Abstract
ESR1 has been listed in the Human Obesity Gene Map as candidate gene associated with obesity. Thus, in this study, we investigated the effect of the ESR1 rs1884051 polymorphism on obesity-related variables, together with their modulations by dietary intake in Korean men. The obesity-related variables and dietary intake of 3,039 Korean men aged 40-59 years from KoGES database were analyzed. Body weight (P = 0.007), BMI (P = 0.003), waist-hip ratio (= 0.011), fat body mass (P = 0.010), and body fat percentage (P = 0.040) were significantly lower in subjects with the minor T allele of ESR1 rs1884051 than in subjects carrying the C allele. Moreover, the rs1884051 T allele was associated with a decreased risk of obesity prevalence (P = 0.040). Among the subjects whose total energy intake was below the median, carrier of the minor T allele of ESR1 rs1884051 had a lower BMI (P = 0.003) when compared with subjects carrying the C allele. In addition, among subjects whose plant protein intake was above the median, carrier of the minor T allele of ESR1 rs1884051 had a lower BMI (P = 0.044) compared with subjects carrying the C allele. Our findings demonstrate that there is a significant association between the ESR1 rs1884051 variant and obesity-related variables and this association can be potentially modified by dietary energy and plant protein intake.
Keywords: ESR1 gene, polymorphism, BMI, obesity, dietary intake
Introduction
Obesity is a major health concern worldwide and predisposes subjects to a high risk of premature mortality through an increased risk of many chronic diseases, including type 2 diabetes, cardiovascular diseases, metabolic syndromes, and cancers [1,2]. The primary cause of obesity is known as an accumulation of excessive body fat resulting from an imbalance of energy intake over physical activity [3]. However, genetic variations have recently been estimated to account for 40-70% of the population variance in obesity [4,5]. In other words, obesity is regarded as a complex multifactorial disease, which is affected by genetic variation as well as environmental factors, especially diet. Genetic variations may influence on individuals to show different predispositions over obesity through controlling a balance between energy intake and expenditure [6,7].
Estrogen and its receptors are essential for sexual development and reproductive function, but also play a role in protection against obesity and regulation of food intake [8,9]. The estrogen receptor 1 (ESR1) gene encodes an estrogen receptor, which is a ligand-activated transcription factor composed of several important domains for hormone binding, DNA binding, and activation of transcription [9].
Recently the ESR1 has been listed in the Human Obesity Gene Map as candidate gene associated with obesity [10]. The ESR1 was expressed primarily by adipocytes and it attenuates the lipolytic action in subcutaneous fat through up-regulation of the number of antilipolytic alpha 2A-adrenergic receptors [11]. ESR1 polymorphisms have been reported to be associated with obesity-related variables, such as BMI and waist circumference [12-15]. It was reported earlier that rs9340799 (XbaI in intron 1) of ESR1 was related with waist circumference and BMI in middle aged Japanese women but not in men [12]. In the Framingham Heart Study (FHS), ESR1 polymorphisms, rs223469 (PvuII in intron 1) and rs9340799 (XbaI in intron 1) were associated with waist circumference and rs1801132 (C1335G in exon 4) was associated with BMI in men, but not in women [13]. However, rs2234693 and rs9340799 of ESR1 were not associated with obesity in Swedish women [14] and Chinese population [15]. These inconsistencies might be explained by the differences in ESR1 from races and sex-dependent genetic contributions; some of these variations might correspond to functionally important mutations, but those found in other ethnic and gender groups might not. Furthermore, the interaction between genetic variation and dietary intake might affect the phenotype, which might be the source of the inconsistent results observed in these previous studies.
Therefore, we investigated the influence of ESR1 rs1884051 genotype on obesity-related phenotypes in the middle-aged Korean men, who participated in the Korean Genome Epidemiology Study (KoGES). We also evaluated the interaction between the ESR1 rs1884051 genotype and dietary intake in relation with body mass index (BMI).
