Abstract
Objective
To determine the role of the common hepatic branch of the abdominal vagus on the beneficial effects of Roux-en-Y gastric bypass (RYGB) on weight loss, food intake, food choice, and energy expenditure in a rat model.
Background
Although, changes in gut hormone patterns are the leading candidates in RYGB’s effects on appetite, weight loss and reversal of diabetes, a potential role for afferent signaling through the vagal hepatic branch potentially sensing glucose levels in the hepatic portal vein has recently been suggested in a mouse model of RYGB.
Methods
Male Sprague-Dawley rats underwent either RYGB alone (RYGB; n = 7), RYGB + common hepatic branch vagotomy (RYGB + HV; n =6), or sham procedure (Sham; n = 9), and body weight, body composition, meal patterns, food choice, energy expenditure, and fecal energy loss were monitored up to 3 months after intervention.
Results
Both RYGB and RYGB + HV significantly reduced body weight, adiposity, meal size, and fat preference, and increased satiety, energy expenditure, and respiratory exchange rate compared with sham procedure, and there were no significant differences in these effects between RYGB and RYGB + HV rats.
Conclusions
Integrity of vagal nerve supply to the liver, hepatic portal vein, and the proximal duodenum provided by the common hepatic branch is not necessary for RYGB to reduce food intake and body weight, or increase energy expenditure. Specifically, it is unlikely that a hepatic portal vein glucose sensor signaling RYGB-induced increased intestinal gluoneogenesis to the brain depends on vagal afferent fibers.
Keywords: Bariatric surgery, vagus nerve, rat, meal patterns, meal size, satiety ratio, intermeal interval, energy expenditure, adiposity, fat preference, common hepatic branch
Introduction
Roux-en-Y gastric bypass surgery is the most effective treatment for morbid obesity and, in addition, appears to improve obesity-associated type-2 diabetes before significant weight loss has occurred. While a number of mechanisms for these remarkable RYGB-induced beneficial effects have been proposed, none have been convincingly confirmed. The leading hypotheses implicate RYGB-induced exaggerated release of the lower gut hormones GLP-1 and PYY and reduced ghrelin secretion from the stomach, acting on both peripheral and central nervous targets to ameliorate glucose homeostasis and suppress food intake 1–3. However, given the wealth of other hormones (CCK, enterostatin, obestatin), metabolic substrates (glucose, lipids), and other factors (NAPE, OEA, ApoAIV) secreted by the gastrointestinal tract and with known or potential effects on food intake and energy balance regulation 4–8, it is highly likely that the beneficial effects of RYGB are not due to one single hormone, but depend on a more complex change in gut-to-brain communication. Furthermore, most of these hormones and factors can communicate to the brain not only through the circulation 9–12, but also through vagal and spinal afferent nerve fibers 13–17 innervating the gastrointestinal tract and associated organs. Vagal afferent fibers are also important for communicating gastrointestinal distension to the brain, controlling meal size and gastrointestinal transit time18–21. Therefore, vagal integrity may play a crucial role in RYGB-induced reduction of meal size and food intake, improved glucose homeostasis, and sustained body weight loss.
Given the surgical separation of the small gastric pouch from the rest of the stomach, it is clear that RYGB denervates most of the bypassed stomach, as the major vagal and sympathetic innervation is supplied through the gastroesophageal junction and left gastric artery 22. However, innervation of the gastric pouch and most of the intestines, pancreas, hepatic portal vein, and liver remains intact after RYGB 22, 23 and may mediate critical signals to the brain. The vagal (parasympathetic) portion of this remaining innervation is provided by the ventral and dorsal gastric branches innervating the gastric pouch, the ventral (accessory) and dorsal celiac branches innervating the small and large intestines, and the common hepatic branch innervating parts of the proximal duodenum, antral stomach, pyloric sphincter, pancreas, hepatic portal vein, and liver 22.
The common hepatic branch of the ventral vagus has been implicated in a number of important physiological functions. Sensory fibers traveling in this branch have been shown to be crucial for mediating effects of intestinal 24–29 and hepatic 30–32 signals on food intake and glucose homeostasis, while efferent (preganglionic motor) fibers traveling through this branch have been shown to mediate effects of various hypothalamic and caudal brainstem manipulations (chemical and electrical stimulation) on hepatic glucose production and pancreatic insulin secretion 33–36. More specifically, sensory vagal fibers in the common hepatic branch innervate the portal hepatic vein 37 where they may sense the level of glucose 38, and selective sensory denervation of the hepatic portal vein by means of local application of capsaicin rapidly rescued the hypophagic phenotype of mice with a duodenal bypass operation 39. However, because capsaicin destroys all primary afferent nerve fibers, it is not clear whether vagal or dorsal root afferents innervating the hepatic portal vein are critical for the rescue.
The aim of the present study was to test a role for the common hepatic branch, known to provide the vagal component of the sensory innervation of the hepatic portal vein, in RYGB-induced hypophagia and body weight loss. We hypothesized that if the vagal sensory innervation of the hepatic portal vein carries signals critical for RYGB-induced early satiety and hypophagia, removal of the vagal common hepatic branch should attenuate or block these effects.
Material and Methods
Animals and housing
All protocols involved in this study were approved by the Institutional Animal Care and Use Committee at the Pennington Biomedical Research Center in accordance with guidelines established by the National Institutes of Health.
Male Sprague-Dawley rats, 3 months old and weighing ~200 g (Harlan Industries, Indianapolis, IN), were housed individually in wire-mesh cages at a constant temperature of 21–23° C with a 12hr light-dark cycle (lights on 07:00, off at 19:00). Food and water were provided ad libitum except before treatments or tests. For 16–20 weeks, rats were on a three-choice diet consisting of normal laboratory chow (Kcal%: Carb, 58; Fat, 13.5; Prot, 28.5, # 5001, Purina LabDiet, Richmond IN ), high-fat diet (Kcal%: Carb, 20; Fat, 60; Prot, 20, D12492, Research Diets, New Brunswick, NJ), and chocolate-flavored Ensure (Kcal%: Carb, 64; Fat, 21.6; Prot, 14.4, Abbott Ross, Columbus, OH), with each of the diets containing sufficient minerals and vitamins. They were then randomly assigned to either sham surgery, RYGB, or RYGB plus HVX, after which different diets were used as indicated below.
Roux-en-Y gastric bypass surgery and selective common hepatic branch vagotomy
Details of the RYGB surgical procedure have been reported earlier 40, 41. Briefly, the procedure resulted in a gastric pouch of about 20% of the total gastric volume, connected to a 15 cm-long Roux limb, a 25 cm-long common limb, and a roughly 40 cm-long biliopancreatic limb.
