Abstract
Cardiolipin, the signature phospholipid of mitochondria, is a dimer that is important for a diverse range of mitochondrial activities beyond the process of ATP production. Thus not surprisingly, derangements in cardiolipin metabolism are now appreciated to contribute to an assortment of pathological conditions. A comprehensive inventory of enzymes involved in cardiolipin biosynthesis and remodeling was just recently obtained. Post-biosynthesis, the acyl chain composition of cardiolipin is modified by up to three distinct remodeling enzymes that produce either a homogenous tissue-specific mature form of cardiolipin or alternatively, “bad” cardiolipin that has been linked to mitochondrial dysfunction. Here, we initially focus on the newly identified players in cardiolipin metabolism and then shift our attention to how changes in cardiolipin metabolism contribute to human disease.
The Signature Phospholipid
A signature dish is a unique recipe that can by itself identify its culinary master. In the same vein, the presence of the phospholipid cardiolipin in the membranes of an organelle identifies that organelle as a mitochondrion. Because of this capacity, cardiolipin is commonly referred to as the signature phospholipid of the powerhouse of the cell. Indeed, most of the cardiolipin in a cell is associated with mitochondrial membranes, especially the inner membrane (IM) [1]. This is no accident as the IM is where cardiolipin is synthesized [2–5]. Still, in contrast to most phospholipids that are produced in defined compartments, most notably the endoplasmic reticulum (ER), and then disseminate throughout the cellular endomembrane system, cardiolipin remains firmly associated with mitochondrial membranes. This mitochondrial enrichment has been used to promote the hypothesis that cardiolipin is critical for mitochondrial production of ATP via oxidative phosphorylation (OXPHOS), although other arguments can be made (Box 1). Indeed, cardiolipin is only found in membranes (bacterial and mitochondrial) that generate an electrochemical gradient subsequently used to produce ATP. That cardiolipin is dispensable for OXPHOS, at least in the yeast Saccharomyces cerevisiae, is perhaps surprising; that this process functions more robustly and with higher efficiency in the presence of cardiolipin is not [2, 4, 6, 7]. Moreover, emerging evidence indicates that cardiolipin is involved in numerous distinct mitochondrial activities in addition to OXPHOS (Figure 1; and excellently reviewed in [2–4, 8]).
BOX 1. The hidden cost of energy?
Mitochondria were acquired in an endosymbiotic event. Over time, most of the original mitochondrial genome has been transferred to the nucleus, and the biology of this organelle fully integrated into both cellular and organismal metabolism. However, evidence of the mitochondrion’s perhaps contentious origins persists. Cardiolipin is one such clue. The immune system comprises the innate and the adaptive immune systems. In contrast to the flexibility of the adaptive immune system, the innate immune system recognizes hard-wired structures that are unique to potential hostile invaders. As cardiolipin is only found in mitochondrial and bacterial membranes, and given that eukaryotic cardiolipin is normally hidden from the immune system, cardiolipin is an attractive candidate pathogen-associated molecular pattern. Indeed, the prevalence of anti-cardiolipin antibodies in patients suffering from anti-phospholipid syndrome indicates that cardiolipin is recognized by the immune system [60]. The impetus for these anti-cardiolipin antibodies and whether the antibodies are generated against mitochondrial or bacterial cardiolipin are not known. Additional evidence of the ability of the immune system to present and respond to cardiolipin is mounting. CD1 molecules bind and present lipid antigens to immune effector cells, including T cells. Human and murine CD1d molecules can bind cardiolipin [61, 62] and the latter are capable of stimulating cardiolipin-reactive, CD1d-restricted γδ T cells normally present in healthy mice [62]. The presentation of mitochondrial cardiolipin by CD1d indicates that this mitochondrial lipid gains access to the secretory pathway or alternatively, perhaps CD1d molecules survey autophagosomes that have engulfed mitochondria. Bacterial-derived cardiolipin could gain access to CD1d molecules either directly on the cell surface, following engulfment of bacteria into phagosomes, or perhaps by the action of the recently described cardiolipin pump, ATP8B1 [63]. One common theme in immune responses is threshold. Perhaps mitochondrial cardiolipin is normally presented by CD1d in quantities below the threshold for initiating an immune response. In fact, another consequence of the action of MitoPLD, a phospholipase D molecule located on the mitochondrial OM that cleaves cardiolipin to phosphatidic acid [64], may be to ensure that only low levels of cardiolipin exit mitochondria under physiologic conditions. Thus, it is tempting to speculate that cardiolipin, which promotes efficient production of mitochondrial energy, may be kept in mitochondria to hide it from the immune system and thereby prevent a potential autoimmune response.
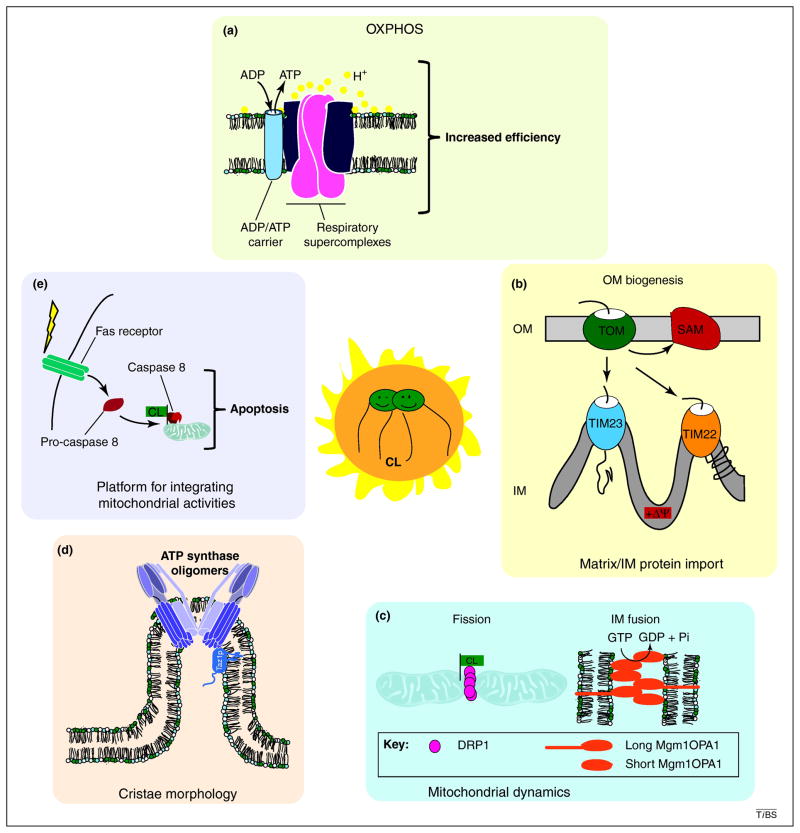
Figure 1. Cardiolipin, the center of mitochondrial physiology.
