Abstract
A wide variety of pathologies can produce focal lesions within the spleen. These are being more frequently encountered as imaging technology improves. It is vital that radiologists are aware of these pathologies to enable accurate diagnosis. The role of ultrasound contrast in splenic disease will be discussed and illustrated with cases likely to be encountered by general and abdominal radiologists.
Keywords: Spleen, Contrast, Ultrasound
Introduction
Ultrasound contrast agents have been shown to be extremely accurate for detection and characterisation of focal hepatic lesions. Given that isolated splenic pathology is relatively uncommon compared with focal hepatic lesions, the spleen has received less attention. However a number of studies related to the spleen have recently been published and show promising results. The purpose of this article is to provide an introduction to contrast-enhanced ultrasound of the spleen, including a review of the evidence and an illustration of common pathologies.
Ultrasound contrast agents
Modern ultrasound contrast agents are composed of perflutren gas within a stabilising lipid shell. These agents oscillate at a harmonic frequency, and this harmonic can be isolated to produce an image that is almost solely related to the contrast agent with little input from the underlying tissue. Ultrasound contrast agents (UCAs) are administered intravenously, are purely intravascular due to their size of 5–10 microns and lack the extracellular phase of CT and MRI contrast agents. Following injection, continuous scanning can be performed, and the phases of contrast are broadly divided into arterial (10–25 s), portal venous (30–120 s) and late phases (over 120 s) [1], although some variability occurs depending upon the patient’s cardiac function. Further injections can be performed after a delay of 5–10 min. Usually only a small proportion of each dose is injected. For example, Definity (Lantheus, North Billerica, MA) is packaged in a 1.5 ml vial, but typical injections are 0.2–0.4 cc each followed by a 5–10 ml saline flush. SonoVue (Bracco Milano, Italy) is a sulphur hexofluride agent licensed for use in Europe and is typically diluted in 5 cc of normal saline before being administered in 1.0–2.5 cc aliquots. Splitting the dose allows multiple runs to be performed per vial and also minimises attenuation of deep structures that can occur with high contrast doses. Further information regarding UCAs is available in Guidelines produced by the European Federation of Societies for Ultrasound in Medicine and Biology (EFSUMB) [2].
The strength of ultrasound contrast agents is their ability to depict both the macrocirculation and the microcirculation. Signal is obtained simply due to the presence of the contrast. It does not matter if the bubble is moving quickly, slowly or is stationary. By comparison Doppler ultrasound can only assess the macrocirculation.
UCAs can be safely administered in renal impairment and are not nephrotoxic [3]. Contraindications include recent myocardial infarction, unstable angina, and recent deterioration in cardiac failure, along with unstable arrhythmias, pulmonary hypertension, and left to right or bidirectional cardiac shunts [3].
Lesion detection
In the liver UCAs have been shown to have a sensitivity of approximately 80% for detecting hepatic metastases [4], which is comparable to multiphase multidetector CT. By comparison, metastatic disease to the spleen is uncommon, occurring in 1% of advanced visceral malignancy [5] and so UCAs do not have a practical role in screening the spleen in patients with visceral tumours. The spleen is involved in 30–40% of patients with systemic lymphoma [6, 7], and if UCAs are to have a role in lesion detection, this is the most likely indication. It has been shown that ultrasound has a higher sensitivity than positron emission tomography and CT for detecting splenic involvement in systemic lymphoma [8]. However a recent study [9] found that UCAs did not offer a significant advantage over B-mode ultrasound alone. Based upon the current body of evidence there appears to be no role for UCAs in lesion detection in the spleen.
Lesion characterisation
B mode accuracy in characterising focal splenic lesions is only moderate at approximately 50% [10], and Doppler ultrasound is of little assistance. In a study examining 98 aetiologically proven focal splenic lesions [11], Doppler ultrasound was disappointing with 68.4% of lesions appearing ‘avascular’, including 40.0% of metastases, 18.7% of lymphomas and 72.8% of primary splenic tumours and so did not allow accurate differentiation of benign and malignant pathologies.