Subjects and Methods
Subjects
3,039 Korean men aged between 40 and 59 years who participated in the Korean Genome Epidemiology Study (KoGES) in 2001 were used in this study [16]. The KoGES was performed as a cohort study to investigate chronic diseases (diabetes, hypertension, osteoporosis, obesity and metabolic syndrome) in adults aged 40-69 years old. The subjects were recruited from two community-based epidemiological studies in the rural Ansung and urban Ansan communities from 2001 to 2008. All subjects were of Korean ancestry. Among the 10,038 subjects in the KoGES, 3,039 men that were between 40 and 59 years old and had completed the dietary analysis were selected. This protocol used in this study was approved by the Institutional Review Board of Ewha Womans University, Seoul, South Korea.
Anthropometric variables
Body weight, height, and waist circumference were measured using a standardized procedure: height without shoes was measured using an anthropometer, weight in light clothes was determined using a weighting scale, and waist circumference over the unclothed abdomen at a minimal diameter was measured using non-stretchable standard tape. Body composition (lean body mass, fat body mass, body fat percentage, and waist/hip ratio) was measured using an In-body 3.0 (Biospace Co., Ltd, Seoul, Korea). BMI was calculated by dividing the weight in kilograms by the height in meters squared. Obesity was defined as BMI ≥ 25 kg/m2 and body fat percentage > 25%, which was based on the WHO Asia-Pacific Area criterion for obesity [17].
Blood biochemical measurements
Venous blood was collected after an overnight fast, and all plasma samples were subjected to biochemical measurements. Fasting glucose, fasting insulin, total cholesterol, HDL cholesterol, and triglycerides were measured using a Hitachi 7600 Automatic Analyzer (Hitachi, Tokyo, Japan). The level of LDL cholesterol was calculated using the following equation, described by Friedewald, for individuals with a plasma triglyceride levels < 400 mg/dl [18]: LDL-cholesterol = [Total cholesterol - {HDL-cholesterol - (Triglycerides/5)}].
Dietary analysis
Dietary intake was estimated through a food frequency questionnaire (FFQ) that was developed and validated in the KoGES [19]. The correlation coefficients of nutrient intakes between the FFQ at the end of the study and the 12-day diet records showed 0.43 (protein), 0.40 (fat), and 0.64 (carbohydrate) for age, sex, de-attenuated and energy-adjusted data. The questionnaire consisted of questions on 103 food items, which were combined into the 23 nutrients used in the Korean food composition table. The data was analyzed to give an average daily dietary intake using the Computer-Aided Nutritional Analysis Program (CAN Pro) 3.0, which is a nutrient database developed by the Korean Nutrition Society [20]. The protein (total, animal, and plant), fat (total, animal, and plant), and carbohydrate intake were given as percentages of the total daily energy intake.
Genotyping and SNP selection
Genomic DNA was extracted from whole blood and genotyped on an Affymetrix Genome-Wide Human SNP Array 5.0 [16]. Among 39 SNPs in and around ESR1 were found from the KoGES, 10 SNPs were first eliminated if they deviated from Hardy-Weinberg equilibrium (P < 0.05). Then, SNPs with minor allele frequencies below 0.05 were eliminated in the analysis. Lastly, ESR1 rs1884051, which presented the significant associations with obesity-related phenotypes, was studied.
