Abstract
Sulfated low molecular weight lignins (LMWLs), designed as oligomeric mimetics of low molecular weight heparins (LMWHs), have been found to bind in exosite II of thrombin (Abdel Aziz et al. Biochem. Biophys. Res. Commun. 413 (2011) 348–352). To assess whether sulfated LMWLs recognize other heparin-binding proteins, we studied their effect on serine proteases of the coagulation, inflammatory and digestive systems. Using chromogenic substrate hydrolysis assay, sulfated LMWLs were found to potently inhibit coagulation factor XIa and human leukocyte elastase, moderately inhibit cathepsin G and not inhibit coagulation factors VIIa, IXa, and XIIa, plasma kallikrein, activated protein C, trypsin, and chymotrypsin. Competition studies show that UFH competes with sulfated LMWLs for binding to factors Xa and XIa. These results further advance the heparin-mimicking property of sulfated LMWLs and will aid the design of anticoagulants based on their novel scaffold.
1. Introduction
Anticoagulants are the mainstay in the treatment and prevention of thromboembolism. The most widely used anticoagulants include the heparins (unfractionated heparin (UFH) and low molecular weight heparin (LMWH)) and the coumarins (warfarin). Although highly successful in managing clinical conditions, the heparins and coumarins suffer from a number of problems including an enhanced bleeding risk, complications arising from an immunologic reaction, and food-drug and/or drug-drug interactions [1–3]. Additional problems such as high response inconsistency, narrow window of efficacy, poor oral bioavailability of the heparins, the necessity to monitor with high frequency, possibility of natural or intentional contamination and high cost to benefit ratio further introduce difficulty in administering safe anticoagulation therapy. Newer anticoagulants have been introduced in the clinic, e.g., fondaparinux, dabigatran, and rivaroxaban, but these continue to suffer from enhanced bleeding risk [1].
To develop better molecules as regulators of coagulation, we recently designed structural variants of lignin, a naturally occurring biopolymer, as functional macromolecular mimetics of LMWHs [4–6]. These designed oligomers, referred to as sulfated low molecular weight lignins (LMWLs), are polydisperse and heterogeneous preparations in a manner similar to LMWHs. For example, sulfated LMWLs are composed of oligomeric chains of varying lengths and contain many different inter-monomeric linkages such as β – O4, β –5, β –β and 5–5 (Fig. 1) [4]. Sulfated LMWLs are multiply charged oligomers decorated with carboxylate and sulfate groups, which mimic the polyanionic scaffold of LMWHs.
Figure 1.
Sulfated low molecular weight lignins (LMWLs) are complex three-dimensional oligomers obtained from enzymatic condensation of 4-hydroxycinnamic acid monomers using horseradish peroxidase followed by chemical sulfation using sulfur trioxide. The oligomers primarily contain β-O-4, β-5 and β-β inter-residue linkages (shown shaded). Other less common linkages, e.g., 5-5, are also present (not shown). X1, X2 and X3 are substituents at the α-position and may be– H, –OH, or –OSO3Na. Z1, Z2 and Z3 may be –H or –COONa. R3 and R5 may be either –H or –OMe depending upon the starting monomer, as shown. These variations generate a large number of sequences.
Yet, there are important differences too. Sulfated LMWLs are unlike LMWHs with regard to the nature of their backbone. Whereas sulfated LMWLs possess a hydrophobic, aromatic backbone, heparins possess a saccharide backbone. Further, whereas sulfated LMWLs contain an average of one negatively charged group for every three monomers [4], LMWHs contain nearly 3 – 3.5 anionic groups for every three monosaccharides [7,8]. The combination of an aromatic scaffold with a limited number of carboxylate and sulfate groups appears to be responsible for interesting physico-chemical properties [9]. In addition, sulfated LMWLs have been found to exhibit novel protein recognition properties [10,11]. Recent work using site directed mutants has shown that CDSO3 binds in exosite II of thrombin to effect inhibition [12], a property not exhibited by heparins, although they also bind in same binding site.