The importance of cardiolipin (CL) to normal mitochondrial function is now appreciated to be multifaceted [2–4, 8]. (i) Although not absolutely required, cardiolipin significantly improves the efficiency and adaptability of the OXPHOS machinery by at least 2 distinct mechanisms. First, cardiolipin stabilizes higher order assemblies of respiratory complexes, called supercomplexes, and is required for the association of the major ADP/ATP carrier with respiratory supercomplexes [2, 7, 8]. This is predicted to increase both the efficiency of electron flow and ADP/ATP exchange. Second, cardiolipin functions as a proton trap that restricts the flow of protons [75]. (ii) One consequence of reduced functionality of the electron transport chain in the absence of cardiolipin is the generation of a weaker electrochemical gradient (ΔΨ). This negatively impacts the biogenesis of proteins destined for the mitochondrial matrix and/or IM via translocases in the IM (TIM22 and TIM23) [2]. Interestingly, cardiolipin is also required for the biogenesis of proteins destined for the outer membrane (OM; import of OM proteins is membrane potential-independent) presumably by stabilizing the OM translocases, TOM and SAM (sorting and assembly machinery) [1]. (iii) Mitochondria are highly dynamic organelles with their shape and cellular numbers dictated by balanced fission and fusion events. IM fusion, performed by dynamin-related GTPases, Mgm1p in yeast and OPA1 in mammals, requires a balance of long and short isoforms. Cardiolipin restricts the short isoforms to cardiolipin-enriched membranes and promotes sMgm1p GTPase activity, which is required for IM fusion [76, 77]. Mitochondrial division is mediated by the dynamin-related GTPase, DRP1 (Dnm1p in yeast). Overexpression of a DRP1 variant defective in cardiolipin binding alters mitochondrial morphology consistent with it functioning as a dominant-negative allele [78]. (iv) The importance of cardiolipin and perhaps mature cardiolipin to cristae morphology is highlighted by the repeated observation in multiple cardiolipin-deficient models of mitochondria with abnormal IM ultrastructure [7, 49, 52–56]. (v) The presence of cardiolipin in membranes identifies those membranes as being mitochondrial. Thus, not surprisingly, the ability to recognize and/or bind cardiolipin-containing membranes is one method through which diverse cellular activities impinge at the level of the mitochondrion. For instance, cardiolipin promotes apoptosis by serving as a recruitment platform for caspase 8 downstream of Fas receptor signaling [79].
Cardiolipin is a lipid dimer consisting of two phosphatidyl groups bridged by a glycerol. Thus, cardiolipin has four attached acyl chains, distinguishing it from typical phospholipids which have two acyl chains. Besides cardiolipin synthase, which displays limited acyl chain selectivity [9], none of the other cardiolipin biosynthetic enzymes has any acyl chain specificity. As such, the final acyl chain composition of cardiolipin is not generated during its biosynthesis but is instead established through deacylation-reacylation/transacylation reactions [10]. Collectively, these remodeling mechanisms generate a final molecular form of cardiolipin that is homogenous with respect to its acyl chain composition, displays symmetry across the two chiral centers of cardiolipin, and is cell-type and tissue specific [10–13]. That is, the final molecular form of cardiolipin is not the same in different organisms or even in different tissues within the same organism, suggesting that the different molecular forms of cardiolipin observed in different cells and tissues are tailored to match the functional requirements and/or energetic demands of that cell/tissue [11, 14]. A major unresolved question is whether cardiolipin molecules with different acyl chain compositions differ functionally in any manner. Alterations in the normal biosynthesis and remodeling of cardiolipin have recently been associated with numerous pathological situations including the first inborn error of cardiolipin metabolism, Barth syndrome (BTHS) [10, 12]. Although all of the players have probably not been identified, proteins responsible for each step in the cardiolipin biosynthetic pathway as well as three potential cardiolipin remodeling pathways are known. The purpose of this review is to discuss the recently completed inventory of cardiolipin biosynthetic and remodeling machinery and detail how derangements in cardiolipin metabolism, in particular cardiolipin remodeling, contribute to human disease.
Recent additions to the pathway
Cardiolipin biosynthesis is proprietary to the mitochondrion. Recently, the enzymes responsible for a key step in the cardiolipin biosynthetic pathway, the dephosphorylation of phosphatidylglycerolphosphate, and the initiation of the cardiolipin remodeling cascade were molecularly identified.
Phosphatidylglycerolphosphate phosphatase
With the identification of Gep4p (genetic interaction with prohibitins) and PTPMT1 (PTP localized to the Mitochondrion 1) as the phosphatidylglycerolphosphate phosphatases [15, 16], each step in the cardiolipin biosynthetic pathway has now been assigned to a specific enzyme (Fig. 2). The ultimate identification of Gep4p and PTPMT1 as phosphatidylglycerolphosphate phosphatases relied on drastically different strategies. Gep4p was originally identified in a screen in yeast for genetic interactors of prohibitins (Box 2) [17]; subsequent to this screen, Gep4p was determined to function directly in the cardiolipin biosynthetic pathway as the phosphatidylglycerolphosphate phosphatase [15]. A gep4 null (Δgep4) strain contains low, but detectable, levels of cardiolipin and phosphatidylglycerol, accumulates phosphatidylglycerolphosphate, has destabilized respiratory supercomplexes, and is unable to grow on carbon sources that require a functional OXPHOS system. Further, recombinant Gep4p dephosphorylates phosphatidylglycerolphosphate to phosphatidylglycerol in vitro and this activity depends on an intact phosphatase motif.
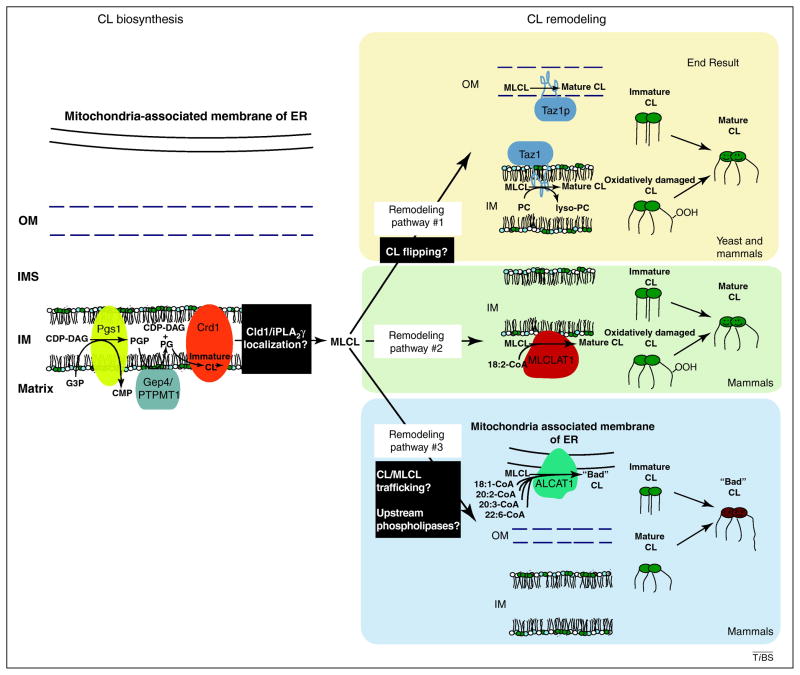
Figure 2. Overview of cardiolipin biosynthesis and remodeling.
