Abstract
Hexavalent chromium [Cr(VI)] is a human carcinogen that results in the generation of reactive oxygen species (ROS) and a variety of DNA lesions leading to cell death. Epigallocatechin-3-gallate (EGCG), the major polyphenol present in green tea, possesses potent antioxidative activity capable of protecting normal cells from various stimuli-induced oxidative stress and cell death. Here we demonstrated that co-treatment with EGCG protected human normal bronchial epithelial BEAS-2B cells from Cr(VI)-induced cell death in a dose-dependent manner. Cr(VI) induces apoptosis as the primary mode of cell death. Co-treatment of BEAS-2B cells with EGCG dose-dependently suppressed Cr(VI)-induced apoptosis. Fluorescence microscopic analyses and quantitative measurement revealed that EGCG significantly decreased intracellular levels of ROS induced by Cr(VI) exposure. Using a well-established K+/SDS precipitation assay, we further showed that EGCG was able to dose-dependently reduce DNA-protein cross-links (DPC), lesions that could be partially attributed to Cr(VI)-induced oxidative stress. Finally, analyses of Affymetrix microarray containing 28,869 well-annotated genes revealed that, among the 3412 genes changed more than 1.5-fold by Cr(VI) treatment, changes of 2404 genes (70%) were inhibited by pretreatment of EGCG. Real-time PCR confirmed the induction of 3 genes involved in cell death and apoptosis by Cr(VI), which was eliminated by EGCG. In contrast, Cr(VI) reduced the expression of 3 genes related to cellular defense, and this reduction was inhibited by EGCG. Our results indicate that EGCG protects BEAS-2B cells from Cr(VI)-induced cytotoxicity presumably by scavenging ROS and modulating a subset of genes. EGCG, therefore, might serve as a potential chemopreventive agent against Cr(VI) carcinogenesis.
Keywords: Chromate, EGCG, BEAS-2B cells, Cell death, Apoptosis, Reactive oxygen species, DNA protein cross-links, Gene expression
Introduction
Hexavalent chromium [Cr(VI)] is commonly used in numerous industries including stainless steel welding, chrome plating, electroplating, leather tanneries and pigment manufacturing (Langard, 1990; Langard, 1993; Simonato et al., 1991). Non-occupational exposure to Cr(VI) compounds may occur from cigarette smoke, automobile emissions, landfills and hazardous waste disposal sites (Langard, 1990; O'Brien et al., 2003). Occupational exposure to Cr(VI) is found in approximately half a million industrial workers in the United States and several million worldwide. Inhalation is a common form of Cr(VI) exposure, and results in a number of serious respiratory effects, including pulmonary fibrosis, chronic bronchitis, lung cancer, and other illnesses (Baruthio, 1992; Deschamps et al., 1995; Franchini et al., 1983; Ishikawa et al., 1994). Bronchial epithelial cells line the airways of the lung and are therefore directly exposed to inhaled Cr(VI) found in fumes and dusts. Upon inhalation, Cr(VI) enters cells through a sulfate/phosphate anion transport system and is reduced to lower oxidation states such as Cr(V), Cr(IV) and Cr(III) by cellular reductants including glutathione and ascorbate (Shi et al., 1999; Shi and Dalal, 1989). During this process, molecular oxygen is reduced to superoxide anion (·O2−), which is further converted to hydrogen peroxide (H2O2) via dismutation. The resultant intermediates react with H2O2 to generate hydroxyl radicals via a Haber-Weiss- or Fenton-like reaction (Shi et al., 1994). Thus, during the one-electron reduction of Cr(VI), along with the reduced intermediates, a spectrum of ROS is generated that causes diverse cytotoxic and genotoxic effects (Shi et al., 1994). For instance, Cr(VI) has been shown to induce chromosomal aberrations, mutations, transformation of cultured mammalian cells (De Flora et al., 1990) and a variety of DNA lesions such as base modifications, single-strand breaks, double-strand breaks, Cr-DNA adducts, DNA-DNA crosslinks , and DNA-protein cross-links (DPC). The Cr(VI)-induced DNA damage can in turn impact DNA replication, transcription and translation, resulting in altered gene expression, as well as lead to cell death (Luo et al., 1996; Shi et al., 1992; Standeven and Wetterhahn, 1991; Sugden and Stearns, 2000). Apoptotic cell death, unlike necrosis, is part of the normal control of growing cell populations, in physiological cell turnover, and in embryonal development. However, apoptosis can also be triggered inappropriately when, after exposure to genotoxic chemicals, damage to genetic material exceeds capacity for repair (Waalkes et al., 2000). Cr(VI)-induced apoptosis has been shown in numerous different cell lines (Azad et al., 2008; Bagchi et al., 2000; Banu et al., 2011; Flores and Perez, 1999; Gambelunghe et al., 2006; Russo et al., 2005; Son et al., 2011b; Vasant et al., 2001).
Epigallocatechin-3-gallate (EGCG) comprises approximately 60% of the catechins in green tea, and has antioxidative, anti-inflammatory, and anti-carcinogenic properties (Higdon and Frei, 2003). EGCG has been reported to display its potent antioxidative property because it possesses two triphenolic groups in the structure (Jin et al., 2001). Several studies have shown that EGCG protects against various stimuli-induced oxidative stress and apoptosis in vitro (Choi et al., 2001; Jin et al., 2001; Jung et al., 2007; Koh et al., 2004; Nie et al., 2002; Sheng et al., 2007; Sheng et al., 2010; Yao et al., 2008) and in an animal studies (Filip et al., 2011). In Cr(VI) and TPA treated Jurkat cells, EGCG is an efficient ˙OH radical scavenger with a reaction rate constant comparable to several well recognized antioxidants, such as ascorbate, glutathione and cysteine. Moreover, EGCG is also a scavenger of ·O2− radicals and can inhibit Cr(VI)-induced DNA damage (Shi et al., 2000). However, no study has been conducted in normal lung cells, which are target cells for Cr(VI)-induced lung injury. Therefore, in this study, we investigated the protective effects of EGCG on Cr(VI)-induced cytotoxicity in human normal bronchial epithelial BEAS-2B cells. This cell line is a well-established model to study Cr(VI)-induced apoptosis and cell death (Gambelunghe et al., 2006; Wang et al., 2004). We found that EGCG protected BEAS-2B cells from Cr(VI)-induced cell death including apoptosis in a dose-dependent manner. We also showed that EGCG significantly decreased intracellular levels of ROS induced by Cr(VI) exposure. Moreover, EGCG was also able to dose-dependently reduce DNA-protein cross-links (DPC), lesions that could be partially attributed to Cr(VI)-induced oxidative stress, using Epstein-Barr virus-transformed human Burkitt's lymphoma (EBV-BL) cells as a model system (Costa et al., 1997). Finally, microarray analyses showed that EGCG modulated changes of gene expression in Cr(VI)-treated BEAS-2B cells, including genes involved in cell death and cellular defense. Our data provide clear support on the protective effects of EGCG against Cr(VI)-induced cytotoxicity.
Materials and methods
Chemicals and reagents
Potassium chromate (K2CrO4) was obtained from J. T. Baker Chemical Co. (Phillipsburg, NJ). Radioactive 51CrO42− was purchased from Perkinelmer Inc. (Waltham, MA). EGCG (Sigma, St. Louis, MO) was dissolved in autoclaved water as a 10mM stock solution. The reagent was further diluted in cell culture media immediately before use. H2O2 and 3-(4, 5-dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide (MTT) were purchased from Sigma (St. Louis, MO). 6-carboxy-2', 7'-dichlorodihydrofluoresce in diacetate, di(acetoxymethyl ester) (H2DCFDA) was purchased from Invitrogen (Carlsbad, CA).
Cell culture
Cells were grown at 37 °C in an incubator with a humidified atmosphere containing 5% CO2. BEAS-2B cells (Li et al., 2009) and EBV-BL cells (Costa et al., 1997) were cultured in Dulbecco's Modified Eagle Medium (DMEM, Invitrogen) and RPMI 1640 medium (Invitrogen), respectively. All media were supplemented with 10% fetal bovine serum (FBS, ATLAS Biological, Fort Collins, CO) and 1% penicillin/streptomycin (Grand Island, NY). BEAS-2B cells were passaged at 80–90% confluence by trypsinization. All treatments were administered when cell density reached approximately 70–80% confluence.
Cytotoxicity assays
Cell cytotoxicity was determined by colony formation and MTT assays. For the colony formation assay, 4×104 BEAS-2B cells were seeded into each well of a 24-well plate and allowed to attach overnight. Cells were then exposed to K2CrO4 and/or EGCG for the selected time. After trypsinization, three hundred cells were then reseeded into each of three dishes (100-mm diameter), and grown for 2 weeks. Surviving colonies were stained with Giemsa stain and counted. For the MTT assay, BEAS-2B cells were seeded into a 96-well plate with a density of 5,000 cells per well and were allowed to attach for 24 h. Cells were then exposed in six replicates to 10 μM Cr(VI) in the presence or absence of different concentrations of EGCG (5–100 μM) for 24 h. After the exposure, the media were removed, and cells were rinsed with phosphate-buffered saline. MTT was then added to each well at a final concentration of 0.5mg/ml and incubated for an additional 4 h at 37°C. Media were then removed and 150 μl dimethyl sulfoxide (DMSO) was added to dissolve the formazan crystals. The absorbance was then read at 590 nm on a SpectraMax microplate reader (Molecular devices, CA).