A relatively large study by Stang et al. [10] examined 147 focal splenic lesions and used four readers, two blinded to all information apart from B mode and CEUS, and two readers who were given clinical information in addition to the images. Fully blinded reporters had an accuracy of 51 and 43% on B mode, which increased to 83 and 81% following CEUS. The readers with access to clinical information performed better on B mode with an accuracy of 70 and 74%, which further increased to 92 and 91% following CEUS. This study shows that to correctly classify focal splenic pathologies, it is vital to know the patient’s clinical context and to administer contrast. Overall the non-clinically blinded readers had sensitivity, specificity, positive and negative predictive value of 100% (79 of 79 malignant lesions), 83.8% (57 of 68 benign lesions), 87.8% (79 of 90 lesions) and 100% (57 of 57 lesions) respectively, which is similar to the results for 18F-FDG PET/CT [12]. These results have been supported by another study involving 48 patients with 75 focal splenic lesions [13] that found a sensitivity, specificity and accuracy of 91.1, 95.0 and 92.0% respectively. The diagnostic utility of CEUS of the spleen is further supported by other investigators [14].
There are essentially three patterns of enhancement of splenic lesions [10, 15]:
Lesions that enhance in the arterial phase and rapidly washout
Lesions that enhance in the arterial phase with enhancement continuing into the parenchymal and late phases or with late phase washout
Lesions that do not enhance at any stage
The first pattern is a malignant enhancement pattern while patterns 2 and 3 are seen in benign lesions. Based upon the experience with liver imaging [16, 17], the high specificity of late phase washout as described in the current studies is likely to decline in the future, albeit to a small degree as atypical lesions breaking the rules are described. The key to differentiating benign from malignant washout is related to timing, with malignant lesions washing out rapidly and benign pathologies generally showing later, more