The biosynthesis of cardiolipin (CL) occurs in the mitochondrion. Phosphatidylglycerolphosphate synthase (Pgs1p) catalyzes the first and committed step in cardiolipin biosynthesis producing the short-lived phosphatidylglycerolphosphate (PGP) from the condensation of cytidine 5′-diphosphate-diacylglycerol (CDP-DAG) and glycerol-3-phosphate (G3P). PGP is dephosphorylated to phosphatidylglycerol (PG) by a phosphatase, identified recently in yeast as Gep4p and even more recently in mammals as the phylogenetically unrelated PTPMT1 [15, 16]. When cardiolipin synthase, Crd1p, forms CL from PG and another molecule of CDP-DAG, the result is immature cardiolipin characterized by a random assortment of attached acyl chains that are saturated and variable in length. Acyl chain remodeling is responsible for the final molecular composition of mature cardiolipin which is typically defined by the symmetric incorporation of unsaturated longer fatty acyl chains [10–12]. The remodeling process is initiated by a phospholipase, in yeast the cardiolipin deacylase Cld1p [21] and in mammals the calcium-independent iPLA2γ [24–26], which remove an acyl chain from cardiolipin generating the remodeling intermediate, monolysocardiolipin (MLCL). The regeneration of cardiolipin from monolysocardiolipin is accomplished by up to three distinct proteins, tafazzin (Taz1p), monolysocardiolipin acyltransferase 1 (MLCLAT1), and acyl-CoA:lysocardiolipin acyltransferase-1 (ALCAT1). Whereas MLCLAT1 and ALCAT1 utilize acyl-CoA as the acyl chain donor for the reacylation of MLCL [33, 38, 39], Taz1p is a transacylase that takes an acyl chain from another phospholipid, preferentially phosphatidylcholine (PC) or phosphatidylethanolamine, and adds it to monolysocardiolipin [28]. Also notable is that the acyl chain specificity of these three enzymes is not the same. Tafazzin lacks acyl chain specificity [29], MLCLAT1 prefers 18:2 linoleoyl-CoA [33, 34], and ALCAT1 utilizes acyl-CoA loaded with diverse long-chain, unsaturated acyl chains [38, 39]. Black boxes highlight currently undefined processes/information. Amazingly, each cardiolipin remodeling enzyme resides in a distinct subcellular compartment suggestive of distinct functional outcomes. Whereas Taz1p and MLCLAT1 remodeling generates, and perhaps maintains, the tissue-specific, homogenous mature cardiolipin, ALCAT1 remodeling produces “bad” cardiolipin that is associated with pathologic processes.
BOX 2. New players, functions needed.
Several proteins that impact the accumulation/abundance of cardiolipin in mitochondria have recently been identified in yeast, none of which directly participate in cardiolipin biosynthesis or remodeling. Many were identified by virtue of the mitochondrion’s need for cardiolipin or phosphatidylethanolamine [65], another structural phospholipid. Structural lipids are characterized by a mismatch in the size of their headgroups relative to their attached acyl chains. Cone-shaped phospholipids, including cardiolipin and phosphatidylethanolamine, have small heads and big tails whereas inverted cone-shaped lipids have proportionately big heads. Phosphatidylethanolamine is made by several distinct pathways, one of which is catalyzed by a mitochondrial IM resident, phosphatidylserine decarboxylase 1 [3]. In yeast, only the mitochondrial pathway of phosphatidylethanolamine biosynthesis is synthetically lethal with cardiolipin synthase [65]. Interestingly, whereas the prohibitin 1 deletion strain was the host that identified many novel genes that impact the accumulation of cardiolipin and/or phosphatidylethanolamine, the absence of prohibitin 1 by itself does not alter the mitochondrial phospholipid profile [17]. Prohibitins are evolutionarily conserved proteins that are integral to IM, form large multimeric ring-shaped complexes, and appear to serve a scaffolding function for a range of protein complexes that in sum total are important for the proper organization, integrity, and composition of mitochondrial membranes [66]. Besides Gep4p, additional proteins identified that modulate mitochondrial cardiolipin levels include Ups1p [17, 67], a soluble IMS protein that assembles with Mdm35p [68, 69], Mdm32p, an IM protein that is required for the maintenance of mitochondrial nucleoids and mitochondrial distribution and morphology [70], and Mdm34p, an integral OM protein that together with additional proteins, mediates the association of mitochondrial and ER membranes [71]. Such ER–mitochondria contact sites are important for cellular phospholipid metabolism, notably trafficking of precursors and products between these two major phospholipid synthesizing organelles, as well as cellular calcium homeostasis [3, 71]. In addition, the abundance of cardiolipin is somehow influenced by Fmp30p, an integral IM resident [72]. Finally, Tam41p plays a critical role in cardiolipin biosynthesis at a very early step in the pathway [73]. How these proteins specifically regulate cardiolipin biosynthesis, degradation, precursor accumulation, and/or trafficking is actively being studied and will significantly contribute to our understanding of cardiolipin metabolism in the near future.
Until recently, the molecular function and preferred substrate(s) of PTPMT1, a member of the PTEN (phosphatase and tensin homolog) family that resides in mitochondria [18], were a mystery. Ptpmt1−/− mice die in utero prior to embryonic day 8.5 [16]. Therefore, the activity and/or substrate of PTPMT1 are required for life. Ptpmt1−/− mouse embryonic fibroblasts (MEFs) grow slowly, display OXPHOS defects, exhibit significantly reduced complex I levels, and contain fewer mitochondria that are bigger and have altered IM (cristae) morphology [16]. Cardiolipin and phosphatidylglycerol levels are reduced, albeit not totally absent, relative to control MEFs, and phosphatidylglycerolphosphate accumulates. Recombinant PTPMT1 dephosphorylates phosphatidylglycerolphosphate in vitro in a reaction that, based on a comparison of the structures of the substrate-bound and unbound phosphatase domain of PTPMT1, involves a large conformational change [19].
Gep4p is only found in fungi and plants whereas PTPMT1 is widely evolutionarily conserved. Yet, these two unrelated proteins are both anchored to the matrix-side of the IM and catalyze the same reaction [15, 16, 18]. The ability of PTPMT1 to rescue a Δgep4 strain underscores their functional homology [16]. The localization of both proteins to the matrix face of the IM is notable because cardiolipin synthase is known to synthesize cardiolipin in the context of this IM leaflet [20] even though cardiolipin is present on both sides of the IM and, to a lesser extent, the outer membrane (OM) [1].
Cardiolipin phospholipases
Cardiolipin remodeling is initiated by the removal of a single acyl chain resulting in monolysocardiolipin. In yeast, cardiolipin remodeling is completed by tafazzin (Taz1p), a transacylase that takes an acyl chain from another phospholipid and attaches it to monolysocardiolipin thus regenerating intact cardiolipin. Cld1p (cardiolipin deacylase 1) functions upstream of Taz1p in the remodeling of cardiolipin [21]. Whereas Δtaz1 yeast has reduced cardiolipin and elevated monolysocardiolipin, Δcld1Δtaz1 yeast lack detectable monolysocardiolipin and accumulate normal levels of cardiolipin [21]. Furthermore, the cardiolipin that accumulates in Δcld1 mitochondria is enriched in palmitic acid (C16:0) instead of palmitoleic acid (C16:1) and oleic acid (C18:1) found in wild type yeast cardiolipin. However, two observations suggest that Cld1p may participate in additional metabolic pathways. First, the growth phenotype of the Δcld1Δtaz1 strain is more severe than either single mutant. Second, Cld1p can hydrolyze phospholipids in addition to cardiolipin.
In Drosophila melanogaster and mammals, calcium-independent phospholipase A2 enzymes are at least partially responsible for the initiation of cardiolipin remodeling. In flies, deletion of iPla2-Via partially restores the monolysocardiolipin:cardiolipin ratio (alterations in this ratio are a biochemical hallmark of the absence of Taz1p activity [10, 22]) and rescues the male sterility of TAZ−/− flies [23]. In mice, a direct role in cardiolipin remodeling has only been provided for the calcium-independent phospholipase, iPLA2γ [24–26]. Whole body deletion of Ipla2 (gamma), the gene encoding iPLA2γ, is not, as in the case of Ptpmt1−/−[16], embryonic lethal [24–26]. Therefore, the absence of cardiolipin remodeling per se is not as detrimental as the complete loss of cardiolipin. However, there are consequences associated with deletion of Ipla2 (gamma). Specifically, Ipla2 (gamma)−/− mice display several bioenergetic alterations including a reduced tolerance to cold and exercise, conditions that demand mitochondrial activity [25]. In the myocardium of Ipla2 (gamma)−/− mice, there is a specific reduction in cardiolipin content and change in molecular forms of cardiolipin; other lipids are unaffected [25]. These observations strongly implicate iPLA2γ as participating in cardiolipin remodeling, presumably by deacylating cardiolipin to monolysocardiolipin, although this needs to be formally demonstrated. Interestingly, the absence of iPLA2γ in the brain, while resulting in an altered distribution of cardiolipin molecular species, increases the absolute levels of cardiolipin [24]. The presence of some cardiolipin with a normal mature acyl chain composition in the knockout mice indicates that phospholipases in addition to iPLA2γ also participate in cardiolipin remodeling. Where Cld1p and iPLA2γ reside within mitochondria has not been demonstrated. As such, it is presently not possible to properly integrate their respective activities with the downstream reacylation of monolysocardiolipin that can be mediated by up to three different enzymes each with a distinct subcellular residence.
