTACG-3′). Deficiency of Lama1 mRNA expression was confirmed by RT-PCR using E85 primer and 5′-CACACAGAGCAAATTCCATGA-3′. Both male and female mice were used for analyses.
4.2. Locomotion analyses
Mice were tested for neuropathic defects with the tail suspension test at 8–15 weeks of age. Mice were suspended for 5 min and observed. For the rotarod analysis, mice were trained for 5 min on a rod rotating at 10 rpm. This training was performed 2 times at 45-min intervals. After the training, a trial was performed on a rod that was accelerated linearly from 10 to 35 rpm over 5 min, and the time taken to fall from the rod was analyzed. The footprint analysis was modified as described previously (Karimi-Abdolrezaee et al., 2006). The mice were trained to walk on a narrow paper-covered runway (50 cm long and 5.5 cm wide). Their forelimbs and hindlimbs were dipped in black and red ink, respectively, and the mice walked on the paper 3 times. For the measurements, the first and last 10 cm of the prints were excluded. If the mouse stopped in the middle of the track, the trial was repeated. The data were analyzed by calculating the average of all steps per print in all trials.
4.3. Histological analysis
Mice at 8 weeks age were fixed by perfusion with 4% paraformaldehyde (PFA) under anesthesia. Samples of brain tissue were fixed in 4% PFA and embedded in paraffin. Sections (4 microns thick) were deparaffinized with xylene, hydrated in a graded series of ethanol, and washed with PBS. Tissue sections were stained with Luxol Fast Blue.
4.4. Immunohistochemistry
The brains were fixed with 4% PFA in 0.1 M PBS, pH 7.4 overnight at 4°C and then cryoprotected in 30% sucrose in PBS for 72 h at 4°C. Sections (20 μm) were cut with a cryostat and mounted onto glass slides. For calbindin staining, the brain was again fixed by perfusion with 4% PFA in 0.1 M PBS, pH 7.4 but sections (50 μm) were prepared using a vibratome. For immunostaining, the frozen sections were air-dried and washed with PBS. The paraffin-embedded sections were deparaffinized and incubated with either proteinase K (DAKO) for GFAP or 10 mM citrate buffer, pH 6.0 under microwave irradiation. The sections were incubated with 0.1% Triton X-100 in PBS for 15 min. After washing, the sections were blocked with blocking buffer (5% normal donkey serum and 2% BSA in PBS) for 30 min, and incubated with dilutions of the primary antibody in the blocking buffer overnight at 4°C. The following primary antibodies were used: rabbit anti-laminin α1 (Lama1) (Sasaki et al., 2002); rabbit anti-laminin α2 (Lama2), rabbit anti-laminin α3 (Lama3), rabbit anti-laminin α4 (Lama4), rabbit anti-laminin α5 (Lama5) (a gift from Dr. Sasaki); rat anti-nidogen-1 (Millipore); mouse anti-phospho-histone 3 (Ser10)(Cell Signaling); rat anti-BrdU (Serotec); rabbit anti-GFAP (DAKO); rabbit anti-Pax6 (Covance); mouse anti-radial glial cell marker-2 (Millipore) and anti-calbindin D28K (Millipore). The slides were washed with PBS and incubated with the secondary antibodies, Alexa 488 donkey anti-rabbit (Molecular Probes), Cy3 donkey anti-mouse, and Cy3 donkey anti-rat (Jackson Immuno) for 1 h. The slides were washed with PBS, coverslipped with mounting medium containing DAPI, and observed using a Zeiss 510 confocal laser microscope. For connexin staining, the frozen sections were fixed in acetone and incubated with primary antibodies in 0.2% gelatin PBS for 3 h. The primary antibodies included rabbit anti-connexin 26 (Invitrogen), monoclonal mouse anti-connexin 43 (Invitrogen), and rat monoclonal anti-laminin γ1 (Lamc1) chain (Millipore). The secondary antibodies were AlexaFluor 488 goat anti-rabbit, AlexaFluor 488 and 546 goat anti-mouse (Invitrogen), and Cy™5-conjugated donkey anti-rat (Jackson ImmunoResearch Laboratories) and were incubated for 1 h. In each experiment, several sections were incubated without the primary antibody to serve as controls. When the primary antibody was omitted from the staining, no immunoreactivity was observed.
