Abstract
The ataxia-telangiectasia mutated (ATM) protein kinase is best known for its role in the DNA damage response, but recent findings suggest that it also functions as a redox sensor that controls the levels of reactive oxygen species in human cells. Here, we review the evidence supporting the conclusion that ATM can be directly activated by oxidation, as well as various observations from ATM-deficient patients and mouse models that point toward the importance of ATM in oxidative stress responses. We also discuss the roles of this kinase in regulating mitochondrial function and metabolic control through its action on tumor suppressor p53, AMP-activated protein kinase (AMPK), mammalian target of rapamycin (mTOR) and hypoxia-inducible factor-1 (HIF-1), and how the regulation of these enzymes may be affected in ATM-deficient patients and in cancer cells.
The protein kinase ataxia-telangiectasia mutated (ATM)
Ataxia-telangiectasia (A-T) is a rare autosomal recessive disorder caused by deficiency of the ataxia-telangiectasia mutated (ATM) protein kinase. Most A-T patients lack functional ATM protein due to missense or nonsense mutations in the ATM gene, which result in truncated or unstable ATM variants [1]. ATM has a key role in coordinating the cellular response to DNA damage and, consequently, A-T patients are hypersensitive to the effects of radiation and are predisposed to cancer development, primarily within the immune system. A-T is characterized by cerebellar degeneration, progressive ataxia, primary immunodeficiency and an increased incidence of lymphoid tumorigenesis and type II diabetes [2, 3]. The manifestation of A-T phenotypes within the immune system likely results from defects in the processing of physiological DNA strand breaks associated with immune cell development in the absence of ATM [4]. Although ATM activation has primarily been viewed as a response to DNA damage, several recent studies have demonstrated that ATM can be activated independently from DNA damage through redox-dependent mechanisms and participates in a diverse set of signaling pathways involved in metabolic regulation and cancer. In this review, we summarize recent findings that reveal novel functions for the protein kinase ATM, and discuss the potential implications and questions raised by these studies.
ATM and the DNA damage response
ATM is a member of the phosphatidylinositol 3 kinase-like kinase (PIKK) family of Ser/Thr-protein kinases, which includes ATR (ataxia-telangiectasia and Rad3-related), DNA-PKcs (DNA-dependent protein kinase catalytic subunit) and mTOR (mammalian target of rapamycin), among others [5]. Proteins in the PIKK family contain a conserved kinase domain (KD), a FRAP-ATM-TRRAP (FAT) domain, and a FAT-C terminal (FATC) domain near the C-terminus (Figure 1). ATM, ATR and DNA-PKcs associate with DNA and coordinate the cellular responses to DNA damage by activating distinct DNA repair and signaling pathways [6]. DNA damage elicits the interaction of specific sensor proteins with ATM, ATR and DNA-PKcs, leading to the activation of these protein kinases and their recruitment to the damaged sites [7]. ATR, through interactions with ATR-interacting protein (ATRIP), is activated by single-stranded DNA regions that are associated with stalled replication forks and are coated by replication protein A (RPA) [8]. Both DNA-PKcs and ATM are activated by double-strand DNA breaks (DSBs), but they initiate different DNA repair pathways. DNA-PKcs is recruited to DSBs and is activated through interactions with the Ku heterodimer (see Glossary), which binds to DNA ends [9]. The activated DNA-PKcs then triggers the repair of DSBs through the nonhomologous end joining DNA repair pathway. By contrast, ATM is recruited to DSBs and activated by DNA damage through interactions with the MRE11-RAD50-NBS1 (MRN) complex, which is bound to DNA ends at the site of the break [10]. The activated ATM is important for the initiation of DNA end resection that is a prelude to DNA repair via the homologous recombination pathway [11]. The specific factors dictating the choice between the two DSB repair pathways — either Ku/DNA-PKcs (nonhomologous end joining) or MRN/ATM (homologous recombination) — are not well understood.
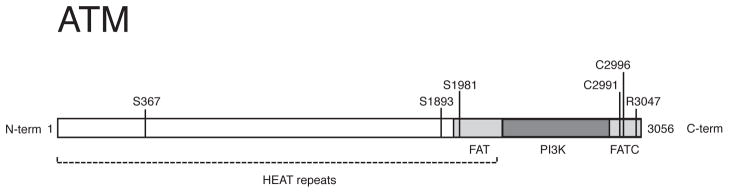
Figure 1.
The ATM protein kinase. The C-terminal PI3 kinase-like kinase domain (KD), spanning aa ~2712 to 2962, is flanked by the domains FAT (FRAP, ATM and TRRAP; ~ aa 1960 to 2566) and FATC (FRAP, ATM and TRRAP C-terminal; ~ aa 2963 to 3056). The N-terminal region of the FATC domain has also been termed the “PIKK-regulatory domain” [5]. The location of autophosphorylation sites in ATM (S367, S1893, S1981 and S2996) [12, 24, 87] is shown. The N-terminus of ATM is composed of HEAT (Huntingtin, elongation factor 1A, protein phosphatase 2A A-subunit, TOR) repeats [88].
Considerable progress has been made in detailing the mechanism of ATM activation by DSBs. ATM is a 350 kDa protein that exists as an inactive dimer and undergoes autophosphorylation and monomerization following DSB induction [12]. Although the exact role of ATM autophosphorylation remains unclear (Box 1), ATM monomerization, activation, and recruitment to DSBs requires the interaction with the NBS1 protein within the MRN complex [13–15]. Once bound at the DSB site, ATM initiates a signaling cascade that regulates cell cycle checkpoints, DNA repair and chromatin structure [16, 17]. For example, ATM regulates the G1/S cell cycle checkpoint through p53 phosphorylation at Ser15 [18] and activates checkpoint kinase 2 (CHK2) through phosphorylation at Thr68 [19]. ATM activation can also trigger the induction of apoptosis or senescence through p53 phosphorylation [18, 20–23], and many other ATM targets affect checkpoint activation in mammalian cells [16]. Furthermore, several hundred putative ATM substrates have been identified in a global proteome analysis of ATM/ATR phosphorylation events following ionizing radiation (IR) treatment [17]. Many ATM targets identified in this study have known roles in the DNA damage response or cell cycle control, whereas other targets operate in other pathways or do not have characterized functions. In addition, over 90% of the phosphorylation and dephosphorylation events during DNA damage do not occur at consensus ATM recognition sites, yet ~60% of the events are dependent on ATM kinase activity [24]; this suggests that there is a complex cascade of phosphorylation events that is ATM-dependent yet not directly catalyzed by ATM. The interplay between ATM and other kinases downstream in the DNA damage response has been reviewed recently [25].
Box 1. ATM autophosphorylation.
ATM autophosphorylation at Ser1981 is considered a hallmark of ATM activation [12], although additional DSB-induced ATM autophosphorylation sites (Ser367, Ser1893 and Ser2996) are required for ATM signaling in human cells [87, 94]. ATM autophosphorylation is rapidly induced following DNA damage, and mutation of the autophosphorylation sites disrupts ATM signaling in human cells [87, 94]. However, the functional significance of ATM autophosphorylation is unclear because in vitro studies and mouse models of ATM variants have shown that ATM autophosphorylation is not required for ATM activation by DNA damage or oxidation[14, 43, 95, 96]. ATM autophosphorylation at Ser1981 is necessary only for the retention of ATM at DSB sites but is not required for ATM recruitment to the DNA break site [97], suggesting that ATM autophosphorylation is functionally significant downstream of the initial activation.