Three sulfated LMWLs, CDSO3, FDSO3 and SDSO3 (Fig. 1), have been designed based on the 4-hydroxy cinnamic acid base monomer. Structurally, the molecules appear to be similar, yet the electronic and steric properties of the hydroxy (—OH) and methoxy (—OCH3) substituents introduce considerable variation in the overall oligomeric composition. For example, SDSO3 is completely devoid of the β-5 linkage, which dominates in CDSO3, due to the presence of methoxy groups at both 3- and 5-positions. Such microscopic variances introduce differences in protein binding properties. In fact, chromogenic substrate assays have shown that all three sulfated LMWLs inhibit thrombin, factor Xa and plasmin, but the potencies are significantly different [5,11].
Considering the similarities, we reasoned that sulfated LMWLs may recognize many proteins that bind heparins, while exhibiting a functional difference, i.e., inhibition, at the same time. Thus, we studied direct inhibition of serine proteases belonging to the coagulation (factors VIIa, IXa, XIa and XIIa, activated protein C (APC) and plasma kallikrein (PK)) and inflammation (human leukocyte elastase (HLE) and cathepsin G) pathways that are known to bind heparin. In addition, three other serine proteases, trypsin, chymotrypsin, and porcine pancreatic elastase (PPE) were also included in the study to assess the possibility of non-heparin mimicking action. Sulfated LMWLs were found to potently inhibit factor XIa (fXIa) and HLE, moderately inhibit cathepsin G and not inhibit APC, PK, fVIIa, fIXa, fXIIa, PPE, trypsin, and chymotrypsin. In combination with our previous results showing potent thrombin and factor Xa inhibition and moderate plasmin inhibition by sulfated LMWLs, these results show significant selectivity of recognition. Competitive inhibition studies showed that heparin competes with sulfated LMWLs for binding to factors Xa and XIa suggesting significant functional similarity. These results advance the heparin mimicking action of sulfated LMWLs to a significant extent and are expected to aid the design of better anticoagulants based on their novel scaffold.
2. Materials and Methods
2.1 Proteins, Chemicals and Reagents
Three sulfated LMWLs, CDSO3, FDSO3 and SDSO3 (Fig. 1), were synthesized in two steps from 4-hydroxycinnamic acid monomers, caffeic acid, ferulic acid and sinapic acid, respectively, using chemo-enzymatic synthesis developed by Monien et al [4]. Human plasma proteinases including APC, fVIIa, fIXa, fXa, fXIa, plasmin and α-thrombin, were purchased from Haematologic Technologies (Essex Junction, VT). Human chymotrypsin, human PK, bovine trypsin, and PPE were purchased from Sigma (St. Louis, MO). FXIIa was purchased from Enzyme Research Laboratories (South Bend, IN). Cathepsin G and HLE was purchased from Elastin Products Company (Owensville, MO).
Chromogenic substrates Spectrozyme TH (H-D- hexahydrotyrosol-Ala-Arg-p-NA) (p-NA = p- nitroanilide), Spectrozyme FVIIa (methanesulfonyl -D-cyclohexylalanyl-butyl-Arg-p-NA), Spectrozyme FIXa (D-Leu-Phe-Gly-Arg-p-NA), Spectrozyme FXa (methoxy-carbonyl-D-cyclohexylglycyl-Gly- Arg-p-NA), Spectrozyme FXIIa (D-cyclohydro- tyrosyl-Gly-Arg-p-NA), Spectrozyme TRY (carbo- benzoxy-Gly-D-Ala-p-NA), Spectrozyme P.Kal (H- D-Pro-hexahydrotyrosyl-Arg-p-nitro-anilide), Spectrozyme PCa (H-D-(γ-carbobenzoxyl)-Lys-Pro- Arg-p-NA) and Spectrozyme PL (H-norleucyl- hexahydrotyrosyl-lysine-4-NA) were purchased from American Diagnostica (Greenwich, CT). Substrates N-succinyl-Ala-Ala-Pro-Phe-p-NA (cathepsin G), N-methoxysuccinyl-Ala-Ala-Pro-Val- p-NA (HLE) and N-succinyl-Ala-Ala-Ala-p-NA (PPE) were purchased from Sigma (St. Louis, MO). UFH (MR ~15,000 Da) was purchased from Sigma (St. Louis, MO). All other chemicals were analytical reagent grade from either Sigma Chemicals (St. Louis, MO) or Fisher (Pittsburgh, PA) and used without further purification.