The monolysocardiolipin remodelers
At least three distinct enzymes have the capacity to attach an acyl chain to monolysocardiolipin: tafazzin, monolysocardiolipin acyltransferase 1 (MLCLAT1), and acyl-CoA:lysocardiolipin acyltransferase-1 (ALCAT1). Having the ability to attach an acyl chain onto monolysocardiolipin does not necessitate a role in cardiolipin biogenesis. Although most attention to date has been given to the role of acyl chain remodeling in the biogenesis of mature cardiolipin, the process of cardiolipin remodeling may additionally be harnessed to fix oxidatively damaged cardiolipin and/or to change the molecular form of existing cardiolipin to modulate mitochondrial function, processes about which significantly less is known. Clearly, the best evidence for a direct role in the generation of mature molecular forms of cardiolipin exists for tafazzin. Because much less is known about the other two remodeling enzymes, the exact role of MLCLAT1 and ALCAT1 in cardiolipin metabolism is not firmly established although some insights have recently been garnered for both.
Tafazzin
Yeast tafazzin, Taz1p, is an integral interfacial membrane protein that associates with both the IM and OM always facing the intermembrane space (IMS) [27]. Due to the nature of its membrane association (membrane anchor inserts into but not through the bilayer), Taz1p-mediated cardiolipin remodeling is predicted to occur in the context of leaflets exposed to the IMS [4, 27]. Topologically, this implies that newly synthesized cardiolipin, produced in the matrix leaflet of IM [20], must flip to the opposite side. How this occurs is not known. Tafazzin is a transacylase [28]. Transacylases utilize phospholipids as acyl chain donors and are bidirectional. Presumably, directionality is enforced by acyltransferases that restore the generated acyl donor lyso-phosphatidylcholine to phosphatidylcholine, thus removing the possible substrate for the reverse reaction. Importantly, tafazzin is capable of remodeling all four acyl chain positions in cardiolipin through a series of forward and reverse transacylations [29]. Interestingly, this process does not require the active participation of a phospholipase, but does necessitate that a phospholipase generates a lysolipid (monolysocardiolipin or lyso-phosphatidylcholine) as the input material. In the absence of acyl chain specificity and with the ability to modify assorted phospholipids, how then does tafazzin-mediated remodeling contribute to the establishment of the mature molecular form of cardiolipin? One possibility is that differences in the free energy of different molecular forms of cardiolipin together with the acyl chain composition of available donor lipids results in the generation of a fairly homogenous cardiolipin pool even in the absence of tafazzin acyl chain specificity [30]. This could explain why expression of human TAZ1 in a Δtaz1 yeast strain results in production of mature yeast cardiolipin and not mature human cardiolipin [31]. Additionally, the specificity of phospholipases may contribute to the final form of cardiolipin by preferentially initiating remodeling of newly synthesized or partially remodeled cardiolipin [21, 32].
MLCLAT1
The protein responsible for the monolysocardiolipin acyltransferase activity originally identified in pig liver mitochondria [33] was recently identified using a clever biochemical strategy involving a final affinity purification employing a monolysocardiolipin-adriamycin column [34]. The recovered peptides matched the α subunit of the porcine trifunctional protein (TFP). TFP is a stoichiometric heterodimer of α and β subunits that catalyzes three steps in the β-oxidation of long-chain fatty acids in the mitochondrial matrix. Homology searches revealed a hit for a human protein that is identical to the C-terminal 59 kDa (537 amino acids) of human TFPα. Curiously, in spite of this absolute conservation with TFPα, the absence of an initiator methionine in the previously unidentified human protein (accession number AAX93141), and the fact that both genes are encoded on chromosome 2, it was concluded that the new 59 kDa protein was distinct from TFPα and designated as MLCLAT1. This conclusion needs to be revisited. TFPα is a bonafide mitochondrial protein [35] that can bind cardiolipin [36], associates with the matrix-facing IM leaflet, and is thus positioned to mediate cardiolipin remodeling without any additional trafficking steps. It also contains a cleavable presequence whose removal generates a predicted 79 kDa protein that is similar in size to that detected in pig liver mitochondria using a MLCLAT1 antisera [34]. Further, in silico analyses predict a cytoplasmic localization of the 59 kDa MLCLAT1. In contrast to TFPβ, recombinant TFPα is soluble when expressed alone suggesting that it may function independent of its association with TFPβ [36]. Finally, mitochondria contain numerous proteases that can generate multiple isoforms from a single polypeptide with distinct functional capacities. Thus, whether MLCLAT1 and TFPα are the products of the same or distinct genes is an open question. This is an important issue as mutations in TP-ALPHA (gene that encodes TFPα) are associated with β-oxidation defects in humans [37]. Interestingly, patients with TP-ALPHA mutations suffer from cardiomyopathy and skeletal myopathy, phenotypes that are also observed in BTHS patients who lack normal cardiolipin remodeling due to mutations in TAZ1 [12].
Regardless of the aforementioned details, the involvement of MLCLAT1 in cardiolipin remodeling is substantiated by all available evidence [33, 34]. Of the three cardiolipin remodeling enzymes, MLCLAT1 alone has the combined acyl chain and lyso-lipid specificity to participate only in cardiolipin metabolism and potentially explain the mature molecular form of cardiolipin in many tissues. Moreover, MLCLAT1 can physically associate with monolysocardiolipin and possibly cardiolipin [34, 36]. Knockdown of MLCLAT1 in HeLa cells decreases the rate of incorporation of linoleate (18:2) into cardiolipin [34]. Finally, overexpression of the 59 kDa MLCLAT1 in TAZ1-deficient lymphoblasts increases incorporation of linoleic acid into cardiolipin as well as total cardiolipin mass. Thus, MLCLAT1 has the capacity to participate in cardiolipin remodeling but future studies are needed to define its relative contribution to this process.
ALCAT1
From its first description, the role of ALCAT1 in cardiolipin remodeling was dubious due to its localization in the mitochondrial associated membrane (MAM), a specialized compartment of the ER [38–40]. Biochemically, ALCAT1 reacylates lyso-lipids, in addition to monolysocardiolipin, and preferentially incorporates CoA loaded with long-chain, unsaturated fatty acyl chains [38, 39]. Recent work has identified an unanticipated role for ALCAT1 in cardiolipin metabolism [40]. Overexpression of ALCAT1 in a murine myoblast cell line reduces the total cardiolipin pool, decreases the amount of mature cardiolipin, and increases the proportion of cardiolipin containing long-chain polyunsaturated fatty acids, including docosohexaenoic acid, commonly associated with mitochondrial dysfunction [5]. ALCAT1 overexpression increases the rate of mitochondrial ATP production and exacerbates the production of reactive oxygen species (ROS) during oxidative stress. Curiously, overexpression of ALCAT1 partially uncouples mitochondrial respiration due to proton leakage and yet hyperpolarizes the mitochondrial IM. Importantly, endogenous ALCAT1 expression and enzyme activity is increased in two mouse models of metabolic disease suggesting that ALCAT1 upregulation may drive the mitochondrial dysfunction associated with these pathologies. Consistent with this possibility, Alcat1−/− mice are protected from diet-induced obesity. Notably, the amount of mature cardiolipin (containing 18:2 linoleic acid) is increased, as is the total amount of cardiolipin, in hearts of Alcat1−/− mice. Interestingly, MLCLAT1 expression is upregulated in Alcat1−/− mice and downregulated upon ALCAT1 overexpression. Thus, these data suggest that in contrast to the cardiolipin remodeling by TAZ1 and perhaps MLCLAT1, which establish and potentially maintain the acyl chain composition of mature cardiolipin, remodeling of monolysocardiolipin by ALCAT1 signals mitochondrial dysfunction through the generation of “bad” cardiolipin [40]. Although the upstream phospholipase(s) required for ALCAT1 remodeling has not been determined, a role for iPLA2γ is suggested by the observation that Ipla2 (gamma)−/− mice are also protected from diet-induced obesity [26].