Statistical analysis
All statistical analyses were performed using SPSS provided in the Windows software (version 17.0; SPSS Inc, Chicago, IL) with a level of significance at P < 0.05. Allele frequencies were calculated by allele counting and the departure from Hardy-Weinberg equilibrium was calculated using the chi-square test. To determine the most appropriate model, we tested different genetic models (dominant, additive and recessive). A recessive model, which carriers of 1 or 2 copies of the major allele (n = 2,387) were grouped and compared with minor allele homozygotes (n = 652), was used. Data for continuous variables was presented as mean values ± SEM (standard error of mean). Generalized linear models were applied to compare variables on the basis of the ESR1 rs1884051 genotype after adjustment for covariates. Potential confounding variables were either statistically significant in univariate analyses or known to be potentially important factors related to genotype or BMI, such as age, hypertension, diabetes, thyroid gland disease, dyslipidemia, smoking, and alcohol consumption. The effect of ESR1 rs1884051 genotype on the risk of obesity (as BMI and body fat percentage) was examined using logistic regression analysis, after adjustment for covariates, to estimate the odds ratios (ORs) and 95% confidence intervals (CI). To determine the interaction between the ESR1 rs1884051 genotype and intake of macronutrients, subjects were categorized by comparing their level of total energy intake and the percentage of energy they obtained from protein (total, animal, and plant), fat (total, animal, and plant), and carbohydrate with the median levels for these parameters. A multivariate interaction model was used to evaluate the interactions between the ESR1 rs1884051 genotype and dietary macronutrient intake, and their effect on BMI after adjustment for covariates.
Results
Anthropometric and biochemical characteristics
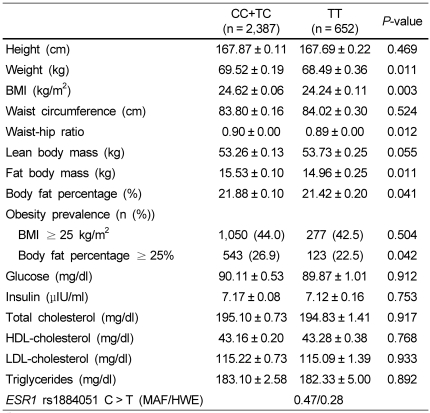
The anthropometric and biochemical characteristics of the 3,039 subjects are listed in Table 1.
Table 1.
Anthropometric data and blood profiles of subjects with the ESR1 rs1884051 genotype in Korean men
1)The data represent the means ± SEM and P values after adjustment for age, hypertension, diabetes, thyroid gland disease, dyslipidemia, smoking, and alcohol consumption.
2)If triglycerides were < 400 mg/dl, LDL-cholesterol = [Total cholesterol-{HDL-cholesterol + (triglyceride/5)}].
In the recessive model, the ESR1 rs1884051 polymorphism was significantly associated with the obesity-related variables, after adjustment for age, hypertension, diabetes, thyroid gland disease, dyslipidemia, smoking, and alcohol consumption (Table 1). Subjects with homozygous minor T allele of ESR1 rs1884051 had a significantly lower weight (CC+TC 69.52 ± 0.06 kg vs. TT 68.49 ± 0.36 kg, P = 0.011), BMI (CC+TC 24.62 ± 0.06 kg/m2 vs. TT 24.24 ± 0.11 kg/m2, P = 0.003), waist-hip ratio (CC+TC 0.90 ± 0.00 vs. TT 0.89 ± 0.00, P = 0.012), fat body mass (CC+TC 15.53 ± 0.10 kg 14.96 ± 0.25 kg, P = 0.011), and body fat percentage (CC+TC 21.88 ± 0.10% vs. TT 21.42 ± 0.20%, P = 0.041) when compared to those carrying the C allele.
The biochemical characteristics, such as the plasma level of glucose, insulin, cholesterol (total, HDL, and LDL), and triglycerides, of the subjects did not differ among the ESR1 rs1884051 polymorphism (Table 1).
The genotype distribution of SNP in ESR1 rs1884051 was in the Hardy-Weinberg equilibrium (P = 0.28), and the minor allele frequency (MAF) of polymorphism in ESR1 rs182052 was 0.47 (Table 1).