2.2 Quantitative Measurement of Enzyme Inhibition Potential of Sulfated LMWLs
Direct inhibition of human coagulation enzymes by sulfated LMWLs was measured through chromogenic substrate hydrolysis assays, following our earlier report [5]. For these assays, 10 μL sulfated LMWL at concentrations ranging from 0.035 to 10,000 μg/mL was diluted with 930 μL of the appropriate buffer in PEG 20,000-coated polystyrene cuvettes. Following the preparation of the sulfated LMWL solution, 10 μL of the proteinase solution was added to the cuvette and incubated for 60 seconds. Following incubation, 50 μL of the appropriate chromogenic substrate was rapidly added to create a final volume of 1000 μL. The residual enzyme activity was determined from the initial rate of increase in absorbance at 405 nm. Special care was taken to ensure that the initial rate was measured at less than 10% consumption of substrate. The conditions used for each enzyme were as follows: 20 mM Tris-HCl buffer, pH 7.4, containing 100 mM NaCl, 2.5 mM CaCl2 and 0.1 % polyethylene glycol (PEG) 8000 at 25°C for thrombin [5]; 20 mM Tris-HCl buffer, pH 7.4, containing 100 mM NaCl, and 0.1 % PEG 8000 at 25°C for fXa and fXIa [5,13,14]; 50 mM Tris-HCl buffer, pH 7.4, containing 150 mM NaCl and 0.1% PEG 8000 at 25 °C for plasmin [11]; 100 mM HEPES buffer, pH 8, containing 100 mM NaCl and 10 mM CaCl2 at 37° C for fIXa [15]; 25 mM HEPES buffer, pH 7.4, containing 100 mM NaCl and 5 mM CaCl2 at 25°C for fVIIa [16]; 50 mM Tris-HCl, 150 mM NaCl, 2 mM CaCl2, 0.1% BSA, pH 7.6 at 25°C for fXIIa [14]; 50 mM Tris-HCl buffer, pH 8.0, containing 125 mM NaCl and 10 mM CaCl2 at 37°C for APC [17,18]; 20 mM Tris-HCl, pH 7.5, 150 mM NaCl, 2 mM CaCl2 and 0.4% BSA at 37°C for kallikrein and PPE [18,19]; 20 mM Tris-HCl, pH 7.5, 150 mM NaCl, 2 mM CaCl2 and 0.4% BSA at 25°C for chymotrypsin [19]; 50 mM Tris-HCl, pH 7.5, 150 mM NaCl, 2.5 mM CaCl2 and 0.1% PEG 8000 at 25°C for trypsin [19]; and 50 mM Tris-HCl, 50 mM NaCl, pH 8.0 for HLE and cathepsin G [20]. Enzyme concentrations in these experiments ranged from 2 nM (fXa) to 212 nM (cathepsin G). Chromogenic substrate concentrations were 50 μM (Spectrozyme FXa, Spectrozyme PL), 100 μM (Spectrozyme FVIIa, Spectrozyme FXIIa, N-succinyl-Ala-Ala-Pro-Val-p-NA, Spectrozyme TRY, N-succinyl-Ala-Ala-Ala-p-NA), 200 μM (Spectrozyme PCa, Spectrozyme FIXa, Spectrozyme P.Kal), and 1000 μM (N-succinyl- Ala-Ala-Pro-Phe-p-NA). FXIa was assayed using 500 μM Spectrozyme FXa. Relative residual proteinase activity at each concentration was calculated using the activity measured under otherwise identical conditions, except for the absence of the sulfated LMWL. Logistic equation 1 was used to fit the dose-dependence of residual proteinase activity to obtain IC50, Y0, YM and HS.
| (Eq. 1) |
In this equation, YM and Y0 are the maximum and minimum values of the fractional residual thrombin activity; IC50 is the concentration of the inhibitor that results in 50% inhibition of enzyme activity, and HS is the Hill slope.
