Abstract
Background
Orthostatic hypotension (OH) is characterized by an abnormal autonomic response to upright posture. Activating autoantibodies to β1/2-adrenergic (AAβ1/2AR) and M2/3 muscarinic receptors (AAM2/3R) produce vasodilative changes in the vasculature that may contribute to OH.
Methods and Results
IgG from 6 patients with idiopathic OH harboring autoantibodies and from 10 healthy control subjects were examined for: 1) β1AR and M2R activity with a perfused Purkinje fiber assay and PKA assay in H9c2 cells, and 2) vasodilator β2AR and M3R activity using a pressurized cremaster resistance arteriole assay. Changes of IgG activity with and without propranolol, atropine, and L-NAME were used to estimate AAβAR, AAM2R and AAM3R activation of their respective functions. All 6 patients had elevated ELISA titers to at least one of the receptors compared to controls. βAR-mediated contractility activity and M2R activity were increased in 5 of the 6 patients. IgG from all 6 patients produced a direct vasodilator effect upon cremaster arterioles. βAR and nitric oxide synthase blockade led to near normalization of IgG-induced vasodilation.
Conclusion
Aβ1/2AR and AAM2/3R are present in some patients with idiopathic OH compatible with an in vivo effect. These autoantibodies and their cardiovascular effects provide new mechanistic insights into the pathophysiology of OH.
Keywords: autonomic receptor, vasodilation, activating autoantibodies, nitric oxide synthase
Introduction
Orthostatic hypotension (OH) of varying severity afflicts up to 2% of the adult population (1). The control of blood pressure (BP) is complex and requires integration of multiple systems to achieve transition from the recumbent to the upright posture. It is not surprising that clinicians are at a disadvantage in identifying the etiology of such patients. There exist a high percentage of patients assigned to the category of “idiopathic” autonomic dysfunction. In the course of our study of patients with conditions associated with the presence of activating autoantibodies to the β1/2-adrenergic and M2 muscarinic G-protein coupled receptors (GPCR), we observed several with coexisting OH. Some had sufficient underlying cardiac or other potential causes for orthostasis, others did not. We have several patients with no apparent etiology, yet were greatly bothered by their orthostatic symptoms and who sought medical assessment.
The autonomic system is intrinsically involved in a primary or secondary fashion in all cases of OH (2, 3). Pathophysiologic changes include peripheral vasodilation with or without an ability to respond to changes in posture by an increase in the cardiac output. These changes are generally attributed to anatomical, metabolic or pharmacological-induced changes in the central or peripheral neural system (2, 3). These may include metabolic or genetic alterations in autonomic control systems ranging from preganglionic sites to post synaptic fibers (4). To date, there have been a limited number of OH patients with a documented autoimmune etiology. These include autoantibodies directed toward neural elements in a group of subjects with autoimmune autonomic ganglionopathy (AAG) (5, 6). Up to 50% of these subjects have measurable blocking autoantibodies to the ganglionic post synaptic α3 nicotinic acetylcholine receptor.
We hypothesized and now report a new category of patients wherein autoantibodies directly activate autonomic receptors on the vascular endothelium and/or smooth muscle leading to peripheral vasodilation and OH. This is based on our observation in such patients of circulating autoantibodies which demonstrate in vitro activation of muscarinic M2/3 receptors (M2/3R) and/or the β1 and especially β2-adrenergic receptors (β1/2R).
We have examined a group of patients several of whom, but not all, have a history of an autoimmune disorder. Many were found to harbor autoantibodies variably directed toward β1/2AR and M2/3R and demonstrated OH without apparent cause. We have used sera and IgG purified from 6 of these subjects to demonstrate with in vitro techniques these autoantibodies are physiologically active and mechanistically capable of causing or enhancing peripheral vasodilation (mediated by β2AR and/or M3R activation) or inhibiting a compensatory rise in pulse rate (M2R). The co-presence of these different autoantibodies may contribute to distinctive patterns of autonomic dysfunction in these patients. We provide evidence for at least three patterns of clinical variability based on a dominance of β1/2-adrenergic activity, of muscarinic M3R activation of endothelial nitric oxide synthase (eNOS) activity and for muscarinic M2R inhibition of pulse rate and cardiac responsiveness to upright posture.
