Abstract
The C-terminal regions (CT) of Pfam PF04740 proteins share significant sequence identity with the toxic CdiA-CT effector domains of contact-dependent growth inhibition (CDI) systems. In accord with this homology, we find that several PF04740 CT domains inhibit cell growth when expressed in E. coli. This growth inhibition is specifically blocked by antitoxin proteins encoded downstream of each PF04740 gene. The YobL-CT, YxiD-CT and YqcG-CT domains from B. subtilis 168 have cytotoxic RNase activities, which are neutralized by the binding of cognate YobK, YxxD and YqcF antitoxin proteins, respectively. Our results show that PF04740 proteins represent a new family of toxin/antitoxin pairs that are widely distributed in Gram-positive bacteria.
Keywords: Bacillus, growth inhibition, Pfam PF04740, ribonuclease, RNase, toxin/antitoxin proteins
1. Introduction
Bacteria express a variety of systems that allow them to cooperate and compete with one another in the environment. Contact-dependent growth inhibition (CDI) is one such mechanism by which some Gram-negative bacteria inhibit the growth of neighboring cells upon direct cell-to-cell contact [1–3]. CDI is mediated by the CdiB/CdiA two-partner secretion system. CdiA is the effector protein of CDI, and its growth inhibition activity is contained within the C-terminus (CdiA-CT). CdiA-CT sequences are highly variable between CDI systems, implying that a variety of toxins are deployed by inhibitor cells. All CDI loci also encode immunity proteins, which specifically bind and inactivate cognate CdiA-CT toxins to prevent autoinhibition. We recently reported on the striking similarities between CdiA and Rhs (rearrangement hotspot) proteins, noting that some members of these two families share C- terminal toxin domains [4]. Rhs systems also encode immunity proteins that specifically neutralize the toxic effects of cognate Rhs-CT domains [4]. During these studies, we discovered that CdiA-CT sequences are also shared with other protein families, including MafB proteins from Neisseria species, Ala-Pro rich proteins from Mycobacterium species, and the Pfam PF04740 family of putative transposases from Gram-positive bacteria. Each of these families is characterized by a conserved N-terminal region and variable C-terminal sequences. The observed homologies suggest that MafB, Ala-Pro and PF04740 proteins contain variable C-terminal toxin domains.
Here, we present evidence that PF04740 proteins constitute a family of polymorphic toxins. PF04740 proteins are present in many species of Bacillus and Listeria as well as some Clostridium and Streptococcus species. Most of these species contain multiple PF04740 proteins, each carrying a distinct CT region. Moreover, the complement of PF04740 proteins varies considerably between different strains of the same species. For example, B. pumilus ATCC 7061 and B. pumilus SAFR-032 each contain five PF04740 proteins, but only one CT region is common to both strains. We examined the function of PF04740 CT domains from B. subtilis 168 and B. cereus ATCC 14579, and found that these proteins inhibit cell growth when expressed in E. coli. This growth inhibition was specifically blocked by cognate antitoxin proteins, which are encoded downstream of each PF04740 family member. Analysis of nucleic acids from inhibited cells revealed that several PF04740 CT domains possess RNase activities. Taken together these results indicate that the PF04740 family constitutes a new family of toxin-antitoxin proteins.
2. Materials and Methods
2.1 Plasmids, bacterial strains and growth conditions
All bacterial strains and plasmids are listed in Table S1. The ΔclpX and ΔclpA alleles were obtained from the Keio collection [5] and transduced into strain X90 using bacteriophage P1. The genes encoding PF04740 CT domains and associated antitoxin genes were amplified from genomic DNA by PCR and ligated into expression plasmids. PF04740 CT/antitoxin gene pairs were cloned into plasmid pET21S [4] for the overproduction and purification of CT domains and His6-tagged antitoxin proteins (Table S1). Gene pairs were also cloned into a derivative of plasmid pCH450 that fuses an in-frame ssrA(DAS) coding sequence to the antitoxin genes (Table S1) [4,6]. These latter constructs were used to test for growth inhibition activity in E. coli clp+ cells. Individual antitoxin genes were cloned into plasmid pTrc2 [7] for use in growth rescue experiments. All PCR primer sequences and plasmid sequences are available upon request. E. coli strains were cultured at 37 °C with aeration in LB medium supplemented with the appropriate antibiotics (150 μg/mL ampicillin and 12.5μg/mL tetracycline) to maintain plasmids. Synthesis of CT domains and ssrA(DAS)-tagged antitoxins was induced with 0.2% L-arabinose.