ATM and oxidative stress
Consistent with the well established role of ATM within the DNA damage response, cells derived from A-T patients display defective cell cycle checkpoints, radiosensitivity, and chromosomal instability [16, 26]. However, the global DSB repair capability of ATM-deficient cells is only partially attenuated, most likely due to functional redundancy between ATM and DNA-PKcs in the repair of DSBs. In fact, only ~10% of DSBs, most of them associated with regions of heterochromatin, require ATM for repair [27].
It has long been suggested that several key features of the A-T disorder, primarily the cerebellar degeneration and ataxia, indicate the existence of ATM functions that are independent from the checkpoint functions of the DNA damage response. Postmitotic neurons of the cerebellum are less dependent on the DNA damage response-associated cell cycle regulation mediated by ATM. Although ATM is considered primarily localized to the nucleus, a minority fraction of the protein has been shown to be present in the cytoplasm of various cell types. In the neurons of the cerebellum, ATM has been reported to be equally distributed between the nucleus and the cytoplasm [28–32], further suggesting additional roles for ATM outside of the nucleus.
Over the past two decades, evidence has accumulated linking ATM deficiency to increased oxidative stress in cells, which is thought to play a key role in neurodegeneration, metabolic dysregulation, and oncogenesis [33]. Early studies showed that ATM-deficient cells have a reduced antioxidant response [29, 33, 34] and are sensitive to treatment with oxidizing agents [35–37]. ATM-deficient mice display increased levels of reactive oxygen species (ROS) and signs of oxidative stress within the central nervous system, particularly the cerebellum, which is the primary site of degeneration in A-T [38, 39]. Hematopoietic stem cell failure in an ATM-deficient mouse model was linked to the induction of the p16-Rb stress pathway and was preventable by treatment with antioxidants [40]. Several studies have demonstrated that treatment of ATM-deficient cells with antioxidants alleviates proliferation defects and inhibits the activation of stress-associated signaling pathways that are initiated by oxidative stress caused by ATM loss [40–42]; these results suggest that increased ROS production contributes to the AT phenotype independently from defects in DNA repair. In the next section, we discuss several studies that provide biochemical and functional evidence of a direct role for ATM in the cellular response to oxidative stress.
ATM activation by oxidation
Recent studies have identified a novel mechanism of ATM activation through direct oxidation [43, 44]. When ATM is activated by DSBs, the protein undergoes monomerization and requires free DNA ends and MRN; by contrast, oxidized ATM is an active dimer in which the two monomers are covalently linked by intermolecular disulfide bonds. In vitro experiments demonstrated direct ATM activation in the presence of hydrogen peroxide (H2O2) independently from both DNA and MRN [43]. A change of Ser1981 to alanine (which prevents ATM autophosphorylation) did not affect p53 phosphorylation by oxidized ATM, which indicates that autophosphorylation is not required for ATM activation by oxidation. Treatment of primary human fibroblasts with low H2O2 concentrations resulted in active ATM that phosphorylated p53 and CHK2 without an accompanying increase in phosphorylation of histone H2AX, a common marker of DSB induction. Furthermore, the heterochromatin protein KAP1, which is phosphorylated by ATM following DSB induction, remained unphosphorylated following H2O2 treatment. These findings indicate that the substrate specificity of ATM when activated by oxidation is probably different from that of ATM when activated by DNA damage and oxidation. Residue Cys2991, located in the C-terminal FATC domain, is critical for ATM activation by oxidation, as a C2991L variant is unable to be activated by H2O2 but can be activated normally by MRN and DNA [43]. Moreover, when expressed in lymphocytes derived from A-T patients, the C2991L variant is unable to induce apoptosis in response to H2O2 treatment, demonstrating a functional role for oxidized ATM. A form of ATM with a truncated C-terminal region (R3047X), identified in several AT patients, can also be fully activated by DNA and MRN, but not by oxidation [43]. Although patients harboring the R3047X variant develop ataxia, their cells exhibit less sensitivity to radiation when compared to the classic A-T phenotype [45, 46], and one R3047X patient did not develop immunodeficiency [47]. Thus, a subset of clinical features associated with A-T might be primarily due to defects in redox regulation by ATM, whereas other features such as the immune defects might solely result from an impaired DNA damage response.
A-T has a pleiotropic phenotype that affects multiple systems, and it is likely that many clinical features arise or are exacerbated from the synergistic effects of a defective DNA damage response and oxidative stress in the absence of ATM. Cerebellar atrophy is a cardinal feature of both A-T and ataxia-telangiectasia-like disorder (ATLD), which is caused by mutations in the gene encoding MRE11, suggesting that the defective DNA damage response associated with ATM deficiency might be sufficient to induce the neurological pathology associated with AT, but the compounded oxidative stress and DNA repair defects in A-T patients would potentially increase the rate and severity of neurodegeneration. Purkinje cells in the cerebellum experience high levels of metabolic demand and oxidative stress [48], thus any loss of redox control may be particularly detrimental in this cell type. Other DNA repair deficiencies, such as ataxia with oculomotor apraxia 1 (AOA1) and spinocerebellar ataxia with axonal neuropathy 1 (SCAN1), also cause cerebellar degeneration [49], which suggests that DNA damage may ultimately be responsible for the loss of cerebellar function. In the case of ATM, clearly further studies are needed to evaluate the physiological role of ATM activation through oxidation and the systemic effects of its absence with regards to the A-T phenotype, including the development of a knock-in mouse model expressing C2991L or R3047X variants.
The discovery of ATM activation by oxidation raises important questions concerning the degree of overlap between the group of ATM substrates that are phosphorylated following DNA damage and those that are phosphorylated following oxidative stress. However, separating ATM activity mediated through oxidation from that mediated by DNA damage is difficult, as oxidative stress and ROS production usually induce DNA damage, and therefore ATM is often exposed to both DNA damage and oxidation simultaneously. Although an ATM variant has been identified that is defective in its ability to be activated by oxidation while remaining competent in DNA repair [43], identification of an ATM variant with the reverse features would be useful to confirm whether ATM is directly being activated by oxidation and not by subsequent DNA damage in the cell. Interestingly, oxidative stress disrupts DNA binding by MRN and therefore inhibits ATM activation by MRN and DNA [44], suggesting that oxidation may be the only operational pathway of ATM activation under conditions of high ROS concentrations. This finding points to a complicated interplay between oxidized ATM and DSB-activated ATM. Since IR treatment induces both ROS production and DSBs, the roughly 700 ATM targets identified in the previously mentioned global proteome analysis probably represent targets from both the DNA repair and oxidation pathways. Although the identified targets are mostly comprised of proteins involved in DNA replication, DNA repair and cell cycle control, multiple proteins involved in insulin signaling were also identified [17]; suggesting that ATM functions in the regulation of metabolic signaling pathways (Figure 2), possibly through activation by direct oxidation, as discussed in the next section.
