Abstract
DNA damage response upon UV radiation involves a complex network of cellular events required for maintaining the homeostasis and restoring genomic stability of the cells. As a new class of players involved in DNA damage response, the regulation and function of microRNAs in response to UV remain poorly understood. Here we show that UV radiation induces a significant increase of miR-22 expression, which appears to be dependent on the activation of DNA damage responding kinase ATM (ataxia telangiectasia mutated). Increased miR-22 expression may result from enhanced miR-22 maturation in cells exposed to UV. We further found that tumor suppressor gene phosphatase and tensin homolog (PTEN) expression was inversely correlated with miR-22 induction and UV-induced PTEN repression was attenuated by overexpression of a miR-22 inhibitor. Moreover, increased miR-22 expression significantly inhibited the activation of caspase signaling cascade, leading to enhanced cell survival upon UV radiation. Collectively, these results indicate that miR-22 is an important player in the cellular stress response upon UV radiation, which may promote cell survival via the repression of PTEN expression.
Keywords: UV, miR-22, PTEN, ATM
1. Introduction
DNA damage is constantly induced by various endogenous and exogenous genotoxic agents, such as reactive oxygen species from cellular metabolism and ultraviolet (UV) radiation from the sunlight. Unrepaired DNA damage may lead to genomic instability, gene mutation/deletion and chromosome translocation, resulting in tumorigenesis and aging. To counteract the detrimental effects of genomic lesions, cells have evolved a comprehensive mechanism, called DNA damage response (DDR), which involves DNA damage sensing, cell cycle arrest, DNA repair, as well as cell senescence and apoptosis when the damage is excessive [1,2]. DDR is orchestrated primarily by two apical kinases, ATM and ATR, which coordinate the cellular response by either directly phosphorylating proteins involved in regulating cell cycle and DNA repair, or indirectly modulating gene transcription through downstream transcription factors such as p53 and NF-κB [3,4]. Recently, microRNAs (miRNAs) are identified as an new category of important players involved in gene regulation at the post-transcriptional level upon DNA damage [5].
MicroRNAs are a class of small non-coding RNAs (~20–24 nucleotides), which primarily bind to the 3′-untranslated region (3′-UTR) of target mRNA and negatively regulate gene expression at posttranscriptional level [6]. Most miRNA genes, which are located in introns of protein-coding genes or intergenic regions, are transcribed by RNA polymerase II. The expression of miRNAs can also been regulated during their biogenesis which involves RNase III enzyme-dependent miRNA processing. The miRNA gene transcripts, termed primary miRNA, can be recognized and cleaved by Drosha/DGCR8 microprocessor complex to generate precursor miRNA. Subsequently, precursor miRNA associates with exportin-5, which directs their exportation from the nucleus. In cytoplasm, precursor miRNA are further processed by Dicer and the mature form of miRNA can be loaded into RNA-induced silencing complex (RISC) along with Argonaute (Ago) proteins to repress target gene expression [5]. MiRNAs may regulate canonical DDR by repressing critical components of DDR signaling cascade, such as ATM, BRCA1, p53 and Cyclin D/E, whereas the expression of miRNAs may be also regulated by DDR [5,7].
It was reported that genotoxic stimulation, such as radiomimetic drug treatment and UVR, can alter the miRNA expression profile through modulating miRNA transcription and maturation [8,9]. However, the function of individual miRNA in regulating DDR upon specific genotoxic stress remains to be further elucidated. Here we show UVR significantly increased the expression of miR-22 in an ATM-dependent fashion. Upregulation of miR-22 may protect cells from UVR-induced apoptosis via repressing PTEN expression.
2. Materials and Methods
2.1. Cell Culture, Plasmids and Reagents
Human embryonic kidney cell line HEK293T and Mouse embryonic fibroblast cells (wild-type and ATM-deficient) were maintained in DMEM medium containing 10% inactivated fetal bovine serum. Human keratinocyte cell line HaCaT were grown in DMEM/F-12 medium supplemented with 10% fetal bovine serum. All cell lines were maintained in the presence of penicillin (100 IU/mL) and streptomycin (100 mg/mL) at 37°C with 5% CO2. The expression construct of pre-miR-22 was from Origene (Rockville, MD). Control (#26164) sponge construct obtained from Addgene and the strategy to generate mir-22 sponge have been described in a previous report [10]. miR-22-luciferase reporter was generated by insertion of miR-22-target sequence downstream of Luc2 gene sequence of pmirGLO Dual-Luciferase reporter construct (Promega, Madison, WI) following manufacturer’s instruction. Antibodies against PTEN or Tubulin were from Santa Cruz Biotechnology (Santa Cruz, CA). Antibodies against pATM (S1981), pChk1 (s317), Chk1, pAkt (T308), PARP1, caspase-8 or cleaved caspase-3 were from Cell Signaling Technology (Danvers, MA). UV radiation was carried out with GS Gene Linker UV Chamber Crosslinker (BioRad, Hercules, CA).