2.2 Toxin-antitoxin binding studies
PF04740 CT domains and antitoxin proteins were purified as native complexes, then isolated under denaturing conditions and refolded as described [1,4]. Purified YobL-CT or YeeF-CT was mixed with either YobK-His6 or YezG-His6 (10μM final concentration) in binding buffer [20 mM sodium phosphate (pH 7.0), 400 mM NaCl, 0.05% Triton X-100, 14 mM β-ME] and incubated for 30 min at room temperature. An aliquot of the mixture was removed for analysis by SDS-PAGE. Ni2+-nitrilotriacetic acid (NTA) agarose resin was then added and incubated at 4 °C for 1.5 hr. The resin was collected by centrifugation and the supernatant removed as the unbound fraction. After washing with binding buffer, resin-bound proteins were eluted with binding buffer supplemented with 250 mM imidazole.
2.3 RNA isolation and analysis
B. subtilis 168 and E. coli lysates were used for in vitro CT activity assays. Cells were grown to mid-log phase, harvested by centrifugation, washed once with ice-cold S30 buffer [10 mM Tris-acetate (pH 7.0), 60 mM ammonium acetate, 10 mM magnesium acetate] and then frozen at −80 °C. Thawed E. coli cells were broken by passage through a French press at 20,000 psi. B. subtilis cells were first treated with 2 mg/mL lysozyme in S30 buffer for one hr before lysis by French press. Lysates were cleared by centrifugation at 30,000 × g and adjusted to an RNA concentration of 100 μg/mL for in vitro RNase assays. Purified PF04749 CT domains and/or antitoxin proteins were added to lysates at 1 μM final concentration. Reactions were incubated at 37 °C for 1 hr, then extracted with guanidinium isothiocyanate–phenol to isolate RNA for gel analysis [8].
3. Results
3.1. Identification of PF04740 CT domains
BLAST analysis using CdiA-CT toxin domains revealed a new class of homologues designated as Pfam PF04740 proteins (http://pfam.sanger.ac.uk/family/PF04740). As an example, the CdiA-CTEC869 from E. coli O157:H7 strain EC869 shares sequence identity with the C-terminal regions of YwqJA (Uniprot B4AIN5) from Bacillus pumilus ATCC 7061 and Lin1451 (Q92BU3) from Listeria innocua strain Clip11262 (Fig. S1A). PF04740 proteins are annotated as transposases, but Zhang et al. have recently postulated that these proteins have toxic nuclease activities based on bioinformatics [9]. The regions downstream of ywqJA and lin1451 both encode small proteins (BAT_3673 (B4AIN4) and Lin1450 (Q92BU4), respectively) with weak homology to the CdiIEC869 immunity protein (Fig. S1B). These observations suggest that the genes encoding PF04740 proteins are associated with downstream immunity/antitoxin genes.
In general, Bacillus species contain several members of the PF04740 family. For example, B. subtilis strain 168 contains six predicted PF04740 proteins: YobL (O34330), YokI (O31998), YeeF (O31506), YqcG (P45942), YwqJ (P96722) and YxiD (P42296). These proteins share a conserved N-terminal region, but the C-terminal 120–150 residues are variable (Fig. S2), analogous to CdiA and Rhs proteins [1,4]. Moreover, the CT region of YeeF shares sequence identity with CdiA-CTs from Burkholderia multivorans (B9CIC5, B9BNB6), Pantoea ananatis (D4GL28) and Providencia alcalifaciens (B6XC53); and the CT region of YxiD is related to the toxic RhsB-CT (E0SIS2) from Dickeya dadantii 3937 [4]. Taken together, these observations suggest that PF04740 proteins carry a variety of toxic CT domains.