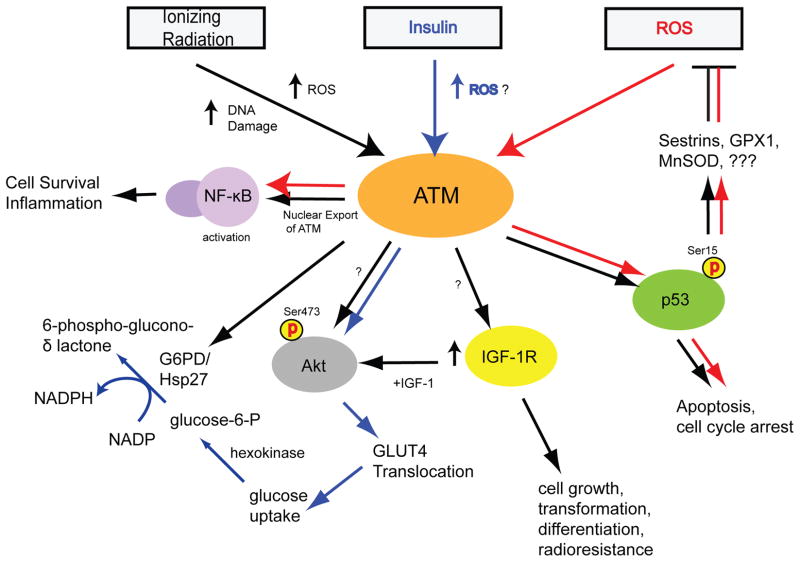
Figure 2.
ATM interaction with metabolic signaling pathways. ATM is required for NF-κB activation following exposure to ionizing radiation (IR) and reactive oxygen species (ROS) via a mechanism that requires the export of ATM from the nucleus to the cytoplasm [61]. ATM-deficient cells exhibit reduced levels of insulin-like growth factor-I receptor (IGF-IR), a defect that is rescued by expression of ATM cDNA [52, 89]. However, the mechanisms linking ATM to IGF-IR expression are unknown. ATM is required for full activation of AKT in response to insulin and IR treatment [53–55, 90, 91], which induces AKT phosphorylation at Ser473 and translocation of glucose transporter 4 (GLUT4) through unknown mechanisms. Inhibition of this pathway induces apoptosis in cancer cells with high AKT activity [55]. Increased activity of glucose-6-phosphate dehydrogenase (G6PD) is observed in the presence of ATM, which promotes NADPH formation and reduction of glutathione [78]. In response to IR treatment and elevated ROS levels, ATM phosphorylates p53 at Ser15 [22]. ROS-induced p53 phosphorylation either induces apoptosis or reduces ROS levels through upregulation of sestrin proteins (which regenerate peroxiredoxins [56, 57]), glutathione peroxidase 1 (GPX1) and manganese superoxide dismutase (MnSOD) [92]. Mutation of Ser15 in p53 to Ala15 results in elevated ROS levels, leading to insulin resistance and impaired glucose metabolism [56]. Signaling pathways initiated by IR, insulin, and ROS are indicated by black, blue, and red arrows, respectively.
ATM and insulin signaling
A-T patients have an increased risk for developing type 2 diabetes and display growth impairments associated with insulin resistance and glucose intolerance [50, 51]. Diabetic complications are not considered a primary characteristic associated with A-T due to the late onset and the fact that most A-T patients succumb to the disease early in life. However, several studies have demonstrated a relationship between ATM and metabolic signaling pathways. For example, the expression of insulin-like growth factor-I receptor (IGF-IR) is reduced in ATM-deficient cells, and the radiosensitivity of A-T cells following IR treatment is affected by IGF-IR expression levels; both effects can be rescued by ATM cDNA expression [52]. ATM also functions upstream of the AKT kinase in response to insulin stimulation and IR treatment [53]. AKT is activated via the phosphoinositide 3-kinase (PI3K) signaling pathway following insulin stimulation, and promotes cell proliferation and survival. ATM is indirectly required for AKT phosphorylation at Ser473 and for translocation of the cell surface glucose transporter 4 (GLUT4) in response to insulin stimulation [54]. Inhibition of ATM activity was recently shown to inhibit cell proliferation and induce apoptosis in cancer cell lines with overactive AKT [55].
Recent evidence indicates that the effects of ATM on insulin function and glucose metabolism may be mediated through p53 phosphorylation [56]. Deletion of the p53-encoding gene, or its mutation to generate p53 variants that lack the primary ATM phosphorylation site, results in elevated ROS levels, glucose intolerance, insulin resistance, reduced AKT phosphorylation and reduced expression of Sestrin proteins, which are involved in the regulation of intracellular antioxidants [56, 57]. These effects were rescued by the addition of dietary antioxidants, suggesting that ATM affects insulin function and glucose metabolism by regulating intracellular ROS levels through p53 phosphorylation. As oxidative stress is a known contributor to the onset of diabetes [58], elevated ROS levels in the absence of ATM or p53 phosphorylation would likely disrupt insulin signaling pathways and affect glucose homeostasis.
ATM has also been shown to phosphorylate the 4EBP1 protein, which binds and represses the translation initiation factor eIF-4E. Exposure of cells to insulin stimulates ATM-mediated phosphorylation of 4EBP1 on Ser111 [59], which releases the binding of eIF-4E and promotes cell growth. Ser111 on 4EBP1 was also identified as an ATM target after IR exposure [17]; thus, oxidative stress or DNA damage associated with the treatment also induces 4E-BP1 phosphorylation.
The existence of cytoplasmic functions for ATM has previously been suggested, and several recent studies have provided compelling evidence in support of a functional role for cytoplasmic ATM in signaling pathways related to metabolism and metabolic stress. In response to IR treatment, ATM activates nuclear factor kappa-B (NF-κB) signaling, which promotes cell survival and has been linked to metabolic disorders such as type 2 diabetes [60, 61]. Multiple post-translational modifications of the NF-κB essential modulator (NEMO) are induced by ATM activation in the nucleus, followed by translocation of NEMO and ATM to the cytoplasm, activation of IκB kinase (IKK) via TGFβ activated kinase (TAK1), and release of I-κBα from NF-κB [61]. Although most of these studies characterized responses to DNA damage, NEMO modification has also been observed under conditions of oxidative stress [61].