All the nerves crossing the gastric cut-line were transected by the cutting stapler, leading to partial denervation of the pyloric sphincter, proximal duodenum, and pancreas through branches of the gastric vagi 22, 35. However, the vagal and sympathetic supply to the gastric pouch (gastric branches), and most of the intestines, pancreas, and liver (vagal celiac and hepatic branches) remained intact.
Hepatic branch vagotomy in addition to RYGB was performed after completion of the jejuno-jejunostomy and before stomach separation, by cutting the common hepatic branch traveling along the hepatoesophageal artery with a high temperature cautery device (Aaron Medical, St. Petersberg, FL). The entire neurovascular bundle was gently lifted from the underlying tissue with a microhook after making it clearly visible by applying slight pull to the stomach and mobilizing the liver lobes laterally with moist gauze pads (Fig. 1). The lifted bundle was then cauterized immediately lateral to the microhook, with care not to damage the nearby esophagus. In addition, successful ablation was verified at the end of the experiment by dissecting the critical area under binocular guidance. While the hepato-esophageal artery and the common hepatic branch could be identified in RYGB and sham-operated rats, there were no suspicious neural connections left between the esophagus and the area of the liver hilus in any of the RYGB + HV rats (note that there is no conclusive retrograde tracing verification test for the common hepatic branch as is the case for the other abdominal vagal branches).
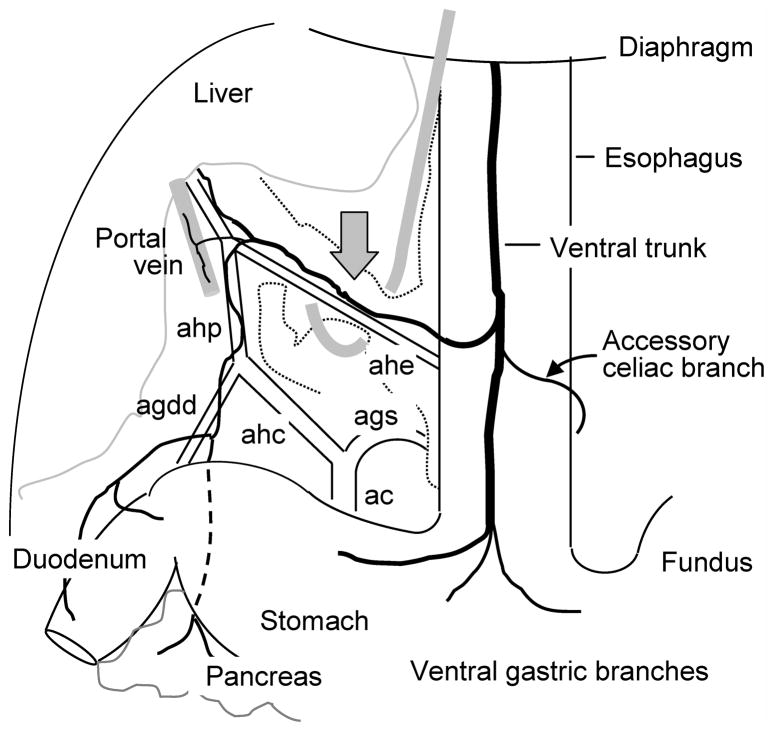
Fig. 1.
Schematic diagram showing the site of common hepatic branch vagotomy. The diagram is a ventral view of the rat abdomen and based on traced vagal branches and blood vessel supply37. The neurovascular bundle containing the hepatoesophageal artery (ahe) and the common hepatic branch were slightly lifted by means of a microhook and cauterized with a high temperature micro-cautery device near the gray arrow. The common hepatic branch further divides into fascicles running along the hepatic artery proper and portal vein, to innervate the hepatic hilus region, and the gastroduodenal artery, innervating the proximal duodenum and pancreas. Abbreviations: ahp = common hepatic artery; ac = celiac artery; ags = left gastric artery; agdd = gastroduodenal artery.
Sham-surgery consisted of the same procedure as for RYGB, except that the transected jejunum was re-anastomosed, one small incision in the jejunum 25 cm from ileocecal valve and one in the gastric fundus were sutured closed, and the cutting stapler was laid over the stomach without firing. Thus, a similar amount of surgical trauma was inflicted, but the normal flow of nutrients was preserved in sham-operated rats.
Measurement of body weight and body composition
Body weight was monitored daily for the first two weeks, and then was recorded weekly. Body composition was also measured before, and 8 – 10 weeks after surgery by using a Minispec LF 90 NMR Analyzer (Bruker Corporation, The Woodlands, TX). This method uses whole body magnetic resonance relaxometry in unanesthetized rodents with excellent linearity and reproducibility 42.
Measurement of food intake, preference, and meal patterns
All procedures were identical to the ones reported earlier, and only a brief summary is provided here. After withholding food and water for the first 24h after surgery, all rats were given access to water and chocolate Ensure for days 2–10, and a choice of high-fat chow, regular fat chow, and Ensure, for the next two weeks. For the remainder of the study a choice between high-fat and regular fat chow was offered. Ensure intake was measured by weighing the drinking bottles, and intake of high-fat and regular fat pellets was measured by weighing them separately at the beginning and end of an observation period, taking spillage into account. Percent fat preference was calculated as calories from high-fat diet/calories from high-fat plus low-fat diets × 100.
To measure meal patterns in the period of 12 – 18 days after surgery, Ensure lick behavior was assessed over 3 consecutive days by means of a lick sensor (Vital View, Mini Mitter, Bend, OR). Initiation of a meal was defined as ≥3 licks with interlick intervals of < 250 ms 43, and the end of a meal was defined by the start of a period of > 5 min without licking 44. Meal duration was calculated by subtracting the time of the first lick from the time of the last lick in a meal. Meal size was calculated on the basis of the number of licks in a meal and the average calculated lick size (total amount of Ensure consumed divided by the total number of licks over 24 hours). The satiety ratio (min/g) was calculated by dividing the intermeal intervals by the amount (g) of food consumed in the preceding meal.
Measurement of energy expenditure
Ten weeks after surgery, animals were adapted for 3 days to the special chambers, and energy expenditure and respiratory exchange rate were measured during three consecutive days under ad libitum food access conditions (Comprehensive Lab Animal Monitoring System [CLAMS]; Columbus Instruments, Columbus, OH).
Measurement of fecal energy loss
Feces were collected over a period of 3 days, weighed, and dried in a convection oven at 80 °C. Gross fecal energy content (kcal/g) was determined by bomb calorimetry in the Analytical Laboratory of Kansas State University (Manhattan, KS).