3. Results and Discussion
3.1 Sulfated LMWLs Inhibit a Select Group of Heparin Binding Proteases
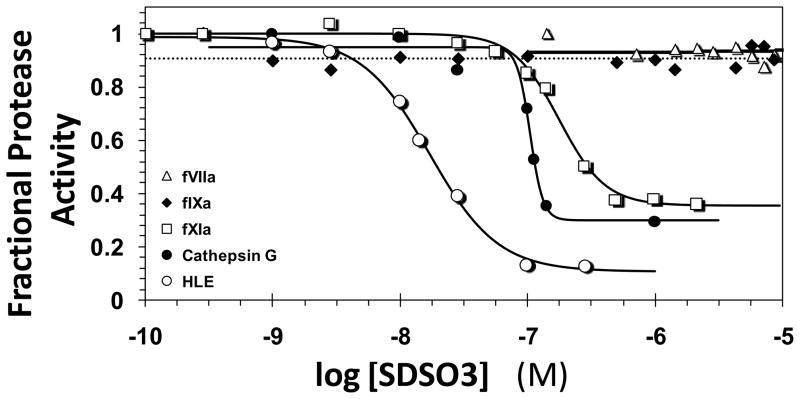
Figure 2 shows representative profiles of the residual enzyme activity as a function of SDSO3 concentration. The residual activity of cathepsin G, HLE, and fXIa progressively decreased with increasing SDSO3 concentration, while it remained essentially invariant for fVIIa and fIXa. The decrease in activity was fitted by the logistic dose – response equation 1 to derive the IC50, the concentration of SDSO3 that results in 50% reduction in activity. CDSO3 and FDSO3, the other two sulfated LMWLs, exhibited similar inhibition profiles, albeit with altered potencies (profiles not shown). Equation 1 also provides HS, the Hill slope. Although HS is typically thought of as an indicator of cooperativity of interaction for homogeneous species, ascribing this phenomenon to inhibition induced by sulfated LMWLs is likely to be error-prone. The structural complexity of sulfated LMWLs may induce multiple binding modes on the protein surface resulting in multiple occupancies, which could affect the HS. Thus, HS values are not interpreted in this analysis. Finally, the efficacy of inhibition (YM – Y0) can also be derived from equation 1. For the three sulfated LMWLs, this value was found to be in the range of 70–95 %. This is a nearly quantitative efficacy that does not seem to be affected by either the inhibitor or the enzyme and hence is not discussed further.
Figure 2.
Typical semi-log profile of direct enzyme inhibition by sulfated LMWLs. Shown is the inhibition of human leukocyte elastase (HLE, ○), cathepsin G (■), factor XIa (fXIa, □), factor IXa (fIXa, ◆) and factor VIIa (fVIIa, △) by SDSO3 as described under ‘Experimental Procedures’. Solid lines represent sigmoidal dose-response fits of Eq. 1 to the HLE, cathepsin G and fXIa data to obtain values of IC50, HS, Y0 and YM.
Sulfated LMWLs inhibit fXIa with IC50 in the range of 22 176 nM suggesting that all three molecules are potent direct inhibitors (Table 1). This potency of inhibition is comparable to that observed against thrombin and fXa [5], and higher than that against plasmin [11]. Among the three, CDSO3 was found to be 4.7 and 8.0-fold more potent than FDSO3 and SDSO3 in inhibiting fXIa, respectively. This order of activity is essentially identical to that observed with thrombin, fXa and plasmin [5,11] suggesting striking parallels in the recognition of enzymes of the coagulation system.
Table 1.
IC50 (in nM) of Sulfated LMWLs Inhibiting Heparin-Binding Serine Proteases.a
| Proteases | CDSO3 | FDSO3 | SDSO3 | UFH |
|---|---|---|---|---|
| aPCb | >12,000 | >10,000 | >11,000 | NIc |
| Cathepsin G | 232±10d | 91±7 | 105±7 | 42±7 |
| Chymotrypsin | >10,000 | >10,000 | >10,000 | NI |
| Factor XIa | 22±2d | 105±11 | 176±11 | NI |
| Factor XIIa | >15,000 | >12,000 | >14,000 | NI |
| HLEb | 11±2d | 9±2 | 17±1 | 1.1±0.1 |
| Kallikrein | >10,000 | >10,000 | >10,000 | NI |
| PPEb | >10,000 | >10,000 | >10,000 | NDe |
| Trypsin | >10,000 | >10,000 | >10,000 | NI |
| Factor IXaf | 3,380±64d | 490±16 | >28,500 | NI |
| Factor VIIaf | >29,000 | >23,000 | >28,000 | NI |
| Factor Xaf | 34±5d | 74±8 | 121±26 | NI |
| Plasming | 240±30d | 760±20 | 1,290±60 | NI |
| Thrombinf | 18±2d | 29±2 | 94±4 | NI |
The IC50 values were measured through chromogenic substrate hydrolysis assays as described in Experimental Procedures.