ATM regulation of AMPK
A p53-independent signaling pathway has recently been identified that is initiated by ROS-dependent activation of ATM in the cytoplasm and regulates protein synthesis and autophagy [62]. Following treatment with H2O2, ATM-dependent phosphorylation of the LKB1 tumor suppressor at Thr366 was shown to mediate activation of AMP-activated protein kinase (AMPK), a key sensor and regulator of cellular energy homeostasis [62]. Activation of AMPK can also occur independently from LKB1 and was shown to be ATM-dependent in response to treatment with 5-aminoimidazole-4-carboxamide-1-beta-4-ribofuranoside (AICAR) [63] or insulin-like growth factor 1 (IGF-1) [64]. AMPK monitors intracellular energy status in the form of alterations in AMP and ADP concentrations, and redirects cellular metabolism from anabolic and energy-consuming processes (i.e. protein synthesis and cell division) to catabolic and energy-producing pathways (i.e. autophagy, glucose uptake and fatty acid oxidation) [65]. Activated AMPK represses mTORC1 signaling through phosphorylation of the tuberous sclerosis complex 2 (TSC2) tumor suppressor [62, 66]. Rapamycin, a potent inhibitor of mTORC1 activity, reduces ROS levels in ATM-null lymphoblasts [62] and delays the onset of thymic lymphomas in ATM−/− mice [67], indicating that unregulated mTOR activity in the absence of ATM contributes to oxidative stress and cancer predisposition that are associated with ATM deficiency. mTOR functions in the cellular response to nutrient availability by regulating protein translation and cellular growth in response to extracellular signals mediated by growth factors and insulin [68]. Elevated mTOR activity in response to excess nutrients leads to oxidative stress through the upregulation of mitochondrial oxygen consumption [69] and subsequent insulin resistance [70]. The activation of growth factor signaling pathways in response to oncogene activation also upregulates mTOR activity, promoting cell growth and proliferation leading to cancer [71]. Therefore, negative regulation of mTOR activity through ATM activation by oxidative stress provides a possible sensory node capable of regulating metabolic stress and mediating tumor suppression (Figure 3).
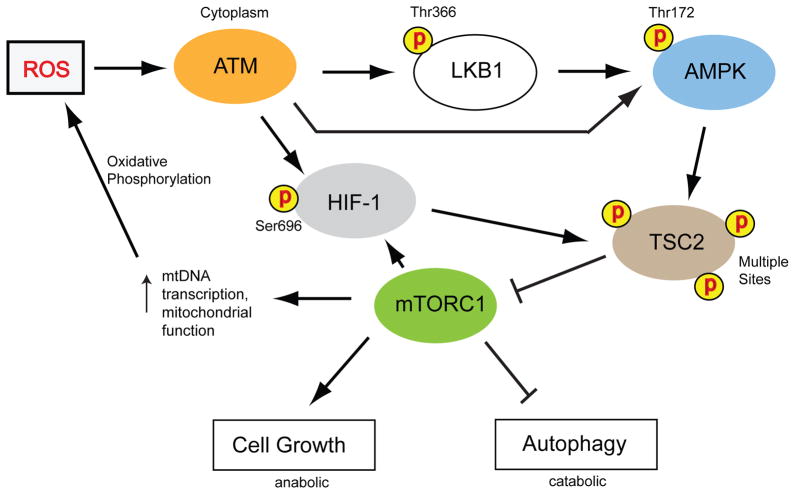
Figure 3.
ATM regulates mTORC1 activity through AMPK in response to elevated ROS levels. Elevated ROS levels activate ATM, which in turn phosphorylates and activates LKB1 at Thr366, which then phosphorylates and activates AMPK at Thr172 [62]. AMPK is also activated by IGF-1 [64] and the AMPK activator AICAR [63] in an ATM-dependent and LKB1-independent manner. AMPK phosphorylates TSC2 at multiple sites, which results in TCS2 activation, and then active TCS2 inhibits mTORC1 activity [66]. In addition, ATM directly phosphorylates HIF-1α (one of the subunits of HIF-1), which also promotes TSC2 activity and therefore blocks mTORC1 [82]. Moreover, mTORC1 promotes the expression of HIF-1 [82] and, as part of the larger mTOR complex, regulates cellular growth and protein synthesis. Inhibition of mTORC1 activity by TSC2 induces autophagy, a catabolic process that functions as a cellular salvaging pathway during periods of reduced energy supplies within the cell. Autophagy also functions as a tumor suppression pathway by inhibiting cellular growth. Unregulated mTORC1 activity might lead to cancer by promoting excessive cellular growth and cell division. In addition, high levels of mTORC1 activity result in increased ROS production in mitochondria by increasing oxidative metabolism through expression of the mitochondrial transcriptional regulator PGC-1α and other mechanisms [82, 93]. Therefore, ATM activation by the excess ROS generated by high mTORC1 activity may function as a feedback mechanism to regulate mTORC1 activity.
ATM has recently been identified as a functional target of metformin, a widely used drug in the treatment of type 2 diabetes [72]. Metformin reduces insulin resistance and increases glucose uptake in skeletal muscle, but the mechanism of its action is not fully understood. Metformin treatment activates AMPK in an LKB1-dependent fashion, probably by inhibiting complex 1 of the electron transport chain (ETC) [73]. A recent genome-wide association study investigating human genome variants associated with the glycemic response to metformin in patients with type 2 diabetes identified alleles at the ATM locus that were associated with a positive response to metformin [72]. Moreover, ATM seems to function upstream of metformin-induced AMPK activation, as treatment of rat hepatoma cells with an ATM inhibitor reduced AMPK activation and phosphorylation following metformin treatment [72]. In addition, metformin activates ATM in tumor cell lines and induce phosphorylation of CHK2 [74], which is a target of ATM following oxidation [43]. Interestingly, immunofluorescence analysis of ATM autophosphorylation at Ser1981in these tumor cell lines did not show distinct nuclear foci (which are often associated with DSB induction), but instead showed a diffuse nuclear staining pattern [74]. ATM activation by metformin could be mediated through oxidation, given the demonstrated inhibition of the ETC by metformin [75]. The major source of intracellular ROS production is the ETC within the mitochondria, where the incomplete reduction of molecular oxygen results in the production of superoxide during normal metabolism. Hypoxia, nutrient excess and disruption of the ETC are all adverse stress conditions that increase ROS production within the mitochondria. Therefore, the effects of metformin on ATM and AMPK activation could be mediated, at least in part, through elevated ROS levels subsequent to inhibition of the mitochondrial ETC.
ATM also regulates mitochondrial function in normal cells, as overall mitochondrial respiratory activity is reduced in A-T cell lines and can be rescued by treatment with an antioxidant or by expression of wild-type ATM [76]. A-T cells have lower activity of cytochrome c oxidase than normal cells, which could explain their reduced respiratory activity; interestingly, treatment of normal cells with an ATM inhibitor also results in reduced cytochrome c oxidase activity [77].
ATM and the regulation of glutathione homeostasis
Reduced glutathione is the major antioxidant in mammalian cells and is converted from a reduced form (GSH) to an oxidized form (GSSG) upon reaction with ROS or oxidized thiols. Regeneration of reduced glutathione is catalyzed by glutathione reductase, which requires the cofactor NADPH. ATM promotes overall antioxidant capacity in Xenopus egg extracts and in human cells by stimulating glucose-6-phosphate dehydrogenase activity in the pentose phosphate pathway, leading to increased NADPH production and nucleotide synthesis [78]. Reduced levels of NADPH may be relevant to the A-T clinical phenotype because A-T cells exhibit lower rates of GSH synthesis [34]. In addition, depletion of GSH or inhibition of the pentose phosphate pathway sensitizes neurons to oxidative stress and induces apoptosis [79].