Effect of the ESR1 rs1884051 genotype on the risk of obesity prevalence
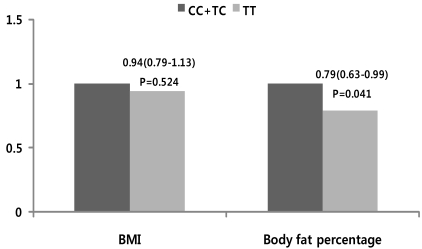
We analyzed whether the ESR1 rs1884051 genotype affected the risk of obesity prevalence based on different criteria for obesity using logistic regression analysis after adjustment for age, hypertension, diabetes, thyroid gland disease, dyslipidemia, smoking, and alcohol consumption (Fig. 1). No association between ESR1 rs1884051 genotype and the prevalence of obesity, which was defined as BMI ≥ 25 kg/m2, was observed (CC+TC 44.0% vs. TT 42.5%, P = 0.504, Table 1). Also, the effect of the ESR1 rs1884051 genotype on the risk of obesity prevalence was not detected (Fig. 1). When obesity prevalence was defined as body fat percentage > 25%, the prevalence of obesity was significantly lower in subjects with the homozygous T allele of ESR1 rs1884051 than in those carrying the C allele, CC+TC: 26.9% and TT: 22.5% respectively, after adjustment for covariates (Table 1). Moreover, the subjects with the minor T allele of ESR1 rs1884051 had a significantly decreased risk of obesity than those carrying the C allele (OR = 0.79, 95% CI = 0.63-0.99, P = 0.041, Fig. 1).
Fig. 1.
Odd Ratio (OR) and 95% confidence interval (95% CI) for obesity based on obesity-related phenotypes (BMI and body fat percentage) of subject with the ESR1 rs1884051 genotype in Korean men. Odds ratios (95% CI) in the aspect of the risk allele using a recessive model (CC+TC vs. TT). Cutoffs for obesity were based on WHO Asia-Pacific Area criteria for obesity (BMI ≥ 25 kg/m2 and body fat percentage > 25%).
The effects of interaction between the ESR1 rs1884051 genotype and dietary intake on BMI
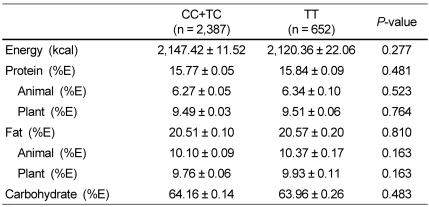
Dietary intake was analyzed to examine whether the ESR1 rs1884051 genotype affected dietary macronutrient intake (Table 2). The polymorphism of ESR1 rs1884051 was not associated with the mean values of the total energy intake. Similarly, the percentage of energy obtained from protein (total, animal, and plant), fat (total, animal, and plant), and carbohydrate consumption were independent of the ESR1 rs1884051 genotype.
Table 2.
Dietary intake of subjects with the ESR1 rs1884051 genotype in Korean men
1)The data represent the means ± SEM and P values after adjustment for age, hypertension, diabetes, thyroid gland disease, dyslipidemia, smoking, and alcohol consumption.
2)Total daily energy intake, together with protein, fat, and carbohydrate intake, was derived from the FFQ (Food frequency questionnaire).