Stands for aPC = activated protein C, HLE = human leukocyte elastase, PPE = porcine pancreatic elastase.
No inhibition.
Errors represent ± 1 S. E.
Not determined.
Taken from ref. 5
Taken from ref. 11.
Sulfated LMWLs also directly inhibit HLE and cathepsin G with IC50 in the range of 9–17 nM and 91–232 nM, respectively (Table 1). This implies that sulfated LMWLs are considerably more potent against HLE than against fXIa, thrombin, fXa and plasmin, while their potency against cathepsin G is moderate. Interestingly, the IC50 variation among the three sulfated LMWLs for inhibiting HLE and cathepsin G is minimal (< 2-fold change). Such dependence most probably arises from a primarily electrostatic component (sulfate and carboxylate groups), which remains essentially equal for the three sulfated LMWLs.
These results are strikingly parallel to the interaction of heparin with serine proteases. Although heparin does not directly inhibit thrombin, fXa, and fXIa, its binding to these enzymes plays a crucial role in ternary complexation with antithrombin [21-23]. With regard to HLE and cathepsin G, heparin is known to be a potent, direct inhibitor in a manner similar to sulfated LMWLs (Table 1) [24,25].
To evaluate the effect of sulfated LMWLs on other serine proteases, inhibition of fXIIa, aPC, and PK was studied. Likewise, non-coagulation enzymes including trypsin, chymotrypsin, and PPE, were also studied. For each of these enzymes, concentrations of sulfated LMWLs as high as 10 μM (sometimes considerably higher) did not reduce the hydrolysis of chromogenic substrates (Table 1, Fig. 3). This indicates that sulfated LMWLs preferentially target a select group of heparin-binding serine proteases.
Figure 3.
Comparison of direct enzyme inhibition potencies (IC50, nM) of the three sulfated LMWLs, CDSO3, FDSO3 and SDSO3. The IC50 was measured as described in ‘Experimental Procedures’ using chromogenic substrate hydrolysis assay under optimal buffer and pH conditions. The IC50 of fXIIa, fVIIa, aPC, kallikrein and trypsin were found to be greater than 10 μM (see Table 1).
3.2 Sulfated LMWLs Compete with Heparin in Binding to Coagulation Enzymes
Previous studies indicated that CDSO3 does not interact with the hirudin-binding site (exosite I) of thrombin, but binds in or near the heparin-binding site, called exosite II, of thrombin [5,12]. The interaction of heparin with other coagulation factors has not been studied in detail, although it is assumed that most homologous enzymes would bind this polysaccharide in a manner similar to thrombin. This may be true for fXa, which is known to possess a homologous exosite II [26]. However, the situation changes with fXIa, which is known to possess two heparin-binding sites [13,28]. Thus, a question arises as to how well sulfated LMWLs mimic heparin binding to these enzymes?
To address this question, competitive inhibition studies were performed. Thus, if a sulfated LMWL binds in or near the heparin-binding site, the potency of its inhibition is expected to decrease in the presence of UFH. A wide range of affinities have been reported in the literature for UFH binding to coagulation enzymes. This is primarily because of the heterogeneity present in the heparin preparations. For example, the affinity of fXa, fIIa and fIXa for heparin has been measured in the range of 0.4–10 μM [15,28,29], while that of fXIa has been found to be ~10 nM [13,28]. To assess competition between sulfated LMWLs and UFH, these affinities were used.