2.2. RNA extraction and quantitative real-time PCR (qPCR)
Total RNA was extracted from cells using the TRIZOL (Invitrogen, Carlsbad, CA) and then converted to first-strand cDNA using Superscript III transcriptase (Invitrogen). For microRNA analysis, total RNA was poly (A) tailed using poly (A) polymerase (Ambion) as described previously [11] before reverse transcription. The small noncoding RNA U6 and housekeeping gene GAPDH were used as an internal control for miR-22 and PTEN quantitation respectively, and gene expression was quantified as previously described [12]. The sequences of gene specific primers used for qPCR are shown as follows: PTEN:Forward 5'-GCTATGGGATTTCCTGCAGAA-3', Reverse 5'-GGCGGTGTCATAATGTCTTTCA-3'; miR-22: Forward 5’- AAGCTGCCAGTTGAAGAACTGT- 3’, Reverse 5'-GCGAGCACAGAATTAATACGAC-3'; GAPDH: Forward 5'-GGATTTGGTCGTATTGGG-3, Reverse 5'-GTGGCTGGGGCTCTACTTC-3'.
2.3. Immunoblotting
Total cell extracts were prepared and subjected to immunoblotting as described previously [13]. The quantitation of Immunoblotting band intensity was carried out with Odyssey system using IRDye 680/800-labled secondary antibodies (Li-Cor, Lincoln, NE).
2.4. Luciferase Assay
HEK293T cells were transfected with pmirGlo Dual-Luciferase construct harboring miR-22 target sequence alone or along with construct encoding pre-miR-22. After 36 h, cells were treated and lyzed with passive lysis buffer and the activity of Firefly Luciferase and Renilla Luciferase in the lysates were measured with the dual Luciferase Assay System (Promega, Madison, WI).
2.5. Cell survival Assay
HaCaT cells were mock transfected or transfected with pre-miR-22. After 36 h, cells were exposed to UVC (20 J/m2) and harvested at time indicated after UV treatment. Cells were then stained with trypan blue and live cell percentage was obtained with TC10 Automated Cell Counter (Bio-Rad, Hercules, CA). Data from three independent experiments were pooled and plotted as shown.
2.6. Statistical Analysis
The results were presented as Mean ± SD, and analyzed with Student’s t-test. P<0.05 was denoted as statistically significant.
3. Results
3.1. UV radiation significantly upregulates miR-22 expression
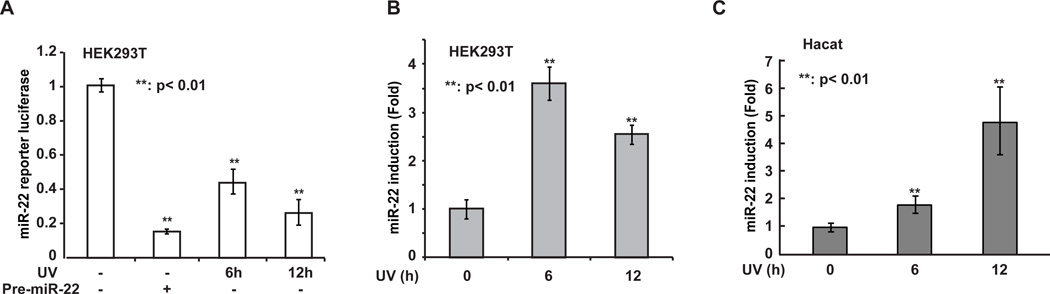
In an effort to explore the impact of UV radiation on miRNA regulation, we examined the expression of a group of selected miRNAs with quantitative real-time PCR (qPCR) in cells exposed to UVC treatment. Interestingly, miR-22 was found to be significantly upregulated upon UV radiation. To confirm our results from the initial screen, we generated a luciferase reporter construct in which luciferase expression is under control of a miR-22 recognition element inserted in its 3’-UTR region. Overexpression of a pre-miR-22 construct was able to remarkably decrease the luciferase signal in human embryonic kidney HEK293T cells, indicating that the reporter system is sensitive to the miR-22 level in the cells (Fig. 1A). We found the luciferase activity was significantly reduced at 6 h and 12 h in HEK293T cells after UV exposure, compared with mock-treated cells (Fig. 1A). Consistently, the expression of miR-22, measured by qPCR, was significantly increased in UV-treated HEK293T cells (Fig. 1B). We also observed the similar upregulation of miR-22 expression in human keratinocyte HaCaT cells upon UV treatment (Fig. 1C). Collectively, these results indicate that miR-22 expression is significantly increased in cells exposed to UV radiation.