4.5. Slice culture
Slice culture of cerebellum tissue was established as described previously (Streit et al., 1993). Cerebella from postnatal day 10 (P10) mice were sliced into 300 μm thick pieces using a tissue chopper (Muromachi Kikai Co., Ltd). The slices were put onto Millicell tissue culture inserts (Millipore) and incubated with 5 mM bromodeoxyuridine (BrdU) in culture medium comprising 50% Minimum essential medium, 25% Hanks’ balanced salt solution, and 25% horse serum (Invitrogen) for 30 min at 37° C in 5% CO2. The slices were washed with the culture medium and cultured with or without 5 μg/ml laminin-1 (Sigma). After incubation, the slices were frozen and cut in a sagittal orientation. The sections were fixed with 4% PFA in 0.1 M PBS, pH 7.4, and incubated with 0.5% Triton X-100 in PBS for 15 min. The sections were subsequently incubated with 2 N HCl for 30 min at 37° C, neutralized with 0.1 M borate buffer for 10 min and then stained as described above.
4.6. Western blotting
Lysate from the cerebellum was prepared in 50 mM Tris-HCl, 150 mM NaCl, 1 mM EDTA, 1 mM EGTA, 1% Triton X-100, 1.5 mM MgCl2, 10% glycerol, complete mini protease inhibitor mixture (Roche), 1 mM PMSF, 1 mM Na3VO4, and 100 mM NaF, pH 7.4. The lysate was subjected to SDS-PAGE using 4–12% gradient gels (Invitrogen). For immunoblotting, the gels were transferred onto PVDF membranes, blocked with 5% non-fat milk (Cell Signaling), and incubated with primary antibodies in 5% BSA overnight at 4° C. The primary antibodies used were mouse anti-phospho Akt (Ser473), rabbit anti-Akt, phospho-Src family kinase (Tyr416), rabbit anti-ILK1 (Cell Signaling), rabbit anti-FAK (Tyr397), mouse anti-FAK, mouse anti-GAPDH, mouse anti-Src family kinase (Millipore), and rabbit anti-integrin β1 (Santa Cruz).
4.7. Electron microscopy
The cerebellum of P0 mice was dissected and fixed with 2.5% glutaraldehyde in phosphate buffer (pH 7.4). The cerebellum of adult mice was dissected and perfusion-fixed with 2% PFA/2% glutaraldehyde in phosphate buffer (pH 7.4). The cerebellum was postfixed with 1% OsO4 in PBS for 1 hour at 4°C, dehydrated through a graded series of ethanol, and embedded in epoxy resin. Ultrathin sections were stained with 4% uranyl acetate and lead citrate and then examined with a JEM1230 (JOEL) electron microscope.
4.8. Statistical analyses
Data are presented as the mean and SD. The minimum level of statistical significance was set at P=0.05. Differences were analyzed using the two-sided Student’s t test with unequal variance.
Supplementary Material
Highlights.
Laminin a1 (Lama1) knockout mice in the epiblast lineage developed behavioral disorders and displayed impaired formation of the cerebellum. Deficiency of Lama1 in the pial basement membrane of the meninges resulted in a decrease in the proliferation and migration of granule cell precursors, disorganization of Bergmann glial fibers and endfeet. Lama1 is required for cerebellar development and functions.
Acknowledgments
We thank Glenn Longenecker and Ashok B. Kulkarni for their help in creating the mutant mice, Takako Sasaki for the laminin α1 antibody, and Hynda K. Kleinman for critical reading. This work was supported by the Intramural Program of the NIDCR, National Institutes of Health (Y.Y.) and grants from the Ministry of Education, Culture, Sports Science and Technology of Japan (17082008 and 2230023 to E. A-H.).