Methods
Patient Selection
Thirty six patients had been referred for evaluation of symptomatic OH in the endocrinology clinics of the VAMC and OU Health Sciences Center. These subjects were screened by ELISA for autoantibodies as part of their examination. Six subjects with evidence for one or more ELISA-positive autoantibodies were selected for more detailed study to determine if these autoantibodies had the potential activity that would impair their ability to compensate for the physiological changes in peripheral resistance (vasodilation) or alter their cardiac output when standing upright. Patients with evident secondary hypotension from administration of antihypertensive drugs or overt neurological diseases associated with OH were excluded. There was no discrete neurological cause of their OH. One patient (#106) was included with a diagnosis of type 1 diabetes mellitus. This patient had minimal diabetic peripheral sensory neuropathy but had evidence for autonomic dysfunction. A second subject (#118) subsequently was diagnosed with coeliac disease which responded to dietary gluten restriction. A third (#159) had been diagnosed with systemic lupus erythematosis and was in remission without pharmacological therapy. A fourth (#112) has an autoimmune hemolytic anemia and is in partial remission after splenectomy. Thus, 4/6 had an associated autoimmune dyscrasia that demonstrated a predilection for production of autoantibodies. Data from these 6 subjects are summarized in Table 1.
Table 1.
Clinical characteristics and autoantibody titers of the patients
| Patient | Age (yrs) |
Sex | BP (mm Hg) |
Pulse (bpm) |
ECI (L/min/m2) |
SVR (dynes/sec/cm5) |
NE Response (pg/ml) |
Cold Pressor BP (Pulse) B = baseline P = post |
ELISA Titers Anti-β1AR Anti-β2AR |
ELISA Titers Anti-M2R Anti-M3R |
|---|---|---|---|---|---|---|---|---|---|---|
| 106 | 73 | M | R 138/94 | R 78 | ND | ND | ND | ND | 1:3,200 | 1:400 |
| U 102/64 | U 88 | 1;3,200 | 1:6,400 | |||||||
| 112 | 71 | M | R 128/84 | R 50 | R 2.6 | R 1775 | R 356 | B 132/84 (70) | 1:3,200 | 1:400 |
| U 112/70 | U 60 | U 2.1 | U 1790 | U 678 | P 158/84 (70) | 1:3,200 | 1:3,200 | |||
| 118 | 50 | F | R 97/72 | R 48 | R 2.9 | R 2066 | R 303 | B 133-84 (52 | 1:1,600 | 1;1,600 |
| U 68/52 | U 68 | U 2.1 | U 2494 | U 567 | P 153/97 (53) | 1:1,600 | 1:3,200 | |||
| 156 | 57 | F | R 118/78 | R 84 | ND | ND | ND | ND | 1:3,200 | 1:3,200 |
| U 102/62 | U 112 | 1:6,400 | 1:1,600 | |||||||
| 159 | 19 | F | R 114/76 | R 86 | R 3.5 | R 1138 | R 167 | B 110/67 (77) | 1;12,800 | 1:12,800 |
| U 94/65 | U 128 | U 2.4 | U 1550 | U 483 | P 118/67 (77) | 1:6,400 | 1:1,600 | |||
| 236 | 53 | F | R 126/68 | R 70 | R 3.0 | R 1277 | R 311 | B 112/76 (64) | 1:25,600 | 1:1,600 |
| U 102/52 | U 85 | U 2.5 | U 1364 | U 455 | P 122/82 (72) | 1:12,800 | 1:1,600 |
BP, blood pressure; ECI, estimated cardiac index; SVR; systemic vascular resistance; NE, norepinephrine; R, recumbent; U, upright; ND, not determined; B, basal; P, peak
BP and heart rates were determined from each following a 5 min period of recumbency and after 5 and 10 min of upright posture. Two patients had difficulty with prolonged upright posture after 5 min and were allowed to sit with their legs in the dependent position for the remainder of the 10 min. Four of the six patients underwent additional testing including a 25 min rest period with an indwelling venous cannula for subsequent measurement of postural changes of norepinephrine. A radial artery pulse wave transducer (HDI/PulseWave CR-2000, Hypertension Diagnostic Inc, Egan, MN) for indirect measurement of estimated cardiac index (ECI) and total peripheral resistance (TPR) was placed distally on the same arm so as to not interfere with the blood collection, while the BP cuff was located on the contralateral arm. After the posture test was completed, the patient sat quietly for 30 min and then underwent a standardized cold pressor test with hand exposure to ice water for 30 seconds. BP was recorded for 5 min. Two subjects did not undergo the full study as they moved away from this location and could not return. Each patient was consented using forms approved by the OUHSC IRB and by the VAMC Research and Development Committee.
ELISA
The enzyme-linked immunosorbent assay (ELISA) was performed as previously described (7). Briefly, microtiter plates were coated with human β1AR, β2AR, or M2R expressed in CHO cell membranes (PerkinElmer, Waltham, WA) at 10 μg/ml in coating buffer. A peptide corresponding to the second extracellular loop (ECL2) of human M3R (aa 213-228) was synthesized (Molecular Biology Proteomics Facility, OUHSC) and used to detect M3R autoantibodies. Controls included serum alone, secondary antibody conjugate alone, 1% BSA alone, and purified pooled human IgG (Sigma). Values were read at 60 min with serum dilutions (titers) assigned when the optical density (OD) reaches 0.1 units.