ATM activation during hypoxia
Transient periods of low oxygen (hypoxia) are common in solid tumors and are often followed by cycles of reoxygenation. ATM is activated under hypoxic conditions in an MRN-independent manner and in the absence of DNA damage, and this phosphorylated ATM is found in a diffuse pattern in the nucleus [80]. The mechanism of ATM activation is not clear: although acute hypoxia induces release of ROS from the mitochondria [81], this was found not to be essential for ATM activation under these conditions [80]. In addition, ATM is a direct regulator of the transcription factor complex HIF-1, a heterodimer of HIF-1α and HIF-1β subunits that regulates metabolism, mitochondrial function, and angiogenesis under hypoxic conditions [82, 83]. One of the target genes for HIF-1 is REDD1, the product of which binds to 14-3-3 proteins and dissociates these proteins from TSC2; this allows TSC2 to inhibit mTORC1 [84]. ATM phosphorylation of HIF-1α on Ser696 stabilizes the protein under hypoxic conditions, promoting mTORC1 inhibition and growth suppression [82]. Thus, ATM modulates TSC2 activity and, therefore, mTOR activity in at least two distinct ways: through AMPK and through 14-3-3/REDD1 (via HIF-1α).
ROS and DNA damage
Nearly all cancer cells show some form of aneuploidy, a hallmark of genomic instability. Interestingly, aneuploidy increases ROS levels in a mouse model with a defective spindle assembly checkpoint [85]. Although the reasons for this are not very clear, the increase in ROS levels induces p53 expression in an ATM-dependent manner and causes oxidative nucleotide damage in DNA that can be blocked by antioxidant treatment. The loss of either p53 or ATM dramatically accelerates the onset of tumor development in these mice [85]. In addition, oxidative DNA lesions and chromosomal abnormalities in ATM-deficient mouse embryonic stem cells can be suppressed by antioxidant treatment, and the histone H2AX has a role in repair of these lesions [86]. These and other observations emphasize the importance of ATM as a tumor suppressor, both through its roles in the DNA damage response as well as its newly-discovered effects on redox homeostasis.
Concluding remarks and future directions
Considerable progress has been made in understanding the roles of ATM in the cell; however, several questions remain unanswered (Box 2). Taken together, recent data indicate that ATM is important not only for DNA damage responses but also for maintenance of cellular redox homeostasis. Of relevance to our understanding of the A-T disorder, these findings suggest that ATM loss leads to an overall increase in ROS levels, which likely results in the loss of neurones and other oxidation-sensitive cell populations (either directly, or indirectly through an increase in DNA damage). The ability of antioxidant treatment to delay and reduce tumor growth that is associated with the absence of ATM in mice suggests that the tumor suppressor function of ATM is at least in part due to its effects on oxidative stress.
Box 2. Outstanding questions.
What degree of overlap exists between the set of proteins targeted by ATM when activated by DNA damage and the set of proteins targeted by ATM when activated by oxidation?
What is the overall contribution of ATM signaling through p53 in metabolic regulation and intracellular redox balance?
What are the respective contributions to neurodegeneration and cancer predisposition of the defective DNA damage response and the oxidative stress that are associated with ATM deficiency?
Can the therapeutic targeting of ATM activation provide beneficial effects in metabolic disorders, neurodegenerative disorders and cancer prevention?
In addition, there may be an ATM-dependent branch of cellular redox homeostasis that remains to be characterized. The p53 protein has an important role in this regulation, but there are also effects through the LKB1-AMPK-mTOR pathway, the pentose phosphate pathway, and perhaps directly through mitochondrial enzymes (Figure 2). A better understanding of the molecular mechanisms underlying these observations and their relationship to the overall effects of ATM on metabolism and insulin signaling is necessary. The large number of ATM targets that have already been identified, some of which have no known function in either DNA damage or stress responses, highlights the broad scope of ATM signaling throughout the cell and demonstrates the need for further studies to directly test the function of ATM modification of these targets. Furthermore, there may be other unknown targets of ATM that are specific to the oxidation response; if so, then identification and characterization of these targets will be crucial to our understanding of the redox control mechanisms that are regulated by ATM.
The C2991L and R3047X ATM variants are useful reagents to dissect the role of ATM in DNA damage from its role in response to oxidation. In addition, it would be very useful to generate a mutant ATM allele that specifically ablates the DNA damage response but leaves the oxidation pathway intact. In theory, a double mutant lacking both pathways of ATM activation should be functionally equivalent to a kinase-deficient ATM allele if there are only two distinct mechanisms of activating ATM. The ubiquitous effects of oxidative stress on cancer, aging, diabetes, and a range of neurological disorders suggests that ATM may play roles in many areas of human health that were not previously recognized. The generation of specific reagents to characterize ATM function would aid in understanding the role of this important protein kinase in these disorders and in determining if ATM may be a useful target for clinical intervention.
Glossary
- Autophagy
a catabolic process in which the lysosomal machinery recycles intracellular components
- Homologous recombination
a pathway of DNA double-strand break repair that uses the genetic information from a sister chromatid or a homolog in order to retain the genetic information at the break site. It primarily occurs during the S and G2 phases of the cell cycle and requires DNA end processing
- Ku heterodimer
a protein complex that binds to DNA ends at the site of double-strand breaks and is required for the non-homologous end joining DNA repair pathway
- MRE11-RAD50-NBS1 (MRN) complex
a protein complex that functions in the detection and repair of DNA double-strand breaks and signaling through the ATM protein kinase. It promotes resection of the 5′ strand at DNA breaks and recruits and activates ATM at these sites
- mTOR complex 1 (mTORC1)
a protein complex composed of mammalian target of rapamycin (mTOR) and other four proteins. mTORC1 regulates protein synthesis, cell growth and cell division