3.2. PF04740 CT domains have toxic RNase activity
Based on homology to CDI and Rhs systems [1,4], we predicted that PF04740 proteins are encoded as toxin-antitoxin pairs. To test this hypothesis, we focused on family members in B. subtilis 168. We cloned the B. subtilis yobLK and yxiD-yxxD gene pairs under control of the PBAD promoter on a plasmid vector that appends the ssrA(DAS) peptide tag onto the C-terminus of the predicted YobK and YxxD antitoxin proteins. Induction of both constructs arrested the growth of wild-type E. coli, but not E. coli ΔclpXΔ clpA mutant cells (Fig. 1), which are unable to degrade ssrA(DAS)-tagged proteins efficiently [10]. These results indicate that YobK and YxxD block the growth inhibitory activities of YobL and YxiD, respectively. We isolated RNA from growth-arrested cells and found that rRNA was cleaved upon activation of YobL and YxiD (Fig. 2). The YxiD-CT has a consensus HNH nuclease motif [11,12], which was mutated (His548Ala) to test whether RNase activity is required for growth inhibition. The resulting protein lacked RNase activity and was no longer toxic to E. coli cells (Figs. 1 & 2). YobL-CT has a similar nuclease motif and mutation of His562 to alanine ablated RNase activity and growth inhibition (Figs. 1 & 2). We also tested YqcG-CT and the CT of BC_0920 from Bacillus cereus ATCC 14579, both of which lack nuclease motifs. These CT domains inhibited E. coli growth and led to RNA degradation (Fig. 3 and not shown), indicating that several PF04740 CT domains have toxic RNase activities.
Figure 1. YobL-CT and YxiD-CT inhibit E. coli growth.
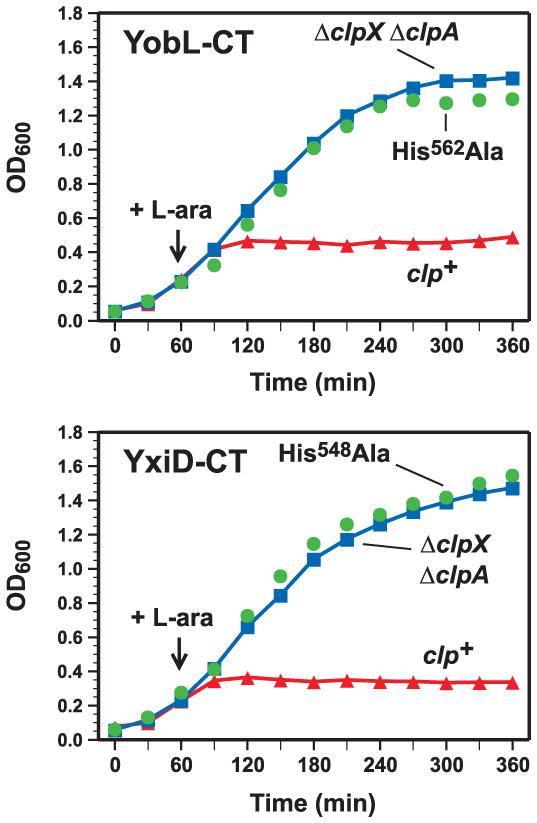
A) YobL-CT inhibits cell growth. YobL-CT/YobK-DAS synthesis was induced in wild-type E. coli (clp+) and ΔclpXΔ clpA cells with L-arabinose at the indicated time and cell growth monitored by optical density at 600 nm (OD600). A construct expressing YobL-CT with the His562Ala mutation (full-length numbering) was also tested. B) YxiD-CT inhibits cell growth. YxiD-CT/YxxD-DAS synthesis was induced in wild-type E. coli (clp+) and ΔclpX ΔclpA cells and cell growth monitored as described in panel A. A construct expressing YxiD-CT with the His548Ala mutation (full-length numbering) was also tested.