Measurement of intracellular ROS
ROS was measured by a fluorimetric assay using H2DCFDA as the probe. Briefly, cells were seed into black 96-well plates (Costar, Corning, NY) at a density of 1.5 × 104 cells per well and incubated for 24 h. Cells were then washed once with HBSS (HyClone, Fisher Scientific, NJ), and treated with10 μM carboxy H2DCFDA for 1 hour. After 3 times wash with HBSS, cells were treated with increasing concentrations of Cr(VI) in the presence or absence of EGCG. After 24 h incubation, the fluorescence was measured using an excitation of 485 nm and emission of 530 nm, on the SpectraMax microplate reader. Hydrogen peroxide (100 μM) was used as a positive control. For visualisation of the intracellular fluorescence, 5×104 cells were seeded into each well of a 4-well chamber-slide (Nunc, Fisher Scientific, NJ) and treated with 5 μM Cr(VI) in the presence or absence of 25 μM EGCG. Fluorescence and bright-field images were recorded from a minimum of 3 areas per sample with a Leica TSC SP5 microscope.
Flow cytometry analysis of cellular DNA content
~106 cells were seeded in 10 cm dishes. The next day, cells were treated with Cr(VI) in the presence or absence of various concentrations of EGCG for 24 h. Both adherent and floating cells were then collected, washed, and fixed in ice-cold 70% ethanol at 4 °C overnight, and stained with 1ml of 50 μg/ml propidium iodide containing 50 μg/ml RNase A at room temperature for 30 min in the dark. DNA content was analyzed using flow cytometry (Epics XL FACS, Beckman-Coulter, Miami, FL). Apoptotic cells have a higher amount of sub-diploid DNA which accumulates in the pre-G1 position of the cell cycle profile.
Western blot
Cells were seeded at a density of 1 × 106 per10-cm dish. The next day, cells were treated with Cr(VI) in the presence or absence of different concentrations of EGCG for 24h. Both adherent and floating cells were then collected and washed twice with ice-cold PBS and lysed in ice-cold radioimmunoprecipitation assay (RIPA) buffer (50 mM Tris-HCl, PH 7.4, 1% NP-40, 0.25% Na-deoxycholate, 150 mM NaCl, 1 mM EDTA) supplemented with protease inhibitor cocktail (Roche Applied Sciences, Indianapolis, IN) for 30 min on ice. The supernatants were collected by centrifugation at 14,000 ×g for 15 min at 4 °C. The protein concentration was measured using Bio-Rad DC protein assay (Bio-Rad, Hercules, CA), and 30 μg protein extracts were separated by 9–12% SDS-PAGE gel and transferred to polyvinylidene difluoride (PVDF) membranes (Bio-Rad). Immunoblotting was performed using poly (ADP-ribose) polymerase (PARP) (1:500, Santa Cruz Biotechnology, Santa Cruz, CA), caspase-3 (1:200, Cell Signaling Technology, Danvers, MA) and HRP-conjugated anti-rabbit secondary antibody (Santa Cruz). The specific signals were detected by enhanced chemiluminescence using ECL reagent (Amersham Biosciences, Piscataway, NJ). The intensity of signals was quantified using ImageJ software (version 1.37v; National Institutes of Health). The loading of protein in each lane was assessed by α-tubulin antibody (Sigma, St. Louis, MO).
Measurement of DPC
DPCs were measured by the K+/SDS precipitation assay as previously described (Costa et al., 1997; Zhang et al., 2002; Zhitkovich and Costa, 1992) with little modification. EBV-BL cells were adjusted to a concentration of 3.5×105 cells/ml and incubated overnight. Following treatment with selected concentrations of Cr(VI) in the presence or absence of EGCG for 24 h, cells were collected by centrifugation and washed twice with PBS. Cells were finally resuspended in ice-cold PBS at a concentration of 7.5 × 105 cells/ml. Cells were then lysed in 0.5% SDS, 20 mM Tris-HCl (pH 7.5), 1mM phenylmethyl-sulfonyl fluoride (PMSF) solution and placed at −70 °C. After thawing, the mixture was vigorously passed four times through a 22-gauge needle to shear DNA. Then, 0.5 ml of 100 mM KCl, 20 mM Tris-HCl (pH 7.5) was added and the content was vortexed for 5 s at maximal speed and heated at 65 °C for 10 min, followed by inverting the mixture three times and incubation on ice for 10 min. The precipitate was collected by centrifugation at 4,400 rpm for 5 min at 4 °C. The supernatant was saved and the pellet was resuspended in 100 mM KCl, 20 mM Tris-HCl (pH 7.5) by brief vortexing at the highest setting. The heating, cooling, and centrifugation steps were repeated for a total of three washes. The final pellet was resuspended in 100 mM KCl, 20 mM Tris-HCl (pH 7.5) containing 10 mM EDTA (pH 8.0) and 0.4 mg/ml proteinase K, and incubated at 50°C overnight. To achieve more complete precipitation of residual SDS 4 mg/ml BSA was added to each tube and the samples were placed on ice for 1 h. The tubes were centrifuged at 4,400 rpm for 20 min at 4 °C and the supernatant was taken to determine the quantity of DNA. The DNA content of the supernatants from the washes and the proteinase K-digested K+/SDS pellet were measured on the SpectraMax microplate reader using PicoGreen dye (emission 535 nm, excitation 485 nm). The percentage of cross-linked DNA is the ratio of SDS-precipitable DNA to total DNA.
The amount of DNA-bound Cr in DPC was determined by inclusion of radiolabeled 51Cr(VI). Cells were seeded as mentioned above and treated with selected concentrations of Cr(VI) and/or EGCG in the presence of radiolabeled 51Cr for 24 h. Following the treatment, the cells were collected and assayed for DNA-protein cross-links. The DNA released from the proteinase K-digested K+/SDS pellet was divided into two parts. One part was quantitated for cross-linked DNA, and the other part was transferred to a scintillation tube and counted for radioactivity. The percentage of DNA-bound Cr is the ratio of radioactivity in SDS-precipitable DNA to that in total DNA.
cDNA Microarray
For microarray analysis, 4×105 BEAS-2B cells were seeded into 6 cm dishes and pretreated with EGCG (25 μM) overnight and then exposed to Cr(VI) (10 μ) for 24 h. Total RNA was isolated using Trizol (Invitrogen) according to the manufacturer's protocol. cRNA probes were synthesized and labeled using GeneChip Whole Transcript cDNA Synthesis and Amplification Kit and Terminal Labeling Kit (Affymetrix), and then hybridized to Affymetrix GeneChip Human Gene 1.0 ST Array which contains 28,869 well annotated genes. Data from the array were analyzed using GeneSpring version 11.0 (Agilent Technologies). Functional annotation was analyzed with the Gene Ontology (GO) classification system using DAVID software (http://david.abcc.ncifcrf.gov/home.jsp).
Real-time PCR
Total RNA was isolated using Trizol (Invitrogen). cDNA was synthesized from 1 μg RNA using the Superscript III kit (Invitrogen). Quantitative real-time PCR analysis was performed using SYBR green PCR Master Mix (Applied Biosystems) in an ABI prism 7300HT system (Applied Biosystems). All PCR reactions were performed in triplicate. The relative gene expression level, normalized to GAPDH expression, was calculated by −ΔΔCt. The results were presented as fold change compared to the level expressed in the untreated cells.
Statistical analysis
Each experiment was performed two or three times and representative data are shown. Data in the graph are given as mean values ± standard deviation (SD) of the mean. Statistical differences were calculated by two-tailed Student's t test and P<0.05 was considered as statistically significant.
Results
EGCG reduces Cr(VI)-induced cytotoxicity
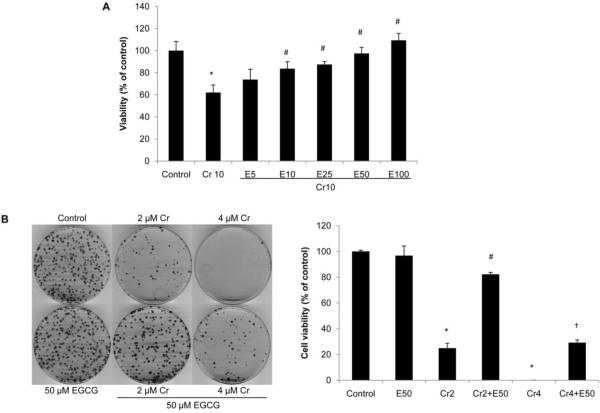
While low concentrations of EGCG have been shown to inhibit DNA damage induced by reactive oxygen and nitrogen species, higher concentrations of the compound may itself result in damage to cellular DNA (Johnson and Loo, 2000). We first assessed the effect of EGCG on cell viability of BEAS-2B cells by colony formation assay. Treatment of BEAS-2B cells with EGCG for 24 h, at doses 5–100 μM did not cause any loss of cell viability (data not shown). Next, to determine the protective effect of EGCG on Cr(VI)-induced cytotoxicity, BEAS-2B cells were treated with 10 μM Cr(VI) in the presence or absence of 5–100 μM EGCG for 24 h and cell viability was examined by MTT assay. As shown in Fig. 1A, treatment of cells with 10 μM Cr(VI) reduced cell viability to 62.1% of the control cells (P < 0.05). The presence of increasing concentrations of EGCG resulted in dose-dependent increases of cell viability, with a significant increase starting at 10 μM. Interestingly, 100 μM EGCG completely blocked Cr(VI)-induced cell death and increased cell viability slightly (109.3±2.6%) compared with the untreated control cells. To further investigate the long term protection of EGCG against Cr(VI)-induced cell death, we performed a colony formation assay, in which cells were exposed to 2 or 4 μM Cr(VI) in the presence or absence of 50 μM EGCG. These doses of Cr(VI) were selected because they were shown to be highly toxic in this assay (Sun et al., 2011). As shown in Fig. 1B, cells treated with EGCG alone reached confluency, as did the untreated cells. However, in dishes treated with Cr(VI), there were few surviving colonies. EGCG co-treatment markedly enhanced the colony forming ability of BEAS-2B cells treated with Cr(VI). Taken together, these observations indicate that Cr(VI) exposure reduced the viability of BEAS-2B cells, and this reduction of viability was inhibited by co-treatment with EGCG (Fig. 1).