- Non-homologous end joining (NHEJ)
a pathway of DNA double-strand break repair that utilizes the direct ligation of broken ends at the break site, with minimal end processing
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Zhang N, et al. Isolation of full-length ATM cDNA and correction of the ataxia-telangiectasia cellular phenotype. Proc Natl Acad Sci U S A. 1997;94:8021–8026. doi: 10.1073/pnas.94.15.8021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lavin MF, et al. Current and potential therapeutic strategies for the treatment of ataxia-telangiectasia. Br Med Bull. 2007;81–82:129–147. doi: 10.1093/bmb/ldm012. [DOI] [PubMed] [Google Scholar]
- 3.Ristow M. Neurodegenerative disorders associated with diabetes mellitus. J Mol Med (Berl) 2004;82:510–529. doi: 10.1007/s00109-004-0552-1. [DOI] [PubMed] [Google Scholar]
- 4.Bredemeyer AL, et al. ATM stabilizes DNA double-strand-break complexes during V(D)J recombination. Nature. 2006;442:466–470. doi: 10.1038/nature04866. [DOI] [PubMed] [Google Scholar]
- 5.Lempiainen H, Halazonetis TD. Emerging common themes in regulation of PIKKs and PI3Ks. EMBO J. 2009;28:3067–3073. doi: 10.1038/emboj.2009.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ciccia A, Elledge SJ. The DNA damage response: making it safe to play with knives. Mol Cell. 2010;40:179–204. doi: 10.1016/j.molcel.2010.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Falck J, et al. Conserved modes of recruitment of ATM, ATR and DNA-PKcs to sites of DNA damage. Nature. 2005;434:605–611. doi: 10.1038/nature03442. [DOI] [PubMed] [Google Scholar]
- 8.Cimprich KA, Cortez D. ATR: an essential regulator of genome integrity. Nat Rev Mol Cell Biol. 2008;9:616–627. doi: 10.1038/nrm2450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mahaney BL, et al. Repair of ionizing radiation-induced DNA double-strand breaks by non-homologous end-joining. Biochem J. 2009;417:639–650. doi: 10.1042/BJ20080413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lee JH, Paull TT. Activation and regulation of ATM kinase activity in response to DNA double-strand breaks. Oncogene. 2007;26:7741–7748. doi: 10.1038/sj.onc.1210872. [DOI] [PubMed] [Google Scholar]
- 11.You Z, et al. CtIP links DNA double-strand break sensing to resection. Mol Cell. 2009;36:954–969. doi: 10.1016/j.molcel.2009.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bakkenist CJ, Kastan MB. DNA damage activates ATM through intermolecular autophosphorylation and dimer dissociation. Nature. 2003;421:499–506. doi: 10.1038/nature01368. [DOI] [PubMed] [Google Scholar]
- 13.Lee JH, Paull TT. Direct activation of the ATM protein kinase by the Mre11/Rad50/Nbs1 complex. Science. 2004;304:93–96. doi: 10.1126/science.1091496. [DOI] [PubMed] [Google Scholar]
- 14.Lee JH, Paull TT. ATM activation by DNA double-strand breaks through the Mre11-Rad50-Nbs1 complex. Science. 2005;308:551–554. doi: 10.1126/science.1108297. [DOI] [PubMed] [Google Scholar]
- 15.Uziel T, et al. Requirement of the MRN complex for ATM activation by DNA damage. EMBO J. 2003;22:5612–5621. doi: 10.1093/emboj/cdg541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lavin MF. Ataxia-telangiectasia: from a rare disorder to a paradigm for cell signalling and cancer. Nat Rev Mol Cell Biol. 2008;9:759–769. doi: 10.1038/nrm2514. [DOI] [PubMed] [Google Scholar]
- 17.Matsuoka S, et al. ATM and ATR substrate analysis reveals extensive protein networks responsive to DNA damage. Science. 2007;316:1160–1166. doi: 10.1126/science.1140321. [DOI] [PubMed] [Google Scholar]
- 18.Barlow C, et al. Atm selectively regulates distinct p53-dependent cell-cycle checkpoint and apoptotic pathways. Nat Genet. 1997;17:453–456. doi: 10.1038/ng1297-453. [DOI] [PubMed] [Google Scholar]
- 19.Matsuoka S, et al. Ataxia telangiectasia-mutated phosphorylates Chk2 in vivo and in vitro. Proc Natl Acad Sci U S A. 2000;97:10389–10394. doi: 10.1073/pnas.190030497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Banin S, et al. Enhanced phosphorylation of p53 by ATM in response to DNA damage. Science. 1998;281:1674–1677. doi: 10.1126/science.281.5383.1674. [DOI] [PubMed] [Google Scholar]
- 21.Bartkova J, et al. Oncogene-induced senescence is part of the tumorigenesis barrier imposed by DNA damage checkpoints. Nature. 2006;444:633–637. doi: 10.1038/nature05268. [DOI] [PubMed] [Google Scholar]
- 22.Canman CE, et al. Activation of the ATM kinase by ionizing radiation and phosphorylation of p53. Science. 1998;281:1677–1679. doi: 10.1126/science.281.5383.1677. [DOI] [PubMed] [Google Scholar]
- 23.Zhan H, et al. Ataxia telangiectasia mutated (ATM)-mediated DNA damage response in oxidative stress-induced vascular endothelial cell senescence. J Biol Chem. 2010;285:29662–29670. doi: 10.1074/jbc.M110.125138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bensimon A, et al. ATM-dependent and -independent dynamics of the nuclear phosphoproteome after DNA damage. Sci Signal. 2010;3:rs3. doi: 10.1126/scisignal.2001034. [DOI] [PubMed] [Google Scholar]
- 25.Bensimon A, et al. Beyond ATM: the protein kinase landscape of the DNA damage response. FEBS Lett. 2011;585:1625–1639. doi: 10.1016/j.febslet.2011.05.013. [DOI] [PubMed] [Google Scholar]
- 26.Lavin MF, Shiloh Y. The genetic defect in ataxia-telangiectasia. Annu Rev Immunol. 1997;15:177–202. doi: 10.1146/annurev.immunol.15.1.177. [DOI] [PubMed] [Google Scholar]
- 27.Goodarzi AA, et al. ATM signaling facilitates repair of DNA double-strand breaks associated with heterochromatin. Mol Cell. 2008;31:167–177. doi: 10.1016/j.molcel.2008.05.017. [DOI] [PubMed] [Google Scholar]
- 28.Lim DS, et al. ATM binds to beta-adaptin in cytoplasmic vesicles. Proc Natl Acad Sci U S A. 1998;95:10146–10151. doi: 10.1073/pnas.95.17.10146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Watters D, et al. Localization of a portion of extranuclear ATM to peroxisomes. J Biol Chem. 1999;274:34277–34282. doi: 10.1074/jbc.274.48.34277. [DOI] [PubMed] [Google Scholar]
- 30.Barlow C, et al. ATM is a cytoplasmic protein in mouse brain required to prevent lysosomal accumulation. Proc Natl Acad Sci U S A. 2000;97:871–876. doi: 10.1073/pnas.97.2.871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Boehrs JK, et al. Constitutive expression and cytoplasmic compartmentalization of ATM protein in differentiated human neuron-like SH-SY5Y cells. J Neurochem. 2007;100:337–345. doi: 10.1111/j.1471-4159.2006.04254.x. [DOI] [PubMed] [Google Scholar]
- 32.Li J, et al. Cytoplasmic ATM in neurons modulates synaptic function. Curr Biol. 2009;19:2091–2096. doi: 10.1016/j.cub.2009.10.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Barzilai A, et al. ATM deficiency and oxidative stress: a new dimension of defective response to DNA damage. DNA Repair (Amst) 2002;1:3–25. doi: 10.1016/s1568-7864(01)00007-6. [DOI] [PubMed] [Google Scholar]