Fig. 1.
UV radiation significantly upregulated miR-22 expression. (A) HEK293T cells were transiently transfected with pmirGLO Dual-Luciferase miR-22 reporter construct alone, or along with pCMV-pre-miR-22. After 36 hours, cells were treated with UV (20J/m2) as indicated, and luciferase activity was quantified at 6h or 12h after the treatment. The histogram represents the normalized relative luciferase activity (Fluc/Rluc) of three independent experiments; shown as mean ± SD. (B and C) Quantitative real time-PCR analysis of miR-22 expression at 6h or 12h after UV treatment (20J/m2) in HEK293T (B) and HaCaT cells (C). Fold change of miR-22 expression was normalized to untreated cells and shown as mean ± SD.
3.2. UV radiation-induced miR-22 upregulation is ATM-dependent
UV-induced DNA damage has been shown to trigger a cell-cycle-dependent relocalization of Ago2 into stress granules and alter the expression of various miRNAs in a partially ATM/ATR independent fashion [9]. Meanwhile, miRNA biogenesis in MEF cells appeared to be upregulated in response to treatment with radio-mimetic drug, depending on ATM kinase activation [8]. Interestingly, increased miR-22 expression upon genotoxic stimulation was abrogated in ATM-deficient MEF cells [8]. In UV-induced DDR, ATR is believed to be the dominant responding kinase to coordinated cellular responses to replication stress [14]. However, ATM may also play an important role in regulating DNA damage response to UV radiation. To explore whether ATM plays a role in miR-22 upregulation in human cells upon UV radiation, we examined ATM activation upon UV exposure. As shown in Fig. 2A, ATM phosphorylation at Ser1981, an ATM activation marker, was readily detected in cells treated by UV radiation, which is consistent with our previous observation that treatment with replication stress-inducing agents, such as UV and hydroxyurea, also activates ATM, although with a slower kinetics compared with ATR [12].
Fig. 2.
UV-induced miR-22 upregulation is ATM-dependent. (A) HaCat and HEK293T cells were treated with UV (20J/m2), Cells were harvested at indicated timepoints and total cell extracts were subjected to Western blot using antibodies as indicated. (B) HEK293T cells were transfected with Luciferase miR-22 reporter. Cells were left untreated or treated with UV in the presence or absence of Ku55933 (10 µM). Luciferase activity was quantified at 6 h after UV radiation. Data from three independent experiments were pooled and shown as mean ± SD. **: p<0.01. (C) ATM wild type (+/+) and deficient (−/−) MEF cells were mock treated or treated with UV (20J/m2), miR-22 expression was examined with qPCR at 12h after UV treatment. **: p<0.01.
To examine whether ATM activity is required for miR-22 upregulation by UV, we measured miR-22 reporter luciferase activity in HEK293T cells upon UV radiation in the presence or absence of an ATM-specific inhibitor KU55933. We found that the significant decrease of luciferase activity upon UV radiation was attenuated in cells treated with KU55933 (Fig. 2B). Moreover, UV treatment induced a significant increase of miR-22 expression in wild-type MEF cells, but not in ATM-deficient MEF cells (Fig. 2C). These observations were consistent with a previous report that miR-22 induction by UV was inhibited by caffeine which may also block ATM activation [9]. Furthermore, the kinetics of miR-22 induction upon UV treatment in HaCat and HEK293T cells correlated with the kinetics of ATM activation in the respective cells. UV induced a quicker ATM activation in HEK293T cells compared with that in HaCat cells, although no significant difference in kinetics of ATR activation, marked by Chk1 phosphorylation, was detected between the two cell lines (Fig. 2A). Consistently, the robust miR-22 induction was detected earlier after UV treatment in HEK293T cells than in HaCat cells (Fig. 1B and 1C). Taken together this evidence indicates that ATM is indispensable for miR-22 upregulation upon UV radiation.