IgG Preparation
IgG was purified from the patient or healthy control sera using the NAb Protein A/G Spin Kit (Pierce Biotechnology, Rockford, IL).
Contractility Bioassay
Contractility assays were performed to estimate βAR and M2R activity using isolated canine Purkinje fibers as described (7, 8). Free running Purkinje fibers from canine ventricles were perfused with normal Tyrode’s solution at 36±0.1°C in a perfusion chamber mounted in vitro. Contractions were recorded before, with IgG equivalent to 1:100 serum dilutions and following washout. IgG plus atropine (100 nmol/L) then was assayed to determine βAR activation attributable to the anti-βAR component of the IgG. IgG also was tested in the presence of dual βΑR and M2R blockade using nadolol (100 nmol/L) and atropine (100 nmol/L). The βAR agonist isoproterenol (10 nmol/L) served as a positive control. Contractility indices were recorded and analyzed offline using pClamp 9.2 (Axon Instruments, Foster City, CA) and Sigmastat statistical software and calculated as the mean of 15 consecutive contraction cycles during baseline and during the agonist/antagonist response.
Protein Kinase A Assay
Assays to determine protein kinase A (PKA) activity were performed as described (7) using serum from the patient. Briefly, 1 × 107 H9c2 cells were plated in T75 culture flasks. Sera (1:100 dilution) with and without atropine (100 nmol/L) were incubated with cells for 1 hr before reactions were stopped by ice-cold PBS. Protein kinase activity was measured using a SignaTECT PKA assay kit (Promega, Madison, WI). For inhibition of autoantibody-induced PKA activity, the β-blocker nadolol (100 nmol/L) was added. The βAR agonist isoproterenol (100 nmol/L) was used as a positive control. The specific activity of the enzyme was determined in pmol/L per minute per μg for each sample and the results were presented as percentages above the basal level of PKA activity.
Isolated Vessel Pressure Myography
This was performed to estimate the effect of IgG from each patient on β2AR and M3R-induced vasodilation as well as to screen for other concurrent agonists that might cause vasoconstriction. Cremaster resistance arterioles (70-80 μm) were surgically removed from anesthetized rats (180-250 g) and placed in a temperature-controlled (<4°C) dissection chamber containing a physiological salt solution. A ~2 mm segment of the main intramuscular arteriole was microdissected, transferred to a 5.0 mL temperature-regulated superfusion chamber and cannulated at each end with glass pipettes (external diameter ~75 μm) (9, 10). Vessel segments were gradually pressurized to 70 mmHg and warmed to 34°C. The vessel preparation was positioned on the stage of an inverted microscope (Olympus IX70) equipped with a video-based imaging system. Measurements of internal vessel diameter were made using electronic video calipers (Texas A&M University, College Station, TX). After equilibration and development of steady-state myogenic tone, all arterioles (except control) were perfused with the specified IgG agonist and cumulative 3-point concentration-response curves were constructed. After equilibrium the perfusate was switched to one containing an identical maximal effective concentration of IgG and 1 μmol/L propranolol for 5 min and the effect on vessel diameter recorded. At that point, 100 μmol/L L-NAME was added to the IgG and propranolol and their cumulative effects recorded until no further change in diameter was observed. The data are reported as percentage of the maximal Ca2+-free passive diameter (maximal dilation) at 70 mmHg measured at the end of each preparation in order to normalize the values.
Statistics
Normally distributed data are expressed as mean ± standard deviation. Contractility values were normalized to their respective baseline values. Comparison between normally distributed groups was performed using the Student’s t-test with a Bonferroni correction to adjust for the multiple comparisons. A p value of <0.05 was considered statistically significant.
Results
Clinical Details
Four females and two males with OH without documented cause are included in the study since they demonstrated significant levels of ELISA positive autoantibodies that were of interest. Their clinical data are shown in Table 1. Each had recurrent episodes of OH by history and documented by repeated testing. β-blockade was discontinued for >24 hrs prior to testing in one patient. Two (#112 and 118) had a resting pulse rate frequently <50 bpm and four had a disproportionately impaired pulse response to upright posture. Two (#156 and 159) had a rapid pulse response to standing and had significant hypotension distinguishing them from classical postural tachycardia syndrome (POTS) wherein the BP is frequently maintained, albeit with a marked tachycardia associated with symptoms compatible with adrenergic hyperactivity (11). Four were available for indirect estimation of their cardiac index and systemic vascular resistance. Each subject demonstrated a significant decrease in their estimated cardiac index with upright posture and their estimated systemic vascular resistance failed to increase appropriately. The plasma norepinephrine concentration at rest in each of these four subjects was within normal limits and rose appropriately with upright posture. All four patients tested had a positive cold pressor response.