Fig. 1.
EGCG reduces Cr(VI)-induced cytotoxicity. A, BEAS-2B cells were exposed to 10 μM Cr(VI) and increasing concentrations of EGCG (5–100 μM) for 24 h. MTT assay showed EGCG dose-dependently increased cell viability in Cr(VI)-treated cells. Values are Mean ± SD (n = 6). *P < 0.01 vs control, #P < 0.01 vs Cr10. B, BEAS-2B cells were treated with 2 μM or 4 μM Cr(VI) with or without 50 μM EGCG for 24h, reseeded and cultured in drug-free medium for an additional two weeks and stained with Giemsa. The colonies were counted and plotted as Mean ± SD (n = 3). *P < 0.01 vs control, #P < 0.01 vs Cr2, †P < 0.01 vs Cr4.
EGCG inhibits Cr(VI)-induced apoptosis
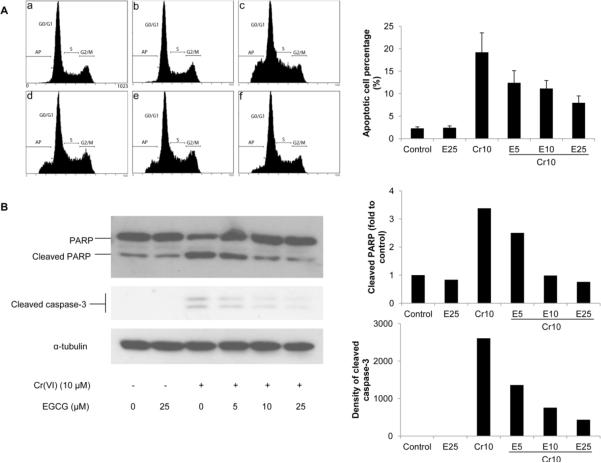
Cr(VI) compounds induce apoptosis as the primary mode of cell death (Carlisle et al., 2000). To investigate whether EGCG can suppress Cr(VI)-induced apoptotic cell death, BEAS-2B cells were treated with 10 μM Cr(VI) in the presence or absence of 5–25 μM EGCG for 24 h, and apoptosis was examined by flow cytometric analysis of cellular DNA content. Treatment with 10 μM Cr(VI) significantly increased the number of sub-G1 phase cells with hypodiploid DNA, indicating that Cr(VI) exposure induced apoptosis (19.2±4.3% vs 2.3±0.3% of control, Fig. 2A). Co-treatment with EGCG suppressed the induction of apoptosis in response to Cr(VI) exposure in a dose-dependent manner, resulting in 12.4%, 11.2%, and 7.8% of apoptotic cells, respectively (Fig. 2A). Activation of caspase-3 and nuclear poly (ADP-ribose) polymerase (PARP) are important terminal events that promote apoptosis of cells (Jiang and Wang, 2004). Therefore we examined the effect of EGCG on the activation of caspase-3 and PARP by Cr(VI) exposure. Western blot analyses showed that Cr(VI) exposure significantly induced cleavage of caspase-3 and PARP, and EGCG mitigated the effect of Cr(VI) in a dose-dependent manner (Fig. 2B). Together these data suggested that EGCG is able to protect BEAS-2B cells from Cr(VI)-induced apoptosis by inhibiting activation of apoptotic effectors (Fig. 2).
Fig. 2.
EGCG inhibits Cr(VI)-induced apoptosis. BEAS-2B cells were treated with 10 μM Cr(VI) and increasing concentrations of EGCG (5–25 μM) for 24 h. A, The cells were collected and stained with propidium iodide. DNA content was analyzed by flow cytometry, and a representative cell cycle profile was shown in the upper panel. (a) control, (b) 25 μM EGCG, (b) 10 μM Cr(VI), (d) 10 μM Cr(VI) + 5 μM EGCG, (e) 10 μM Cr(VI) + 10 μM EGCG, and (f) 10 μM Cr(VI) + 25 μM EGCG. The bottom panel is the apoptotic cell percentage. B, The cells were lysed and cleavage of PARP and caspase-3 were measured by Western blot. The left panels are representative blots while the right panels are the densitometric data.
EGCG acts as a ROS scavenger
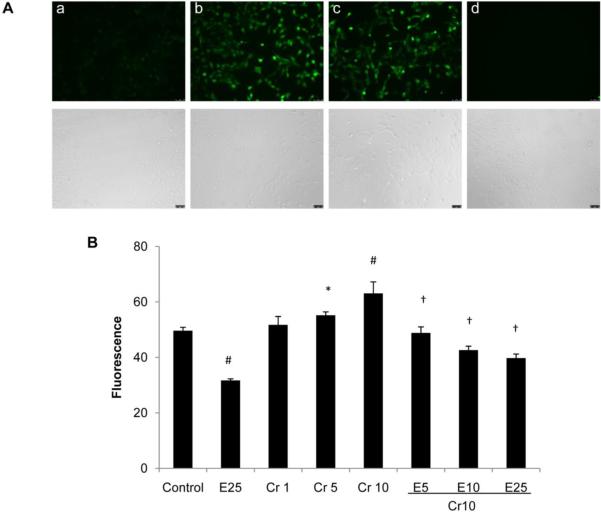
Previous studies implicated ROS in the activation of Cr(VI)-induced apoptosis and carcinogenicity (Bagchi et al., 2001; Ye et al., 1999; Zhang et al., 2001). In human lung epithelial H460 cells, addition of antioxidants such as N-acetyl cysteine (a general antioxidant) and catalase (H2O2 scavenger) inhibited Cr(VI)-induced apoptosis, confirming the involvement of ROS in Cr(VI)-induced apoptosis. On the other hand, EGCG has been recognized as an antioxidant (Nagle et al., 2006). Therefore, whether EGCG is capable of scavenging Cr(VI)-induced ROS accumulation is of interest in this investigation. Using carboxy-2', 7'-H2DCFDA as a probe, generation of intracellular ROS, which is proportional to the activity of oxidized product of DCFDA, carboxy-2', 7'-dichlorofluorescein (DCF), was measured. Intracellular ROS production in the BEAS-2B cells was easily visualized using a fluorescence microscope, as shown in Fig. 3 for a 2 h exposure of 100 μM H2O2 or 5 μM Cr(VI). EGCG, at a concentration of 25 μM, completely abolished Cr(VI)-induced ROS production, as indicated by the decrease in DCF fluorescence intensity (Fig. 3). The fluorescence was quantified using a plate reader, which provides an average of the statistically variable response of individual cells (Elbekai and El-Kadi, 2005). We observed a significant reduction in intracellular ROS level with treatment of 25 μM EGCG alone (Fig. 4). On the other hand, Cr(VI) treatment caused a dose-dependent increase in ROS production over control (Fig. 4). More importantly, DCF intensity was decreased in a dose-dependent manner upon treatment with EGCG and Cr(VI) (Fig. 4), indicating that EGCG inhibited Cr(VI)-induced cell death by acting as a potent ROS scavenger.
Fig. 3.
EGCG inhibits Cr(VI)-induced intracellular ROS generation. A, BEAS-2B cells were simultaneously treated with Cr(VI) and EGCG for 2 h and fluorescence micrographs were taken. (a) negative control, (b) positive control (100 μM H2O2), (c) 5 μM Cr(VI), and (d) 5 μM Cr(VI)+ 25 μM EGCG. Bright-field images are shown below the fluorescence micrographs. B, BEAS-2B cells were treated with increasing doses of Cr(VI) (1, 5, and 10 μM) and EGCG (5, 10, and 25 μM) for 24 h. The fluorescence was quantified using a plate reader. Values are shown as Mean ± SD (n = 3). *P < 0.05 vs control, #P < 0.01 vs control, †P < 0.01 vs Cr10.
Fig. 4.