3.3. PTEN expression is repressed by miR-22 upon UV radiation
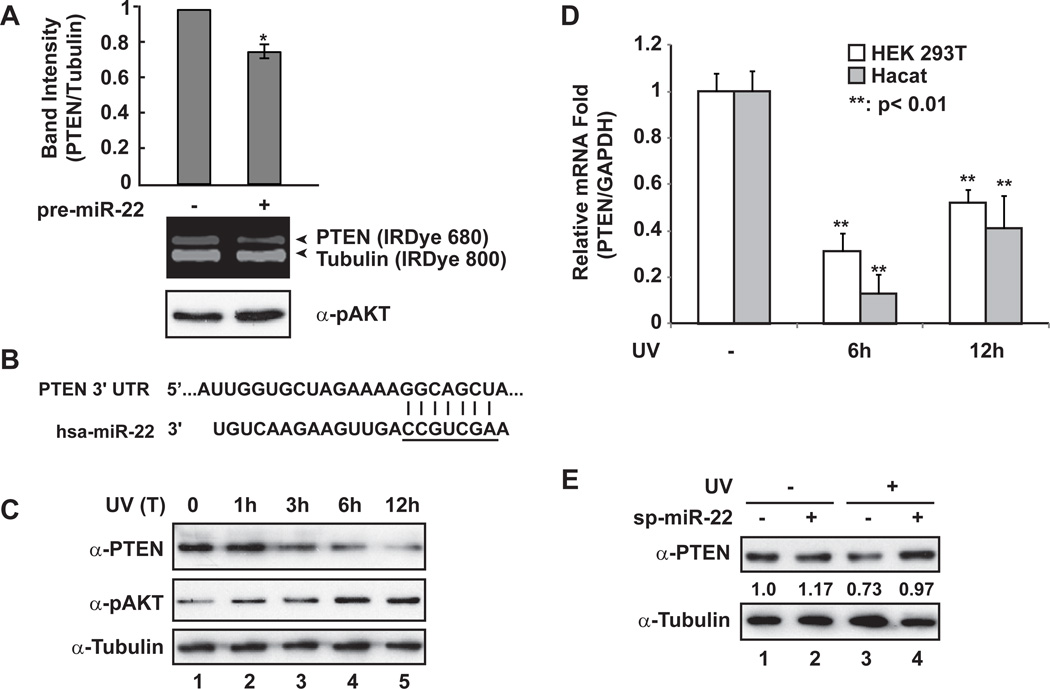
Based on seed region complementarity, hundreds of genes can be predicted as potential targets for a given miRNA. However, the specific gene set repressed by a miRNA may be cell type specific, and depend on diverse upstream signaling pathways. It was shown that miR-22 may function as either oncogene or tumor-suppressor through downregulating various gene targets, such as PTEN, CDK6, SIRT1, Sp1, and EVI-1 [15,16,17,18], depending on the context of malignancies examined. In our effort to determine the potential target genes of UV-induced miR-22, we found that increased miR-22 correlated with reduced expression of PTEN (Fig. 3A), whose inhibition has been linked to carcinogenesis of skin cancer upon UV radiation [19].
Fig. 3.
PTEN expression was repressed by miR-22 upon UV radiation. (A) HEK293 cells were transiently transfected with control or pre-miR-22. After 36 h, whole cell lysates were prepared and immunoblotted with anti-PTEN, anti-pT308 AKT and anti-Tubulin antibodies. Immunoblots were visualized with IRDye-labeled secondary antibodies as shown and quantified with Odyssey imagining system. *: p<0.05. (B) miR-22 recognition element within 3’-UTR of PTEN gene is complementary to miR-22 seed region (underlined). (C) HaCaT cells were treated with UV (20J/m2), and cells were harvested after treatment at the indicated times. Whole cell lysates were used for immunoblotting with antibodies against PTEN, pAKT and Tubulin. (D) HEK293T and HaCaT cells were treated with UV (20J/m2). PTEN and GAPDH mRNA level at 6 h or 12 h after UV treatment were analyzed with qRT-PCR, and the fold change of relative mRNA expression was shown as mean ± SD. (E) HEK293T cells were transfected with miR-22 sponge inhibitor as indicated. 36 h later, cells were mock treated or treated with UV (20J/m2). Cells were harvested at 6 h after treatment and whole cell lysates were subjected to immunoblotting with indicated antibodies. Immunoblot data were obtained and quantified as in (A).
We identified one miR-22 recognition element within 3’-UTR of human PTEN gene (Fig. 3B), which is highly conserved among vertebrates (www.Targetscan.org), indicating that miR-22-dependent repression of PTEN may be evolutionarily preserved. We found PTEN expression in HaCaT cells exposed to UV was decreased in a time-dependent manner, which inversely correlated with an increase in AKT activation (Fig. 3C). Consistently, we detected a significant decrease of PTEN mRNA level in both HEK293T and HaCaT cells upon UV radiation (Fig. 3D). Furthermore, the decrease of PTEN expression in response to UV radiation was rescued by expressing a miR-22 sponge inhibitor in HEK293T cells (Fig. 3E), suggesting that miR-22 played a critical role in PTEN repression in cells upon UV treatment.