Statistical analyses
Body weight, single-diet intake, and total caloric intake across days were analyzed by two-way repeated measures ANOVA, with treatment as a between-subject factor and days as a repeated within-subject factor, followed by Bonferroni’s post-hoc multiple comparison tests. Body composition, water intake, food preference, meal pattern parameters, fecal energy, energy expenditure, and respiratory exchange rate were analyzed by one way ANOVA followed by Bonferroni’s post-hoc multiple comparison tests.
Results
Body weight and body composition
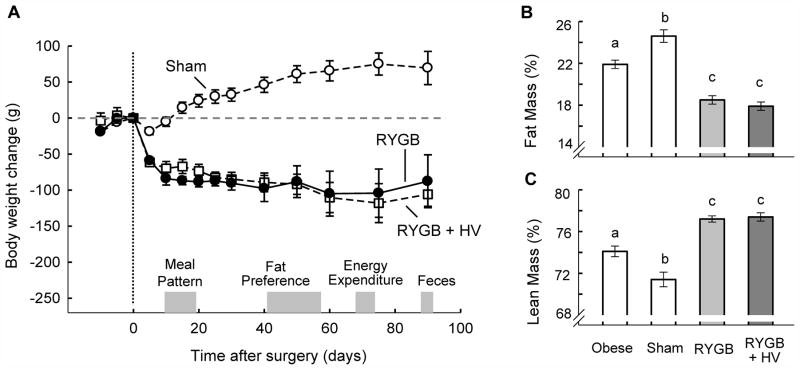
The body weight curves for both RYGB and RYGB + HV rats showed a similar rapid decline over the first 10 days followed by a slower decline until about 60 days and a steady level thereafter (Fig. 2A). In contrast, sham-operated rats showed only a very transient dip in body weight and then continued to gain additional weight.
Fig. 2.
Body weight change (A) and body composition 3 months after surgery (B,C) of rats with Roux-en-Y gastric bypass surgery (RYGB, n = 7, open squares), rats with Roux-en Y gastric bypass surgery plus common hepatic branch vagotomy (RYGB + HV, n = 6, closed circles), sham-operated rats (n = 9, open circles). Bars that do not share the same letters are significantly (p < 0.05) different from each other (based on ANOVA followed by Bonferrroni-adjusted multiple comparisons). The times of measurements of meal patterns, fat preference, energy expenditure, and fecal energy content are indicated by gray bars.
Body weight changes were well-reflected in body fat mass and adiposity index, with both RYGB and RYGB + HV rats exhibiting significantly reduced adiposity but sham-operated controls further increased adiposity 2 months after surgery, compared with pre-operative values (Fig. 2B). Percent lean mass was also not different between RYGB and RYGB + HV rats (Fig. 2C).
Food and water intake
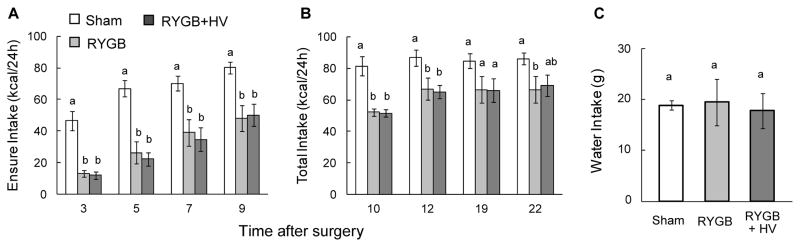
Intake of Ensure during the first 9 days and total intake (Ensure + HF + chow) during days 10–22 was significantly reduced in both RYGB and RYGB + HV rats compared with sham-operated rats after surgery (Fig. 3A,B). Separate ANOVAs for the two periods revealed significant effects of treatment (F[2,57] = 19.78, p < 0.001 and F[2,57] = 7.67, p < 0.005, as well as day (F[3,57] = 43.73, p < 0.001 and F[3,57] = 4.59, p < 0.01) for Ensure and Ensure + HF + chow, respectively. Initially, the suppression was > 50% but then moderated to about 15–20%, 3 weeks post-surgery. There was no significant interaction in either period of observation. Importantly, there was no significant difference in food intake between RYGB and RYGB + HV rats overall within either time period (t[19] = 0.26, and t[19] = 0.013, n.s.) and at any time point. Water intake measured 5 weeks post-surgery was similar for all 3 groups (Fig. 3C).
Fig. 3.
Food and water intake of sham-operated (white bars, n = 9), RYGB (dark grey bars, n = 7), and RYGB + HV rats (black bars, n = 6). (A) Intake of Ensure for days 3–9 post-surgery. (B) Total calorie intake with choice of Ensure, high-fat diet, and chow, for days 10–22 post-surgery. (C) Water intake measured on 5 consecutive days, five weeks post-surgery, when on 2-choice diet.
Liquid meal patterns
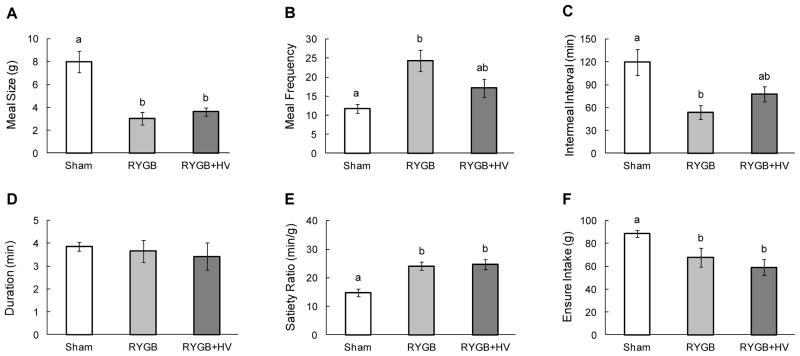
As assessed 12 – 18 days after surgery, meal size was drastically reduced by about 60 – 70 % in both RYGB and RYGB + HV rats compared with sham controls (Fig. 4A). Rats in both groups attempted to compensate for the reduced meal size by eating more meals as indicated by increased meal frequency and reduced intermeal intervals (Fig. 4B,C). While the increase in meal frequency and reduction in intermeal interval in RYGB rats were about 2-fold and significant, they were less pronounced in RYGB + HV rats and did not reach statistical significance (p = 0.20 and p = 0.15, respectively). The differences in meal frequency and intermeal interval between RYGB and RYGB + HV rats also did not reach statistical significance (p = 0.089 and p = 0.88, respectively). There were no differences in meal duration (Fig. 4D), indicating that the eating rate (ml/min) in RYGB and RYGB + HV rats was slower compared with sham-operated rats.
Fig. 4.
Liquid meal patterns assessed 12–18 days after RYGB (dark grey bars, n = 6), RYGB + HV (black bars, n = 6), and sham-surgery (white bars, n = 8). Average meal size (A), meal frequency (B), intermeal interval (C), meal duration (D), satiety ratio (E), and total intake (F). Bars that do not share the same letters are significantly different from each other (p<0.05), based on two-way ANOVA).