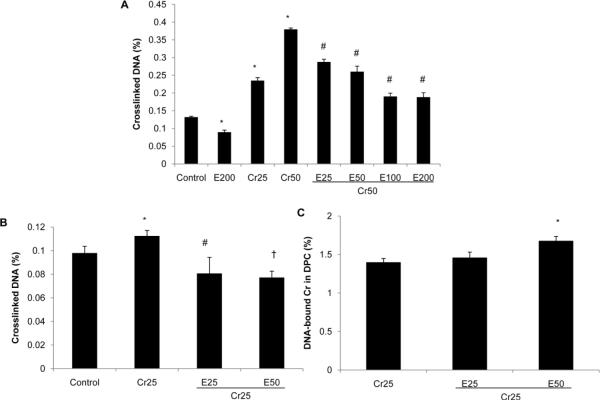
EGCG decreases Cr(VI)-induced DNA-protein cross-links (DPC). EBV-BL cells were treated with selected concentrations of Cr(VI) and EGCG in the presence or absence of radiolabeled 51Cr(VI) for 24 h. Cells were then collected and assayed for DPC as described in the Materials and Methods section. The percentage of cross-linked DNA is the ratio of SDS-precipitable DNA to total DNA. The percentage of DNA-bound Cr is the ratio of radioactivity in SDS-precipitable DNA to that in total DNA. Values are Mean ± SD (n = 3). A, Cr(VI) significantly increased DPC and addition of EGCG dose-dependently decreased Cr(VI)-induced DPC formation. *P < 0.01 vs control, #P < 0.01 vs Cr50. B, EGCG abrogated Cr(VI)-induced DPC formation in EBV-BL cells. *P < 0.05 vs control, #P < 0.01 vs Cr25, †P < 0.01 vs Cr25. C, EGCG does not inhibit Cr(III) cross-linking to DNA. *P < 0.01 vs Cr25.
EGCG inhibits Cr(VI)-induced DPC
Cr(VI) can induce DPC via either Cr(III)-mediated cross-linking reactions or oxidative mechanisms. To investigate the effects of EGCG on Cr(VI)-induced DPC, a well-established K+/SDS assay was employed. This assay is based on the selective precipitation of protein cross-linked DNA by K+/SDS. The anionic detergent SDS binds proteins but not DNA. The addition of K+ ions produces a K+/SDS precipitate, which can be recovered by low speed centrifugation. Protein-free DNA remains in the supernatant whereas protein cross-linked DNA is found in the SDS pellet. Repeated washes and high temperature heating steps ensure the dissociation of DNA from noncovalently bound proteins. The percentage of the SDS-precipitable DNA represents a quantitative measure of the number of DPC. Instead of using BEAS-2B cells, we chose EBV-BL cells as a culture system, which have been used to show that a number of chemicals are capable of inducing DNA-protein cross-links (Costa et al., 1997). As can be seen from Fig. 5A, cells treated with 25 and 50 μM Cr(VI) for 24 h exhibited increased DPC as compared with the control cells. Addition of EGCG caused a dose-dependent decrease in Cr(VI)-induced DPC formation (Fig. 5A). To test whether the protective effect of EGCG is associated with its antioxidative property or via interfering with Cr(III)-mediated cross-linking to DNA, cells were treated with 25 μM Cr(VI) and/or EGCG in the presence of radiolabeled 51Cr(VI). While EGCG was capable of abrogating Cr(VI)-induced DPC formation (Fig. 5B), it was inefficient in disrupting Cr(III) cross-linking to DNA as indicated by the similar percentage of DNA-bound Cr (Fig. 5C). These results suggest that Cr(VI) induced DPC formation partially by oxidative mechanisms, and the formation of these crosslinks was attenuated by EGCG.
Fig. 5.
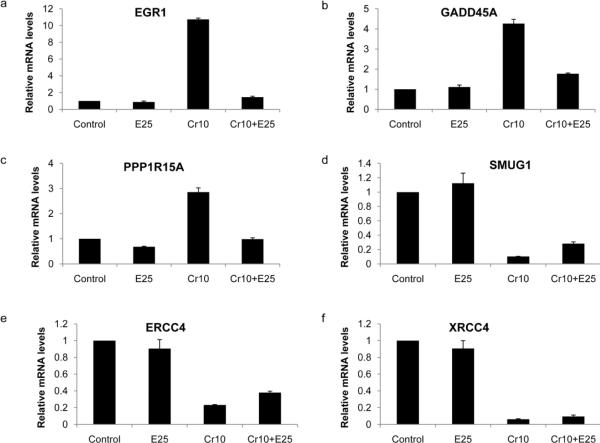
Confirmation of expression levels of selected genes by quantitative RT-PCR. BEAS-2B cells were treated with 10 μM Cr(VI) in the presence of absence of 25 μM EGCG for 24 h. Total RNA was extracted and expression levels of (a) EGR1, (b) GADD45A, (c) PPP1R15A, (d) SMUG1, (e) ERCC4, and (f) XRCC4 were analyzed by quantitative RT-PCR. Relative gene expression level, normalized to GAPDH expression, was presented as fold change to the level expressed in the control cells. Values are shown as Mean ± SD (n = 3).
EGCG modulates changes of gene expression profile in response to Cr(VI)treatment
In order to understand the effect of EGCG on gene expression changes induced by Cr(VI) exposure, we utilized microarray analyses. BEAS-2B cells were pretreated with EGCG (25 μM) overnight and then exposed to Cr(VI) (10 μM ) for 24 h. One sample each from the treatments was subjected to microarray analyses using Affymetrix Human Gene 1.0 ST Array. Untreated BEAS-2B cells were used as the control. The GeneSpring version 11.0 was used to filter gene expression levels. Of the 28,869 genes analyzed by the arrays, 3412 genes were changed more than 1.5-fold in Cr(VI)-treated cells as compared with the control cells. Of this number of genes, changes of 2404 genes were alleviated by pretreatment of EGCG. After elimination of the unannotated genes, a total of 2132 genes, including 569 up-regulated genes and 1563 down-regulated genes in response to Cr(VI) exposure, were selected for further analysis (Supplementary Table 1). We next performed Gene Ontology and pathway analysis using DAVID functional annotation software, to gain further information on the biological relevance of the EGCG regulated genes. Among the 569 up-regulated genes, the group with highest degree of significance and number of genes was transcription regulator activity (n = 71, p = 4.2 × 10−9) in Molecular Function, followed by regulation of transcription (n = 109, p = 6.8 × 10−9) in Biological Process; the most significant KEGG pathway involved was ribosome (n = 16, p = 6.3 × 10−10). Among the 1563 down-regulated genes, the group with highest degree of significance and number of genes was chromosome in Cellular Component (n = 82, p = 4.4 × 10−14); the most significant KEGG pathway involved was ubiquitin mediated proteolysis (n = 29, p = 1.1 × 10−6), endocytosis (n = 31, p = 5.4 × 10−5), and pathways in cancer (n = 46, p = 6.9 × 10−5). The complete list of the functional categories and terms is presented in Supplementary Table 2 and 3.
We next selected six genes whose functional annotation gives clues for their potential involvement in cell death and cellular defense even if their expression did not change a great deal in response to the treatments (Table 1). Messenger RNA expression of the selected genes was examined by quantitative real-time PCR (Fig.6). While the extent of the gene expression level varied somewhat between real-time PCR and microarray analyses, the direction of change was confirmed for all genes analyzed. EGR1 (early growth response 1) is a zinc-finger transcription factor that belongs to a group of early response genes. EGR1 has been implicated in the control of cell death, growth and transformation (Ahmed, 2004; Thiel and Cibelli, 2002; Thyss et al., 2005). PPP1R15A (protein phosphatase 1, regulatory (inhibitor) subunit 15; syn. GADD34) is an important protein phosphatase involved in cell death pathways (Adler et al., 1999; Hollander et al., 2001). GADD45A (growth arrest and DNA-damage-inducible, alpha), which can be induced by p53 dependent or independent pathway, is associated with cell-cycle regulation, apoptosis, DNA repair and genomic stability (Hildesheim and Fornace, 2002). Numerous studies have found a correlation between up-regulation of GADD45 and apoptotic induction (O'Reilly et al., 2000; Powolny et al., 2003; Thyss et al., 2005). These three cell death related genes were increased 10.7-, 4.4-, and 2.9-fold, respectively, in response to Cr(VI) exposure. This intensive induction was strongly inhibited by EGCG, which reduced the fold change to 1.5-, 1.3-, and 1.6-, respectively (Fig. 6 a–c). On the other hand, a set of genes involved in cellular defense were decreased by Cr(VI) treatment, and the decreases were inhibited by EGCG (Fig. 6 d–f). SMUG1 (single-strand-selective monofunctional uracil-DNA glycosylase 1) is a glycosylase that removes uracil from single- and double-stranded DNA in nuclear chromatin, thus contributing to base excision repair (Darwanto et al., 2009). XRCC4 (X-ray repair complementing defective repair in Chinese hamster cells 4) functions together with DNA ligase IV and the DNA-dependent protein kinase in the repair of DNA double-strand break by non-homologous end joining and the completion of V(D)J recombination events. ERCC4 (excision repair cross-complementing rodent repair deficiency, and complementation group 4) is involved in nucleotide excision repair (NER), which has been shown to play a pivotal role in the survival of mammalian cells after Cr(VI) exposure (O'Brien et al., 2005; Reynolds et al., 2004). The loss of ERCC4 increases the sensitivity toward Cr(VI) lethality and impairs the removal of Cr-DNA adducts (Reynolds et al., 2004).
Table 1.