Presence of ELISA Positive Autoantibodies
Sera were examined by ELISA for autoantibodies directed against β1AR, β2AR, M2R and M3R. These data are shown in Table 1 for each subject. Normal control subjects generally have ELISA titers of ≤1:1,600 for anti-≤1/2AR and β1:400 for anti-M2/3R. Bioactivity is not usually observed in these “normal” samples and attributed to either low affinity autoantibodies or is directed to an inactive site on the receptor. One such “normal” volunteer had elevated bioactivity and on posture testing was found to have an asymptomatic but abnormal BP/pulse response and was therefore not included in the normal subjects of this study.
Effect of Serum IgG on βAR and M2R-Mediated Purkinje Fiber Contractility
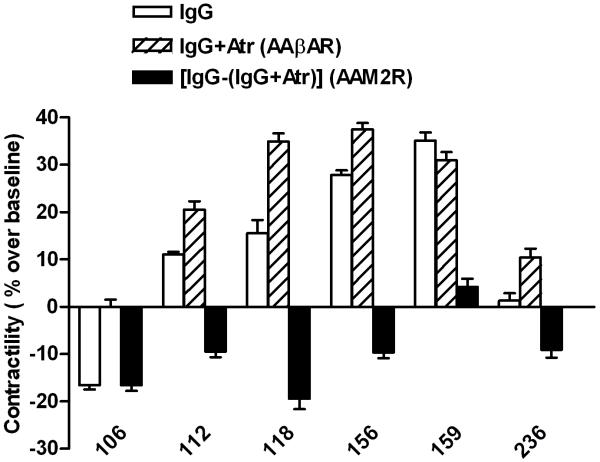
IgG purified from the sera of 6 subjects demonstrated significant levels of bioactivity for βAR and/or M2R as measured by their effects on contractility of perfused canine Purkinje fibers (Figure 1A). Five of 6 patients demonstrated significant levels of bioactivity associated with the βAR inasmuch as they demonstrated a positive effect on contractility either with IgG alone and/or following IgG plus atropine blockade. IgG from patient 106, although possessing autoantibodies to β1/2AR by ELISA, demonstrated a significant inhibitory contractile effect of IgG alone consistent with M2R activation. When atropine was added, no βAR contractile activity was revealed with this assay. In a separate study (data not shown), this patient demonstrated a significant increase in specific β2AR activity and low β1AR activity. The non-selective β1/2AR blocker nadolol also did not alter IgG activity in this patient. Four of 6 patients demonstrated a significant concurrence of activating autoantibodies to both βAR and M2R. The sixth patient demonstrated modest βAR and M2R-mediated contractile activity, but had relatively little net activity by IgG alone. The mean values for IgG alone for all 6 patients, for M2R contractile activity as measured in the presence of the β-blocker nadolol, and for βAR activity when IgG was given in the presence of atropine are shown in Figure 1B. The mean values for all 6 were significantly different from baseline and from control subjects. βAR activity (% over baseline) was elevated in 5 of 6 patients (26.8±5.0%, n=5, vs. control 1.0±1.0%, n=10, p<0.0001). M2R activity was increased in 5 of the 6 patients (12.8±2.1%, n=5 vs. control 0.6±1.3%, n=10, p=0.0002). The values are comparable to the maximal effect of the βAR isoproterenol positive control.
Figure 1. Effects of IgG from the 6 orthostatic hypotension patients on Purkinje contractility.
A. Each value represents the mean ± SD of 15 consecutive contractions and was measured once the preparation had stabilized. The data are expressed as percent above basal levels of the buffer control. The IgG alone shows net activity. An increase in contractility over baseline with IgG plus muscarinic blocker atropine (Atr) represented the βAR effect of IgG (AAβAR). The difference in IgG effect on contractility between the presence and absence of atropine was a surrogate marker of the M2R inhibitory effect of IgG (AAM2R). The co-presence of atropine demonstrates that βAR activity was impaired partially by concurrent M2R inhibitory effects in 4 of the 6 patients. Patient 106 has marked M2R dominance while patient 159 is βAR dominant. B. The mean effect of IgG from the 6 patients on Purkinje contractility. The βAR activity of the IgG was measured as effect of IgG plus atropine (Atr) and M2R activity as IgG plus the non-selective β-blocker nadolol (Nad). The effect of IgG from 10 control subjects and the positive isoproterenol (ISO) control are also shown.