Figure 2. YobL-CT and YxiD-CT are cytotoxic RNases.
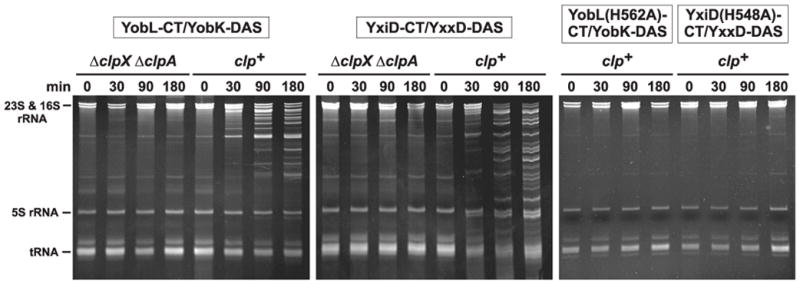
The synthesis of CT toxins and ssrA(DAS)-tagged antitoxin proteins was induced in wild-type E. coli (clp+) and ΔclpX ΔclpA cells as described in Methods. Constructs expressing YobL-CT with the His562Ala mutation and YxiD-CT with the His548Ala mutation were also expressed in wild-type E. coli (clp+). Cells were collected at the indicated times after induction and RNA isolated for gel analysis. The gel migration positions of rRNAs and tRNAs are indicated.
Figure 3. PF04740 CT domains have RNase activities.
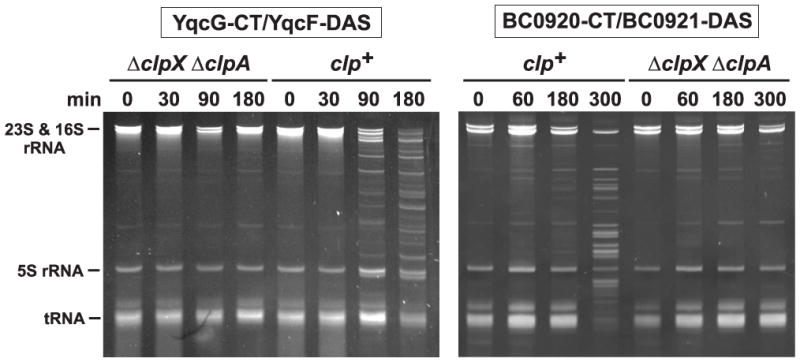
PF04740 CTs and ssrA(DAS)-tagged antitoxin proteins was induced in wild-type E. coli (clp+) and ΔclpX Δ clpA cells as described in Methods. Cells were collected at the indicated times after induction and RNA isolated for gel analysis. The gel migration positions of rRNAs and tRNAs are indicated.
3.3. Cognate antitoxin proteins block PF04740 activity
To determine if PF04740 antitoxins specifically block only cognate toxin activity, we tested antitoxins for the ability to rescue cells from PF04740 CT-mediated growth inhibition. We introduced arabinose-inducible yobL/yobK-DAS, yxiD/yxxD-DAS, yqcG/yqcF-DAS and yokI/yokJ-DAS constructs into E. coli cells that carry plasmid-borne yobK, yxxD, yqcF or yokJ antitoxin genes, and selected for transformants on LB agar supplemented with L-arabinose. Stable transformants were only obtained when cells carried the cognate antitoxin gene (Fig. 4). For example, plasmid-borne yobK permitted transformation of the yobL/yobK-DAS construct, but not the other three toxin constructs (Fig. 4). These results indicate that the B. subtilis YobK, YxxD, YqcF and YokJ proteins all possess antitoxin function that is specific for their cognate PF04740 toxins.
Figure 4. Antitoxins specifically neutralize the toxicity of cognate PF04740 CT domains.

Wild-type E. coli clp+ cells producing the YokJ, YobK, YxxD or YqcF antitoxins (labeled columns) were incubated with the indicated PF04740 CT/DAS-tagged antitoxin expression plasmids (labeled rows), then plated onto LB agar plates (supplemented with antibiotics and L-arabinose) to select transformants.