The greater reduction of meal size compared with intermeal interval resulted in significantly greater satiety ratios in both RYGB and RYGB + HV rats compared with sham-operated rats (Fig. 4E), and the lack of complete compensation for reduced meal size by increased meal frequency resulted in significantly reduced total intake of Ensure during the observation period (Fig. 4F).
Importantly, for none of the meal pattern parameters were there any significant differences between RYGB and RYGB + HV rats.
Food choice
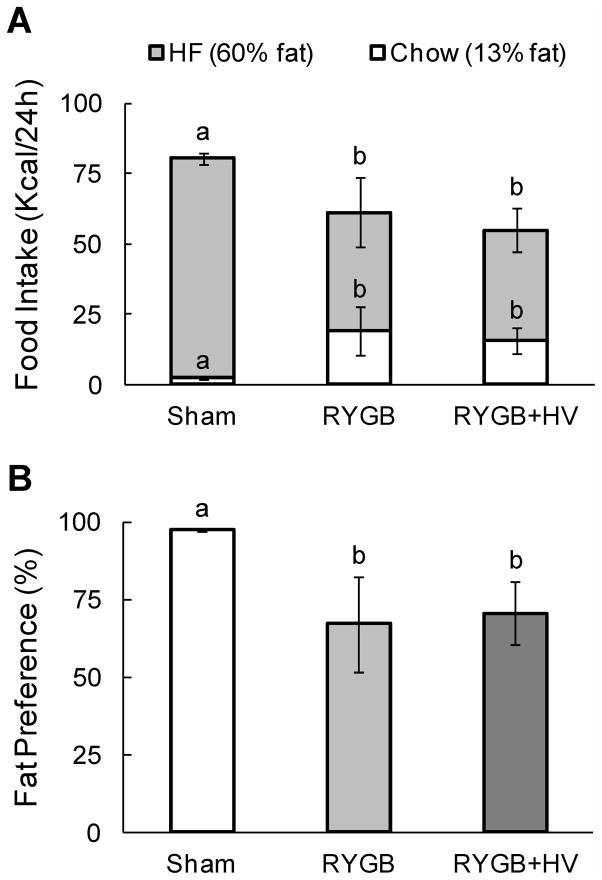
Preference for high-fat vs. low-fat pellets was tested 6 – 9 weeks after surgery. While sham-operated rats highly preferred high-fat over low-fat, this preference was significantly lower in both RYGB and RYGB + HV rats, with similar reduction (Fig. 5B). In both surgical groups, intake of energy from high-fat pellets was significantly decreased and intake of energy from low-fat pellets was significantly increased. Total energy intake from both diets was significantly reduced by about 25% (F[2,16] = 13.47, p < 0.001).
Fig. 5.
Food choice and fat preference of RYGB and sham-operated, obese and lean rats. (A) Total calorie intake from chow and high fat diet in two-choice paradigm. (B) Relative fat preference. Bars that do not share the same letters are significantly different from each other (p<0.05), based on two-way ANOVA.
Energy expenditure and respiratory exchange rate
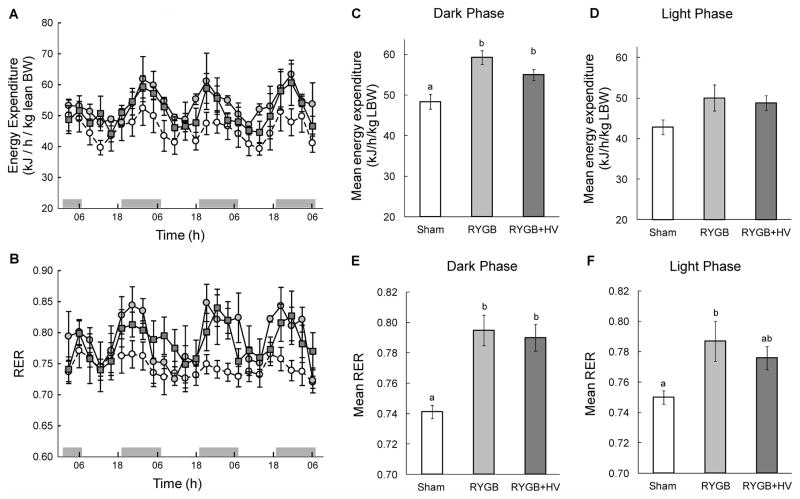
Ten weeks after the surgery, three consecutive days of measuring energy expenditure in the CLAMS system showed increased energy expenditure throughout the light/dark cycle in both RYGB and RYGB + HV rats compared with sham-operated rats, if expressed per lean body mass (Fig. 6A). The mean energy expenditure across the 3 days was about 22% and 14% higher during the dark period and 17% and 14% during the light period for RYGB and RYGB + HV, respectively, but only the increase during the dark period was statistically significant (RYGB vs. Sham: t[9] = 4.45, p < 0.05; RYGB+HV vs. Sham: t[9] = 2.93, p = 0.05; Fig. 6C,D). Importantly, there was no significant difference between RYGB and RYGB+HV (t[9] = 1, 68, n.s.). No significant effect was found when energy expenditure was expressed per total body weight or per body weight to the 0.75 power.
Fig. 6.
Energy expenditure (EE) and respiratory exchange rate (RER) measured during three consecutive days, 10 weeks after RYGB (open squares and dark gray bars, n = 6), RYGB + HV (filled circles and black bars, n = 6), or sham surgery (open circles and white bars, n = 8). A, B: Diurnal variation of energy expenditure expressed per lean body mass (A) and RER (B). C–F: Average dark (C,E) and light phase (D,F) energy expenditure and RER over 3 days. Bars that do not share the same letters are significantly different from each other (p<0.05), based on separate one-way ANOVAs followed by Tukey’s multiple comparisons tests.
Similarly, the respiratory exchange rate was increased throughout the light/dark cycle in both RYGB and RYGB + HV rats compared with sham-operated rats, indicating enhanced oxidation of carbohydrates (Fig. 6B). The mean RER across the 3 days was about 7.2% and 6.6% (both p < 0.05) higher during the dark period and 4.9% (p < 0.05) and 3.5% (p < 0.99, n.s.) during the light period for RYGB and RYGB + HV, respectively. Importantly, there was no difference between RYGB and RYGB+HV during the dark (t[9] = 0.45, n.s.) and light period (t[9] = 0.93, n.s.; Fig. 6E,F).