3.4. The increased miR-22 expression inhibits apoptosis in response to UV radiation
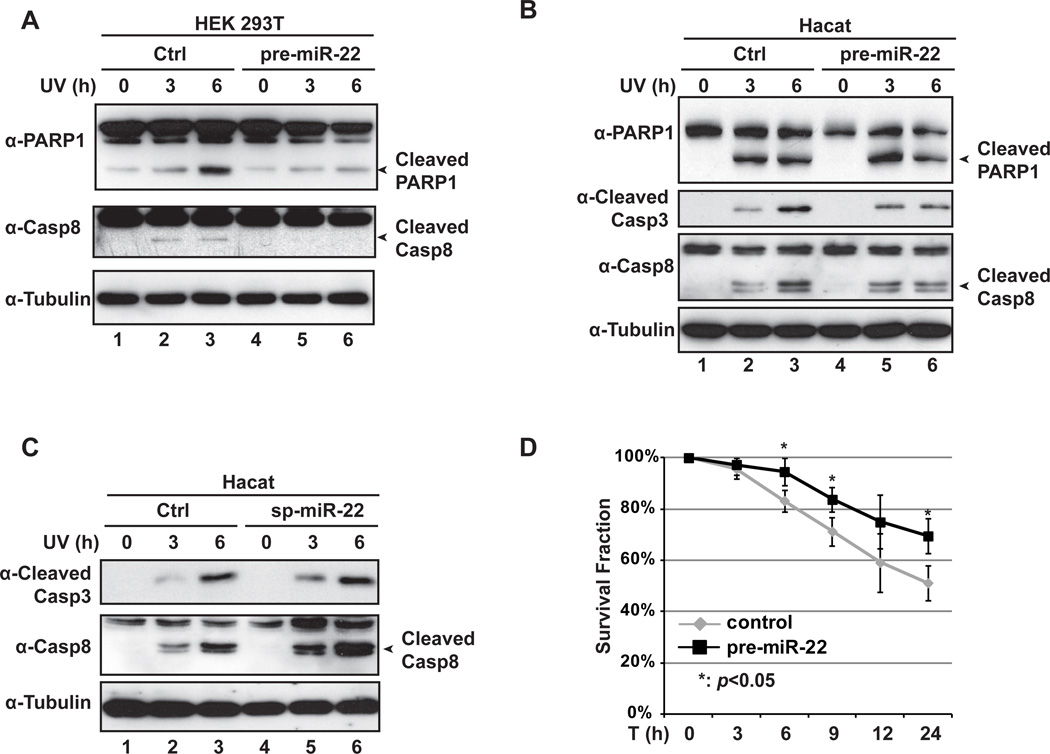
PTEN is a well-known tumor suppressor gene involved in regulating phosphatidylinositol 3-kinase (PI-3K)/Akt signaling pathway, and PTEN suppression was shown to promote survival of epidermal keratinocytes upon UV radiation [19]. Since miR-22 upregulation significantly repressed PTEN expression (Fig. 3), we postulated that increase of miR-22 may protect cells from UV-induced apoptosis. As we expected, overexpression pre-miR-22 in HEK293T cells significantly inhibited PARP-1 and caspase-8 cleavage upon UV radiation (Fig. 4A). Similarly, UV-induced cleavage of PARP-1, caspase-8 and caspase-3 was also attenuated by pre-miR-22 transfection in HaCaT cells (Fig. 4B). Moreover, the inhibition of caspase activation was prominent at 3 h after UV treatment in HEK293T cells transfected with pre-miR-22, while the same anti-apoptotic effect of pre-miR-22 was not observed in HaCaT cells until 6 h after UV exposure. This observation is consistent with the qPCR results of miR-22 quantification in Fig. 1, which suggested that UV-induced upregulation of miR-22 occurred with a slower kinetics in HaCaT cells when compared to HEK293T cells (Figs. 1B and 1C).
Fig. 4.
miR-22 inhibits apoptosis and promotes survival in cells exposed to UV radiation. (A) HEK293T cells were transiently transfected with control or pre-miR-22. Transfected cells were left untreated or treated with UV (20J/m2). Cells were collected after UV treatment at time as indicated. Whole cell lysates were immunoblotted with anti-PARP1, anti-Caspase-8 and anti-Tubulin antibodies. (B) HaCaT cells were treated and analyzed as in (A). Whole cell lysates were immunoblotted with indicated antibodies. (C) HaCaT cells were transfected with control or miR-22 sponge. After 36 h, Cells were treated with UV and analyzed as in (B) with indicated antibodies. (D) HaCaT cells were transiently transfected with control or pre-miR-22. Transfected cells were treated with UV, and live cell population were quantified at time indicated. The cell survival fraction data from three independent experiments were pooled and shown as mean ± SD. **: p<0.05.