Selected genes potentially involved in cell death and cellular defense.
| Affym etrix ID | Gene symbo 1 | Gene name | EGCG vs control | Cr(VI) vs control | EGCG+ Cr(VI) vs control |
|---|---|---|---|---|---|
| 8108370 | EGR1 | early growth response 1 | 1.5404166 | 6.124425 | 4.2347093 |
| 8030128 | PPP1R15A | protein phosphatase 1, regulatory (inhibitor) subunit 15A | 1.1806267 | 5.6823635 | 4.4210606 |
| 7902227 | GADD45A | growth arrest and DNA-damage-inducible, alpha | 1.0291786 | 2.729948 | 2.3178353 |
| - | - | ||||
| 8106730 | XRCC4 | X-ray repair complementing defective repair in Chinese hamster cells 4 | 1.4269797 | 4.0261154 | −3.4437976 |
| - | - | ||||
| 7963741 | SMUG1 | single-strand-selective monofunctional uracil-DNA glycosylase 1 | 1.4213992 | 2.653778 | −2.4583664 |
| - | |||||
| 7993298 | ERCC4 | excision repair cross-complementing rodent repair deficiency, complementation group 4 | 1.2749481 | 1.963632 | −1.4473683 |
Discussion
Although the precise molecular mechanisms of Cr(VI)-induced cytotoxicity are not yet fully known, ROS formation is most likely a key factor (Bagchi et al., 2001; Ye et al., 1999). Therefore, an antioxidant might play a chemopreventive role. EGCG, which has been shown to have strong antioxidative activity, is an attractive candidate due to its lack of significant toxicity in normal cells (Ahmad et al., 1997). The colony formation assay in the present study also showed that EGCG did not affect noncancerous BEAS-2B cells (data not shown). In our study, EGCG attenuated Cr(VI)-induced ROS accumulation in a dose-dependent manner, demonstrating its direct scavenging effect of free radicals. This effect can be attributed to its molecular structure, with the para-hydroxyl and galloyl groups responsible for the antioxidant effects (Tachibana et al., 2004; Umeda et al., 2008). In agreement with its scavenging effect, EGCG dose-dependently reduced cell death and apoptosis in Cr(VI)-treated BEAS-2B cells, which are consistent with other in vitro studies on cardiomyocytes (Sheng et al., 2007; Sheng et al., 2010), neuronal cells (Choi et al., 2001; Jin et al., 2001; Jung et al., 2007; Koh et al., 2004; Nie et al., 2002), and human lens epithelial cells (Yao et al., 2008), as well as an animal study which showed that a subcutaneous application of EGCG protects mouse skin cells against UVB-induced apoptosis in vivo (Filip et al., 2011). Apoptosis was originally viewed as a normal physiologic process by which correct functional cellular population dynamics are maintained through the apoptotic loss of cell populations carrying abnormal genetic information. However, defects in apoptosis regulatory mechanisms allow the damaged cells to escape from cell death and to proliferate, thus contributing to carcinogenesis (Shi et al., 2004). Indeed, the DNA damage response such as DNA double-strand break-induced apoptosis precedes the increase in DNA replication and cell proliferation in a parallel study of the progression of early premalignant and malignant lesions in human urinary bladder and lung (Bartek et al., 2007). It has been suggested that apoptosis must be considered as a component of Cr(VI)-induced multistage carcinogenesis (Manning et al., 1994). The carcinogenic process of Cr(VI) exposure is expected to occur in cycles of DNA damage response→apoptosis→cell-cycle-arrest/DNA repair, followed by an increase in unregulated DNA-replication as well as cell division and cancer development (Chiu et al., 2010). Therefore, our findings support the notion that EGCG has the potential to act as a preventive agent against Cr(VI) carcinogenesis. Future studies in transformed cells or established cancer cell lines would be valuable in elucidating the anti-carcinogenic effect of EGCG.
Importantly, using EBV-BL cells as a culture system, the present study demonstrates that EGCG was also able to inhibit Cr(VI)-induced formation of DPC. Although the biological significance of DPC in general is poorly understood, these bulky lesions have long been assumed to be genotoxic. A likely impediment of DNA replication by Cr-induced DPC has been suggested to lead to gross genetic rearrangements (Costa et al., 1993), mutations (Zhitkovich et al., 1998) or S-phase specific DNA double-strand breaks (Ha et al., 2004). In principle, Cr(VI) can induce DPC via either direct Cr(III)-mediated cross-linking reactions or oxidative mechanisms throuogh the formation of oxidized DNA bases Formation of advanced products of guanine oxidation by Cr(VI) (Slade et al., 2005) is one of the potential routes to protein cross-linking via oxidative mechanisms. Studies in CHO cells showed that about 50% of Cr(VI)-induced DPC were sensitive to disruption by EDTA (Miller and Costa, 1989), indicative of a major role of Cr(III) in DNA-protein cross-linking. However, experimentally very similar work using human MOLT4 lymphoma cells detected only a very small effect of EDTA (Mattagajasingh and Misra, 1999), which has been interpreted as evidence for oxidative linkages in DPC formation. In the present study, EGCG was shown to inhibit DPC formation in Cr(VI)-treated EBV-BL cells in a dose-dependent manner, while it does not inhibit Cr(III)-mediated cross-linking reactions. This result further indicates that EGCG is capable of protecting cells from oxidative stress-related lesions induced by Cr(VI) exposure.
Microarray analyses revealed that EGCG modulated 70% of the gene expression changes induced by Cr(VI) exposure. However, whether this modulation was mediated through its ROS scavenging property is unclear. Of note, a high portion of genes which have been shown to be involved in response to oxidative stress were increased by Cr(VI) treatment and were further reverted by pretreatment of EGCG. For instance, dual specificity phosphatases (DUSPs; DUSP5, DUSP6, DUSP7, and DUSP8 in the present study), a group of enzymes that belong to the superfamily of protein-tyrosine phosphatases, participate in the control of MAPK signaling pathway (Jeffrey et al., 2007) which is highly responsive to ROS (Son et al., 2011a) and has been shown to be involved in Cr(VI)-induced growth arrest and apoptosis (Wakeman et al., 2005). AP-1 family genes FosB and ATF3 are key early responders that demonstrate quantitative up-regulation of expression in response to oxidative stress (Chaum et al., 2009) and their activation is inhibited by a well-known antioxidant ascorbate in retinal pigment epithelium cells (Yin et al., 2010). Further studies are warranted to verify their involvement in Cr(VI)-induced cytotoxicity. It is also worth mentioning that, besides simply scavenging ROS, EGCG might also have an effect through several other putative mechanisms. It may function directly by chelating redox-active transition metal ions (Salah et al., 1995) or binding to cellular proteins (Hagerman et al., 2003) or, alternatively, indirectly by inducing phase II and antioxidative enzymes via Nrf2 pathway (Zhang, 2006). However, our findings from the microarray data suggested that EGCG might have limited effects on antioxidative enzymes because GSTA4 and GSTT2 were the only antioxidative genes regulated by EGCG. On the other hand, a series of genes involved in cell death and cellular defense, such as genes related to DNA damage, apoptosis, and DNA repair, were clearly altered by Cr(VI) treatment and EGCG was capable of reducing these changes, which may in turn contribute to its anti-cytotoxic effects as observed above.
It should be noted that although EGCG is intensively studied for its antioxidant properties, because of its low bioavailability, the peak plasma concentrations observed in humans and mice due to oral administration of polyphenols are generally 5–7 μM (Chow et al., 2003; Lambert et al., 2006; Lee et al., 2002). However, in studies with cell lines, most experiments have used EGCG concentrations in the range of 5–100 μM. It is therefore critical to devise strategies to safely increase its in vivo concentrations. Studies using lower in vitro doses which could still be effective are also needed. One possible way to reduce the concentration of EGCG required to exert biological activities, which could be more readily achieved in vivo or in humans, is to prolong the treatment period in cell culture (Shimizu et al., 2005). The other is to combine EGCG with other natural or synthetic compounds having synergistic effects. Further studies are warranted to explore these strategies.
In summary, our data clearly demonstrated that EGCG dose-dependently inhibited Cr(VI)-induced cytotoxicity and oxidative stress in BEAS-2B cells, as well as DNA-protein cross-links related to oxidative stress, at least in part, by scavenging ROS and modulating a subset of genes, supporting the potential use of this naturally occurring agent in the chemoprevention of Cr(VI) carcinogenesis.
Supplementary Material
Highlights.
EGCG protected human normal bronchial epithelial BEAS-2B cells from Cr(VI)-induced cell death and apoptosis. EGCG significantly decreased intracellular levels of ROS induced by Cr(VI) exposure. EGCG reduced DNA-protein cross-links (DPC), lesions that could be partially attributed to Cr(VI)-induced oxidative stress. EGCG modulated 70% of the gene expression changes induced by Cr(VI) exposure70% of changes of gene expression by Cr(VI) treatment were inhibited by pretreatment of EGCG.
Acknowledgments
This work was supported by National Institute of Environmental Health Sciences (NIEHS) grants ES000260, ES010344, ES014454, and ES005512; National Cancer Institute (NCI) grant CA16087; National Center for Research Resources (NCRR) grant RR029893. We would like to thank Yonghui Yu for flow cytometry analyses and Yixin Yao for fluorescence microscopic analyses.
Abbreviations
- EGCG
Epigallocatechin-3-gallate
- Cr(VI)
Hexavalent chromium
- ROS
Reactive oxygen species
- H2O2
Hydrogen peroxide
- ·O2−
Superoxide anion
- K2CrO4
Potassium chromate
- MTT
3-(4, 5-dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide
- H2DCFDA
6-carboxy-2', 7'-dichlorodihydrofluoresce in diacetate, di(acetoxymethyl ester)
- PARP
Poly (ADP-ribose) polymerase
- DPC
DNA-protein cross-links
- EGR1
Early growth response 1
- PPP1R15A
Protein phosphatase 1, regulatory (inhibitor) subunit 15
- GADD45A
Growth arrest and DNA-damage-inducible, alpha
- SMUG1
single-strand-selective monofunctional uracil-DNA glycosylase 1
- XRCC4
X-ray repair complementing defective repair in Chinese hamster cells 4
- ERCC4
Excision repair cross-complementing rodent repair deficiency, and complementation group 4
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest statement None.