Fecal energy content
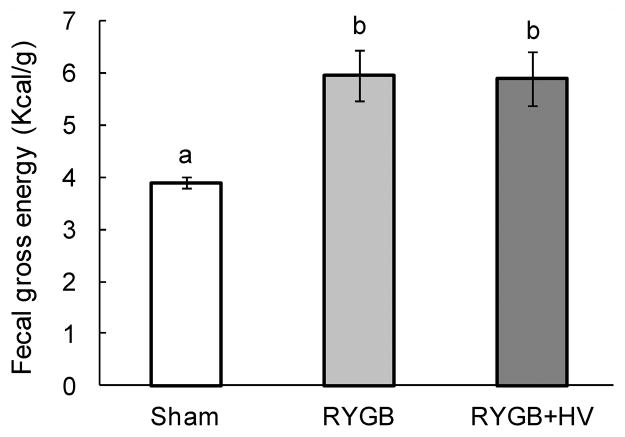
There was a small but significant (F[2,9] = 10.86, p < 0.01) increase of fecal energy content in both RYGB (6.0 ± 0.5 Kcal/g) and RYGB + HV rats (5.9 ± 0.5 Kcal/g), compared with sham-operated rats (3.9 ± 0.1 Kcal/g) (Fig. 7).
Fig. 7.
Fecal energy content assessed 10 weeks after RYGB (dark grey bars, n = 6), RYGB + HV (black bars, n = 6), and sham-surgery (white bars, n = 8). Bars that do not share the same letters are significantly different from each other (p<0.05), based on one-way ANOVA followed by Tukey’s multiple comparisons tests.
Discussion
The absence of any differences in food intake, energy expenditure, body weight, and adiposity between RYGB and RYGB + HV rats suggests that integrity of vagal fibers traveling in the common hepatic branch and innervating parts of the proximal duodenum, pancreas, hepatic portal vein, and liver, is not necessary for the beneficial effects of RYGB on these parameters. Specifically, vagal afferent fibers innervating the glucose sensor in the hepatic portal vein 26, 32 are not important for RYGB-induced reduction of food intake and body weight, and it is unlikely that the rescue of duodenal bypass-induced hypophagia by hepatic portal vein application of capsaicin in mice as reported earlier 39, is due to elimination of increased activity of vagal afferent fibers signaling satiation to the brain.
The common hepatic vagal branch is predominantly sensory and supplies not only the liver but a number of other abdominal targets 37, 45, 46. It has been implicated in a variety of sensory mechanisms such as the detection of nutrients and related factors in the duodenum, hepatic portal vein, and liver 24–32. It also contains some efferent, vagal parasympathetic fibers involved in duodenal, pancreatic, and hepatic functions 35, 36. The outcome of the present study shows that none of these sensory and motor functions are crucial for the effectiveness of RYGB to lower body weight in obese rats. Although selective ablation of sensory fibers would have been the ideal approach, it is difficult to imagine that concomitant ablation of efferent fibers is responsible for the negative outcome in the present experiment. This only could have happened if a food intake stimulatory effect of deafferentation would have been counteracted by an equally strong food intake suppressive effect of deefferentation, and there is no notion in the literature for such an effect.
The lack of a control group with only common hepatic branch vagotomy could also be considered as a limitation of the present study, but it is similarly unlikely that inclusion of such a group would have changed the conclusions. The limited literature describing long-term effects of common hepatic vagotomy on food intake and body weight is controversial. Selective common hepatic vagotomy in SD rats resulted in modest hyperphagia and an increased rate of body weight gain compared to sham vagotomized rats when fed a sweet milk diet 47 or lard 48, but had no significant effects in other studies 49, 50 and in our own unpublished study using a high-fat diet. Therefore, if anything, common hepatic branch vagotomy in the absence of RYGB leads to mild hyperphagia and increased body weight gain, and does not support an appetite suppressive role when combined with RYGB.
The common hepatic branch of the vagus is also not important for the gradual shift in preference from high-fat to low-fat diet, increased energy expenditure, and increased fecal energy content after RYGB. It suggests that vagal afferents and efferents innervating the hepatic portal vein, bile ducts, and proximal duodenum (as well as the few innervating the liver proper) are not providing important information regarding these gradual changes after RYGB. In particular, the increased energy expenditure after RYGB does not depend on this innervation, thus ruling out potential contributions of vagal effects on hepatic functions. Energy expenditure was elevated in both experimental groups compared to sham-operated animals if expressed per lean body mass, with increases statistically significant only during the dark period. Variable effects of RYGB on energy expenditure have been reported, with some studies finding an increase (rat 51–53; human 54 and other studies finding no change or even a decrease (rat 53, 55; human 56, 57. Some of these discrepancies are likely due to differences in type and time after surgery as well as correction for body mass. For example, one study found energy expenditure per rat to be decreased 2 weeks after surgery but unchanged 6 weeks after surgery, compared with sham-operated controls. Considering that by 6 weeks, the bypassed rats had significantly reduced body weight and fat mass, their relative energy expenditure was clearly higher 53. Taken together, existing studies suggest that although there might be an initial decrease as a reaction to the rapid weight loss, energy expenditure over the long term after surgery is higher than expected from the adaptive lowering of energy expenditure found after substantial body weight loss induced by caloric restriction 58.
These results do not rule out participation of the celiac and remaining gastric vagal branches in the beneficial effects of RYGB. The celiac branches which innervate the distal duodenum, jejunum, ileum, cecum, and colon are in an ideal position to mediate chemical and mechanical changes induced by RYGB. Particularly the Roux limb, exposed to large quantities of undigested food, remains innervated by the vagal celiac branches. Among the sensory terminal structures produced by the celiac branches are the intraganglionic laminar endings (IGLEs), found abundantly in the myenteric plexus between the circular and longitudinal smooth muscle layers 59. Similar structures in the stomach have been demonstrated to sense the degree of tension in the gastric wall 60, and it is conceivable that increased tension and stretch of the Roux limb activates IGLEs and their vagal afferent fibers travelling in the celiac branches. Thus, vagal tension sensors in the Roux limb could be responsible for the reduced meal size observed in rats 40, 61 and humans 62. The finding that the threshold for eliciting Roux limb distension-induced sensations were strongly and negatively correlated to the preferred meal size in gastric bypass patients at 6 and 12 months after surgery 62 is consistent with such a role for vagal afferents, although dorsal root/spinal pathways may also participate 63.
Chemosensitivity of IGLEs 22 may also contribute to their increased activity. In addition, mucosal terminals of afferent fibers in the celiac branches 64 are also in an excellent position to detect increased levels of gut hormones 65 and other substances. Finally, innervation of the gastric pouch by the gastric vagal branches remains intact after RYGB and the many stretch sensitive intramuscular endings 66, 67 may mediate signals of increased pouch distension. Future experiments with selective ablation of afferent fibers in these vagal branches will be necessary to determine their role in RYGB induced hypophagia and weight loss.