We further examined the impact of miR-22 inhibition on cell apoptosis upon UV radiation by using a miR-22 sponge inhibitor. Transient transfection of miR- 22 sponge in HaCaT cells remarkably increased caspase activation upon UV treatment (Fig. 4C), further supporting a critical role of miR-22 in antagonizing cell apoptosis by UV radiation. We also found that overexpression of pre-miR-22 in HaCaT cells significantly increased the cell survival in response to UV treatment (Fig. 4D). All these data indicate that upregulation of miR-22 is an important mechanism involved in the pro-survival response of cells upon UV radiation.
4. Discussion
As an active barrier against tumorigenesis, DDR involves a myriad of cellular processes that maintain the genomic stability and eliminate DNA damage. These processes depend on functional modulation of a group of key molecules at transcriptional and post-transcriptional levels. Since miRNAs were found to play an important role in DDR, accumulating evidence has indicated that the expression of miRNAs can be regulated at the transcriptional level and during biogenesis upon DNA damage [5,7]. We found mature miR-22 expression was significantly increased upon UV radiation in HEK293T cells and HaCaT cells (Fig. 1). Although miR-22 was shown to be transcriptionally activated by p53 in genotoxic drug-treated HCT116 cells [20], we did not detect significant change of pri-miR-22 level in either HEK293T (p53 wild type) or HaCaT (p53 mutated) cell lines upon UV radiation (Data not shown), indicating that UV radiation enhanced the expression of miR-22 most likely via promoting miR-22 post-transcriptional processing.
Several proteins involved in DNA damage response have been shown to regulate the biogenesis of miRNAs [5]. It was found that p53 enhanced the post-transcriptional maturation of a number of miRNAs, such as miR-16-1, miR-143 and miR-145, in HCT116 cells upon DNA damage [21]. This enhancement depends on the functional association between p53 and the Drosha processing complex, bridged by DEAD-box RNA helicase p68, which facilitates the processing of primary miRNAs to precursor miRNAs. However, transcriptionally inactive p53 mutants failed to form such a functional assembly between Drosha complex and p68, leading to attenuated miRNA-processing activity [21]. We found significant miR-22 induction by UV in HaCaT cells, which harbor p53 transcriptionally inactive mutations H179Y and R282W (http://www-p53.iarc.fr), suggesting UV-induced miR-22 upregulation may be independent of p53 at either transcriptional or post-transcriptional level. Recently, KSRP was found to be phosphorylated by ATM in response to DNA damage, which promoted KSRP association with Drosha and Dicer, resulting in enhanced miRNA maturation [8]. We found upregulation of miR-22 by UV was ATM-dependent, which is consistent with the observation that caffeine was able to attenuate miR-22 induction upon UV radiation [9]. Further studies will be required to determine the function of KSRP in regulating miR-22 biogenesis upon UV radiation.
miR-22 was found to be increased in human senescent fibroblasts and epithelial cells but decreased in various cancer cell lines [16]. miR-22 may repress cancer progression by inducing cellular senescence through repressing genes involved in the senescence program, including CDK6, SIRT1, and Sp1[16]. Also, other pro-oncogenic genes, such as ERα, EVI-1 and MYCBP, have been identified as miR-22 target genes, which may be involved in miR-22-dependent inhibition of tumor progression [18,22,23]. Nevertheless, a single miRNA may function as either tumor-suppressor or oncogene depending on the gene it targets in the context of different malignancies [24]. Overexpression of miR-22 was found in human prostate cancer samples compared to adjacent normal tissues [15]. A well-known tumor suppressor, PTEN, was shown to be repressed by miR-22 through directly targeting its 3’-UTR, resulting in hyper-activation of AKT and transformation of bronchial epithelial cells [15,17]. Interestingly, miR-22 itself can be upregulated by AKT, suggesting that miR-22 forms a feed-forward circuit in its regulation [17]. miR-22-dependent PTEN repression also effectively protected rat cardiomyocytes from hypertrophy [25]. We found UV-induced miR-22 correlated with decreased PTEN expression. Moreover, expression of a miR-22 sponge inhibitor was able to rescue the PTEN repression and promote cell apoptosis upon UV radiation. In contrast, increase of miR-22 expression significantly inhibited caspase activation and enhanced cell survival in human keratinocytes exposed to UV radiation. These results strongly support a pro-survival role of miR-22 in cellular response to UV exposure. It also implies that miR-22 may function as an oncogene in tumorigenesis of skin cancer upon UV radiation.