References
- Adler HT, Chinery R, Wu DY, Kussick SJ, Payne JM, Fornace AJ, Jr., Tkachuk DC. Leukemic HRX fusion proteins inhibit GADD34-induced apoptosis and associate with the GADD34 and hSNF5/INI1 proteins. Mol. Cell Biol. 1999;19:7050–7060. doi: 10.1128/mcb.19.10.7050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmad N, Feyes DK, Nieminen AL, Agarwal R, Mukhtar H. Green tea constituent epigallocatechin-3-gallate and induction of apoptosis and cell cycle arrest in human carcinoma cells. J. Natl. Cancer Inst. 1997;89:1881–1886. doi: 10.1093/jnci/89.24.1881. [DOI] [PubMed] [Google Scholar]
- Ahmed MM. Regulation of radiation-induced apoptosis by early growth response-1 gene in solid tumors. Curr. Cancer Drug Targets. 2004;4:43–52. doi: 10.2174/1568009043481704. [DOI] [PubMed] [Google Scholar]
- Azad N, Iyer AK, Manosroi A, Wang L, Rojanasakul Y. Superoxide-mediated proteasomal degradation of Bcl-2 determines cell susceptibility to Cr(VI)-induced apoptosis. Carcinogenesis. 2008;29:1538–1545. doi: 10.1093/carcin/bgn137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagchi D, Bagchi M, Stohs SJ. Chromium (VI)-induced oxidative stress, apoptotic cell death and modulation of p53 tumor suppressor gene. Mol. Cell. Biochem. 2001;222:149–158. [PubMed] [Google Scholar]
- Bagchi D, Joshi SS, Bagchi M, Balmoori J, Benner EJ, Kuszynski CA, Stohs SJ. Cadmium- and chromium-induced oxidative stress, DNA damage, and apoptotic cell death in cultured human chronic myelogenous leukemic K562 cells, promyelocytic leukemic HL-60 cells, and normal human peripheral blood mononuclear cells. J. Biochem. Mol. Toxicol. 2000;14:33–41. doi: 10.1002/(sici)1099-0461(2000)14:1<33::aid-jbt5>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- Banu SK, Stanley JA, Lee J, Stephen SD, Arosh JA, Hoyer PB, Burghardt RC. Hexavalent chromium-induced apoptosis of granulosa cells involves selective sub-cellular translocation of Bcl-2 members, ERK1/2 and p53. Toxicol. Appl. Pharmacol. 2011;251:253–266. doi: 10.1016/j.taap.2011.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartek J, Bartkova J, Lukas J. DNA damage signalling guards against activated oncogenes and tumour progression. Oncogene. 2007;26:7773–7779. doi: 10.1038/sj.onc.1210881. [DOI] [PubMed] [Google Scholar]
- Baruthio F. Toxic effects of chromium and its compounds. Biol. Trace Elem. Res. 1992;32:145–153. doi: 10.1007/BF02784599. [DOI] [PubMed] [Google Scholar]
- Carlisle DL, Pritchard DE, Singh J, Patierno SR. Chromium(VI) induces p53-dependent apoptosis in diploid human lung and mouse dermal fibroblasts. Mol. Carcinog. 2000;28:111–118. doi: 10.1002/1098-2744(200006)28:2<111::aid-mc7>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- Chaum E, Yin J, Yang H, Thomas F, Lang JC. Quantitative AP-1 gene regulation by oxidative stress in the human retinal pigment epithelium. J. Cell Biochem. 2009;108:1280–1291. doi: 10.1002/jcb.22358. [DOI] [PubMed] [Google Scholar]
- Chiu A, Shi XL, Lee WK, Hill R, Wakeman TP, Katz A, Xu B, Dalal NS, Robertson JD, Chen C, Chiu N, Donehower L. Review of chromium (VI) apoptosis, cell-cycle-arrest, and carcinogenesis. J. Environ. Sci. Health C Environ. Carcinog. Ecotoxicol. Rev. 2010;28:188–230. doi: 10.1080/10590501.2010.504980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi YT, Jung CH, Lee SR, Bae JH, Baek WK, Suh MH, Park J, Park CW, Suh SI. The green tea polyphenol (−)-epigallocatechin gallate attenuates beta-amyloid-induced neurotoxicity in cultured hippocampal neurons. Life Sci. 2001;70:603–614. doi: 10.1016/s0024-3205(01)01438-2. [DOI] [PubMed] [Google Scholar]
- Chow HH, Cai Y, Hakim IA, Crowell JA, Shahi F, Brooks CA, Dorr RT, Hara Y, Alberts DS. Pharmacokinetics and safety of green tea polyphenols after multiple-dose administration of epigallocatechin gallate and polyphenon E in healthy individuals. Clin. Cancer Res. 2003;9:3312–3319. [PubMed] [Google Scholar]
- Costa M, Zhitkovich A, Harris M, Paustenbach D, Gargas M. DNA-protein cross-links produced by various chemicals in cultured human lymphoma cells. J. Toxicol. Environ. Health. 1997;50:433–449. doi: 10.1080/00984109708984000. [DOI] [PubMed] [Google Scholar]
- Costa M, Zhitkovich A, Toniolo P. DNA-protein cross-links in welders: molecular implications. Cancer Res. 1993;53:460–463. [PubMed] [Google Scholar]
- Darwanto A, Theruvathu JA, Sowers JL, Rogstad DK, Pascal T, Goddard W, 3rd, Sowers LC. Mechanisms of base selection by human single-stranded selective monofunctional uracil-DNA glycosylase. J. Biol. Chem. 2009;284:15835–15846. doi: 10.1074/jbc.M807846200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Flora S, Bagnasco M, Serra D, Zanacchi P. Genotoxicity of chromium compounds. A review. Mutat. Res. 1990;238:99–172. doi: 10.1016/0165-1110(90)90007-x. [DOI] [PubMed] [Google Scholar]
- Deschamps F, Moulin JJ, Wild P, Labriffe H, Haguenoer JM. Mortality study among workers producing chromate pigments in France. Int. Arch. Occup. Environ. Health. 1995;67:147–152. doi: 10.1007/BF00626345. [DOI] [PubMed] [Google Scholar]
- Elbekai RH, El-Kadi AO. The role of oxidative stress in the modulation of aryl hydrocarbon receptor-regulated genes by As3+, Cd2+, and Cr6+ Free Radic. Biol. Med. 2005;39:1499–1511. doi: 10.1016/j.freeradbiomed.2005.07.012. [DOI] [PubMed] [Google Scholar]
- Filip A, Daicoviciu D, Clichici S, Mocan T, Muresan A, Postescu ID. Photoprotective Effects of 2 Natural Products on Ultraviolet B-Induced Oxidative Stress and Apoptosis in SKH-1 Mouse Skin. J. Med. Food. 2011;14:761–766. doi: 10.1089/jmf.2010.0142. [DOI] [PubMed] [Google Scholar]
- Flores A, Perez JM. Cytotoxicity, apoptosis, and in vitro DNA damage induced by potassium chromate. Toxicol. Appl. Pharmacol. 1999;161:75–81. doi: 10.1006/taap.1999.8779. [DOI] [PubMed] [Google Scholar]
- Franchini I, Magnani F, Mutti A. Mortality experience among chromeplating workers. Initial findings. Scand. J. Work Environ. Health. 1983;9:247–252. doi: 10.5271/sjweh.2413. [DOI] [PubMed] [Google Scholar]
- Gambelunghe A, Piccinini R, Abbritti G, Ambrogi M, Ugolini B, Marchetti C, Migliorati G, Balducci C, Muzi G. Chromium VI-induced apoptosis in a human bronchial epithelial cell line (BEAS-2B) and a lymphoblastic leukemia cell line (MOLT-4) J. Occup. Environ. Med. 2006;48:319–325. doi: 10.1097/01.jom.0000197859.46894.7d. [DOI] [PubMed] [Google Scholar]
- Ha L, Ceryak S, Patierno SR. Generation of S phase-dependent DNA double-strand breaks by Cr(VI) exposure: involvement of ATM in Cr(VI) induction of gamma-H2AX. Carcinogenesis. 2004;25:2265–2274. doi: 10.1093/carcin/bgh242. [DOI] [PubMed] [Google Scholar]
- Hagerman AE, Dean RT, Davies MJ. Radical chemistry of epigallocatechin gallate and its relevance to protein damage. Arch. Biochem. Biophys. 2003;414:115–120. doi: 10.1016/s0003-9861(03)00158-9. [DOI] [PubMed] [Google Scholar]
- Higdon JV, Frei B. Tea catechins and polyphenols: health effects, metabolism, and antioxidant functions. Crit. Rev. Food Sci. Nutr. 2003;43:89–143. doi: 10.1080/10408690390826464. [DOI] [PubMed] [Google Scholar]
- Hildesheim J, Fornace AJ., Jr. Gadd45a: an elusive yet attractive candidate gene in pancreatic cancer. Clin Cancer Res. 2002;8:2475–2479. [PubMed] [Google Scholar]