Abbreviations
- CKO
conditional Lama1 knockout
- CNS
central nervous system
- Cont
control
- ECM
extracellular matrix
- EGL
external granule layer
- GCPs
distorted the layer; granule cell precursors
- IGL
internal granule layer
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alpy F, Jivkov I, Sorokin L, Klein A, Arnold C, Huss Y, Kedinger M, Simon-Assmann P, Lefebvre O. Generation of a conditionally null allele of the laminin alpha1 gene. Genesis. 2005;43:59–70. doi: 10.1002/gene.20154. [DOI] [PubMed] [Google Scholar]
- Andrae J, Afink G, Zhang XQ, Wurst W, Nister M. Forced expression of platelet-derived growth factor B in the mouse cerebellar primordium changes cell migration during midline fusion and causes cerebellar ectopia. Mol Cell Neurosci. 2004;26:308–321. doi: 10.1016/j.mcn.2004.02.004. [DOI] [PubMed] [Google Scholar]
- Aumailley M, Bruckner-Tuderman L, Carter WG, Deutzmann R, Edgar D, Ekblom P, Engel J, Engvall E, Hohenester E, Jones JC, Kleinman HK, Marinkovich MP, Martin GR, Mayer U, Meneguzzi G, Miner JH, Miyazaki K, Patarroyo M, Paulsson M, Quaranta V, Sanes JR, Sasaki T, Sekiguchi K, Sorokin LM, Talts JF, Tryggvason K, Uitto J, Virtanen I, von der Mark K, Wewer UM, Yamada Y, Yurchenco PD. A simplified laminin nomenclature. Matrix Biol. 2005;24:326–332. doi: 10.1016/j.matbio.2005.05.006. [DOI] [PubMed] [Google Scholar]
- Barnabe-Heider F, Miller FD. Endogenously produced neurotrophins regulate survival and differentiation of cortical progenitors via distinct signaling pathways. J Neurosci. 2003;23:5149–5160. doi: 10.1523/JNEUROSCI.23-12-05149.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaess S, Graus-Porta D, Belvindrah R, Radakovits R, Pons S, Littlewood-Evans A, Senften M, Guo H, Li Y, Miner JH, Reichardt LF, Muller U. Beta1-integrins are critical for cerebellar granule cell precursor proliferation. J Neurosci. 2004;24:3402–3412. doi: 10.1523/JNEUROSCI.5241-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chuong CM, Crossin KL, Edelman GM. Sequential expression and differential function of multiple adhesion molecules during the formation of cerebellar cortical layers. J Cell Biol. 1987;104:331–342. doi: 10.1083/jcb.104.2.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark BA, Barbour B. Currents evoked in Bergmann glial cells by parallel fibre stimulation in rat cerebellar slices. J Physiol. 1997;502 (Pt 2):335–350. doi: 10.1111/j.1469-7793.1997.335bk.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edmondson JC, Hatten ME. Glial-guided granule neuron migration in vitro: a high-resolution time-lapse video microscopic study. J Neurosci. 1987;7:1928–1934. doi: 10.1523/JNEUROSCI.07-06-01928.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards MM, Mammadova-Bach E, Alpy F, Klein A, Hicks WL, Roux M, Simon-Assmann P, Smith RS, Orend G, Wu J, Peachey NS, Naggert JK, Lefebvre O, Nishina PM. Mutations in Lama1 disrupt retinal vascular development and inner limiting membrane formation. J Biol Chem. 2010;285:7697–7711. doi: 10.1074/jbc.M109.069575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekblom P, Lonai P, Talts JF. Expression and biological role of laminin-1. Matrix Biol. 2003;22:35–47. doi: 10.1016/s0945-053x(03)00015-5. [DOI] [PubMed] [Google Scholar]
- Goldowitz D, Hamre K. The cells and molecules that make a cerebellum. Trends Neurosci. 1998;21:375–382. doi: 10.1016/s0166-2236(98)01313-7. [DOI] [PubMed] [Google Scholar]
- Graus-Porta D, Blaess S, Senften M, Littlewood-Evans A, Damsky C, Huang Z, Orban P, Klein R, Schittny JC, Muller U. Beta1-class integrins regulate the development of laminae and folia in the cerebral and cerebellar cortex. Neuron. 2001;31:367–379. doi: 10.1016/s0896-6273(01)00374-9. [DOI] [PubMed] [Google Scholar]
- Groszer M, Erickson R, Scripture-Adams DD, Lesche R, Trumpp A, Zack JA, Kornblum HI, Liu X, Wu H. Negative regulation of neural stem/progenitor cell proliferation by the Pten tumor suppressor gene in vivo. Science. 2001;294:2186–2189. doi: 10.1126/science.1065518. [DOI] [PubMed] [Google Scholar]