3.4. Antitoxin proteins bind cognate PF04740 proteins and block RNase activity
In CDI systems, CdiI immunity proteins neutralize CdiA-CT domains through direct binding interactions. To determine if antitoxins bind specifically to cognate PF04740 CT domains, we tested whether untagged CT domains co-purify with His6-tagged antitoxins during Ni2+-affinity chromatography. We incubated purified YobK-His6 with either YobL-CT or YeeF-CT and found that only the cognate YobL-CT co-purified with tagged YobK (Fig. 5). Similarly, only the cognate YeeF-CT domain co-purified with YezG-His6 antitoxin during Ni2+-affinity chromatography (Fig. 5). These results show that PF04740 CTs and antitoxins bind one another in a specific manner.
Figure 5. PF04740 CT domains bind specifically to cognate antitoxin proteins.
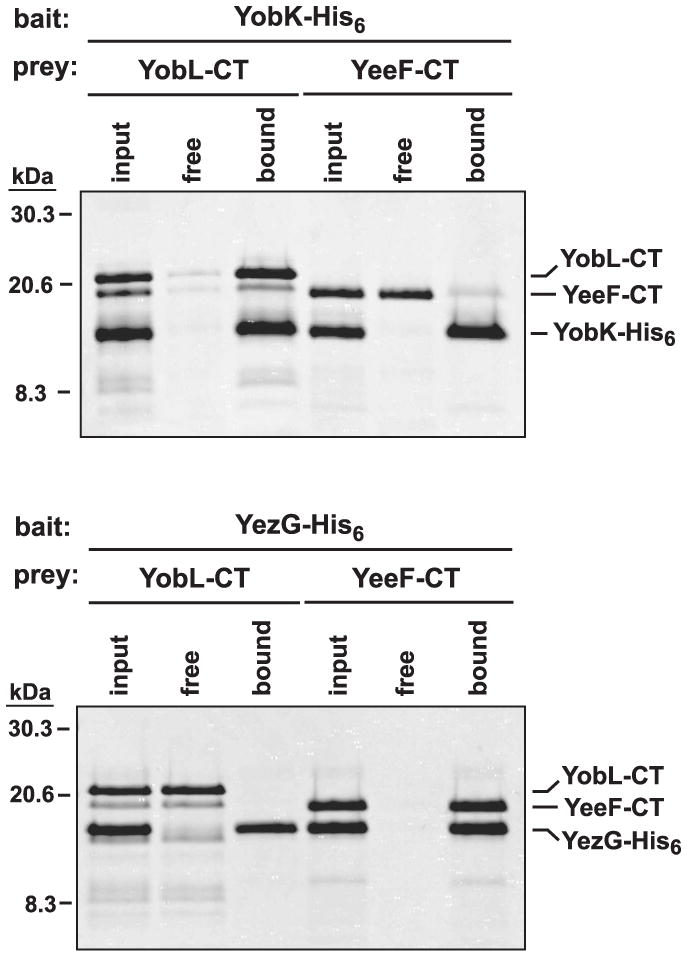
His6-tagged YobK and YezG were used as bait for the co-purification of untagged YobL-CT and YeeF-CT prey domains. CT/antitoxin protein mixtures (input) were incubated with Ni2+-NTA agarose, and the free and resin-bound fractions isolated for analysis by SDS-PAGE. The gel migration positions for molecular weight standards (given in kDa) and each CT/antitoxin pair are indicated.
Finally, we tested whether antitoxins specifically block RNase activity. Purified YobL-CT and YxiD-CT both promoted RNA degradation in E. coli cell lysates (Fig. 6A). Addition of cognate antitoxin protein completely blocked RNase activity (Fig. 6A), consistent with the experiments showing that antitoxin proteins prevent growth inhibition. We also performed these assays with B. subtilis lysates and observed similar RNA degradation (Fig. 6B). Again, RNase activity was blocked by cognate, but not non-cognate antitoxin (Fig. 6B), demonstrating the specificity of antitoxin function.