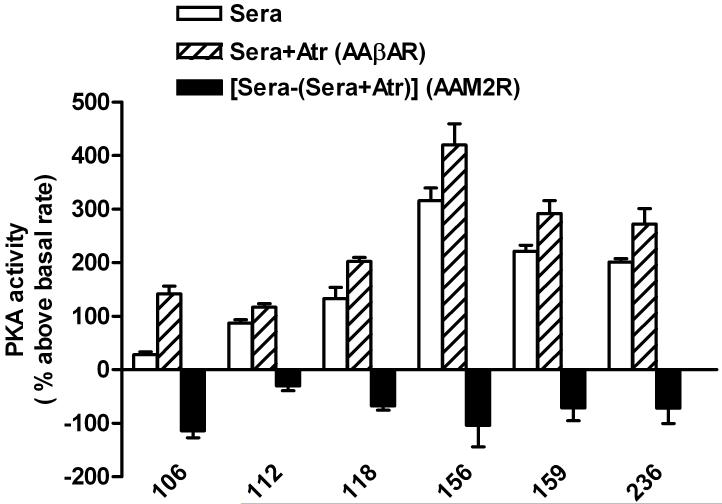
Effect of Serum IgG on PKA activation
Patient sera were obtained when no interfering drugs were present and tested for its ability to activate or inhibit PKA in cultured H9c2 cells in vitro (Figure 2). These data are compared to baseline in the presence of buffer alone and in the presence of atropine. This assay provides an important parameter of cellular function relevant to the intrinsic activity of these autoantibodies. All of the patients demonstrated significant β-activation of PKA. M2R activity was estimated by subtracting the PKA activity in the presence of a high dosage of atropine from the activity in its absence. Significant atropine-sensitive effects were present in all 6 subjects. Patient 106, which demonstrated little positive contractile β1AR activity with the Purkinje contractility assay, significantly activated PKA supporting a role for the elevated β2AR activity. An isoproterenol stimulation, not shown, was performed with each assay as a positive control.
Figure 2. Effects of sera from the 6 orthostatic hypotension patients on PKA activity in H9c2 cells.
The values are expressed as percent above basal levels of PKA in medium control. An increase in PKA activity with sera plus atropine (Atr) represented the βAR effect (AAβAR). The difference in sera effect between the presence and absence of atropine was a surrogate marker of the M2R inhibitory effect (AAM2R). This assay demonstrates a significant impact of muscarinic blockade on PKA activity in 5 of 6 subjects.
Effect of Serum IgG on Skeletal Muscle Arteriole Resistance
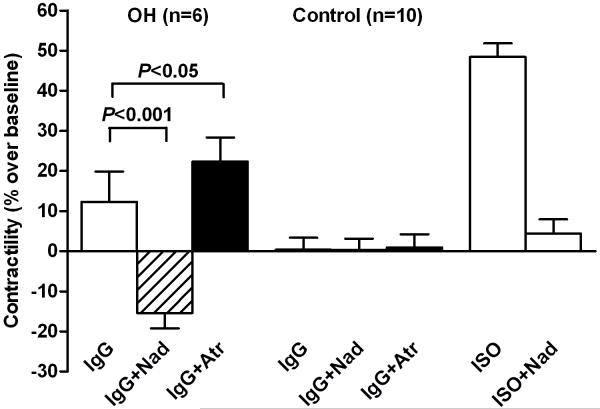
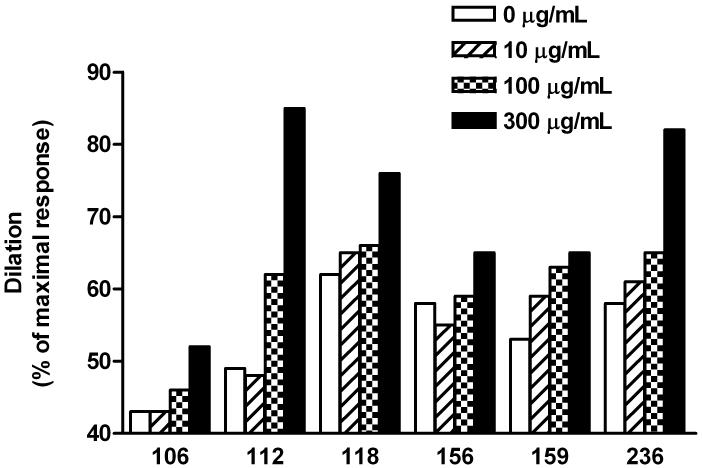
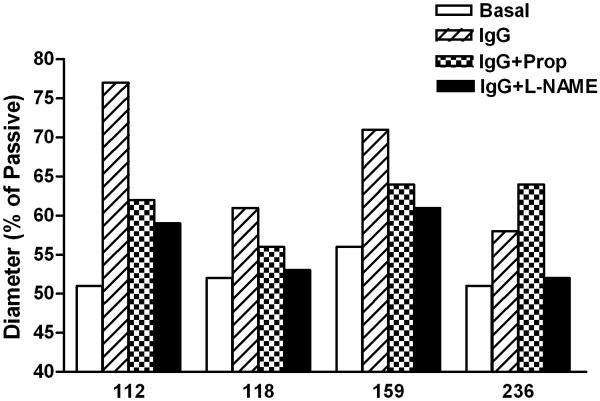
We examined the vasodilator response to a 3-point dosage of IgG from 6 patients on rat cremaster arteriolar diameter (Figure 3). Pooled, dialyzed normal human IgG (Sigma) at 150 and 300 μg/mL did not produce vasodilation. IgG from 3 individual control subjects produced less than 5% vasodilation, which is within the range of changes affected by buffer alone. There was a significant and dosage dependent vasodilator effect from each patient’s IgG during perfusion. The effect of sequential addition of propranolol and L-NAME on vasodilation induced by IgG from 4 OH patients and a representative tracing are shown in Figure 4. There was a significant decrease in vasodilation with non-selective β-blockade and a smaller mean decrease with blockade of nitric oxide synthase by L-NAME. No residual pressor effect was observed in any subject during combined β-blockade and eNOS suppression.
Figure 3. Effects of serum IgG from orthostatic hypotension patients on basal spontaneous myogenic tone of rat cremaster arterioles.