- 34.Meredith MJ, Dodson ML. Imparied glutathione biosynthesis in cultured human ataxia-telangiectasia cells. Cancer Res. 1987;47:4576–4581. [PubMed] [Google Scholar]
- 35.Yi M, et al. Response of fibroblast cultures from ataxia-telangiectasia patients to oxidative stress. Cancer Lett. 1990;54:43–50. doi: 10.1016/0304-3835(90)90089-g. [DOI] [PubMed] [Google Scholar]
- 36.Ward AJ, et al. Response of fibroblast cultures from ataxia-telangiectasia patients to reactive oxygen species generated during inflammatory reactions. Environ Mol Mutagen. 1994;24:103–111. doi: 10.1002/em.2850240205. [DOI] [PubMed] [Google Scholar]
- 37.Shackelford RE, et al. The Ataxia telangiectasia gene product is required for oxidative stress-induced G1 and G2 checkpoint function in human fibroblasts. J Biol Chem. 2001;276:21951–21959. doi: 10.1074/jbc.M011303200. [DOI] [PubMed] [Google Scholar]
- 38.Kamsler A, et al. Increased oxidative stress in ataxia telangiectasia evidenced by alterations in redox state of brains from Atm-deficient mice. Cancer Res. 2001;61:1849–1854. [PubMed] [Google Scholar]
- 39.Quick KL, Dugan LL. Superoxide stress identifies neurons at risk in a model of ataxia-telangiectasia. Ann Neurol. 2001;49:627–635. [PubMed] [Google Scholar]
- 40.Ito K, et al. Regulation of oxidative stress by ATM is required for self-renewal of haematopoietic stem cells. Nature. 2004;431:997–1002. doi: 10.1038/nature02989. [DOI] [PubMed] [Google Scholar]
- 41.Kim J, Wong PK. Oxidative stress is linked to ERK1/2-p16 signaling-mediated growth defect in ATM-deficient astrocytes. J Biol Chem. 2009;284:14396–14404. doi: 10.1074/jbc.M808116200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kurz EU, et al. Doxorubicin activates ATM-dependent phosphorylation of multiple downstream targets in part through the generation of reactive oxygen species. J Biol Chem. 2004;279:53272–53281. doi: 10.1074/jbc.M406879200. [DOI] [PubMed] [Google Scholar]
- 43.Guo Z, et al. ATM activation by oxidative stress. Science. 2010;330:517–521. doi: 10.1126/science.1192912. [DOI] [PubMed] [Google Scholar]
- 44.Guo Z, et al. ATM activation in the presence of oxidative stress. Cell Cycle. 2010:9. doi: 10.4161/cc.9.24.14323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chessa L, et al. Heterogeneity in ataxia-telangiectasia: classical phenotype associated with intermediate cellular radiosensitivity. Am J Med Genet. 1992;42:741–746. doi: 10.1002/ajmg.1320420524. [DOI] [PubMed] [Google Scholar]
- 46.Gilad S, et al. Genotype-phenotype relationships in ataxia-telangiectasia and variants. Am J Hum Genet. 1998;62:551–561. doi: 10.1086/301755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Toyoshima M, et al. Ataxia-telangiectasia without immunodeficiency: novel point mutations within and adjacent to the phosphatidylinositol 3-kinase-like domain. Am J Med Genet. 1998;75:141–144. [PubMed] [Google Scholar]
- 48.Kern JK, Jones AM. Evidence of toxicity, oxidative stress, and neuronal insult in autism. J Toxicol Environ Health B Crit Rev. 2006;9:485–499. doi: 10.1080/10937400600882079. [DOI] [PubMed] [Google Scholar]
- 49.Rass U, et al. Defective DNA repair and neurodegenerative disease. Cell. 2007;130:991–1004. doi: 10.1016/j.cell.2007.08.043. [DOI] [PubMed] [Google Scholar]
- 50.Bar RS, et al. Extreme insulin resistance in ataxia telangiectasia: defect in affinity of insulin receptors. N Engl J Med. 1978;298:1164–1171. doi: 10.1056/NEJM197805252982103. [DOI] [PubMed] [Google Scholar]
- 51.Schalch DS, et al. An unusual form of diabetes mellitus in ataxia telangiectasia. N Engl J Med. 1970;282:1396–1402. doi: 10.1056/NEJM197006182822503. [DOI] [PubMed] [Google Scholar]
- 52.Peretz S, et al. ATM-dependent expression of the insulin-like growth factor-I receptor in a pathway regulating radiation response. Proc Natl Acad Sci U S A. 2001;98:1676–1681. doi: 10.1073/pnas.041416598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Viniegra JG, et al. Full activation of PKB/Akt in response to insulin or ionizing radiation is mediated through ATM. J Biol Chem. 2005;280:4029–4036. doi: 10.1074/jbc.M410344200. [DOI] [PubMed] [Google Scholar]
- 54.Halaby MJ, et al. ATM protein kinase mediates full activation of Akt and regulates glucose transporter 4 translocation by insulin in muscle cells. Cell Signal. 2008;20:1555–1563. doi: 10.1016/j.cellsig.2008.04.011. [DOI] [PubMed] [Google Scholar]
- 55.Li Y, Yang DQ. The ATM inhibitor KU-55933 suppresses cell proliferation and induces apoptosis by blocking Akt in cancer cells with overactivated Akt. Mol Cancer Ther. 2010;9:113–125. doi: 10.1158/1535-7163.MCT-08-1189. [DOI] [PubMed] [Google Scholar]
- 56.Armata HL, et al. Requirement of the ATM/p53 tumor suppressor pathway for glucose homeostasis. Mol Cell Biol. 2010;30:5787–5794. doi: 10.1128/MCB.00347-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sablina AA, et al. The antioxidant function of the p53 tumor suppressor. Nat Med. 2005;11:1306–1313. doi: 10.1038/nm1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Roberts CK, Sindhu KK. Oxidative stress and metabolic syndrome. Life Sci. 2009;84:705–712. doi: 10.1016/j.lfs.2009.02.026. [DOI] [PubMed] [Google Scholar]
- 59.Yang DQ, Kastan MB. Participation of ATM in insulin signalling through phosphorylation of eIF-4E-binding protein 1. Nat Cell Biol. 2000;2:893–898. doi: 10.1038/35046542. [DOI] [PubMed] [Google Scholar]
- 60.Baker RG, et al. NF-kappaB, inflammation, and metabolic disease. Cell Metab. 2011;13:11–22. doi: 10.1016/j.cmet.2010.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Miyamoto S. Nuclear initiated NF-kappaB signaling: NEMO and ATM take center stage. Cell Res. 2011;21:116–130. doi: 10.1038/cr.2010.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Alexander A, et al. ATM signals to TSC2 in the cytoplasm to regulate mTORC1 in response to ROS. Proc Natl Acad Sci U S A. 2010;107:4153–4158. doi: 10.1073/pnas.0913860107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sun Y, et al. AICAR induces phosphorylation of AMPK in an ATM-dependent, LKB1-independent manner. Mol Cell Biochem. 2007;306:239–245. doi: 10.1007/s11010-007-9575-6. [DOI] [PubMed] [Google Scholar]
- 64.Suzuki A, et al. IGF-1 phosphorylates AMPK-alpha subunit in ATM-dependent and LKB1-independent manner. Biochem Biophys Res Commun. 2004;324:986–992. doi: 10.1016/j.bbrc.2004.09.145. [DOI] [PubMed] [Google Scholar]
- 65.Hardie DG, Hawley SA. AMP-activated protein kinase: the energy charge hypothesis revisited. Bioessays. 2001;23:1112–1119. doi: 10.1002/bies.10009. [DOI] [PubMed] [Google Scholar]