In this study, we found miR-22 was upregulated in cells exposed to UV radiation, which protected cells from apoptosis through repressing PTEN expression. This UV stress response may inhibit the acute activation of caspase signaling cascade and provide a time window for cells to repair the DNA damage induced by UV, so as to mitigate the detrimental impact of UV radiation on cells. On the other hand, overexpression or a long-lasting high miR-22 level may contribute to tumorigenesis of skin cancers, such as melanoma, whose pathogenesis is closely associated with solar UV radiation. PTEN loss has been show to promote carcinogenesis of skin cancer upon UV radiation [19], and miR-22-mediated PTEN repression may contribute to this pathological process. A number of miRNAs have been linked to melanoma progression [26], and miRNAs have emerged as novel drug targets for cancer treatment [24]. We envision that miR-22 may serve as a potential therapeutic target for preventing tumorigenesis of skin cancers induced by UV radiation.
Highlights.
-
❖
miR-22 is induced in cells treated with UV radiation.
-
❖
ATM is required for miR-22 induction in response to UV.
-
❖
miR-22 targets 3’-UTR of PTEN to repress its expression in UV treated cells.
-
❖
Upregulated miR-22 inhibits apoptosis in cells exposed to UV.
Acknowledgements
We thank members of Wu Lab for stimulating discussion. G.T. was supported by a scholarship from the China Scholarship Council. This work was supported by NIH (R01CA149251) to Z.W.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bartek J, Lukas J. DNA damage checkpoints: from initiation to recovery or adaptation. Curr Opin Cell Biol. 2007;19:238–245. doi: 10.1016/j.ceb.2007.02.009. [DOI] [PubMed] [Google Scholar]
- 2.Harper JW, Elledge SJ. The DNA Damage Response: Ten Years After. Mol Cell. 2007;28:739–745. doi: 10.1016/j.molcel.2007.11.015. [DOI] [PubMed] [Google Scholar]
- 3.Rashi-Elkeles S, Elkon R, Weizman N, Linhart C, Amariglio N, Sternberg G, Rechavi G, Barzilai A, Shamir R, Shiloh Y. Parallel induction of ATM-dependent pro- and antiapoptotic signals in response to ionizing radiation in murine lymphoid tissue. Oncogene. 2006;25:1584–1592. doi: 10.1038/sj.onc.1209189. [DOI] [PubMed] [Google Scholar]
- 4.Wu ZH, Miyamoto S. Many faces of NF-kappaB signaling induced by genotoxic stress. J Mol Med. 2007;85:1187–1202. doi: 10.1007/s00109-007-0227-9. [DOI] [PubMed] [Google Scholar]
- 5.Wan G, Mathur R, Hu X, Zhang X, Lu X. miRNA response to DNA damage. Trends in Biochemical Sciences. 2011;36:478–484. doi: 10.1016/j.tibs.2011.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004;116:281–297. doi: 10.1016/s0092-8674(04)00045-5. [DOI] [PubMed] [Google Scholar]
- 7.Wouters MD, van Gent DC, Hoeijmakers JH, Pothof J. MicroRNAs, the DNA damage response and cancer. Mutat Res. 2011 doi: 10.1016/j.mrfmmm.2011.03.012. [DOI] [PubMed] [Google Scholar]
- 8.Zhang X, Wan G, Berger FG, He X, Lu X. The ATM Kinase Induces MicroRNA Biogenesis in the DNA Damage Response. Mol Cell. 2011;41:371–383. doi: 10.1016/j.molcel.2011.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pothof J, Verkaik NS, van Ijcken W, Wiemer EAC, Ta VTB, van der Horst GTJ, Jaspers NGJ, van Gent DC, Hoeijmakers JHJ, Persengiev SP. MicroRNA-mediated gene silencing modulates the UV-induced DNA-damage response. Embo J. 2009;28:2090–2099. doi: 10.1038/emboj.2009.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ebert MS, Neilson JR, Sharp PA. MicroRNA sponges: competitive inhibitors of small RNAs in mammalian cells. Nat Meth. 2007;4:721–726. doi: 10.1038/nmeth1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yang CH, Yue J, Fan M, Pfeffer LM. IFN Induces miR-21 through a Signal Transducer and Activator of Transcription 3–Dependent Pathway as a Suppressive Negative Feedback on IFN-Induced Apoptosis. Cancer Research. 2010;70:8108–8116. doi: 10.1158/0008-5472.CAN-10-2579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wu ZH, Miyamoto S. Induction of a pro-apoptotic ATM-NF-kappaB pathway and its repression by ATR in response to replication stress. EMBO J. 2008;27:1963–1973. doi: 10.1038/emboj.2008.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wu ZH, Shi Y, Tibbetts RS, Miyamoto S. Molecular linkage between the kinase ATM and NF-kappaB signaling in response to genotoxic stimuli. Science. 2006;311:1141–1146. doi: 10.1126/science.1121513. [DOI] [PubMed] [Google Scholar]