For fXa, the presence of 10.5 μM UFH increased the IC50 of CDSO3 inhibition from 31 nM to 74 nM, while 27 μM UFH effected a further increase to 170 nM (Fig. 4A). These represent significant decreases in potencies of sulfated LMWLs in the presence of UFH suggesting competitive inhibition. Likewise, 0.27 and 2.7 μM UFH induced an increase in IC50 from 23 to 52 nM (Fig. 4B), respectively, for CDSO3 inhibition of fXIa. This increase is smaller than expected. It is possible that CDSO3 recognizes only one heparin binding site on fXIa of the two available resulting in less than optimal competition. Thus, the results support competitive phenomenon for fXIa also.
Figure 4.
Direct inhibition of factor Xa (A) and factor XIa (B) by CDSO3 in the presence of UFH. The IC50 was measured as described in ‘Experimental Procedures’ in the presence of 0 to 27 μM UFH for fXa and 0 to 2.7 μM for fXIa. See text for details.
Overall, the significant competition observed with UFH for binding to fXa and fXIa, in combination with similar results observed for thrombin and plasmin earlier [5,11], show that sulfated LMWLs appear to mimic heparin in recognizing coagulation enzymes.
3.3 Significance of the Results
Sulfated LMWLs are structurally unique and exhibit potent in vitro and ex vivo anticoagulation [4–6]. This work highlights a key aspect of their functional property – the regulation of a select group of coagulation factors, fXIa, fXa and thrombin. While thrombin and fXa are known as validated drug targets, fXIa has recently received considerable attention as an enzyme worth targeting because of its most involvement in venous thrombosis without significant in bleeding risk [30,31]. Thus, sulfated LMWLs represent powerful leads for discovering selective agents that target fXIa.
This work also shows that sulfated LMWLs inhibit enzymes HLE and cathepsin G in the manner of heparin. Further work will be necessary to identify the mechanism of inhibition and to decipher structural features that contribute to this inhibition. However, the results suggest a strong possibility for the use of sulfated LMWLs in inflammatory conditions.
The significant correspondence between heparin and sulfated LMWLs, and the recent identification of a plausible binding site of sulfated LMWLs on thrombin [12], bodes well for computational design of new macromolecular regulators. This work shows that exosite II-like domains in fXa and fXIa should be targeted in a computational search, which raises a strong possibility of engineering selectivity in such macromolecules for the first time. Thus, this work forms the basis for designing advanced macromolecules as allosteric regulators of coagulation enzymes.
Highlights.
Sulfated LMWLs potently inhibit factor XIa, leukocyte elastase and cathepsin G
Sulfated LMWLs do not inhibit factors VIIa, IXa, and XIIa, activated protein C and plasma kallikrein
Sulfated LMWLs compete with heparin for binding to factors Xa and XIa.
The results will aid the design of anticoagulants based on the sulfated LMWL scaffold.
Acknowledgments
This work was supported by Grants HL090586 and HL107152 from the National Institutes of Health and Grant EIA 0640053N from the American Heart Association National Center
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Henry BL, Desai UR. Anticoagulants. In: Rotella D, Abraham DJ, editors. Burger’s Medicinal Chemistry, Drug Discovery andDevelopment. 7. John Wiley and Sons; NY: 2010. pp. 365–408. [Google Scholar]
- 2.Hirsh J, Anand SS, Halperin JL, Fuster V. Guide to anticoagulant therapy: Heparin : a statement for healthcare professionals from the American Heart Association. Circulation. 2001;103:2994–3018. doi: 10.1161/01.cir.103.24.2994. [DOI] [PubMed] [Google Scholar]