Figure 6. Antitoxin proteins specifically block PF04740 CT RNase activity.
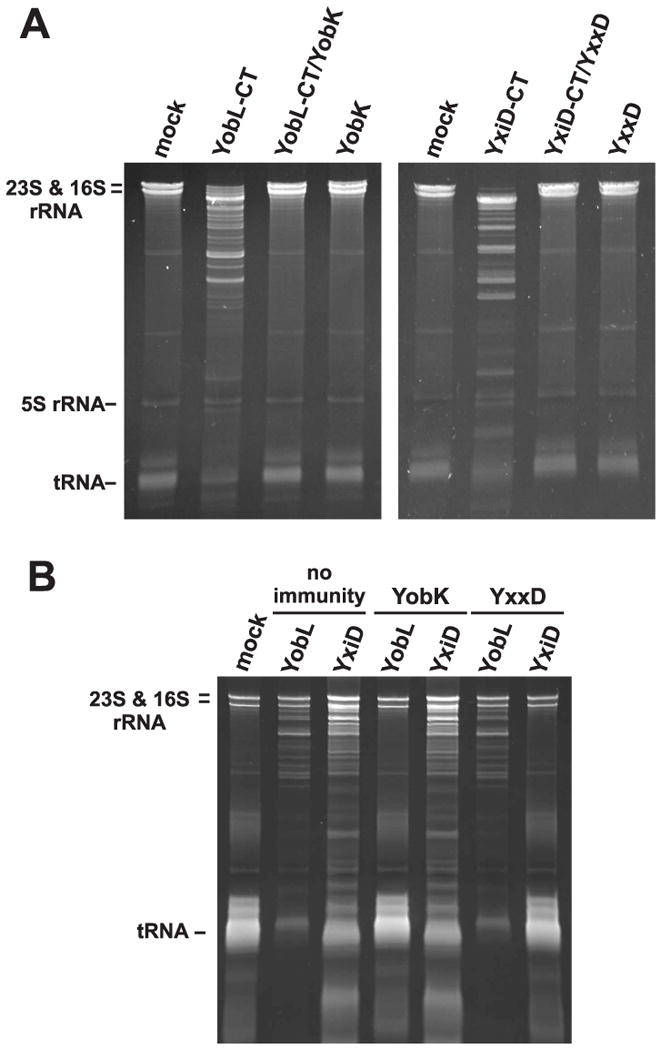
A) Antitoxins block RNase activity in an E. coli lysate. Lysate was treated with buffer (mock) or the indicated purified proteins at 1 μM final concentration. RNA was then isolated for gel analysis. B) Antitoxins block RNase activity in B. subtilis extracts. The lysate was treated as described for panel A. The gel migration positions for rRNAs and tRNAs are indicated.
4. Discussion
Our results suggest that PF04740 proteins represent a new class of bacterial toxin/antitoxin pairs, with each PF04740 gene followed by a specific antitoxin gene. Although the PF04740 CT sequences are quite divergent, several of these domains exhibit similar RNase activities. This characteristic is reminiscent of the toxin-antitoxin (TA) modules found throughout the eubacteria and archaea [13]. TA modules produce small toxins with divergent primary sequences, but many of these toxins are nucleases that cleave mRNA. However, the PF04740 toxin family is distinct from TA modules. TA systems are typically organized with the opposite gene order with the antitoxin encoded upstream of the toxin. Moreover, PF04740 toxins are significantly larger than TA module toxins and contain a conserved N-terminal region that defines the protein family. Our data show that the PF04740 toxin activity resides in the variable CT region, suggesting the N-terminus performs a function required for all family members. The N-terminal region is predicted to form an α-helical coiled-coil, and Zhang et al. have recently predicted that this domain is related of the ESAT-6/WXG100 superfamily [9,14]. The ESAT-6/WXG100 motif is a secretion signal for the type VII secretion systems of Mycobacterium and Bacillus species [15–18]. Though this hypothesis has yet to be confirmed experimentally, it suggests that the PF04740 proteins are secreted into the environment or perhaps delivered to other cells.