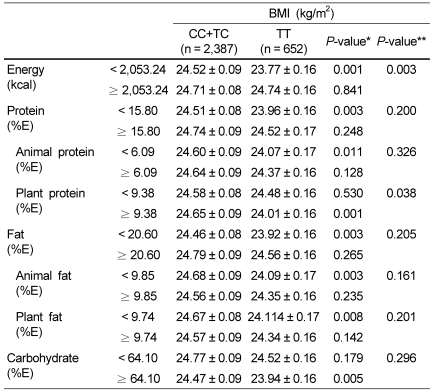
We analyzed whether the interaction between the ESR1 rs1884051 genotype and dietary macronutrient intake affected the BMI (Table 3). We sorted each subject by comparing their total energy intake and the percentage of energy they obtained from protein (total, animal, and plant), fat (total, animal, and plant), and carbohydrate consumption with the respective medians. An interaction between ESR1 rs1884051 genotype and dietary energy intake with respect to BMI was identified (P-interaction = 0.003). The homozygous minor T allele of ESR1 rs1884051 was associated with lower BMI only in subjects who consumed low levels of energy compared to those with carrying the C allele (CC+TC 24.52 ± 0.09 kg/m2 vs. TT 23.77 ± 0.16 kg/m2, P < 0.001). On the other hand, there was no significant difference with respect to BMI between the ESR1 rs1884051 genotype in subjects who consumed high levels of energy. Also, we identified significant interaction between ESR1 rs1884051 genotype and dietary plant protein intake with respect to BMI (P-interaction = 0.038). In the group with high plant protein intake, the subjects with the homozygous minor T allele of ESR1 rs1884051 had a lower BMI than in those carrying the C allele (CC+TC 24.64 ± 0.09 kg/m2 vs. TT 24.01 ± 0.16 kg/m2, P < 0.001). However, in the group with low plant protein intake, the ESR1 rs1884051 genotype did not affect the BMI. In addition, we could not detect an interaction between the ESR1 rs1884051 genotype and the percentage of energy obtained from protein (total and animal), fat (total, animal, and plant), and carbohydrate consumption with respect to BMI.
Table 3.
The effects of the interaction between the ESR1 rs1884051 genotype and dietary intake on BMI in Korean men
1)The data represent the means ± SEM and P values after adjustment for age, hypertension, diabetes, thyroid gland disease, dyslipidemia, smoking, and alcohol consumption (* within genotype, ** within gene-diet interaction).
2)Total daily energy intake, together with protein, fat, and carbohydrate intake, was derived from the FFQ (Food frequency questionnaire).
Discussion
ESR1 on chromosome 6q25 is comprised of 8 exons and 7 introns with a total size of 140 kilobases [4]. The ESR1 rs1884051 polymorphism occurs because of the transversion of C to T at nucleotide 271749 (NM_001122742) in intron 4 of the ESR1 gene, which does not result in amino acid changes. This MAF was different from that observed in a European population (29.7%), but was similar to that observed in other Asian populations (Japanese: 48.9% and Chinese: 45.6%) and in an African (41.7%) population obtained from HapMap data.
In this study, significant associations between obesity-related variables and ESR1 rs1884051 polymorphism were identified. Subjects with the homozygous minor T allele of ESR1 rs1884051 showed significantly lower body weight (P = 0.011), BMI (P = 0.003), waist-hip ratio (P = 0.012), fat body mass (P = 0.011), and body fat percentage (P = 0.041) when compared to subjects carrying the major C allele after adjustment for age, hypertension, diabetes, thyroid gland disease, dyslipidemia, smoking, and alcohol consumption. These results were consistent with earlier studies that examined other ESR1 polymorphisms [12,13,21]. For example, Okura et al. [12] found a similar association with obesity-related variables and the rs9340799 (XbaI) polymorphism in 2,238 Japanese subjects. Older women (60-79 years) with homozygous minor G allele had smaller fat mass by 18% and waist circumference by 6% when compared to those carrying the homozygous major A allele. On the other hand, no difference was found among the rs9340799 genotype for men. The Framingham Heart Study (FHS) investigated the association between the ESR1 polymorphisms and BMI or waist circumference in 1,763 unrelated men and women [13]. In this population, men with the minor homozygous allele for the rs2224693 (PvuII) and rs9340799 (XbaI) had lower waist circumference than those with the major allele. Also, subjects with the minor allele for the rs1801132 (C1335G) had a lower BMI when compared to subjects with the major allele in men, but not women. In addition, Gallagher et al. [21] reported that among 17 ESR1 polymorphisms, rs6902771, rs2431260 and rs2175898 were associated with BMI in African Americans from the Insulin Resistance Atherosclerosis Family Study. Our results provide a new perspective in studying associations between genetic variation of ESR1 rs1884051 and the obesity in middle-aged Korean men.