In conclusion, vagal innervation of the portal hepatic space, proximal duodenum, and pancreas as provided by the common hepatic branch are not important for the beneficial effects of RYGB on food intake, energy expenditure, and body weight. Possible involvement in the beneficial effects of RYGB on glucose homeostasis remains to be determined, as is the role of the other abdominal vagal branches.
Acknowledgments
We thank Laurel Patterson and Leigh Townsend for expert technical assistance and help with editing the manuscript and David Sigalet for help with establishing the rat RYGB model. This research was supported by the National Institutes of Health, Grants DK047348 and DK071082.
Abbreviations
- GLP-1
glucagon-like peptide-1
- PYY
peptide YY
- NAPE
N-acetylphosohatidylethanolamine
- OEA
oleylethanolamide
- ApoAIV
apolipoprotein A-IV
References
- 1.Cummings DE. Endocrine mechanisms mediating remission of diabetes after gastric bypass surgery. Int J Obes (Lond) 2009;33 (Suppl 1):S33–40. doi: 10.1038/ijo.2009.15. [DOI] [PubMed] [Google Scholar]
- 2.Korner J, Inabnet W, Febres G, et al. Prospective study of gut hormone and metabolic changes after adjustable gastric banding and Roux-en-Y gastric bypass. Int J Obes (Lond) 2009;33(7):786–95. doi: 10.1038/ijo.2009.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.le Roux CW, Welbourn R, Werling M, et al. Gut hormones as mediators of appetite and weight loss after Roux-en-Y gastric bypass. Ann Surg. 2007;246(5):780–5. doi: 10.1097/SLA.0b013e3180caa3e3. [DOI] [PubMed] [Google Scholar]
- 4.Schwartz GJ, Fu J, Astarita G, et al. The lipid messenger OEA links dietary fat intake to satiety. Cell Metab. 2008;8(4):281–8. doi: 10.1016/j.cmet.2008.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gaetani S, Fu J, Cassano T, et al. The fat-induced satiety factor oleoylethanolamide suppresses feeding through central release of oxytocin. J Neurosci. 2010;30(24):8096–101. doi: 10.1523/JNEUROSCI.0036-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tso P, Liu M. Apolipoprotein A-IV, food intake, and obesity. Physiol Behav. 2004;83(4):631–43. doi: 10.1016/j.physbeh.2004.07.032. [DOI] [PubMed] [Google Scholar]
- 7.Gillum MP, Zhang D, Zhang XM, et al. N-acylphosphatidylethanolamine, a gut-derived circulating factor induced by fat ingestion, inhibits food intake. Cell. 2008;135(5):813–24. doi: 10.1016/j.cell.2008.10.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Culnan DM, Cooney RN, Stanley B, et al. Apolipoprotein A-IV, a putative satiety/antiatherogenic factor, rises after gastric bypass. Obesity (Silver Spring) 2009;17(1):46–52. doi: 10.1038/oby.2008.428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Arnold M, Mura A, Langhans W, et al. Gut vagal afferents are not necessary for the eating-stimulatory effect of intraperitoneally injected ghrelin in the rat. J Neurosci. 2006;26(43):11052–60. doi: 10.1523/JNEUROSCI.2606-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Reidelberger RD, Hernandez J, Fritzsch B, et al. Abdominal vagal mediation of the satiety effects of CCK in rats. Am J Physiol Regul Integr Comp Physiol. 2004;286(6):R1005–12. doi: 10.1152/ajpregu.00646.2003. [DOI] [PubMed] [Google Scholar]
- 11.Merchenthaler I, Lane M, Shughrue P. Distribution of pre-pro-glucagon and glucagon-like peptide-1 receptor messenger RNAs in the rat central nervous system. J Comp Neurol. 1999;403(2):261–80. doi: 10.1002/(sici)1096-9861(19990111)403:2<261::aid-cne8>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 12.Yamamoto H, Kishi T, Lee CE, et al. Glucagon-like peptide-1-responsive catecholamine neurons in the area postrema link peripheral glucagon-like peptide-1 with central autonomic control sites. J Neurosci. 2003;23(7):2939–46. doi: 10.1523/JNEUROSCI.23-07-02939.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Koda S, Date Y, Murakami N, et al. The role of the vagal nerve in peripheral PYY3-36-induced feeding reduction in rats. Endocrinology. 2005;146(5):2369–75. doi: 10.1210/en.2004-1266. [DOI] [PubMed] [Google Scholar]
- 14.Smith GP, Jerome C, Norgren R. Afferent axons in abdominal vagus mediate satiety effect of cholecystokinin in rats. Am J Physiol. 1985;249(5 Pt 2):R638–41. doi: 10.1152/ajpregu.1985.249.5.R638. [DOI] [PubMed] [Google Scholar]
- 15.Date Y, Murakami N, Toshinai K, et al. The role of the gastric afferent vagal nerve in ghrelin-induced feeding and growth hormone secretion in rats. Gastroenterology. 2002;123(4):1120–8. doi: 10.1053/gast.2002.35954. [DOI] [PubMed] [Google Scholar]
- 16.Batterham RL, Cowley MA, Small CJ, et al. Gut hormone PYY(3-36) physiologically inhibits food intake. Nature. 2002;418(6898):650–4. doi: 10.1038/nature00887. [DOI] [PubMed] [Google Scholar]
- 17.Abbott CR, Monteiro M, Small CJ, et al. The inhibitory effects of peripheral administration of peptide YY(3–36) and glucagon-like peptide-1 on food intake are attenuated by ablation of the vagal-brainstem-hypothalamic pathway. Brain Res. 2005;1044(1):127–31. doi: 10.1016/j.brainres.2005.03.011. [DOI] [PubMed] [Google Scholar]
- 18.Wang GJ, Tomasi D, Backus W, et al. Gastric distention activates satiety circuitry in the human brain. Neuroimage. 2008;39(4):1824–31. doi: 10.1016/j.neuroimage.2007.11.008. [DOI] [PubMed] [Google Scholar]
- 19.Phillips RJ, Powley TL. Gastric volume rather than nutrient content inhibits food intake. Am J Physiol. 1996;271(3 Pt 2):R766–9. doi: 10.1152/ajpregu.1996.271.3.R766. [DOI] [PubMed] [Google Scholar]
- 20.Kissileff HR, Carretta JC, Geliebter A, et al. Cholecystokinin and stomach distension combine to reduce food intake in humans. Am J Physiol Regul Integr Comp Physiol. 2003;285(5):R992–8. doi: 10.1152/ajpregu.00272.2003. [DOI] [PubMed] [Google Scholar]