gradual washout. Examples of splenic haemangiomas demonstrating late phase washout have recently been described in the literature [18] and have been encountered in our practice (Fig. 1). The presence of irregular intralesional vessels is associated with malignant lesions as is a ‘dotted’ appearance in the parenchymal phase [10]. While these findings may support the diagnosis of a malignant lesion, they are not specific and in our experience are subject to interobserver variation, while the binary decision of rapid lesion washout versus no washout is accurate, reproducible and serves as the basis for classifying lesions as malignant or benign respectively.
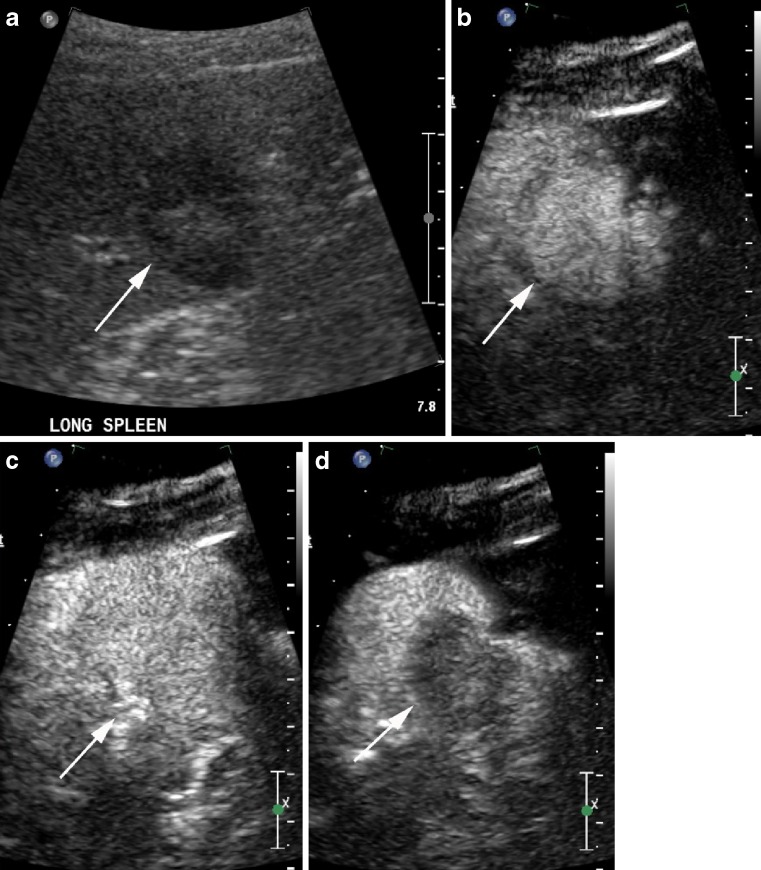
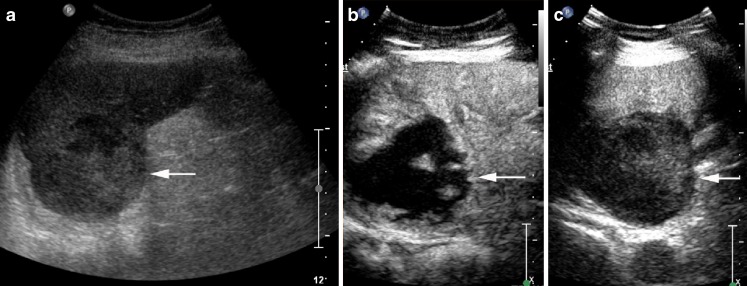
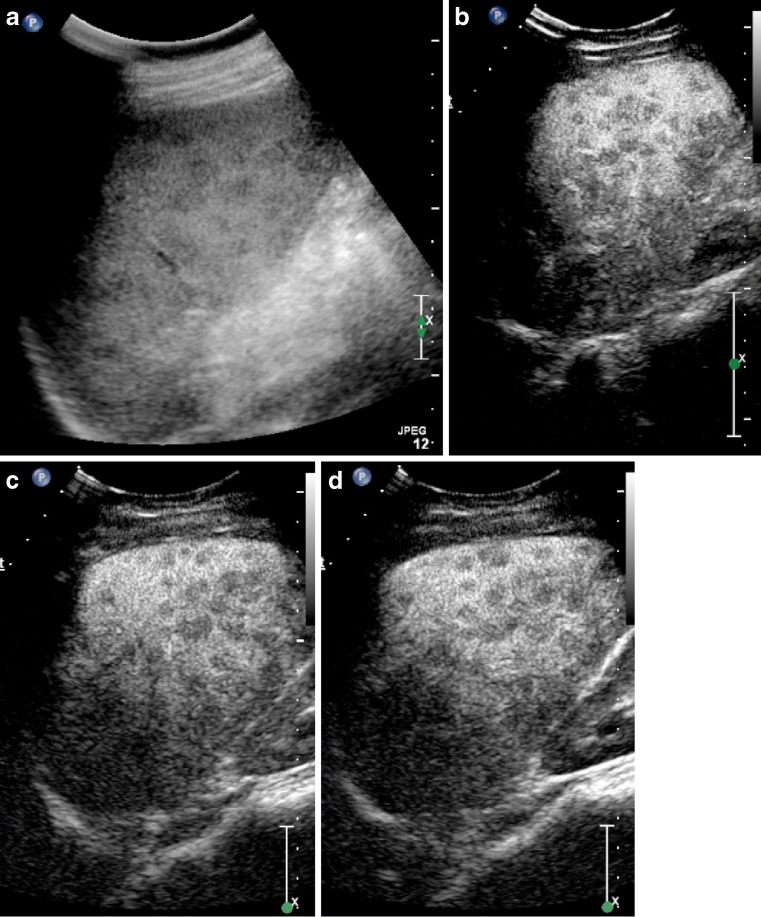
Fig. 1.
Hypoechoic slightly heterogenous splenic haemangioma (arrows) on B mode ultrasound (a), has homogenous enhancement 30 s after contrast administration (b), remains isoenhancing at 2 min (c), but develops washout at 3 min 30 s (d)
Haemangioma
Haemangiomas are the most frequent primary splenic tumour. They are benign and may be either cavernous or capillary and as such may appear hypoechoic or echogenic on B mode ultrasound. With CEUS they typically enhance diffusely in the arterial phase [10]. The peripheral nodular enhancement with an early incomplete ring seen with hepatic haemangiomas is uncommonly encountered with splenic haemangiomas. The enhancement may be homogenous or heterogenous and the lesions retain contrast into the late phase to be isoechoic to normal splenic parenchyma (Fig. 2). Large haemangiomas may retain so much contrast that they obscure the deep aspect of the spleen (Fig. 3). Rarely haemangiomas can show washout in the delayed phase, and in our experience this generally occurs late unlike the early rapid washout seen with malignant lesions. As a technical point, when examining large lesions or deep lesions, it is best to inject only small volumes of UCAs to minimise attenuation in the late phase that may obscure the region of interest.
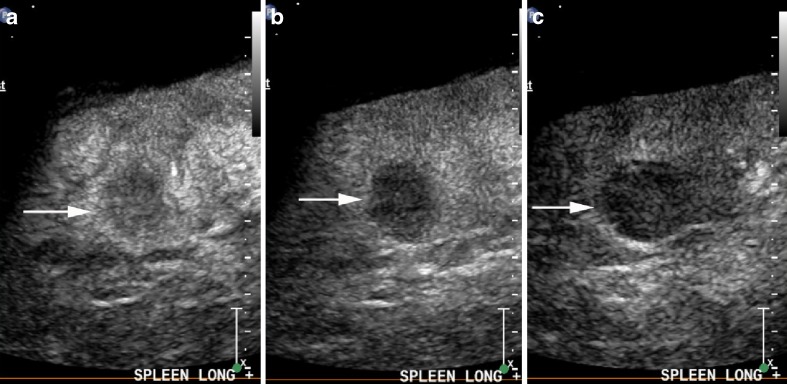
Fig. 2.