Our result showed that the prevalence of obesity, which is defined as body fat percentage > 25%, was significantly lower in subjects with the minor T allele of ESR1 rs1884051 than in subjects carrying the major C allele. Moreover, subjects with homozygous minor T allele had a lower risk of obesity by 79%, when compared to individuals carrying the C allele. Recently, Goulart et al. [22] showed that women with the variant of rs2234693 had a lower prevalence of obesity when compared to those with the wild type allele and the rs2234693 polymorphism was inversely associated with risk of obesity, which was in agreement with our results. These results imply that there is a decrease in the prevalence and risk of obesity in subjects with the ESR1 rs1884051 T allele.
We also analyzed the effect of genetic variations on dietary macronutrient intake. No significant differences in dietary intake of energy and the percentage of energy they obtained from protein (total, animal, and plant), fat (total, animal, and plant), and carbohydrate were observed among subjects with different genotypes of ESR1 rs1884051. These results suggested that genetic variations in ESR1 rs1884051 might not influence dietary macronutrient intake.
In our study, a significant novel interaction between ESR1 rs1884051 genotype and dietary intake of energy with respect to BMI was identified. Among subjects with low energy intake, carriers of the minor T allele of ESR1 rs1884051 had a lower BMI than subjects carrying the C allele, whereas for subjects with a high energy intake, the ESR1 rs1884051polymorphism did not affect BMI. Miyaki et al. [23] found that dietary energy intake affected the association between the Trp64Arg polymorphism of beta 3-adrenergic receptor (ADRB3) and obesity in 295 healthy Japanese men. Among the subjects who had a high energy intake, the variant of ADRB3 was significantly associated with a higher risk of obesity, as compared with the wild type. Moreover, the variant of ADRB3 may reduce the risk of obesity among subject with proper energy intake.
Another study reported that the interaction between dietary nutrient intake and genetic variation has the potential to decrease the risk of obesity [24]. Among subjects who consumed levels of polyunsaturated fatty acid (PUFA) above the median level, the minor T allele of PDZK1_i33968C > T polymorphism was significantly associated with lower BMI, as compared with to non-obese subjects carrying the C allele in the Genetics of Lipid Lowering Drugs and Diet Network (GOLDN) study. Similarly, in our study, a significant novel interaction was observed between ESR1 rs1884051 genotype and the percentage of energy they obtained from plant protein in relation to their effect on BMI. The minor T allele of ESR1 rs1884051 was associated with lower BMI in subjects that consumed a high level of dietary plant protein. On other hand, there was no difference in BMI for subjects that consumed a low level of dietary plant protein between the ESR1 rs1884051 genotypes. These findings suggested that personalized nutritional research and applications should be developed to control the risk of obesity in predisposed individuals. For example, if genetically susceptible individuals choose a diet with low dietary energy intake and high dietary plant protein intake, their risk of obesity can be reduced while improving their health.
This study has several limitations. First, this study was conducted in 3,039 Korean men aged 40-59 years, thus cannot be generalized to other populations. Second, it is generally considered that several SNPs among multiple genes may affect obesity-related variables. As this study was tested only 1 SNP from the single gene, the results have to be confirmed and to be examined interaction between other SNPs from the multiple genes and ESR1 rs1884051 with respect to BMI in future studies.
In conclusion, this study demonstrated that the genetic variation in ESR1 rs1884051 was directly associated with obesity-related variables such as body weight, BMI, waist-hip ratio, fat body mass, and body fat percentage in Korean men. Moreover, the risk of obesity was also affected by variations in ESR1 rs1884051. In particular, with respect to the intake of a low level of dietary energy and high level of dietary plant protein, subjects with the minor T allele of ESR1 rs1884051 had significantly lower BMI than those carrying the C allele. Although these findings will need to be reproduced in further studies, this is the first study to demonstrate the concept of using a personalized approach to control obesity and prevent disease.
Acknowledgments
We thank the Korea Centers for Disease Control and Prevention for making available the data from the KoGES.
Footnotes
This research was supported by grants from the Health Fellowship Foundation, Republic of Korea. The first author was a recipient of Brain Korea 21 project (2010) funding.