Consequences of altered cardiolipin metabolism
Given its importance for numerous mitochondrial and cellular activities, in addition to OXPHOS (Figure 1), it is not surprising that alterations in the abundance and molecular form of cardiolipin are associated with a plethora of pathological states [41], including aging (widely perceived of as a pathology) [42], ischemia and reperfusion [42], heart failure [5, 43], inherited and diabetic cardiomyopathy [12, 44], and cancer [45]. For instance, in a rat model of spontaneous heart failure, variable changes in monolysocardiolipin remodeling enzymes are observed [43]. Interestingly, Mlclat1 expression is increased, Taz1 is decreased, and Alcat1 is unaffected, suggesting that MLCLAT1 expression and activity may be a part of the physiologic response to counteract the acute diminution in mature cardiolipin that is observed in this heart failure model [5]. Due to its high content of unsaturated acyl chains, cardiolipin is known to be susceptible to oxidative attack. Indeed, cardiolipin is oxidatively damaged following an ischemic insult in hearts from old but not adult rats an observation that may explain the decreased bioenergetic capacity observed in older rats [42]. Additionally, the copper overload that occurs in Atp7b−/− mice, a model of Wilson’s disease, a recessive disorder caused by mutations in ATP7B and characterized by excessive tissue copper accumulation, may induce mitochondrial damage subsequent to copper-generated free radical-mediated fragmentation of cardiolipin [46]. Moreover, the acyl chain composition of cardiolipin is altered and there is an increase in the abundance of immature species of cardiolipin in brain tumors that also have impaired OXPHOS activity and efficiency [45], parameters that are influenced by respiratory supercomplexes whose stability is dependent on cardiolipin [2–4, 6, 7]. These findings suggest that the impaired energy metabolism observed in cancer, first described by Otto Warburg, may in part be driven by changes in the metabolism of cardiolipin [45]. Consistent with the notion that deficits in cardiolipin can significantly alter cellular metabolism is the increase in glycolysis that occurs very quickly following deletion of Ptpmt1 [16].
Barth syndrome is an X-linked disease caused by mutations in the TAZ1 gene that encodes the monolysocardiolipin transacylase, TAZ1 [2, 12]. BTHS patients display an acute diminution in tetra-linoleoyl (18:2) cardiolipin that is typically the major molecular species of cardiolipin in the human heart [47]. Patients suffer from cardiac and skeletal myopathies and cyclic neutropenia and major causes of mortality are heart failure and opportunistic infection. Numerous nonsense, frameshift, and splicing mutations have been detected in the patient population, as well as 36 missense mutations (Box 3). In the absence of tafazzin activity there is a decrease in the absolute abundance of cardiolipin, the acyl chain composition of the remaining cardiolipin pool is heterogenous, and monolysocardiolipin, the remodeling intermediate, accumulates [2, 10, 12]. The relative contribution of each of these individual changes in the mitochondrial phospholipid profile to mitochondrial dysfunction and resultant pathophysiology is unresolved. The suppression of many of the phenotypes of TAZ−/− flies by the additional inactivation of the upstream phospholipase, iPla2-Via [23] indicates that the absolute levels of cardiolipin, and not necessarily the final acyl chain composition, are important. By contrast, the severe growth defect of Δcld1Δtaz1 yeast compared to wild type yeast supports the opposite conclusion, that remodeled cardiolipin is better than unremodeled cardiolipin with respect to mitochondrial function [21]. Clearly, this remains an unresolved issue.
BOX 3. Loss-of-function mechanisms of BTHS mutant tafazzins.
Although not formally demonstrated, mutations that impact splicing or introduce a frameshift or stop codon are anticipated to produce aberrant, truncated forms of TAZ. Because Taz1p does not function as an obligate homodimer [49], such products should be null alleles and not function as dominant-negatives. To date, 36 missense mutations have been identified in the BTHS patient population occurring at 26 unique loci. Although there are a few mutation hotspots, including those that affect Arg94 and Gly197, most BTHS mutations are rare events that are distributed throughout the gene and therefore provide little in the way of clues as to the basis for their presumed loss-of-function. Additionally, correlations between particular mutations and the severity of disease are not apparent. Molecular studies in mammalian model systems are presently hamstrung by the lack of appropriate reagents, most notably antibodies. Still, progress on this front has been made utilizing a yeast compendium of BTHS mutations. Many basic processes, including cardiolipin biosynthetic and remodeling pathways are conserved from yeast to humans. Support for this statement is provided by the observation that 20 of the 23 missense mutations that have been individually modeled in the yeast tafazzin ortholog fail to complement a Δtaz1 yeast strain [74]. Of the 8 mutants characterized in detail, three distinct loss-of-function mechanisms have been defined: submitochondrial mislocalization, altered assembly, and complex lability [27, 74]. These findings are interesting for several reasons. First, they emphasize the importance of the correct compartmentalization of Taz1p for cardiolipin remodeling. Second, they imply that the ability to engage in a normal range of interactions is critical for Taz1p activity. And third, they reveal a direct link between the stability of Taz1p quaternary structure and Taz1p-mediated cardiolipin remodeling. Characterization of the remaining yeast BTHS panel should reveal additional loss-of-function mechanisms that could identify targets that can be clinically exploited to the benefit of BTHS patients.
Although mRNA analyses suggest that multiple splice variants of human TAZ1 are expressed [10], how these findings translate to protein expression is presently not known. Upon overexpression, all analyzed mammalian isoforms localize to mitochondria; however, there may be differences in their submitochondrial distribution [48]. In yeast, Taz1p assembles in a range of complexes, two of which involve separate Taz1p–ATP synthase and Taz1p–ADP/ATP carrier interactions [27, 49]. Both Taz1p binding partners are required for OXPHOS and notably, variable respiratory defects are observed in BTHS patient biopsies [12]. These defects could reflect deficits in the function of TAZ1–binding partners in the absence of tafazzin or instead be due to the destabilization of respiratory supercomplexes secondary to overall cardiolipin depletion. Destabilization of respiratory supercomplexes has been detected in BTHS lymphocytes [50] and in some [51], but not all [6], Δtaz1 yeast strains.