- 21.Miranda A, Mickle A, Medda B, et al. Altered mechanosensitive properties of vagal afferent fibers innervating the stomach following gastric surgery in rats. Neuroscience. 2009;162(4):1299–306. doi: 10.1016/j.neuroscience.2009.05.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Berthoud HR, Neuhuber WL. Functional and chemical anatomy of the afferent vagal system. Auton Neurosci. 2000;85(1–3):1–17. doi: 10.1016/S1566-0702(00)00215-0. [DOI] [PubMed] [Google Scholar]
- 23.Berthoud HR. Anatomy and function of sensory hepatic nerves. Anat Rec. 2004;280A(1):827–35. doi: 10.1002/ar.a.20088. [DOI] [PubMed] [Google Scholar]
- 24.Wang PY, Caspi L, Lam CK, et al. Upper intestinal lipids trigger a gut-brain-liver axis to regulate glucose production. Nature. 2008;452(7190):1012–6. doi: 10.1038/nature06852. [DOI] [PubMed] [Google Scholar]
- 25.Rodriguez de Fonseca F, Navarro M, Gomez R, et al. An anorexic lipid mediator regulated by feeding. Nature. 2001;414(6860):209–12. doi: 10.1038/35102582. [DOI] [PubMed] [Google Scholar]
- 26.Mithieux G, Misery P, Magnan C, et al. Portal sensing of intestinal gluconeogenesis is a mechanistic link in the diminution of food intake induced by diet protein. Cell Metab. 2005;2(5):321–9. doi: 10.1016/j.cmet.2005.09.010. [DOI] [PubMed] [Google Scholar]
- 27.Nakabayashi H, Nishizawa M, Nakagawa A, et al. Vagal hepatopancreatic reflex effect evoked by intraportal appearance of tGLP-1. Am J Physiol. 1996;271(5 Pt 1):E808–13. doi: 10.1152/ajpendo.1996.271.5.E808. [DOI] [PubMed] [Google Scholar]
- 28.Ruttimann EB, Arnold M, Hillebrand JJ, et al. Intrameal hepatic portal and intraperitoneal infusions of glucagon-like peptide-1 reduce spontaneous meal size in the rat via different mechanisms. Endocrinology. 2009;150(3):1174–81. doi: 10.1210/en.2008-1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Eisen S, Phillips RJ, Geary N, et al. Inhibitory effects on intake of cholecystokinin-8 and cholecystokinin-33 in rats with hepatic proper or common hepatic branch vagal innervation. Am J Physiol Regul Integr Comp Physiol. 2005;289(2):R456–R462. doi: 10.1152/ajpregu.00062.2005. [DOI] [PubMed] [Google Scholar]
- 30.Langhans W. Role of the liver in the control of glucose-lipid utilization and body weight. Curr Opin Clin Nutr Metab Care. 2003;6(4):449–55. doi: 10.1097/01.mco.0000078993.96795.16. [DOI] [PubMed] [Google Scholar]
- 31.Friedman MI. Control of energy intake by energy metabolism. Am J Clin Nutr. 1995;62(5 Suppl):1096S–1100S. doi: 10.1093/ajcn/62.5.1096S. [DOI] [PubMed] [Google Scholar]
- 32.Niijima A. Glucose-sensitive afferent nerve fibers in the liver and their role in food intake and blood glucose regulation. J Auton Nerv Syst. 1983;9(1):207–20. doi: 10.1016/0165-1838(83)90142-x. [DOI] [PubMed] [Google Scholar]
- 33.Lam TK, Pocai A, Gutierrez-Juarez R, et al. Hypothalamic sensing of circulating fatty acids is required for glucose homeostasis. Nat Med. 2005;11(3):320–7. doi: 10.1038/nm1201. [DOI] [PubMed] [Google Scholar]
- 34.Pocai A, Obici S, Schwartz GJ, et al. A brain-liver circuit regulates glucose homeostasis. Cell Metab. 2005;1(1):53–61. doi: 10.1016/j.cmet.2004.11.001. [DOI] [PubMed] [Google Scholar]
- 35.Berthoud HR, Carlson NR, Powley TL. Topography of efferent vagal innervation of the rat gastrointestinal tract. Am J Physiol. 1991;260(1 Pt 2):R200–7. doi: 10.1152/ajpregu.1991.260.1.R200. [DOI] [PubMed] [Google Scholar]
- 36.Berthoud HR, Fox EA, Powley TL. Localization of vagal preganglionics that stimulate insulin and glucagon secretion. Am J Physiol. 1990;258(1 Pt 2):R160–8. doi: 10.1152/ajpregu.1990.258.1.R160. [DOI] [PubMed] [Google Scholar]
- 37.Berthoud HR, Kressel M, Neuhuber WL. An anterograde tracing study of the vagal innervation of rat liver, portal vein and biliary system. Anat Embryol (Berl) 1992;186(5):431–42. doi: 10.1007/BF00185458. [DOI] [PubMed] [Google Scholar]
- 38.Hevener AL, Bergman RN, Donovan CM. Novel glucosensor for hypoglycemic detection localized to the portal vein. Diabetes. 1997;46(9):1521–5. doi: 10.2337/diab.46.9.1521. [DOI] [PubMed] [Google Scholar]
- 39.Troy S, Soty M, Ribeiro L, et al. Intestinal gluconeogenesis is a key factor for early metabolic changes after gastric bypass but not after gastric lap-band in mice. Cell Metab. 2008;8(3):201–11. doi: 10.1016/j.cmet.2008.08.008. [DOI] [PubMed] [Google Scholar]
- 40.Zheng H, Shin AC, Lenard NR, et al. Meal patterns, satiety, and food choice in a rat model of Roux-en-Y gastric bypass surgery. Am J Physiol Regul Integr Comp Physiol. 2009;297(5):R1273–82. doi: 10.1152/ajpregu.00343.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shin AC, Zheng H, Townsend RL, et al. Meal-induced hormone responses in a rat model of Roux-en-Y gastric bypass surgery. Endocrinology. 2010;151(4):1588–97. doi: 10.1210/en.2009-1332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kunnecke B, Verry P, Benardeau A, et al. Quantitative body composition analysis in awake mice and rats by magnetic resonance relaxometry. Obes Res. 2004;12(10):1604–15. doi: 10.1038/oby.2004.200. [DOI] [PubMed] [Google Scholar]
- 43.Davis JD, Perez MC. Food deprivation- and palatability-induced microstructural changes in ingestive behavior. Am J Physiol. 1993;264(1 Pt 2):R97–103. doi: 10.1152/ajpregu.1993.264.1.R97. [DOI] [PubMed] [Google Scholar]
- 44.Rushing PA, Houpt TA, Henderson RP, et al. High lick rate is maintained throughout spontaneous liquid meals in freely feeding rats. Physiol Behav. 1997;62(5):1185–8. doi: 10.1016/s0031-9384(97)00308-9. [DOI] [PubMed] [Google Scholar]