- Gullberg D, Ekblom P. Extracellular matrix and its receptors during development. Int J Dev Biol. 1995;39:845–854. [PubMed] [Google Scholar]
- Halfter W, Dong S, Yip YP, Willem M, Mayer U. A critical function of the pial basement membrane in cortical histogenesis. J Neurosci. 2002;22:6029–6040. doi: 10.1523/JNEUROSCI.22-14-06029.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall H, Carbonetto S, Schachner M. L1/HNK-1 carbohydrate- and beta 1 integrin-dependent neural cell adhesion to laminin-1. J Neurochem. 1997;68:544–553. doi: 10.1046/j.1471-4159.1997.68020544.x. [DOI] [PubMed] [Google Scholar]
- Hatten ME. Central nervous system neuronal migration. Annu Rev Neurosci. 1999;22:511–539. doi: 10.1146/annurev.neuro.22.1.511. [DOI] [PubMed] [Google Scholar]
- Hayashi S, Lewis P, Pevny L, McMahon AP. Efficient gene modulation in mouse epiblast using a Sox2Cre transgenic mouse strain. Mech Dev. 2002;119(Suppl 1):S97–S101. doi: 10.1016/s0925-4773(03)00099-6. [DOI] [PubMed] [Google Scholar]
- Herrup K, Kuemerle B. The compartmentalization of the cerebellum. Annu Rev Neurosci. 1997;20:61–90. doi: 10.1146/annurev.neuro.20.1.61. [DOI] [PubMed] [Google Scholar]
- Ichikawa N, Iwabuchi K, Kurihara H, Ishii K, Kobayashi T, Sasaki T, Hattori N, Mizuno Y, Hozumi K, Yamada Y, Arikawa-Hirasawa E. Binding of laminin-1 to monosialoganglioside GM1 in lipid rafts is crucial for neurite outgrowth. J Cell Sci. 2009;122:289–299. doi: 10.1242/jcs.030338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imbeault S, Gauvin LG, Toeg HD, Pettit A, Sorbara CD, Migahed L, DesRoches R, Menzies AS, Nishii K, Paul DL, Simon AM, Bennett SA. The extracellular matrix controls gap junction protein expression and function in postnatal hippocampal neural progenitor cells. BMC Neurosci. 2009;10:13. doi: 10.1186/1471-2202-10-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isakson BE, Olsen CE, Boitano S. Laminin-332 alters connexin profile, dye coupling and intercellular Ca2+ waves in ciliated tracheal epithelial cells. Respir Res. 2006;7:105. doi: 10.1186/1465-9921-7-105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karimi-Abdolrezaee S, Eftekharpour E, Wang J, Morshead CM, Fehlings MG. Delayed transplantation of adult neural precursor cells promotes remyelination and functional neurological recovery after spinal cord injury. J Neurosci. 2006;26:3377–3389. doi: 10.1523/JNEUROSCI.4184-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleinman HK, Sephel GC, Tashiro K, Weeks BS, Burrous BA, Adler SH, Yamada Y, Martin GR. Laminin in neuronal development. Ann N Y Acad Sci. 1990;580:302–310. doi: 10.1111/j.1749-6632.1990.tb17939.x. [DOI] [PubMed] [Google Scholar]
- Komuro H, Rakic P. Distinct modes of neuronal migration in different domains of developing cerebellar cortex. J Neurosci. 1998;18:1478–1490. doi: 10.1523/JNEUROSCI.18-04-01478.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lampe PD, Nguyen BP, Gil S, Usui M, Olerud J, Takada Y, Carter WG. Cellular interaction of integrin alpha3beta1 with laminin 5 promotes gap junctional communication. J Cell Biol. 1998;143:1735–1747. doi: 10.1083/jcb.143.6.1735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S, Edgar D, Fassler R, Wadsworth W, Yurchenco PD. The role of laminin in embryonic cell polarization and tissue organization. Dev Cell. 2003;4:613–624. doi: 10.1016/s1534-5807(03)00128-x. [DOI] [PubMed] [Google Scholar]