Tafazzin deficiency is associated with the accumulation of mitochondria with abnormal ultrastructure [49, 52–56]. Membrane bending is hypothesized to involve three potential players: protein scaffolds, structural phospholipids, and lipid-modifying enzymes [57]. Oligomers of the ATP synthase are the scaffolds for IM bending and are necessary [58], but not sufficient [49], for normal cristae ultrastructure. In addition to attached headgroups, another determinant in the overall structure of a lipid is the degree of incorporation of unsaturated fatty acyl chains: unsaturated fatty acids occupy more space than saturated fatty acids. Thus, immature cardiolipin is predicted to have less structural capacity than mature cardiolipin. Finally, as a transacylase, tafazzin has the capacity to alter the local abundance of multiple classes of structural phospholipid [28]. Therefore, the abnormal cristae morphology in the absence of tafazzin could be caused by changes in the abundance and/or molecular form of cardiolipin, the absence of the tafazzin–ATP synthase interaction, and/or the lack of transacylase activity physically attached to the ATP synthase. However, abnormal cristae morphology is not driven simply by reduced cardiolipin levels as altered IM ultrastructure is only detected upon differentiation of Taz−/− embryonic stem cells into cardiomyocytes even though cardiolipin levels are equally impacted in both cell types [52]. Interestingly, Δtaz1 yeast accumulate oxidatively damaged proteins, which indicates an overall increase in ROS, when grown in media requiring mitochondrially-derived ATP [59]. Together these observations suggest that in the absence of Taz1p-mediated remodeling, conditions that require active OXPHOS result in an increased production of ROS, perhaps secondary to alterations in the assembly and efficiency of the respiratory chain, some of which may directly modify cardiolipin. Whether oxidatively damaged cardiolipin drives or is the consequence of alterations in cristae morphology remains to be determined. Changes in the biogenesis of both IM and OM proteins could additionally contribute to ultrastructural abnormalities [1, 2].
A major recent advance was the development of a mouse model of BTHS [54, 56]. This newly generated model utilizes a doxycycline-inducible shRNA to knockdown endogenous TAZ. Tafazzin knockdown reduces the amount of mature cardiolipin, alters the acyl chain composition of remaining cardiolipin, results in accumulation of monolysocardiolipin, and is associated with the appearance of ultrastructurally abnormal mitochondria. Critically, both cardiac and skeletal muscle functions are reduced, consistent with BTHS pathology. Thus, whereas the exact molecular mechanisms responsible for these phenotypic changes have yet to be established, the BTHS mouse model should prove invaluable in acquiring this basic information. BTHS patients additionally suffer from cyclic neutropenia. Unfortunately, it does not appear as if the BTHS mouse model will be suitable to study this extremely intriguing aspect of BTHS pathogenesis [56].
Concluding remarks
Our knowledge of how cardiolipin metabolism is integrated into cellular physiology and how deficits or changes in its metabolism contribute to disease pathogenesis remains rudimentary. With the recent acquisition of a complete biosynthetic and remodeling inventory, future studies can address the regulation of cardiolipin metabolism in and contribution to health and disease. Multiple fundamental questions remain with many exciting chapters to be developed (Box 4). With a comprehensive list of parts in place and the recent establishment of many model systems, including mouse models, forthcoming answers to these and additional questions will undoubtedly further highlight the importance of this signature phospholipid of the mitochondrion.
Box 4. OUTSTANDING QUESTIONS.
How do mitochondria in distinct tissues establish a homogenous pool of cardiolipin that differs with respect to acyl chain composition?
Do different molecular forms of cardiolipin have distinct functional capacities?
Does the process of cardiolipin remodeling repair damaged cardiolipin molecules?
How does cardiolipin and/or monolysocardiolipin gain access to the distinct subcellular compartments in which the monolysocardiolipin remodeling enzymes reside?
Do the same phospholipases provide monolysocardiolipin for all three known remodeling enzymes, or are additional phospholipases also involved?
Do the different remodeling enzymes work on the same or different pools of cardiolipin?
What is the relative importance of each monolysocardiolipin remodeling enzyme for cardiolipin remodeling and mitochondrial function?
What is the physiological consequence of the pathogenic remodeling catalyzed by ALCAT1?
How does cardiolipin metabolism contribute to the mitochondrial dysfunction observed in numerous disease states including BTHS?
Acknowledgments
This work was supported by the American Heart Association 0640076N, CIRM grants RS1-00313 and RB1-01397, and National Institutes of Health Grant R00HL089185 (SMC) and 1R01GM61721 and 1R01 GM073981 (CMK).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Gebert N, et al. Mitochondrial cardiolipin involved in outer-membrane protein biogenesis: implications for Barth syndrome. Curr Biol. 2009;19:2133–2139. doi: 10.1016/j.cub.2009.10.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Joshi AS, et al. Cellular functions of cardiolipin in yeast. Biochim Biophys Acta. 2009;1793:212–218. doi: 10.1016/j.bbamcr.2008.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Osman C, et al. Making heads or tails of phospholipids in mitochondria. J Cell Biol. 2011;192:7–16. doi: 10.1083/jcb.201006159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schlame M, Ren M. The role of cardiolipin in the structural organization of mitochondrial membranes. Biochim Biophys Acta. 2009;1788:2080–2083. doi: 10.1016/j.bbamem.2009.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sparagna GC, Lesnefsky EJ. Cardiolipin remodeling in the heart. J Cardiovasc Pharmacol. 2009;53:290–301. doi: 10.1097/FJC.0b013e31819b5461. [DOI] [PubMed] [Google Scholar]
- 6.Claypool SM, et al. Cardiolipin defines the interactome of the major ADP/ATP carrier protein of the mitochondrial inner membrane. J Cell Biol. 2008;182:937–950. doi: 10.1083/jcb.200801152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mileykovskaya E, Dowhan W. Cardiolipin membrane domains in prokaryotes and eukaryotes. Biochim Biophys Acta. 2009;1788:2084–2091. doi: 10.1016/j.bbamem.2009.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Claypool SM. Cardiolipin, a critical determinant of mitochondrial carrier protein assembly and function. Biochim Biophys Acta. 2009;1788:2059–2068. doi: 10.1016/j.bbamem.2009.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Houtkooper RH, et al. Identification and characterization of human cardiolipin synthase. FEBS Lett. 2006;580:3059–3064. doi: 10.1016/j.febslet.2006.04.054. [DOI] [PubMed] [Google Scholar]
- 10.Houtkooper RH, et al. The enigmatic role of tafazzin in cardiolipin metabolism. Biochim Biophys Acta. 2009;1788:2003–2014. doi: 10.1016/j.bbamem.2009.07.009. [DOI] [PubMed] [Google Scholar]
- 11.Schlame M. Cardiolipin synthesis for the assembly of bacterial and mitochondrial membranes. J Lipid Res. 2008;49:1607–1620. doi: 10.1194/jlr.R700018-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schlame M, Ren M. Barth syndrome, a human disorder of cardiolipin metabolism. FEBS Lett. 2006;580:5450–5455. doi: 10.1016/j.febslet.2006.07.022. [DOI] [PubMed] [Google Scholar]
- 13.Schlame M, et al. Molecular symmetry in mitochondrial cardiolipins. Chem Phys Lipids. 2005;138:38–49. doi: 10.1016/j.chemphyslip.2005.08.002. [DOI] [PubMed] [Google Scholar]