The PF04740 proteins could be secreted for at least two purposes. Firstly, the PF04740 RNases could be released into the environment to scavenge nucleosides for metabolism prior to sporulation. The DegU-DegS regulon controls expression of extracellular degradative enzymes, which are secreted by B. subtilis during the transition from exponential to stationary phase growth [19]. The PF04740 genes yobL, yxiD and ywqJ genes are all members of the DegS-DegU regulon [20,21], consistent with a scavenging function. In addition, the yqcG gene is under Spo0A control [22] and is therefore also expressed during stationary phase. However, if PF04740 proteins function as degradative nucleases, this raises the question of why multiple, sequence-diverse enzymes are secreted when they have nearly identical activities. A second possibility is that PF04740 proteins function in growth competition analogous to CDI systems in proteobacteria [1,3,23]. In this scenario, bacteria with different complements of PF04740 proteins would compete with one another for environmental niches. The resulting selective pressure could perhaps account for the observed diversity in PF04740 toxin/antitoxin sequences. We are actively testing these models to determine the physiological function of these toxin/antitoxin proteins.
Supplementary Material
Highlights.
We identify homologies between the toxin domains of CDI systems and PF04740 proteins.
Several PF04740 C-terminal domains have cytotoxic RNase activities.
The activity of each PF04740 protein is specifically blocked by cognate antitoxin.
We conclude that PF04740 proteins constitute a new toxin-antitoxin family.
Acknowledgments
This work was supported by grant 0642052 (D.A.L.) from the National Science Foundation and grant GM078634 (C.S.H.) from the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Aoki SK, et al. A widespread family of polymorphic contact-dependent toxin delivery systems in bacteria. Nature. 2010;468:439–42. doi: 10.1038/nature09490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aoki SK, Pamma R, Hernday AD, Bickham JE, Braaten BA, Low DA. Contact-dependent inhibition of growth in Escherichia coli. Science. 2005;309:1245–8. doi: 10.1126/science.1115109. [DOI] [PubMed] [Google Scholar]
- 3.Aoki SK, Poole SJ, Hayes CS, Low DA. Toxin on a stick: modular CDI toxin delivery systems play roles in bacterial competition. Virulence. 2011;2:356–9. doi: 10.4161/viru.2.4.16463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Poole SJ, Diner EJ, Aoki SK, Braaten BA, t’Kint de Roodenbeke C, Low DA, Hayes CS. Identification of functional toxin/immunity genes linked to contact-dependent growth inhibition (CDI) and rearrangement hotspot (Rhs) systems. PLoS Genet. 2011;7:e1002217. doi: 10.1371/journal.pgen.1002217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Baba T, et al. Construction of Escherichia coli K-12 in-frame, single-gene knockout mutants: the Keio collection. Mol Syst Biol. 2006;2:2006 0008. doi: 10.1038/msb4100050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Garza-Sanchez F, Shoji S, Fredrick K, Hayes CS. RNase II is important for A-site mRNA cleavage during ribosome pausing. Mol Microbiol. 2009;73:882–97. doi: 10.1111/j.1365-2958.2009.06813.