Each IgG sample was perfused into the cremasteric arteriole after a stable baseline was achieved. A dosage response to buffer (control), 10, 100 and 300 μg/mL of IgG was recorded. The peak response for each IgG concentration is the percentage of the maximal Ca2+-free passive diameter (maximal dilation) for that arteriolar preparation. A 10% increase in dilation is considered significant. All of the samples caused a significant dilation compared to their tonic control value. Three control IgG samples and a dialyzed (to eliminate sodium azide preservative) pooled commercial IgG gave less than 5% change from baseline (data not shown).
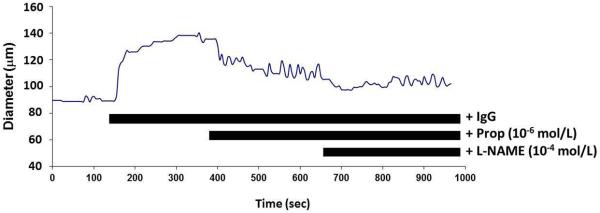
Figure 4. The effects of βAR and eNOS blockade on IgG-mediated resistance arteriole dilatation.
A. Representative tracing of the effect of sequential addition of the β-blocker propranolol (Prop 10−6 mol/L) and nitric oxide synthase inhibitor L-NAME (10−4 mol/L) on IgG-induced arteriolar dilation. B. Data for four of the subjects. Patients 118 and 236 had a return to baseline tone with combined blockade while 112 and 159 failed to return completely to control. No subject had residual vasoconstriction compared to baseline during combined blockade.
Pharmacological Interventions
Pharmacological effects were observed in several subjects during either selective β1AR or muscarinic blockade. Subjects #106, #112 and #118 presented with a muscarinic dominant picture and also had concurrent urinary bladder contractions and frequency of urination. The M3R antagonist oxybutynin, which also has some M1R and M2R activity, led to clinical improvement in their OH with a small pulse rate increase of approximately 5 bpm. Unfortunately the concurrent and unpleasant M1R side effects of this muscarinic blockade (constipation, dry mouth and eyes) led all three to discontinue the medication by 3 months. The use of other muscarinic blockers yielded similar side effects. Two of these muscarinic dominant subjects also had been previously prescribed a β-blocker and reported a dramatic but fortunately short lived bradycardia with rates of 36 and 38 bpm. Subjects #156 and #159 with a β-dominant picture had previously been tried on a selective β-blocker (metoprolol) with a slowing of their tachycardia but with worsening of their orthostasis.
Discussion
BP during upright postural change is maintained by a coordinated autonomic response with rapid and sustained vasoconstriction and/or an increase in cardiac output to maintain blood flow to the brain, heart and peripheral vasculature. Any agent superimposing systemic vasodilation (whether drug-induced or pathological loss of adrenergic tone) or inhibiting cardiac responsiveness (drug or structural damage) will cause or accentuate orthostasis in susceptible subjects.