- 14.Cheng H, et al. Shotgun lipidomics reveals the temporally dependent, highly diversified cardiolipin profile in the mammalian brain: temporally coordinated postnatal diversification of cardiolipin molecular species with neuronal remodeling. Biochemistry. 2008;47:5869–5880. doi: 10.1021/bi7023282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Osman C, et al. A mitochondrial phosphatase required for cardiolipin biosynthesis: the PGP phosphatase Gep4. EMBO J. 2010;29:1976–1987. doi: 10.1038/emboj.2010.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang J, et al. Mitochondrial Phosphatase PTPMT1 Is Essential for Cardiolipin Biosynthesis. Cell Metab. 2011;13:690–700. doi: 10.1016/j.cmet.2011.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Osman C, et al. The genetic interactome of prohibitins: coordinated control of cardiolipin and phosphatidylethanolamine by conserved regulators in mitochondria. J Cell Biol. 2009;184:583–596. doi: 10.1083/jcb.200810189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pagliarini DJ, et al. Involvement of a mitochondrial phosphatase in the regulation of ATP production and insulin secretion in pancreatic beta cells. Mol Cell. 2005;19:197–207. doi: 10.1016/j.molcel.2005.06.008. [DOI] [PubMed] [Google Scholar]
- 19.Xiao J, et al. Structural and functional analysis of PTPMT1, a phosphatase required for cardiolipin synthesis. Proc Natl Acad Sci U S A. 2011;108:11860–11865. doi: 10.1073/pnas.1109290108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schlame M, Haldar D. Cardiolipin is synthesized on the matrix side of the inner membrane in rat liver mitochondria. J Biol Chem. 1993;268:74–79. [PubMed] [Google Scholar]
- 21.Beranek A, et al. Identification of a cardiolipin-specific phospholipase encoded by the gene CLD1 (YGR110W) in yeast. J Biol Chem. 2009;284:11572–11578. doi: 10.1074/jbc.M805511200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Houtkooper RH, et al. Cardiolipin and monolysocardiolipin analysis in fibroblasts, lymphocytes, and tissues using high-performance liquid chromatography-mass spectrometry as a diagnostic test for Barth syndrome. Anal Biochem. 2009;387:230–237. doi: 10.1016/j.ab.2009.01.032. [DOI] [PubMed] [Google Scholar]
- 23.Malhotra A, et al. Role of calcium-independent phospholipase A2 in the pathogenesis of Barth syndrome. Proc Natl Acad Sci U S A. 2009;106:2337–2341. doi: 10.1073/pnas.0811224106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mancuso DJ, et al. Genetic ablation of calcium-independent phospholipase A2{gamma} leads to alterations in hippocampal cardiolipin content and molecular species distribution, mitochondrial degeneration, autophagy, and cognitive dysfunction. J Biol Chem. 2009;284:35632–35644. doi: 10.1074/jbc.M109.055194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mancuso DJ, et al. Genetic ablation of calcium-independent phospholipase A2gamma leads to alterations in mitochondrial lipid metabolism and function resulting in a deficient mitochondrial bioenergetic phenotype. J Biol Chem. 2007;282:34611–34622. doi: 10.1074/jbc.M707795200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mancuso DJ, et al. Genetic ablation of calcium-independent phospholipase A2gamma prevents obesity and insulin resistance during high fat feeding by mitochondrial uncoupling and increased adipocyte fatty acid oxidation. J Biol Chem. 2010;285:36495–36510. doi: 10.1074/jbc.M110.115766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Claypool SM, et al. Mitochondrial mislocalization and altered assembly of a cluster of Barth syndrome mutant tafazzins. J Cell Biol. 2006;174:379–390. doi: 10.1083/jcb.200605043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Xu Y, et al. The enzymatic function of tafazzin. J Biol Chem. 2006;281:39217–39224. doi: 10.1074/jbc.M606100200. [DOI] [PubMed] [Google Scholar]
- 29.Malhotra A, et al. Formation of molecular species of mitochondrial cardiolipin. 1. A novel transacylation mechanism to shuttle fatty acids between sn-1 and sn-2 positions of multiple phospholipid species. Biochim Biophys Acta. 2009;1791:314–320. doi: 10.1016/j.bbalip.2009.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schlame M. Formation of molecular species of mitochondrial cardiolipin 2. A mathematical model of pattern formation by phospholipid transacylation. Biochim Biophys Acta. 2009;1791:321–325. doi: 10.1016/j.bbalip.2009.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vaz FM, et al. Only one splice variant of the human TAZ gene encodes a functional protein with a role in cardiolipin metabolism. J Biol Chem. 2003;278:43089–43094. doi: 10.1074/jbc.M305956200. [DOI] [PubMed] [Google Scholar]
- 32.Rijken PJ, et al. Cardiolipin molecular species with shorter acyl chains accumulate in Saccharomyces cerevisiae mutants lacking the acyl coenzyme A-binding protein Acb1p: new insights into acyl chain remodeling of cardiolipin. J Biol Chem. 2009;284:27609–27619. doi: 10.1074/jbc.M109.016311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Taylor WA, Hatch GM. Purification and characterization of monolysocardiolipin acyltransferase from pig liver mitochondria. J Biol Chem. 2003;278:12716–12721. doi: 10.1074/jbc.M210329200. [DOI] [PubMed] [Google Scholar]
- 34.Taylor WA, Hatch GM. Identification of the human mitochondrial linoleoyl-coenzyme A monolysocardiolipin acyltransferase (MLCL AT-1) J Biol Chem. 2009;284:30360–30371. doi: 10.1074/jbc.M109.048322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Carpenter K, et al. Human liver long-chain 3-hydroxyacyl-coenzyme A dehydrogenase is a multifunctional membrane-bound beta-oxidation enzyme of mitochondria. Biochem Biophys Res Commun. 1992;183:443–448. doi: 10.1016/0006-291x(92)90501-b. [DOI] [PubMed] [Google Scholar]
- 36.Fould B, et al. Structural and functional characterization of the recombinant human mitochondrial trifunctional protein. Biochemistry. 2010;49:8608–8617. doi: 10.1021/bi100742w. [DOI] [PubMed] [Google Scholar]
- 37.Brackett JC, et al. Two alpha subunit donor splice site mutations cause human trifunctional protein deficiency. J Clin Invest. 1995;95:2076–2082. doi: 10.1172/JCI117894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cao J, et al. A novel cardiolipin-remodeling pathway revealed by a gene encoding an endoplasmic reticulum-associated acyl-CoA:lysocardiolipin acyltransferase (ALCAT1) in mouse. J Biol Chem. 2004;279:31727–31734. doi: 10.1074/jbc.M402930200. [DOI] [PubMed] [Google Scholar]
- 39.Cao J, et al. ALCAT1 is a polyglycerophospholipid acyltransferase potently regulated by adenine nucleotide and thyroid status. Am J Physiol Endocrinol Metab. 2009;296:E647–653. doi: 10.1152/ajpendo.90761.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Li J, et al. Cardiolipin remodeling by ALCAT1 links oxidative stress and mitochondrial dysfunction to obesity. Cell Metab. 2010;12:154–165. doi: 10.1016/j.cmet.2010.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chicco AJ, Sparagna GC. Role of cardiolipin alterations in mitochondrial dysfunction and disease. Am J Physiol Cell Physiol. 2007;292:C33–44. doi: 10.1152/ajpcell.00243.2006. [DOI] [PubMed] [Google Scholar]
- 42.Lesnefsky EJ, et al. Enhanced modification of cardiolipin during ischemia in the aged heart. J Mol Cell Cardiol. 2009;46:1008–1015. doi: 10.1016/j.yjmcc.2009.03.007. [DOI] [PubMed] [Google Scholar]