- Hollander MC, Sheikh MS, Yu K, Zhan Q, Iglesias M, Woodworth C, Fornace AJ., Jr. Activation of Gadd34 by diverse apoptotic signals and suppression of its growth inhibitory effects by apoptotic inhibitors. Int. J. Cancer. 2001;96:22–31. doi: 10.1002/1097-0215(20010220)96:1<22::aid-ijc3>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- Ishikawa Y, Nakagawa K, Satoh Y, Kitagawa T, Sugano H, Hirano T, Tsuchiya E. Characteristics of chromate workers' cancers, chromium lung deposition and precancerous bronchial lesions: an autopsy study. Br. J. Cancer. 1994;70:160–166. doi: 10.1038/bjc.1994.268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeffrey KL, Camps M, Rommel C, Mackay CR. Targeting dual-specificity phosphatases: manipulating MAP kinase signalling and immune responses. Nat. Rev. Drug Discov. 2007;6:391–403. doi: 10.1038/nrd2289. [DOI] [PubMed] [Google Scholar]
- Jiang X, Wang X. Cytochrome C-mediated apoptosis. Annu. Rev. Biochem. 2004;73:87–106. doi: 10.1146/annurev.biochem.73.011303.073706. [DOI] [PubMed] [Google Scholar]
- Jin CF, Shen SR, Sr., Zhao BL. Different effects of five catechins on 6-hydroxydopamine-induced apoptosis in PC12 cells. J. Agric. Food Chem. 2001;49:6033–6038. doi: 10.1021/jf010903r. [DOI] [PubMed] [Google Scholar]
- Johnson MK, Loo G. Effects of epigallocatechin gallate and quercetin on oxidative damage to cellular DNA. Mutat. Res. 2000;459:211–218. doi: 10.1016/s0921-8777(99)00074-9. [DOI] [PubMed] [Google Scholar]
- Jung JY, Mo HC, Yang KH, Jeong YJ, Yoo HG, Choi NK, Oh WM, Oh HK, Kim SH, Lee JH, Kim HJ, Kim WJ. Inhibition by epigallocatechin gallate of CoCl2-induced apoptosis in rat PC12 cells. Life Sci. 2007;80:1355–1363. doi: 10.1016/j.lfs.2006.11.033. [DOI] [PubMed] [Google Scholar]
- Koh SH, Kim SH, Kwon H, Kim JG, Kim JH, Yang KH, Kim J, Kim SU, Yu HJ, Do BR, Kim KS, Jung HK. Phosphatidylinositol-3 kinase/Akt and GSK-3 mediated cytoprotective effect of epigallocatechin gallate on oxidative stress-injured neuronal-differentiated N18D3 cells. Neurotoxicology. 2004;25:793–802. doi: 10.1016/j.neuro.2004.02.001. [DOI] [PubMed] [Google Scholar]
- Lambert JD, Lee MJ, Diamond L, Ju J, Hong J, Bose M, Newmark HL, Yang CS. Dose-dependent levels of epigallocatechin-3-gallate in human colon cancer cells and mouse plasma and tissues. Drug. Metab. Dispos. 2006;34:8–11. doi: 10.1124/dmd.104.003434. [DOI] [PubMed] [Google Scholar]
- Langard S. One hundred years of chromium and cancer: a review of epidemiological evidence and selected case reports. Am. J. Ind. Med. 1990;17:189–215. doi: 10.1002/ajim.4700170205. [DOI] [PubMed] [Google Scholar]
- Langard S. Role of chemical species and exposure characteristics in cancer among persons occupationally exposed to chromium compounds. Scand. J. Work Environ. Health. 1993;19(Suppl 1):81–89. [PubMed] [Google Scholar]
- Lee MJ, Maliakal P, Chen L, Meng X, Bondoc FY, Prabhu S, Lambert G, Mohr S, Yang CS. Pharmacokinetics of tea catechins after ingestion of green tea and (-)-epigallocatechin-3-gallate by humans: formation of different metabolites and individual variability. Cancer Epidemiol. Biomarkers Prev. 2002;11:1025–1032. [PubMed] [Google Scholar]
- Li Q, Suen TC, Sun H, Arita A, Costa M. Nickel compounds induce apoptosis in human bronchial epithelial Beas-2B cells by activation of c-Myc through ERK pathway. Toxicol. Appl. Pharmacol. 2009;235:191–198. doi: 10.1016/j.taap.2008.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo H, Lu Y, Shi X, Mao Y, Dalal NS. Chromium (IV)-mediated fenton-like reaction causes DNA damage: implication to genotoxicity of chromate. Ann. Clin. Lab Sci. 1996;26:185–191. [PubMed] [Google Scholar]
- Manning FC, Blankenship LJ, Wise JP, Xu J, Bridgewater LC, Patierno SR. Induction of internucleosomal DNA fragmentation by carcinogenic chromate: relationship to DNA damage, genotoxicity, and inhibition of macromolecular synthesis. Environ. Health Perspect. 1994;102(Suppl 3):159–167. doi: 10.1289/ehp.94102s3159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattagajasingh SN, Misra HP. Analysis of EDTA-chelatable proteins from DNA-protein crosslinks induced by a carcinogenic chromium(VI) in cultured intact human cells. Mol. Cell Biochem. 1999;199:149–162. doi: 10.1023/a:1006910732307. [DOI] [PubMed] [Google Scholar]
- Miller CA, 3rd, Costa M. Analysis of proteins cross-linked to DNA after treatment of cells with formaldehyde, chromate, and cis-diamminedichloroplatinum(II) Mol. Toxicol. 1989;2:11–26. [PubMed] [Google Scholar]
- Nagle DG, Ferreira D, Zhou YD. Epigallocatechin-3-gallate (EGCG): chemical and biomedical perspectives. Phytochemistry. 2006;67:1849–1855. doi: 10.1016/j.phytochem.2006.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nie G, Cao Y, Zhao B. Protective effects of green tea polyphenols and their major component, (-)-epigallocatechin-3-gallate (EGCG), on 6-hydroxydopamine-induced apoptosis in PC12 cells. Redox. Rep. 2002;7:171–177. doi: 10.1179/135100002125000424. [DOI] [PubMed] [Google Scholar]
- O'Brien TJ, Brooks BR, Patierno SR. Nucleotide excision repair functions in the removal of chromium-induced DNA damage in mammalian cells. Mol. Cell Biochem. 2005;279:85–95. doi: 10.1007/s11010-005-8225-0. [DOI] [PubMed] [Google Scholar]
- O'Brien TJ, Ceryak S, Patierno SR. Complexities of chromium carcinogenesis: role of cellular response, repair and recovery mechanisms. Mutat. Res. 2003;533:3–36. doi: 10.1016/j.mrfmmm.2003.09.006. [DOI] [PubMed] [Google Scholar]
- O'Reilly MA, Staversky RJ, Watkins RH, Maniscalco WM, Keng PC. p53-independent induction of GADD45 and GADD153 in mouse lungs exposed to hyperoxia. Am. J. Physiol. Lung Cell Mol. Physiol. 2000;278:L552–559. doi: 10.1152/ajplung.2000.278.3.L552. [DOI] [PubMed] [Google Scholar]
- Powolny A, Takahashi K, Hopkins RG, Loo G. Induction of GADD gene expression by phenethylisothiocyanate in human colon adenocarcinoma cells. J.Cell. Biochem. 2003;90:1128–1139. doi: 10.1002/jcb.10733. [DOI] [PubMed] [Google Scholar]
- Reynolds M, Peterson E, Quievryn G, Zhitkovich A. Human nucleotide excision repair efficiently removes chromium-DNA phosphate adducts and protects cells against chromate toxicity. J. Biol. Chem. 2004;279:30419–30424. doi: 10.1074/jbc.M402486200. [DOI] [PubMed] [Google Scholar]
- Russo P, Catassi A, Cesario A, Imperatori A, Rotolo N, Fini M, Granone P, Dominioni L. Molecular mechanisms of hexavalent chromium-induced apoptosis in human bronchoalveolar cells. Am J Respir Cell Mol Biol. 2005;33:589–600. doi: 10.1165/rcmb.2005-0213OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salah N, Miller NJ, Paganga G, Tijburg L, Bolwell GP, Rice-Evans C. Polyphenolic flavanols as scavengers of aqueous phase radicals and as chain-breaking antioxidants. Arch. Biochem. Biophys. 1995;322:339–346. doi: 10.1006/abbi.1995.1473. [DOI] [PubMed] [Google Scholar]
- Sheng R, Gu ZL, Xie ML, Zhou WX, Guo CY. EGCG inhibits cardiomyocyte apoptosis in pressure overload-induced cardiac hypertrophy and protects cardiomyocytes from oxidative stress in rats. Acta Pharmacol. Sin. 2007;28:191–201. doi: 10.1111/j.1745-7254.2007.00495.x. [DOI] [PubMed] [Google Scholar]
- Sheng R, Gu ZL, Xie ML, Zhou WX, Guo CY. Epigallocatechin gallate protects H9c2 cardiomyoblasts against hydrogen dioxides- induced apoptosis and telomere attrition. Eur. J. Pharmacol. 2010;641:199–206. doi: 10.1016/j.ejphar.2010.05.054. [DOI] [PubMed] [Google Scholar]
- Shi H, Hudson LG, Liu KJ. Oxidative stress and apoptosis in metal ion-induced carcinogenesis. Free Radic. Biol. Med. 2004;37:582–593. doi: 10.1016/j.freeradbiomed.2004.03.012. [DOI] [PubMed] [Google Scholar]