- 45.Berthoud HR, Neuhuber WL. Peripheral and central functional neuroanatomy of sensory and motor innervation of the portal-hepatic axis and biliary system. In: Haussinger DJK, editor. Liver and Nervous System. Lancaster, UK: Kluwer; 1998. pp. 17–33. [Google Scholar]
- 46.Berthoud HR, Neuhuber WL. An anatomical analysis of vagal and spinal afferent innervation of rat liver and associated organs. In: Shimazu T, editor. Liver innervation and the neural control of hepatic function. London: John Libbey; 1996. pp. 31–42. [Google Scholar]
- 47.Kraly FS, Jerome C, Smith GP. Specific postoperative syndromes after total and selective vagotomies in the rat. Appetite. 1986;7(1):1–17. doi: 10.1016/s0195-6663(86)80038-1. [DOI] [PubMed] [Google Scholar]
- 48.la Fleur SE, Ji H, Manalo SL, et al. The hepatic vagus mediates fat-induced inhibition of diabetic hyperphagia. Diabetes. 2003;52(9):2321–30. doi: 10.2337/diabetes.52.9.2321. [DOI] [PubMed] [Google Scholar]
- 49.Bellinger LL. A non-essential role of liver innervation in controlling feeding behavior. Nutrition. 1999;15(6):506. doi: 10.1016/s0899-9007(99)00059-3. [DOI] [PubMed] [Google Scholar]
- 50.Friedman MI, Sawchenko PE. Evidence for hepatic involvement in control of ad libitum food intake in rats. Am J Physiol. 1984;247(1 Pt 2):R106–13. doi: 10.1152/ajpregu.1984.247.1.R106. [DOI] [PubMed] [Google Scholar]
- 51.Bueter M, Lowenstein C, Olbers T, et al. Gastric bypass increases energy expenditure in rats. Gastroenterology. 2010;138(5):1845–53. doi: 10.1053/j.gastro.2009.11.012. [DOI] [PubMed] [Google Scholar]
- 52.Stylopoulos N, Hoppin AG, Kaplan LM. Roux-en-Y gastric bypass enhances energy expenditure and extends lifespan in diet-induced obese rats. Obesity (Silver Spring) 2009;17(10):1839–47. doi: 10.1038/oby.2009.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Nadreau E, Baraboi ED, Samson P, et al. Effects of the biliopancreatic diversion on energy balance in the rat. Int J Obes (Lond) 2006;30(3):419–29. doi: 10.1038/sj.ijo.0803166. [DOI] [PubMed] [Google Scholar]
- 54.Rodieux F, Giusti V, D’Alessio DA, et al. Effects of gastric bypass and gastric banding on glucose kinetics and gut hormone release. Obesity (Silver Spring) 2008;16(2):298–305. doi: 10.1038/oby.2007.83. [DOI] [PubMed] [Google Scholar]
- 55.Meirelles K, Ahmed T, Culnan DM, et al. Mechanisms of glucose homeostasis after Roux-en-Y gastric bypass surgery in the obese, insulin-resistant Zucker rat. Ann Surg. 2009;249(2):277–85. doi: 10.1097/SLA.0b013e3181904af0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Carrasco F, Papapietro K, Csendes A, et al. Changes in resting energy expenditure and body composition after weight loss following Roux-en-Y gastric bypass. Obes Surg. 2007;17(5):608–16. doi: 10.1007/s11695-007-9117-z. [DOI] [PubMed] [Google Scholar]
- 57.del Genio F, Alfonsi L, Marra M, et al. Metabolic and nutritional status changes after 10% weight loss in severely obese patients treated with laparoscopic surgery vs integrated medical treatment. Obes Surg. 2007;17(12):1592–8. doi: 10.1007/s11695-007-9286-9. [DOI] [PubMed] [Google Scholar]
- 58.Schwartz A, Doucet E. Relative changes in resting energy expenditure during weight loss: a systematic review. Obes Rev. 2010;11(7):531–47. doi: 10.1111/j.1467-789X.2009.00654.x. [DOI] [PubMed] [Google Scholar]
- 59.Berthoud HR, Patterson LM, Neumann F, et al. Distribution and structure of vagal afferent intraganglionic laminar endings (IGLEs) in the rat gastrointestinal tract. Anat Embryol (Berl) 1997;195(2):183–91. doi: 10.1007/s004290050037. [DOI] [PubMed] [Google Scholar]
- 60.Zagorodnyuk VP, Chen BN, Brookes SJ. Intraganglionic laminar endings are mechano-transduction sites of vagal tension receptors in the guinea-pig stomach. J Physiol. 2001;534(Pt 1):255–68. doi: 10.1111/j.1469-7793.2001.00255.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Furnes MW, Stenstrom B, Tommeras K, et al. Feeding behavior in rats subjected to gastrectomy or gastric bypass surgery. Eur Surg Res. 2008;40(3):279–88. doi: 10.1159/000114966. [DOI] [PubMed] [Google Scholar]
- 62.Bjorklund P, Laurenius A, Een E, et al. Is the roux limb a determinant for meal size after gastric bypass surgery? Obes Surg. 2010;20(10):1408–14. doi: 10.1007/s11695-010-0192-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Grundy D. Neuroanatomy of visceral nociception: vagal and splanchnic afferent. Gut. 2002;51 (Suppl 1):i2–5. doi: 10.1136/gut.51.suppl_1.i2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Berthoud HR, Kressel M, Raybould HE, et al. Vagal sensors in the rat duodenal mucosa: distribution and structure as revealed by in vivo DiI-tracing. Anat Embryol (Berl) 1995;191(3):203–12. doi: 10.1007/BF00187819. [DOI] [PubMed] [Google Scholar]
- 65.Cox JE. Cholecystokinin satiety involves CCKA receptors perfused by the superior pancreaticoduodenal artery. Am J Physiol. 1998;274(5 Pt 2):R1390–6. doi: 10.1152/ajpregu.1998.274.5.R1390. [DOI] [PubMed] [Google Scholar]
- 66.Phillips RJ, Powley TL. Tension and stretch receptors in gastrointestinal smooth muscle: re-evaluating vagal mechanoreceptor electrophysiology. Brain Res Brain Res Rev. 2000;34(1–2):1–26. doi: 10.1016/s0165-0173(00)00036-9. [DOI] [PubMed] [Google Scholar]
- 67.Fox EA, Phillips RJ, Martinson FA, et al. Vagal afferent innervation of smooth muscle in the stomach and duodenum of the mouse: morphology and topography. J Comp Neurol. 2000;428(3):558–76. doi: 10.1002/1096-9861(20001218)428:3<558::aid-cne11>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]