- 3.Hirsh J, Fuster V, Ansell J, Halperin JL. American Heart Association/American College of Cardiology Foundation guide to warfarin therapy. Circulation. 2003;107:1692–1711. doi: 10.1161/01.CIR.0000063575.17904.4E. [DOI] [PubMed] [Google Scholar]
- 4.Monien BH, Henry BL, Raghuraman A, Hindle M, Desai UR. Novel chemo-enzymatic oligomers of cinnamic acids as direct and indirect inhibitors of coagulation proteinases. Bioorg Med Chem. 2006;14:7988–7998. doi: 10.1016/j.bmc.2006.07.066. [DOI] [PubMed] [Google Scholar]
- 5.Henry BL, Monien BH, Bock PE, Desai UR. A novel allosteric pathway of thrombin inhibition. Exosite II mediated potent inhibition of thrombin by chemo-enzymatic, sulfated dehydropolymers of 4-hydroxycinnamic acids. J Biol Chem. 2007;282:31891–31899. doi: 10.1074/jbc.M704257200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Henry BL, Thakkar JN, Martin EJ, Brophy DF, Desai UR. Characterization of the plasma and blood anticoagulant potential of structurally and mechanistically novel oligomers of 4-hydroxycinnamic acids. Blood Coag Fibrinol. 2009;20:27–34. doi: 10.1097/MBC.0b013e328304e077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gray E, Mulloy B, Barrowcliffe TW. Heparin and low-molecular-weight heparin. Thromb Haemost. 2008;99:807–818. doi: 10.1160/TH08-01-0032. [DOI] [PubMed] [Google Scholar]
- 8.Rabenstein DL. Heparin and heparin sulfate: Structure and function. Nat Prod Rep. 2002;19:312–331. doi: 10.1039/b100916h. [DOI] [PubMed] [Google Scholar]
- 9.Liang A, Thakkar JN, Desai UR. Study of physico-chemical properties of novel highly sulfated, aromatic, mimetics of heparin and heparan sulfate. J Pharm Sci. 2010;99:1207–1216. doi: 10.1002/jps.21908. [DOI] [PubMed] [Google Scholar]
- 10.Henry BL, Connell J, Liang A, Krishnasamy C, Desai UR. Interaction of antithrombin with sulfated, low molecular weight lignins. Opportunities for potent, selective modulation of antithrombin function. J Biol Chem. 2009;284:20897–20908. doi: 10.1074/jbc.M109.013359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Henry BL, Abdel Aziz M, Zhou Q, Desai UR. Sulfated, low molecular weight lignins are potent inhibitors of plasmin, in addition to thrombin and factor Xa: Novel opportunity for controlling complex pathologies. Thromb Haemost. 2010;103:507–515. doi: 10.1160/TH09-07-0454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Abdel Aziz MH, Mosier PD, Desai UR. Identification of the site of binding of sulfated, low molecular weight lignins on thrombin. Biochem Biophys Res Commun. 2011;413:348–352. doi: 10.1016/j.bbrc.2011.08.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yang L, Sun MF, Gailani D, Rezaie AR. Characterization of a heparin-binding site on the catalytic domain of factor XIa: mechanism of heparin acceleration of factor XIa inhibition by the serpins antithrombin and C1-inhibitor. Biochemistry. 2009;48:1517–1524. doi: 10.1021/bi802298r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shi T, Iverson GM, Qi JC, Cockerill KA, Linnik MD, Konecny P, Krilis SA. Beta 2-Glycoprotein I binds factor XI and inhibits its activation by thrombin and factor XIIa: loss of inhibition by clipped beta 2-glycoprotein I. Proc Natl Acad Sci U S A. 2004;101:3939–3944. doi: 10.1073/pnas.0400281101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bedsted T, Swanson R, Chuang YJ, Bock PE, Bjork I, Olson STT. Heparin and calcium ions dramatically enhance antithrombin reactivity with factor IXa by generating new interaction exosites. Biochemistry. 2003;42:8143–8152. doi: 10.1021/bi034363y. [DOI] [PubMed] [Google Scholar]
- 16.Neuenschwander PF, Branam DE, Morrissey JH. Importance of substrate composition, pH and other variables on tissue factor enhancement of factor VIIa activity. Thromb Haemost. 1993;70:970–977. [PubMed] [Google Scholar]
- 17.Yang L, Bae JS, Manithody C, Rezaie AR. Identification of a specific exosite on activated protein C for interaction with protease-activated receptor 1. J Biol Chem. 2007;282:25493–25500. doi: 10.1074/jbc.M702131200. [DOI] [PubMed] [Google Scholar]