- Shi X, Chiu A, Chen CT, Halliwell B, Castranova V, Vallyathan V. Reduction of chromium(VI) and its relationship to carcinogenesis. J. Toxicol. Environ. Health B Crit. Rev. 1999;2:87–104. doi: 10.1080/109374099281241. [DOI] [PubMed] [Google Scholar]
- Shi X, Mao Y, Knapton AD, Ding M, Rojanasakul Y, Gannett PM, Dalal N, Liu K. Reaction of Cr(VI) with ascorbate and hydrogen peroxide generates hydroxyl radicals and causes DNA damage: role of a Cr(IV)-mediated Fenton-like reaction. Carcinogenesis. 1994;15:2475–2478. doi: 10.1093/carcin/15.11.2475. [DOI] [PubMed] [Google Scholar]
- Shi X, Ye J, Leonard SS, Ding M, Vallyathan V, Castranova V, Rojanasakul Y, Dong Z. Antioxidant properties of (-)-epicatechin-3-gallate and its inhibition of Cr(VI)-induced DNA damage and Cr(IV)- or TPA-stimulated NF-kappaB activation. Mol. Cell Biochem. 2000;206:125–132. doi: 10.1023/a:1007012403691. [DOI] [PubMed] [Google Scholar]
- Shi XG, Sun XL, Gannett PM, Dalal NS. Deferoxamine inhibition of Cr(V)-mediated radical generation and deoxyguanine hydroxylation: ESR and HPLC evidence. Arch. Biochem. Biophys. 1992;293:281–286. doi: 10.1016/0003-9861(92)90396-e. [DOI] [PubMed] [Google Scholar]
- Shi XL, Dalal NS. Chromium (V) and hydroxyl radical formation during the glutathione reductasecatalyzed reduction of chromium (VI) Biochem. Biophys. Res. Commun. 1989;163:627–634. doi: 10.1016/0006-291x(89)92183-9. [DOI] [PubMed] [Google Scholar]
- Shimizu M, Deguchi A, Lim JT, Moriwaki H, Kopelovich L, Weinstein IB. (-)-Epigallocatechin gallate and polyphenon E inhibit growth and activation of the epidermal growth factor receptor and human epidermal growth factor receptor-2 signaling pathways in human colon cancer cells. Clin. Cancer Res. 2005;11:2735–2746. doi: 10.1158/1078-0432.CCR-04-2014. [DOI] [PubMed] [Google Scholar]
- Simonato L, Fletcher AC, Andersen A, Anderson K, Becker N, Chang-Claude J, Ferro G, Gerin M, Gray CN, Hansen KS, et al. A historical prospective study of European stainless steel, mild steel, and shipyard welders. Br. J. Ind. Med. 1991;48:145–154. doi: 10.1136/oem.48.3.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slade PG, Hailer MK, Martin BD, Sugden KD. Guanine-specific oxidation of double-stranded DNA by Cr(VI) and ascorbic acid forms spiroiminodihydantoin and 8-oxo-2'-deoxyguanosine. Chem. Res. Toxicol. 2005;18:1140–1149. doi: 10.1021/tx050033y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Son Y, Cheong YK, Kim NH, Chung HT, Kang DG, Pae HO. Mitogen-Activated Protein Kinases and Reactive Oxygen Species: How Can ROS Activate MAPK Pathways? J. Signal. Transduct. 2011a;2011:792639. doi: 10.1155/2011/792639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Son YO, Hitron JA, Cheng S, Budhraja A, Zhang Z, Lan Guo N, Lee JC, Shi X. The dual roles of c-Jun NH2-terminal kinase signaling in Cr(VI)-induced apoptosis in JB6 cells. Toxicol. Sci. 2011b;119:335–345. doi: 10.1093/toxsci/kfq335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Standeven AM, Wetterhahn KE. Possible role of glutathione in chromium(VI) metabolism and toxicity in rats. Pharmacol. Toxicol. 1991;68:469–476. doi: 10.1111/j.1600-0773.1991.tb01272.x. [DOI] [PubMed] [Google Scholar]
- Sugden KD, Stearns DM. The role of chromium(V) in the mechanism of chromate-induced oxidative DNA damage and cancer. J. Environ. Pathol. Toxicol. Oncol. 2000;19:215–230. [PubMed] [Google Scholar]
- Sun H, Clancy HA, Kluz T, Zavadil J, Costa M. Comparison of gene expression profiles in chromate transformed BEAS-2B cells. PLoS One. 2011;6:e17982. doi: 10.1371/journal.pone.0017982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tachibana H, Koga K, Fujimura Y, Yamada K. A receptor for green tea polyphenol EGCG. Nat. Struct. Mol. Biol. 2004;11:380–381. doi: 10.1038/nsmb743. [DOI] [PubMed] [Google Scholar]
- Thiel G, Cibelli G. Regulation of life and death by the zinc finger transcription factor Egr-1. J. Cell Physiol. 2002;193:287–292. doi: 10.1002/jcp.10178. [DOI] [PubMed] [Google Scholar]
- Thyss R, Virolle V, Imbert V, Peyron JF, Aberdam D, Virolle T. NF-kappaB/Egr-1/Gadd45 are sequentially activated upon UVB irradiation to mediate epidermal cell death. EMBO J. 2005;24:128–137. doi: 10.1038/sj.emboj.7600501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umeda D, Yano S, Yamada K, Tachibana H. Green tea polyphenol epigallocatechin-3-gallate signaling pathway through 67-kDa laminin receptor. J. Biol. Chem. 2008;283:3050–3058. doi: 10.1074/jbc.M707892200. [DOI] [PubMed] [Google Scholar]
- Vasant C, Balamurugan K, Rajaram R, Ramasami T. Apoptosis of lymphocytes in the presence of Cr(V) complexes: role in Cr(VI)-induced toxicity. Biochem. Biophys. Res. Commun. 2001;285:1354–1360. doi: 10.1006/bbrc.2001.5335. [DOI] [PubMed] [Google Scholar]
- Waalkes MP, Fox DA, States JC, Patierno SR, McCabe MJ., Jr. Metals and disorders of cell accumulation: modulation of apoptosis and cell proliferation. Toxicol. Sci. 2000;56:255–261. doi: 10.1093/toxsci/56.2.255. [DOI] [PubMed] [Google Scholar]
- Wakeman TP, Wyczechowska D, Xu B. Involvement of the p38 MAP kinase in Cr(VI)-induced growth arrest and apoptosis. Mol. Cell Biochem. 2005;279:69–73. doi: 10.1007/s11010-005-8216-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S, Chen F, Zhang Z, Jiang BH, Jia L, Shi X. NF-kappaB prevents cells from undergoing Cr(VI)-induced apoptosis. Mol. Cell. Biochem. 2004;255:129–137. doi: 10.1023/b:mcbi.0000007269.74532.98. [DOI] [PubMed] [Google Scholar]
- Yao K, Ye P, Zhang L, Tan J, Tang X, Zhang Y. Epigallocatechin gallate protects against oxidative stress-induced mitochondria-dependent apoptosis in human lens epithelial cells. Mol. Vis. 2008;14:217–223. [PMC free article] [PubMed] [Google Scholar]
- Ye J, Wang S, Leonard SS, Sun Y, Butterworth L, Antonini J, Ding M, Rojanasakul Y, Vallyathan V, Castranova V, Shi X. Role of reactive oxygen species and p53 in chromium(VI)-induced apoptosis. J. Biol. Chem. 1999;274:34974–34980. doi: 10.1074/jbc.274.49.34974. [DOI] [PubMed] [Google Scholar]
- Yin J, Thomas F, Lang JC, Chaum E. Modulation of oxidative stress responses in the human retinal pigment epithelium following treatment with vitamin C. J. Cell Physiol. 2010;226:2025–2032. doi: 10.1002/jcp.22532. [DOI] [PubMed] [Google Scholar]
- Zhang DD. Mechanistic studies of the Nrf2-Keap1 signaling pathway. Drug Metab. Rev. 2006;38:769–789. doi: 10.1080/03602530600971974. [DOI] [PubMed] [Google Scholar]
- Zhang Q, Kluz T, Salnikow K, Costa M. Comparison of the cytotoxicity, cellular uptake, and DNA-protein crosslinks induced by potassium chromate in lymphoblast cell lines derived from three different individuals. Biol. Trace Elem. Res. 2002;86:11–22. doi: 10.1385/BTER:86:1:11. [DOI] [PubMed] [Google Scholar]
- Zhang Z, Leonard SS, Wang S, Vallyathan V, Castranova V, Shi X. Cr (VI) induces cell growth arrest through hydrogen peroxide-mediated reactions. Mol. Cell Biochem. 2001;222:77–83. [PubMed] [Google Scholar]
- Zhitkovich A, Costa M. A simple, sensitive assay to detect DNA-protein crosslinks in intact cells and in vivo. Carcinogenesis. 1992;13:1485–1489. doi: 10.1093/carcin/13.8.1485. [DOI] [PubMed] [Google Scholar]
- Zhitkovich A, Voitkun V, Kluz T, Costa M. Utilization of DNA-protein cross-links as a biomarker of chromium exposure. Environ. Health Perspect. 1998;106(Suppl 4):969–974. doi: 10.1289/ehp.98106s4969. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.