We herein report a detailed mechanistic study of sera/IgG obtained from 6 patients with OH, four with and two without a history of an autoimmune dyscrasia, who harbored autoantibodies to β1/2AR and to M2/3R. We have demonstrated with in vitro assays that β2AR and M3R activating autoantibodies are fully capable of inducing significant systemic arteriolar vasodilation. We have demonstrated: 1) they activate their appropriate intracellular messengers including PKA; 2) they alter contractility in cardiac tissue and most importantly in peripheral resistance vessels; and 3) these effects can be blocked in vitro selectively by β-blockade, generalized muscarinic blockade and/or by inhibition of endothelial nitric oxide synthase.
Normally, β1AR and β2AR (to a lesser extent) agonists increase automaticity of the heart but have variable effects on vascular tone. Autonomic orthosteric agonists have a variable vasoconstrictive effect on resistance vessels. This occurs with the α1AR at physiologic levels for norepinephrine but at higher concentrations for epinephrine. Activating β1/2AR autoantibodies are receptor specific and do not cross react with the α1AR as would epinephrine. These activating autoantibodies have a variable effect on vasodilation in resistance arteries (12). β2AR agonists may produce vasodilation in resistance arteries by two different mechanisms. These include: 1) direct vasodilation via activation of cAMP/PKA-mediated dephosphorylation of the smooth muscle myosin (13); and 2) endothelial nitric oxide-mediated vasodilation (14).The effect of M3R activation on eNOS-mediated vasodilation is well established (15, 16). M2R activation, while not directed toward vasodilation, has a profound inhibitory effect on heart rate while β1/2AR is cardioacceleratory.
We have demonstrated three phenotypic patterns of activating autoantibodies within these 6 subjects. All had circulating activating autoantibodies coexistent with OH. Three (#106, #112 and #118) had notable resting bradycardia and/or biochemical evidence consistent with a muscarinic M2R dominant cardiovascular response. Two subjects (#156 and 159) had a resting heart rate that was at the high end of normal and frequently observed tachycardia associated with their orthostasis. These had lesser M2R activity and appear to be β1/2AR dominant in their autoantibody activity. The 6th (#236) had a mixed pattern that was more difficult to characterize, which is not surprising since this patient expressed evidence for activity with all of the autoantibodies. The bioactivity-based tests using Purkinje contractility, the cremaster resistance artery vasodilation and the biochemical assay measuring PKA activation were generally consistent with the patient’s phenotype. All four patients tested demonstrated a significant drop in their cardiac index with upright posture and an inadequate compensatory reserve for their systemic vascular resistance.
Although we did not measure α3 nicotinic acetylcholine receptor antibodies in our patients to rule out AAG, the symptom complex of our patients was somewhat different than described for patients with AAG. Measurement of the circulating norepinephrine response to upright posture was normal in four of our patients in contrast to the very low values measured in patients harboring the α3 acetylcholine receptor blocking antibodies. Since up to half of the patients with a putative diagnosis of AAG failed to demonstrate these α3 blocking antibodies (5), it is possible that activating autoantibodies to the β1/2AR and M2/3R might be coexistent and implicated in that entity as well.
We have for the first time demonstrated the potential role of these activating autoantibodies in mechanistically altering the normal autonomic response to upright posture. Unfortunately, there are no useful animal models for OH in the human. Our initial studies in these subjects demonstrate an intact norepinephrine response to orthostasis, an intact cold pressor responsiveness; but a blunted cardiovascular response. Those subjects with a muscarinic dominant effect had an inadequate cardiac pulse rate response during orthostasis while all subjects had an inadequate response in their estimated peripheral resistance. Final proof for the role of these autoantibodies will consist in effectively blocking them using either existent pharmaceutical agents or autoantibody-specific agents in current development and demonstrating return of a normal physiological response to upright posture. Support for this concept was observed when 3 of our patients with a relative muscarinic dominance and concurrent bladder spasms were treated with the relatively non-selective M3R blocker oxybutynin with some improvement in orthostasis. β-blockade had previously been attempted with β1AR-selective agents in the two patients with a tachycardia prior to our examining them and though their pulse rate slowed, their orthostasis was worsened likely due to inadequate β2AR and M3R blockade. We believe that our studies provide a rational explanation for these pharmacological responses. The combination of βAR and muscarinic blockade has inherent cardiovascular risk and protocols to examine this possibility are under consideration.
Thus, we have demonstrated for the first time that a subgroup of patients with idiopathic OH demonstrates several activating autoantibodies capable of contributing to the pathophysiology of OH. A large epidemiological study to examine the role of these activating autoantibodies in several subgroups with various types of OH is now underway.
Acknowledgments
Adita Blanco, Kathy Alvarez, and Daniel Collier provided technical support.