- 43.Saini-Chohan HK, et al. Cardiolipin biosynthesis and remodeling enzymes are altered during development of heart failure. J Lipid Res. 2009;50:1600–1608. doi: 10.1194/jlr.M800561-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Han X, et al. Alterations in myocardial cardiolipin content and composition occur at the very earliest stages of diabetes: a shotgun lipidomics study. Biochemistry. 2007;46:6417–6428. doi: 10.1021/bi7004015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kiebish MA, et al. Cardiolipin and electron transport chain abnormalities in mouse brain tumor mitochondria: lipidomic evidence supporting the Warburg theory of cancer. J Lipid Res. 2008;49:2545–2556. doi: 10.1194/jlr.M800319-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yurkova IL, et al. Fragmentation of mitochondrial cardiolipin by copper ions in the Atp7b(−/−) mouse model of Wilson’s disease. Chem Phys Lipids. 2011;164:393–400. doi: 10.1016/j.chemphyslip.2011.05.006. [DOI] [PubMed] [Google Scholar]
- 47.Valianpour F, et al. Monolysocardiolipins accumulate in Barth syndrome but do not lead to enhanced apoptosis. J Lipid Res. 2005;46:1182–1195. doi: 10.1194/jlr.M500056-JLR200. [DOI] [PubMed] [Google Scholar]
- 48.Xu Y, et al. Characterization of tafazzin splice variants from humans and fruit flies. J Biol Chem. 2009;284:29230–29239. doi: 10.1074/jbc.M109.016642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Claypool SM, et al. The cardiolipin transacylase, tafazzin, associates with two distinct respiratory components providing insight into Barth syndrome. Mol Biol Cell. 2008;19:5143–5155. doi: 10.1091/mbc.E08-09-0896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.McKenzie M, et al. Mitochondrial respiratory chain supercomplexes are destabilized in Barth Syndrome patients. J Mol Biol. 2006;361:462–469. doi: 10.1016/j.jmb.2006.06.057. [DOI] [PubMed] [Google Scholar]
- 51.Brandner K, et al. Taz1, an outer mitochondrial membrane protein, affects stability and assembly of inner membrane protein complexes: implications for Barth Syndrome. Mol Biol Cell. 2005;16:5202–5214. doi: 10.1091/mbc.E05-03-0256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Acehan D, et al. Distinct effects of tafazzin deletion in differentiated and undifferentiated mitochondria. Mitochondrion. 2009;9:86–95. doi: 10.1016/j.mito.2008.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Acehan D, et al. Cardiolipin affects the supramolecular organization of ATP synthase in mitochondria. Biophys J. 2011;100:2184–2192. doi: 10.1016/j.bpj.2011.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Acehan D, et al. Cardiac and skeletal muscle defects in a mouse model of human Barth syndrome. J Biol Chem. 2011;286:899–908. doi: 10.1074/jbc.M110.171439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Acehan D, et al. Comparison of lymphoblast mitochondria from normal subjects and patients with Barth syndrome using electron microscopic tomography. Lab Invest. 2007;87:40–48. doi: 10.1038/labinvest.3700480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Soustek MS, et al. Characterization of a Transgenic Short Hairpin RNA-Induced Murine Model of Tafazzin Deficiency. Hum Gene Ther. 2011;22:865–871. doi: 10.1089/hum.2010.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chernomordik LV, et al. Membranes of the world unite! J Cell Biol. 2006;175:201–207. doi: 10.1083/jcb.200607083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Paumard P, et al. The ATP synthase is involved in generating mitochondrial cristae morphology. EMBO J. 2002;21:221–230. doi: 10.1093/emboj/21.3.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chen S, et al. Loss of tafazzin in yeast leads to increased oxidative stress during respiratory growth. Mol Microbiol. 2008;68:1061–1072. doi: 10.1111/j.1365-2958.2008.06216.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Tripodi A, et al. Antiphospholipid syndrome: laboratory detection, mechanisms of action and treatment. J Intern Med. 2011;270:110–122. doi: 10.1111/j.1365-2796.2011.02362.x. [DOI] [PubMed] [Google Scholar]
- 61.Cox D, et al. Determination of cellular lipids bound to human CD1d molecules. PLoS One. 2009;4:e5325. doi: 10.1371/journal.pone.0005325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Dieude M, et al. Cardiolipin binds to CD1d and stimulates CD1d-restricted gammadelta T cells in the normal murine repertoire. J Immunol. 2011;186:4771–4781. doi: 10.4049/jimmunol.1000921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ray NB, et al. Dynamic regulation of cardiolipin by the lipid pump Atp8b1 determines the severity of lung injury in experimental pneumonia. Nat Med. 2010;16:1120–1127. doi: 10.1038/nm.2213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Choi SY, et al. A common lipid links Mfn-mediated mitochondrial fusion and SNARE-regulated exocytosis. Nat Cell Biol. 2006;8:1255–1262. doi: 10.1038/ncb1487. [DOI] [PubMed] [Google Scholar]
- 65.Gohil VM, et al. Synthetic lethal interaction of the mitochondrial phosphatidylethanolamine and cardiolipin biosynthetic pathways in Saccharomyces cerevisiae. J Biol Chem. 2005;280:35410–35416. doi: 10.1074/jbc.M505478200. [DOI] [PubMed] [Google Scholar]
- 66.Osman C, et al. Prohibitins and the functional compartmentalization of mitochondrial membranes. J Cell Sci. 2009;122:3823–3830. doi: 10.1242/jcs.037655. [DOI] [PubMed] [Google Scholar]
- 67.Tamura Y, et al. Ups1p and Ups2p antagonistically regulate cardiolipin metabolism in mitochondria. J Cell Biol. 2009;185:1029–1045. doi: 10.1083/jcb.200812018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Potting C, et al. Regulation of mitochondrial phospholipids by Ups1/PRELI-like proteins depends on proteolysis and Mdm35. EMBO J. 2010;29:2888–2898. doi: 10.1038/emboj.2010.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Tamura Y, et al. Mdm35p imports Ups proteins into the mitochondrial intermembrane space by functional complex formation. EMBO J. 2010;29:2875–2887. doi: 10.1038/emboj.2010.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Dimmer KS, et al. Mdm31 and Mdm32 are inner membrane proteins required for maintenance of mitochondrial shape and stability of mitochondrial DNA nucleoids in yeast. J Cell Biol. 2005;168:103–115. doi: 10.1083/jcb.200410030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kornmann B, et al. An ER-mitochondria tethering complex revealed by a synthetic biology screen. Science. 2009;325:477–481. doi: 10.1126/science.1175088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kuroda T, et al. FMP30 is required for the maintenance of a normal cardiolipin level and mitochondrial morphology in the absence of mitochondrial phosphatidylethanolamine synthesis. Mol Microbiol. 2011;80:248–265. doi: 10.1111/j.1365-2958.2011.07569.x. [DOI] [PubMed] [Google Scholar]
- 73.Kutik S, et al. The translocator maintenance protein Tam41 is required for mitochondrial cardiolipin biosynthesis. J Cell Biol. 2008;183:1213–1221. doi: 10.1083/jcb.200806048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Claypool SM, et al. Barth syndrome mutations that cause tafazzin complex lability. J Cell Biol. 2011;192:447–462. doi: 10.1083/jcb.201008177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Haines TH. A new look at Cardiolipin. Biochim Biophys Acta. 2009;1788:1997–2002. doi: 10.1016/j.bbamem.2009.09.008. [DOI] [PubMed] [Google Scholar]
- 76.Ban T, et al. OPA1 disease alleles causing dominant optic atrophy have defects in cardiolipin-stimulated GTP hydrolysis and membrane tubulation. Hum Mol Genet. 2010;19:2113–2122. doi: 10.1093/hmg/ddq088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.DeVay RM, et al. Coassembly of Mgm1 isoforms requires cardiolipin and mediates mitochondrial inner membrane fusion. J Cell Biol. 2009;186:793–803. doi: 10.1083/jcb.200906098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Montessuit S, et al. Membrane remodeling induced by the dynamin-related protein Drp1 stimulates Bax oligomerization. Cell. 2010;142:889–901. doi: 10.1016/j.cell.2010.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Gonzalvez F, et al. Cardiolipin provides an essential activating platform for caspase-8 on mitochondria. J Cell Biol. 2008;183:681–696. doi: 10.1083/jcb.200803129. [DOI] [PMC free article] [PubMed] [Google Scholar]