- Li S, Liquari P, McKee KK, Harrison D, Patel R, Lee S, Yurchenco PD. Laminin-sulfatide binding initiates basement membrane assembly and enables receptor signaling in Schwann cells and fibroblasts. J Cell Biol. 2005;169:179–189. doi: 10.1083/jcb.200501098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mercier F, Hatton GI. Immunocytochemical basis for a meningeo-glial network. J Comp Neurol. 2000;420:445–465. doi: 10.1002/(sici)1096-9861(20000515)420:4<445::aid-cne4>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- Mercier F, Hatton GI. Connexin 26 and basic fibroblast growth factor are expressed primarily in the subpial and subependymal layers in adult brain parenchyma: roles in stem cell proliferation and morphological plasticity? J Comp Neurol. 2001;431:88–104. doi: 10.1002/1096-9861(20010226)431:1<88::aid-cne1057>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- Mills J, Niewmierzycka A, Oloumi A, Rico B, St-Arnaud R, Mackenzie IR, Mawji NM, Wilson J, Reichardt LF, Dedhar S. Critical role of integrin-linked kinase in granule cell precursor proliferation and cerebellar development. J Neurosci. 2006;26:830–840. doi: 10.1523/JNEUROSCI.1852-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miner JH, Li C, Mudd JL, Go G, Sutherland AE. Compositional and structural requirements for laminin and basement membranes during mouse embryo implantation and gastrulation. Development. 2004;131:2247–2256. doi: 10.1242/dev.01112. [DOI] [PubMed] [Google Scholar]
- Miner JH, Yurchenco PD. Laminin functions in tissue morphogenesis. Annu Rev Cell Dev Biol. 2004;20:255–284. doi: 10.1146/annurev.cellbio.20.010403.094555. [DOI] [PubMed] [Google Scholar]
- Muller T, Moller T, Neuhaus J, Kettenmann H. Electrical coupling among Bergmann glial cells and its modulation by glutamate receptor activation. Glia. 1996;17:274–284. doi: 10.1002/(SICI)1098-1136(199608)17:4<274::AID-GLIA2>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- Nagy JI, Li X, Rempel J, Stelmack G, Patel D, Staines WA, Yasumura T, Rash JE. Connexin26 in adult rodent central nervous system: demonstration at astrocytic gap junctions and colocalization with connexin30 and connexin43. J Comp Neurol. 2001;441:302–323. doi: 10.1002/cne.1414. [DOI] [PubMed] [Google Scholar]
- Nagy JI, Ochalski PA, Li J, Hertzberg EL. Evidence for the co-localization of another connexin with connexin-43 at astrocytic gap junctions in rat brain. Neuroscience. 1997;78:533–548. doi: 10.1016/s0306-4522(96)00584-2. [DOI] [PubMed] [Google Scholar]
- Nagy JI, Patel D, Ochalski PA, Stelmack GL. Connexin30 in rodent, cat and human brain: selective expression in gray matter astrocytes, co-localization with connexin43 at gap junctions and late developmental appearance. Neuroscience. 1999;88:447–468. doi: 10.1016/s0306-4522(98)00191-2. [DOI] [PubMed] [Google Scholar]
- O’Shea KS, Rheinheimer JS, Dixit VM. Deposition and role of thrombospondin in the histogenesis of the cerebellar cortex. J Cell Biol. 1990;110:1275–1283. doi: 10.1083/jcb.110.4.1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pons S, Trejo JL, Martinez-Morales JR, Marti E. Vitronectin regulates Sonic hedgehog activity during cerebellum development through CREB phosphorylation. Development. 2001;128:1481–1492. doi: 10.1242/dev.128.9.1481. [DOI] [PubMed] [Google Scholar]
- Qu Q, Smith FI. Alpha-dystroglycan interactions affect cerebellar granule neuron migration. J Neurosci Res. 2004;76:771–782. doi: 10.1002/jnr.20129. [DOI] [PubMed] [Google Scholar]
- Radakovits R, Barros CS, Belvindrah R, Patton B, Muller U. Regulation of radial glial survival by signals from the meninges. J Neurosci. 2009;29:7694–7705. doi: 10.1523/JNEUROSCI.5537-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rakic P. Neuron-glia relationship during granule cell migration in developing cerebellar cortex. A Golgi and electronmicroscopic study in Macacus Rhesus. J Comp Neurol. 1971;141:283–312. doi: 10.1002/cne.901410303. [DOI] [PubMed] [Google Scholar]
- Rakic P, Sidman RL. Weaver mutant mouse cerebellum: defective neuronal migration secondary to abnormality of Bergmann glia. Proc Natl Acad Sci U S A. 1973;70:240–244. doi: 10.1073/pnas.70.1.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki M, Kleinman HK, Huber H, Deutzmann R, Yamada Y. Laminin, a multidomain protein. The A chain has a unique globular domain and homology with the basement membrane proteoglycan and the laminin B chains. J Biol Chem. 1988;263:16536–16544. [PubMed] [Google Scholar]