This publication was made possible by NIH Grant Number P20 RR 024215 from the COBRE Program of the National Center for Research Resources (X.Y.), the Heart Rhythm Institute, OUHSC and the Veterans Administration Research Foundation (D.C.K.) and by individual grants from Will and Helen Webster and Britani T. and Paul E. Bowman, Jr.
Footnotes
Conflict of interest: none.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Low PA. Prevalence of orthostatic hypotension. Clin Auton Res. 2008;18(Suppl 1):8–13. doi: 10.1007/s10286-007-1001-3. [DOI] [PubMed] [Google Scholar]
- 2.Robertson D. The pathophysiology and diagnosis of orthostatic hypotension. Clin Auton Res. 2008;18(Suppl 1):2–7. doi: 10.1007/s10286-007-1004-0. [DOI] [PubMed] [Google Scholar]
- 3.Medow MS, Stewart JM, Sanyal S, Mumtaz A, Sica D, Frishman WH. Pathophysiology, diagnosis, and treatment of orthostatic hypotension and vasovagal syncope. Cardiol Rev. 2008;16:4–20. doi: 10.1097/CRD.0b013e31815c8032. [DOI] [PubMed] [Google Scholar]
- 4.Goldstein DS, Sharabi Y. Neurogenic orthostatic hypotension: a pathophysiological approach. Circulation. 2009;119:139–46. doi: 10.1161/CIRCULATIONAHA.108.805887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vernino S, Low PA, Fealey RD, Stewart JD, Farrugia G, Lennon VA. Autoantibodies to ganglionic acetylcholine receptors in autoimmune autonomic neuropathies. N Engl J Med. 2000;343:847–55. doi: 10.1056/NEJM200009213431204. [DOI] [PubMed] [Google Scholar]
- 6.Vernino S, Hopkins S, Wang Z. Autonomic ganglia, acetylcholine receptor antibodies, and autoimmune ganglionopathy. Auton Neurosci. 2009;146:3–7. doi: 10.1016/j.autneu.2008.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kem DC, Yu X, Patterson E, Huang S, Stavrakis S, Szabo B, et al. Autoimmune hypertensive syndrome. Hypertension. 2007;50:829–34. doi: 10.1161/HYPERTENSIONAHA.107.096750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stavrakis S, Yu X, Patterson E, Huang S, Hamlett SR, Chalmers L, et al. Activating autoantibodies to the beta-1 adrenergic and m2 muscarinic receptors facilitate atrial fibrillation in patients with Graves’ hyperthyroidism. J Am Coll Cardiol. 2009;54:1309–16. doi: 10.1016/j.jacc.2009.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Meininger GA, Zawieja DC, Falcone JC, Hill MA, Davey JP. Calcium measurement in isolated arterioles during myogenic and agonist stimulation. Am J Physiol. 1991;261:H950–9. doi: 10.1152/ajpheart.1991.261.3.H950. [DOI] [PubMed] [Google Scholar]
- 10.Hill MA, Zou H, Davis MJ, Potocnik SJ, Price S. Transient increases in diameter and [Ca(2+)](i) are not obligatory for myogenic constriction. Am J Physiol Heart Circ Physiol. 2000;278:H345–52. doi: 10.1152/ajpheart.2000.278.2.H345. [DOI] [PubMed] [Google Scholar]
- 11.Low PA, Sandroni P, Joyner M, Shen WK. Postural tachycardia syndrome (POTS) J Cardiovasc Electrophysiol. 2009;20:352–8. doi: 10.1111/j.1540-8167.2008.01407.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bakris G. An in-depth analysis of vasodilation in the management of hypertension: focus on adrenergic blockade. J Cardiovasc Pharmacol. 2009;53:379–87. doi: 10.1097/FJC.0b013e31819fd501. [DOI] [PubMed] [Google Scholar]
- 13.Murray KJ. Cyclic AMP and mechanisms of vasodilation. Pharmacol Ther. 1990;47:329–45. doi: 10.1016/0163-7258(90)90060-f. [DOI] [PubMed] [Google Scholar]
- 14.Queen LR, Ferro A. Beta-adrenergic receptors and nitric oxide generation in the cardiovascular system. Cell Mol Life Sci. 2006;63:1070–83. doi: 10.1007/s00018-005-5451-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Eglen RM, Hegde SS, Watson N. Muscarinic receptor subtypes and smooth muscle function. Pharmacol Rev. 1996;48:531–65. [PubMed] [Google Scholar]
- 16.Khurana S, Chacon I, Xie G, Yamada M, Wess J, Raufman JP, et al. Vasodilatory effects of cholinergic agonists are greatly diminished in aorta from M3R−/− mice. Eur J Pharmacol. 2004;493:127–32. doi: 10.1016/j.ejphar.2004.04.012. [DOI] [PubMed] [Google Scholar]