- 66.Shaw RJ. LKB1 and AMP-activated protein kinase control of mTOR signalling and growth. Acta Physiol (Oxf) 2009;196:65–80. doi: 10.1111/j.1748-1716.2009.01972.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kuang X, et al. Deregulation of mTOR signaling is involved in thymic lymphoma development in Atm−/− mice. Biochem Biophys Res Commun. 2009;383:368–372. doi: 10.1016/j.bbrc.2009.04.019. [DOI] [PubMed] [Google Scholar]
- 68.Sengupta S, et al. Regulation of the mTOR complex 1 pathway by nutrients, growth factors, and stress. Mol Cell. 2010;40:310–322. doi: 10.1016/j.molcel.2010.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Schieke SM, et al. The mammalian target of rapamycin (mTOR) pathway regulates mitochondrial oxygen consumption and oxidative capacity. J Biol Chem. 2006;281:27643–27652. doi: 10.1074/jbc.M603536200. [DOI] [PubMed] [Google Scholar]
- 70.Dann SG, et al. mTOR Complex1-S6K1 signaling: at the crossroads of obesity, diabetes and cancer. Trends Mol Med. 2007;13:252–259. doi: 10.1016/j.molmed.2007.04.002. [DOI] [PubMed] [Google Scholar]
- 71.Menon S, Manning BD. Common corruption of the mTOR signaling network in human tumors. Oncogene. 2008;27(Suppl 2):S43–51. doi: 10.1038/onc.2009.352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zhou K, et al. Common variants near ATM are associated with glycemic response to metformin in type 2 diabetes. Nat Genet. 2011;43:117–120. doi: 10.1038/ng.735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Shackelford DB, Shaw RJ. The LKB1-AMPK pathway: metabolism and growth control in tumour suppression. Nat Rev Cancer. 2009;9:563–575. doi: 10.1038/nrc2676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Vazquez-Martin A, et al. Metformin activates an Ataxia Telangiectasia Mutated (ATM)/Chk2-regulated DNA damage-like response. Cell Cycle. 2011;10:1499–1501. doi: 10.4161/cc.10.9.15423. [DOI] [PubMed] [Google Scholar]
- 75.Owen MR, et al. Evidence that metformin exerts its anti-diabetic effects through inhibition of complex 1 of the mitochondrial respiratory chain. Biochem J. 2000;348(Pt 3):607–614. [PMC free article] [PubMed] [Google Scholar]
- 76.Ambrose M, et al. Intrinsic mitochondrial dysfunction in ATM-deficient lymphoblastoid cells. Hum Mol Genet. 2007;16:2154–2164. doi: 10.1093/hmg/ddm166. [DOI] [PubMed] [Google Scholar]
- 77.Patel AY, et al. Ataxia telangiectasia mutated influences cytochrome c oxidase activity. Biochem Biophys Res Commun. 2011;405:599–603. doi: 10.1016/j.bbrc.2011.01.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Cosentino C, et al. ATM activates the pentose phosphate pathway promoting anti-oxidant defence and DNA repair. EMBO J. 2011;30:546–555. doi: 10.1038/emboj.2010.330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Vaughn AE, Deshmukh M. Glucose metabolism inhibits apoptosis in neurons and cancer cells by redox inactivation of cytochrome c. Nat Cell Biol. 2008;10:1477–1483. doi: 10.1038/ncb1807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Bencokova Z, et al. ATM activation and signaling under hypoxic conditions. Mol Cell Biol. 2009;29:526–537. doi: 10.1128/MCB.01301-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Chandel NS, et al. Reactive oxygen species generated at mitochondrial complex III stabilize hypoxia-inducible factor-1alpha during hypoxia: a mechanism of O2 sensing. J Biol Chem. 2000;275:25130–25138. doi: 10.1074/jbc.M001914200. [DOI] [PubMed] [Google Scholar]
- 82.Cam H, et al. mTORC1 signaling under hypoxic conditions is controlled by ATM-dependent phosphorylation of HIF-1alpha. Mol Cell. 2010;40:509–520. doi: 10.1016/j.molcel.2010.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Majmundar AJ, et al. Hypoxia-inducible factors and the response to hypoxic stress. Mol Cell. 2010;40:294–309. doi: 10.1016/j.molcel.2010.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.DeYoung MP, et al. Hypoxia regulates TSC1/2-mTOR signaling and tumor suppression through REDD1-mediated 14–3–3 shuttling. Genes Dev. 2008;22:239–251. doi: 10.1101/gad.1617608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Li M, et al. The ATM-p53 pathway suppresses aneuploidy-induced tumorigenesis. Proc Natl Acad Sci U S A. 2010;107:14188–14193. doi: 10.1073/pnas.1005960107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Zha S, et al. Complementary functions of ATM and H2AX in development and suppression of genomic instability. Proc Natl Acad Sci U S A. 2008;105:9302–9306. doi: 10.1073/pnas.0803520105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kozlov SV, et al. Involvement of novel autophosphorylation sites in ATM activation. Embo J. 2006;25:3504–3514. doi: 10.1038/sj.emboj.7601231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Perry J, Kleckner N. The ATRs, ATMs, and TORs are giant HEAT repeat proteins. Cell. 2003;112:151–155. doi: 10.1016/s0092-8674(03)00033-3. [DOI] [PubMed] [Google Scholar]
- 89.Shahrabani-Gargir L, et al. Ataxia-telangiectasia mutated gene controls insulin-like growth factor I receptor gene expression in a deoxyribonucleic acid damage response pathway via mechanisms involving zinc-finger transcription factors Sp1 and WT1. Endocrinology. 2004;145:5679–5687. doi: 10.1210/en.2004-0613. [DOI] [PubMed] [Google Scholar]
- 90.Fraser M, et al. MRE11 promotes AKT phosphorylation in direct response to DNA double-strand breaks. Cell Cycle. 2011;10:2218–2232. doi: 10.4161/cc.10.13.16305. [DOI] [PubMed] [Google Scholar]
- 91.Khalil A, et al. ATM-dependent ERK signaling via AKT in response to DNA double-strand breaks. Cell Cycle. 2011;10:481–491. doi: 10.4161/cc.10.3.14713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Hussain SP, et al. p53-induced up-regulation of MnSOD and GPx but not catalase increases oxidative stress and apoptosis. Cancer Res. 2004;64:2350–2356. doi: 10.1158/0008-5472.can-2287-2. [DOI] [PubMed] [Google Scholar]
- 93.Cunningham JT, et al. mTOR controls mitochondrial oxidative function through a YY1-PGC-1alpha transcriptional complex. Nature. 2007;450:736–740. doi: 10.1038/nature06322. [DOI] [PubMed] [Google Scholar]
- 94.Kozlov SV, et al. Autophosphorylation and ATM activation: additional sites add to the complexity. J Biol Chem. 2010;286:9107–9119. doi: 10.1074/jbc.M110.204065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Daniel JA, et al. Multiple autophosphorylation sites are dispensable for murine ATM activation in vivo. J Cell Biol. 2008;183:777–783. doi: 10.1083/jcb.200805154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Pellegrini M, et al. Autophosphorylation at serine 1987 is dispensable for murine Atm activation in vivo. Nature. 2006;443:222–225. doi: 10.1038/nature05112. [DOI] [PubMed] [Google Scholar]
- 97.So S, et al. Autophosphorylation at serine 1981 stabilizes ATM at DNA damage sites. J Cell Biol. 2009;187:977–990. doi: 10.1083/jcb.200906064. [DOI] [PMC free article] [PubMed] [Google Scholar]