- 18.Nunes VA, Gozzo AJ, Sampaio MU, Juliano MA, Sampaio CA, Araujo MS. Mapping of human plasma kallikrein active site by design of peptides based on modifications of a Kazal-type inhibitor reactive site. J Protein Chem. 2003;22:533–541. doi: 10.1023/b:jopc.0000005503.20628.4e. [DOI] [PubMed] [Google Scholar]
- 19.Berry CN, Lassalle G, Lunven C, Altenburger JM, Guilbert F, Lalé A, Hérault JP, Lecoffre C, Pfersdorff C, Herbert JM, O'Connor SE. SSR182289A, a novel, orally active thrombin inhibitor: in vitro profile and ex vivo anticoagulant activity. J Pharmacol Exp Ther. 2002;303:1189–1198. doi: 10.1124/jpet.102.040667. [DOI] [PubMed] [Google Scholar]
- 20.Yoshimura T, Barker LN, Powers JC. Specificity and reactivity of human leukocyte elastase, porcine pancreatic elastase, human granulocyte cathepsin G, and bovine pancreatic chymotrypsin with arylsulfonyl fluorides. Discovery of a new series of potent and specific irreversible elastase inhibitors. J Biol Chem. 1982;257:5077–5084. [PubMed] [Google Scholar]
- 21.Olson ST, Chuang YJ. Heparin activates antithrombin anticoagulant function by generating new interaction sites (exosites) for blood clotting proteinases. Trends Cardiovasc Med. 2002;12:331–338. doi: 10.1016/s1050-1738(02)00183-4. [DOI] [PubMed] [Google Scholar]
- 22.Olson ST, Swanson R, Raub-Segall S, Bedsted T, Sadri M, Petitou M, Herault JP, Herbert JM, Bjork I. Accelerating ability of synthetic oligosaccharides on antithrombin inhibition of proteinases of the clotting and fibrinolytic systems. Comparison with heparin and low-molecular-weight heparin. Thromb Haemost. 2004;92:929–939. doi: 10.1160/TH04-06-0384. [DOI] [PubMed] [Google Scholar]
- 23.Yang L, Manithody C, Qureshi SH, Rezaie AR. Contribution of exosite occupancy by heparin to the regulation of coagulation proteases by antithrombin. Thromb Haemost. 2010;103:277–283. doi: 10.1160/TH09-08-0585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Spencer JL, Stone PJ, Nugent MA. New insights into the inhibition of human neutrophil elastase by heparin. Biochemistry. 2006;45:9104–9120. doi: 10.1021/bi060338r. [DOI] [PubMed] [Google Scholar]
- 25.Sissi C, Lucatello L, Naggi A, Torri G, Palumbo M. Interactions of low-molecular-weight semi-synthetic sulfated heparins with human leukocyte elastase and human cathepsin G. Biochem Pharmacol. 2006;71:287–293. doi: 10.1016/j.bcp.2005.10.027. [DOI] [PubMed] [Google Scholar]
- 26.Rezaie AR. Identification of basic residues in the heparin-binding exosite of factor Xa critical for heparin and factor Va binding. J Biol Chem. 2000;275:3320–3327. doi: 10.1074/jbc.275.5.3320. [DOI] [PubMed] [Google Scholar]
- 27.Badellino KO, Walsh PN. Localization of a heparin binding site in the catalytic domain of factor XIa. Biochemistry. 2001;40:7569–7580. doi: 10.1021/bi0027433. [DOI] [PubMed] [Google Scholar]
- 28.Streusand VJ, Björk I, Gettins PG, Petitou M, Olson ST. Mechanism of acceleration of antithrombin-proteinase reactions by low affinity heparin. Role of the antithrombin binding pentasaccharide in heparin rate enhancement. J Biol Chem. 1995;270:9043–9051. doi: 10.1074/jbc.270.16.9043. [DOI] [PubMed] [Google Scholar]
- 29.Carter WJ, Cama E, Huntington JA. Crystal structure of thrombin bound to heparin. J Biol Chem. 2005;280:2745–2749. doi: 10.1074/jbc.M411606200. [DOI] [PubMed] [Google Scholar]
- 30.Schumacher WA, Luettgen JM, Quan ML, Seiffert DA. Inhibition of factor XIa as a new approach to anticoagulation. Arterioscler Thromb Vasc Biol. 2010;30:388–392. doi: 10.1161/ATVBAHA.109.197178. [DOI] [PubMed] [Google Scholar]
- 31.Emsley J, McEwan PA, Gailani D. Structure and function of factor XI. Blood. 2010;115:2569–2577. doi: 10.1182/blood-2009-09-199182. [DOI] [PMC free article] [PubMed] [Google Scholar]