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Holberger LE, Hayes CS. Ribosomal protein S12 and aminoglycoside antibiotics modulate A-site mRNA cleavage and transfer-messenger RNA activity in Escherichia coli. J Biol Chem. 2009;284:32188–200. doi: 10.1074/jbc.M109.062745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Garza-Sanchez F, Janssen BD, Hayes CS. Prolyl-tRNA(Pro) in the A-site of SecM-arrested ribosomes inhibits the recruitment of transfer-messenger RNA. J Biol Chem. 2006;281:34258–68. doi: 10.1074/jbc.M608052200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang D, Iyer LM, Aravind L. A novel immunity system for bacterial nucleic acid degrading toxins and its recruitment in various eukaryotic and DNA viral systems. Nucleic Acids Res. 2011;39:4532–52. doi: 10.1093/nar/gkr036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McGinness KE, Baker TA, Sauer RT. Engineering controllable protein degradation. Mol Cell. 2006;22:701–7. doi: 10.1016/j.molcel.2006.04.027. [DOI] [PubMed] [Google Scholar]
- 11.Hsia KC, Chak KF, Liang PH, Cheng YS, Ku WY, Yuan HS. DNA binding and degradation by the HNH protein ColE7. Structure. 2004;12:205–14. doi: 10.1016/j.str.2004.01.004. [DOI] [PubMed] [Google Scholar]
- 12.Pommer AJ, Kuhlmann UC, Cooper A, Hemmings AM, Moore GR, James R, Kleanthous C. Homing in on the role of transition metals in the HNH motif of colicin endonucleases. J Biol Chem. 1999;274:27153–60. doi: 10.1074/jbc.274.38.27153. [DOI] [PubMed] [Google Scholar]
- 13.Gerdes K, Christensen SK, Lobner-Olesen A. Prokaryotic toxin-antitoxin stress response loci. Nat Rev Microbiol. 2005;3:371–82. doi: 10.1038/nrmicro1147. [DOI] [PubMed] [Google Scholar]
- 14.Iyer LM, Makarova KS, Koonin EV, Aravind L. Comparative genomics of the FtsK-HerA superfamily of pumping ATPases: implications for the origins of chromosome segregation, cell division and viral capsid packaging. Nucleic Acids Res. 2004;32:5260–79. doi: 10.1093/nar/gkh828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Garufi G, Butler E, Missiakas D. ESAT-6-like protein secretion in Bacillus anthracis. J Bacteriol. 2008;190:7004–11. doi: 10.1128/JB.00458-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pallen MJ. The ESAT-6/WXG100 superfamily -- and a new Gram-positive secretion system? Trends Microbiol. 2002;10:209–12. doi: 10.1016/s0966-842x(02)02345-4. [DOI] [PubMed] [Google Scholar]
- 17.Simeone R, Bottai D, Brosch R. ESX/type VII secretion systems and their role in host-pathogen interaction. Curr Opin Microbiol. 2009;12:4–10. doi: 10.1016/j.mib.2008.11.003. [DOI] [PubMed] [Google Scholar]
- 18.Sutcliffe IC. New insights into the distribution of WXG100 protein secretion systems. Antonie Van Leeuwenhoek. 2011;99:127–31. doi: 10.1007/s10482-010-9507-4. [DOI] [PubMed] [Google Scholar]
- 19.Kunst F, Msadek T, Bignon J, Rapoport G. The DegS/DegU and ComP/ComA two-component systems are part of a network controlling degradative enzyme synthesis and competence in Bacillus subtilis. Res Microbiol. 1994;145:393–402. doi: 10.1016/0923-2508(94)90087-6. [DOI] [PubMed] [Google Scholar]
- 20.Mader U, Antelmann H, Buder T, Dahl MK, Hecker M, Homuth G. Bacillus subtilis functional genomics: genome-wide analysis of the DegS-DegU regulon by transcriptomics and proteomics. Mol Genet Genomics. 2002;268:455–67. doi: 10.1007/s00438-002-0774-2. [DOI] [PubMed] [Google Scholar]
- 21.Ogura M, Yamaguchi H, Yoshida K, Fujita Y, Tanaka T. DNA microarray analysis of Bacillus subtilis DegU, ComA and PhoP regulons: an approach to comprehensive analysis of B.subtilis two-component regulatory systems. Nucleic Acids Res. 2001;29:3804–13. doi: 10.1093/nar/29.18.3804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Molle V, Fujita M, Jensen ST, Eichenberger P, Gonzalez-Pastor JE, Liu JS, Losick R. The Spo0A regulon of Bacillus subtilis. Mol Microbiol. 2003;50:1683–701. doi: 10.1046/j.1365-2958.2003.03818.x. [DOI] [PubMed] [Google Scholar]
- 23.Hayes CS, Aoki SK, Low DA. Bacterial contact-dependent delivery systems. Annu Rev Genet. 2010;44:71–90. doi: 10.1146/annurev.genet.42.110807.091449. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