References
- 1.Razquin C, Marti A, Martinez JA. Evidences on three relevant obesogenes: MC4R, FTO and PPARγ. Approaches for personalized nutrition. Mol Nutr Food Res. 2011;55:136–149. doi: 10.1002/mnfr.201000445. [DOI] [PubMed] [Google Scholar]
- 2.Cotran RS, Kumar V, Collins T. Robbins Pathologic Basis of Disease. 6th ed. Philadelphia: W.B. Saunders Company; 1999. [Google Scholar]
- 3.Walker CG, Zariwala MG, Holness MJ, Sugden MC. Diet, obesity and diabetes: a current update. Clin Sci (Lond) 2007;112:93–111. doi: 10.1042/CS20060150. [DOI] [PubMed] [Google Scholar]
- 4.Chung WK, Leibel RL. Considerations regarding the genetics of obesity. Obesity (Silver Spring) 2008;16(Suppl 3):S33–S39. doi: 10.1038/oby.2008.514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wardle J, Carnell S, Haworth CM, Plomin R. Evidence for a strong genetic influence on childhood adiposity despite the force of the obesogenic environment. Am J Clin Nutr. 2008;87:398–404. doi: 10.1093/ajcn/87.2.398. [DOI] [PubMed] [Google Scholar]
- 6.Swithers SE, Martin AA, Davidson TL. High-intensity sweeteners and energy balance. Physiol Behav. 2010;100:55–62. doi: 10.1016/j.physbeh.2009.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Drewnowski A. Obesity, diets, and social inequalities. Nutr Rev. 2009;67(Suppl 1):S36–S39. doi: 10.1111/j.1753-4887.2009.00157.x. [DOI] [PubMed] [Google Scholar]
- 8.Sertic J, Juricic L, Ljubic H, Bozina T, Lovric J, Markeljevic J, Jelakovic B, Merkler M, Reiner Z. Variants of ESR1, APOE, LPL and IL-6 loci in young healthy subjects: association with lipid status and obesity. BMC Res Notes. 2009;2:203. doi: 10.1186/1756-0500-2-203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen HH, Lee WJ, Fann CS, Bouchard C, Pan WH. Severe obesity is associated with novel single nucleotide polymorphisms of the ESR1 and PPARgamma locus in Han Chinese. Am J Clin Nutr. 2009;90:255–262. doi: 10.3945/ajcn.2009.25914. [DOI] [PubMed] [Google Scholar]
- 10.Rankinen T, Zuberi A, Chagnon YC, Weisnagel SJ, Argyropoulos G, Walts B, Pérusse L, Bouchard C. The human obesity gene map: the 2005 update. Obesity (Silver Spring) 2006;14:529–644. doi: 10.1038/oby.2006.71. [DOI] [PubMed] [Google Scholar]
- 11.Pedersen SB, Kristensen K, Hermann PA, Katzenellenbogen JA, Richelsen B. Estrogen controls lipolysis by up-regulating alpha2A-adrenergic receptors directly in human adipose tissue through the estrogen receptor alpha. Implications for the female fat distribution. J Clin Endocrinol Metab. 2004;89:1869–1878. doi: 10.1210/jc.2003-031327. [DOI] [PubMed] [Google Scholar]
- 12.Okura T, Koda M, Ando F, Niino N, Ohta S, Shimokata H. Association of polymorphisms in the estrogen receptor alpha gene with body fat distribution. Int J Obes Relat Metab Disord. 2003;27:1020–1027. doi: 10.1038/sj.ijo.0802378. [DOI] [PubMed] [Google Scholar]
- 13.Fox CS, Yang Q, Cupples LA, Guo CY, Atwood LD, Murabito JM, Levy D, Mendelsohn ME, Housman DE, Shearman AM. Sex-specific association between estrogen receptor-alpha gene variation and measures of adiposity: the Framingham Heart Study. J Clin Endocrinol Metab. 2005;90:6257–6262. doi: 10.1210/jc.2005-0670. [DOI] [PubMed] [Google Scholar]