- 14.Abraham RT. Cell cycle checkpoint signaling through the ATM and ATR kinases. Genes Dev. 2001;15:2177–2196. doi: 10.1101/gad.914401. [DOI] [PubMed] [Google Scholar]
- 15.Poliseno L, Salmena L, Riccardi L, Fornari A, Song MS, Hobbs RM, Sportoletti P, Varmeh S, Egia A, Fedele G, Rameh L, Loda M, Pandolfi PP. Identification of the miR-106b~25 microRNA cluster as a proto-oncogenic PTEN-targeting intron that cooperates withits host gene MCM7 in transformation. Sci Signal. 2010;3:ra29. doi: 10.1126/scisignal.2000594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xu D, Takeshita F, Hino Y, Fukunaga S, Kudo Y, Tamaki A, Matsunaga J, Takahashi R-u, Takata T, Shimamoto A, Ochiya T, Tahara H. miR-22 represses cancer progression by inducing cellular senescence. The Journal of Cell Biology. 2011;193:409–424. doi: 10.1083/jcb.201010100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bar N, Dikstein R. miR-22 Forms a Regulatory Loop in PTEN/AKT Pathway and Modulates Signaling Kinetics. PLoS ONE. 2010;5:e10859. doi: 10.1371/journal.pone.0010859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Patel JB, Appaiah HN, Burnett RM, Bhat-Nakshatri P, Wang G, Mehta R, Badve S, Thomson MJ, Hammond S, Steeg P, Liu Y, Nakshatri H. Control of EVI-1 oncogene expression in metastatic breast cancer cells through microRNA miR-22. Oncogene. 2011;30:1290–1301. doi: 10.1038/onc.2010.510. [DOI] [PubMed] [Google Scholar]
- 19.Ming M, He Y-Y. PTEN: New Insights into Its Regulation and Function in Skin Cancer. J Invest Dermatol. 2009;129:2109–2112. doi: 10.1038/jid.2009.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tsuchiya N, Izumiya M, Ogata-Kawata H, Okamoto K, Fujiwara Y, Nakai M, Okabe A, Schetter AJ, Bowman ED, Midorikawa Y, Sugiyama Y, Aburatani H, Harris CC, Nakagama H. Tumor Suppressor miR-22 Determines p53-Dependent Cellular Fate through Post-transcriptional Regulation of p21. Cancer Res. 2011;71:4628–4639. doi: 10.1158/0008-5472.CAN-10-2475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Suzuki HI, Yamagata K, Sugimoto K, Iwamoto T, Kato S, Miyazono K. Modulation of microRNA processing by p53. Nature. 2009;460:529–533. doi: 10.1038/nature08199. [DOI] [PubMed] [Google Scholar]
- 22.Pandey DP, Picard D. miR-22 Inhibits Estrogen Signaling by Directly Targeting the Estrogen Receptor α mRNA. Mol Cell Biol. 2009;29:3783–3790. doi: 10.1128/MCB.01875-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Xiong J, Du Q, Liang Z. Tumor-suppressive microRNA-22 inhibits the transcription of E-box-containing c-Myc target genes by silencing c-Myc binding protein. Oncogene. 2010;29:4980–4988. doi: 10.1038/onc.2010.241. [DOI] [PubMed] [Google Scholar]
- 24.Oncogene R, Marcucci G, Croce CM. Targeting microRNAs in cancer: rationale, strategies and challenges. Nat Rev Drug Discov. 2010;9:775–789. doi: 10.1038/nrd3179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Xu X-D, Song X-W, Li Q, Wang G-K, Jing Q, Qin Y-W. Attenuation of microRNA-22 derepressed PTEN to effectively protect rat cardiomyocytes from hypertrophy. Journal of Cellular Physiology. 2011 doi: 10.1002/jcp.22852. n/a-n/a. [DOI] [PubMed] [Google Scholar]
- 26.Mueller DW, Bosserhoff AK. Role of miRNAs in the progression of malignant melanoma. Br J Cancer. 2009;101:551–556. doi: 10.1038/sj.bjc.6605204. [DOI] [PMC free article] [PubMed] [Google Scholar]