- Sasaki T, Giltay R, Talts U, Timpl R, Talts JF. Expression and distribution of laminin alpha1 and alpha2 chains in embryonic and adult mouse tissues: an immunochemical approach. Exp Cell Res. 2002;275:185–199. doi: 10.1006/excr.2002.5499. [DOI] [PubMed] [Google Scholar]
- Satz JS, Ostendorf AP, Hou S, Turner A, Kusano H, Lee JC, Turk R, Nguyen H, Ross-Barta SE, Westra S, Hoshi T, Moore SA, Campbell KP. Distinct functions of glial and neuronal dystroglycan in the developing and adult mouse brain. J Neurosci. 2010;30:14560–14572. doi: 10.1523/JNEUROSCI.3247-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheele S, Falk M, Franzen A, Ellin F, Ferletta M, Lonai P, Andersson B, Timpl R, Forsberg E, Ekblom P. Laminin alpha1 globular domains 4–5 induce fetal development but are not vital for embryonic basement membrane assembly. Proc Natl Acad Sci U S A. 2005;102:1502–1506. doi: 10.1073/pnas.0405095102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smyth N, Vatansever HS, Murray P, Meyer M, Frie C, Paulsson M, Edgar D. Absence of basement membranes after targeting the LAMC1 gene results in embryonic lethality due to failure of endoderm differentiation. J Cell Biol. 1999;144:151–160. doi: 10.1083/jcb.144.1.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solecki DJ, Model L, Gaetz J, Kapoor TM, Hatten ME. Par6alpha signaling controls glial-guided neuronal migration. Nat Neurosci. 2004;7:1195–1203. doi: 10.1038/nn1332. [DOI] [PubMed] [Google Scholar]
- Sotelo C. Cellular and genetic regulation of the development of the cerebellar system. Prog Neurobiol. 2004;72:295–339. doi: 10.1016/j.pneurobio.2004.03.004. [DOI] [PubMed] [Google Scholar]
- Streit A, Nolte C, Rasony T, Schachner M. Interaction of astrochondrin with extracellular matrix components and its involvement in astrocyte process formation and cerebellar granule cell migration. J Cell Biol. 1993;120:799–814. doi: 10.1083/jcb.120.3.799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka M. Dendrite formation of cerebellar purkinje cells. Neurochem Res. 2009;34:2078–2088. doi: 10.1007/s11064-009-0073-y. [DOI] [PubMed] [Google Scholar]
- Tanaka M, Yamaguchi K, Tatsukawa T, Nishioka C, Nishiyama H, Theis M, Willecke K, Itohara S. Lack of Connexin43-mediated bergmann glial gap junctional coupling does not affect cerebellar long-term depression, motor coordination, or eyeblink conditioning. Front Behav Neurosci. 2008a;2:1. doi: 10.3389/neuro.08.001.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka M, Yamaguchi K, Tatsukawa T, Theis M, Willecke K, Itohara S. Connexin43 and bergmann glial gap junctions in cerebellar function. Front Neurosci. 2008b;2:225–233. doi: 10.3389/neuro.01.038.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tissir F, Goffinet AM. Reelin and brain development. Nat Rev Neurosci. 2003;4:496–505. doi: 10.1038/nrn1113. [DOI] [PubMed] [Google Scholar]
- Watanabe F, Miyazaki T, Takeuchi T, Fukaya M, Nomura T, Noguchi S, Mori H, Sakimura K, Watanabe M, Mishina M. Effects of FAK ablation on cerebellar foliation, Bergmann glia positioning and climbing fiber territory on Purkinje cells. Eur J Neurosci. 2008;27:836–854. doi: 10.1111/j.1460-9568.2008.06069.x. [DOI] [PubMed] [Google Scholar]
- Wiencken-Barger AE, Djukic B, Casper KB, McCarthy KD. A role for Connexin43 during neurodevelopment. Glia. 2007;55:675–686. doi: 10.1002/glia.20484. [DOI] [PubMed] [Google Scholar]
- Yuasa S. Bergmann glial development in the mouse cerebellum as revealed by tenascin expression. Anat Embryol (Berl) 1996;194:223–234. doi: 10.1007/BF00187133. [DOI] [PubMed] [Google Scholar]
- Yuasa S, Kawamura K, Kuwano R, Ono K. Neuron-glia interrelations during migration of Purkinje cells in the mouse embryonic cerebellum. Int J Dev Neurosci. 1996;14:429–438. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.