- 14.Nilsson M, Dahlman I, Rydén M, Nordström EA, Gustafsson JA, Arner P, Dahlman-Wright K. Oestrogen receptor alpha gene expression levels are reduced in obese compared to normal weight females. Int J Obes (Lond) 2007;31:900–907. doi: 10.1038/sj.ijo.0803528. [DOI] [PubMed] [Google Scholar]
- 15.Jian WX, Yang YJ, Long JR, Li YN, Deng FY, Jiang DK, Deng HW. Estrogen receptor alpha gene relationship with peak bone mass and body mass index in Chinese nuclear families. J Hum Genet. 2005;50:477–482. doi: 10.1007/s10038-005-0281-5. [DOI] [PubMed] [Google Scholar]
- 16.Cho YS, Go MJ, Kim YJ, Heo JY, Oh JH, Ban HJ, Yoon D, Lee MH, Kim DJ, Park M, Cha SH, Kim JW, Han BG, Min H, Ahn Y, Park MS, Han HR, Jang HY, Cho EY, Lee JE, Cho NH, Shin C, Park T, Park JW, Lee JK, Cardon L, Clarke G, McCarthy MI, Lee JY, Lee JK, Oh B, Kim HL. A large-scale genome-wide association study of Asian populations uncovers genetic factors influencing eight quantitative traits. Nat Genet. 2009;41:527–534. doi: 10.1038/ng.357. [DOI] [PubMed] [Google Scholar]
- 17.World Health Organization. The Asia-Pacific Perspective: Redefining Obesity and Its Treatment. Geneva: WHO; 2000. p. 18. [Google Scholar]
- 18.Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18:499–502. [PubMed] [Google Scholar]
- 19.Ahn Y, Kwon E, Shim JE, Park MK, Joo Y, Kimm K, Park C, Kim DH. Validation and reproducibility of food frequency questionnaire for Korean genome epidemiologic study. Eur J Clin Nutr. 2007;61:1435–1441. doi: 10.1038/sj.ejcn.1602657. [DOI] [PubMed] [Google Scholar]
- 20.The Korean Nutrition Society. CAN Pro 3.0 Software. Seoul: The Korean Nutrition Society; 2006. [Google Scholar]
- 21.Gallagher CJ, Langefeld CD, Gordon CJ, Campbell JK, Mychaleckyj JC, Bryer-Ash M, Rich SS, Bowden DW, Sale MM. Association of the estrogen receptor-alpha gene with the metabolic syndrome and its component traits in African-American families: the Insulin Resistance Atherosclerosis Family Study. Diabetes. 2007;56:2135–2141. doi: 10.2337/db06-1017. [DOI] [PubMed] [Google Scholar]
- 22.Goulart AC, Zee RY, Rexrode KM. Estrogen receptor 1 gene polymorphisms and decreased risk of obesity in women. Metabolism. 2009;58:759–764. doi: 10.1016/j.metabol.2009.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Miyaki K, Sutani S, Kikuchi H, Takei I, Murata M, Watanabe K, Omae K. Increased risk of obesity resulting from the interaction between high energy intake and the Trp64Arg polymorphism of the beta3-adrenergic receptor gene in healthy Japanese men. J Epidemiol. 2005;15:203–210. doi: 10.2188/jea.15.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Junyent M, Arnett DK, Tsai MY, Kabagambe EK, Straka RJ, Province M, An P, Lai CQ, Parnell LD, Shen J, Lee YC, Borecki I, Ordovás JM. Genetic variants at the PDZ-interacting domain of the scavenger receptor class B type I interact with diet to influence the risk of metabolic syndrome in obese men and women. J Nutr. 2009;139:842–848. doi: 10.3945/jn.108.101196. [DOI] [PMC free article] [PubMed] [Google Scholar]