Well defined echogenic haemangioma (arrows) on B mode (a) is homogenously slightly hypoenhancing at 20 s (b) and at 90 s (c) and retains contrast into the late phase at 4 min (d)
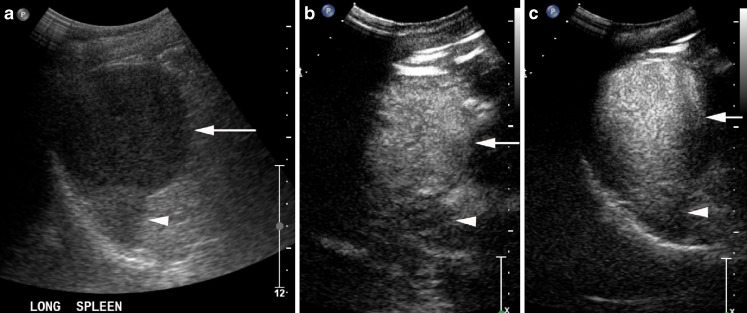
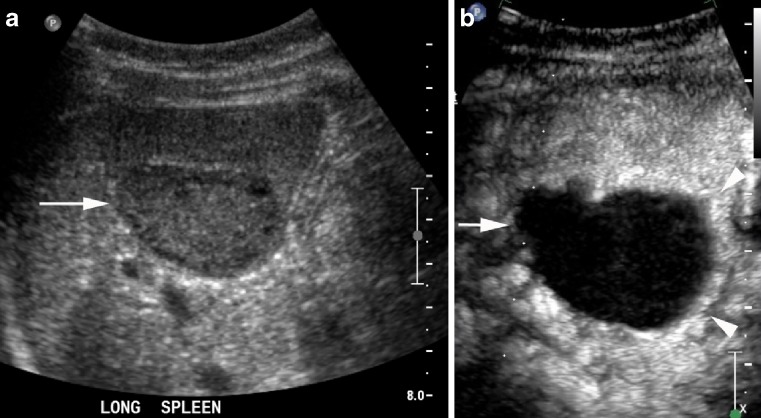
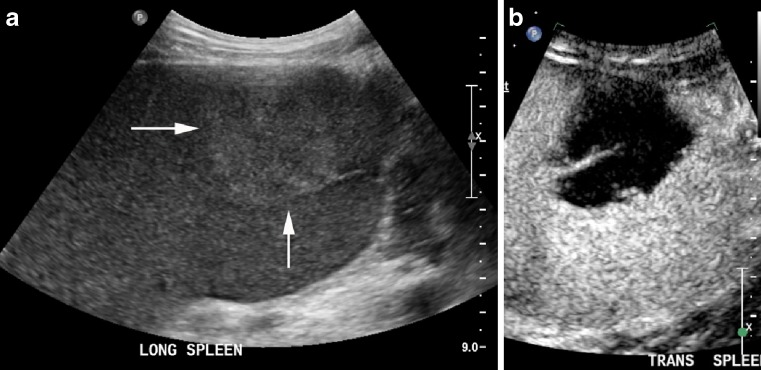
Fig. 3.
Large hypoechoic haemangioma (arrows) on B mode (a) with a small amount of normal spleen at the deep aspect (arrowheads). It has homogenous enhancement at 15 s (b) and retains contrast into the late phase (c)
Lymphoma
As previously mentioned the spleen is involved in 30–40% of cases of systemic lymphoma with a variety of B mode sonographic appearances, including small nodules, large masses and bulky infiltrative disease [19]. Lesions are typically hypoechoic relative to normal splenic parenchyma. In the arterial phase lymphoma may be isoenhancing or hypoenhancing relative to normal spleen [15] and is typically diffuse and with internal irregular vessels [10]. Homogenous arterial enhancement is more typical than heterogenous enhancement [9] (Fig. 4). With increasing delay they become more conspicuous and progressively more hypoenhancing relative to normal spleen [8, 20]. Late phase imaging can demonstrate the microcirculation within elements of the tumour that may not enhance during the early phase (Fig. 5). This microcirculation is usually seen as fine enhancing regions relative to normal splenic parenchyma that lacks the chaotic irregular vessels representing the macrocirculation that may be seen during the arterial phase.
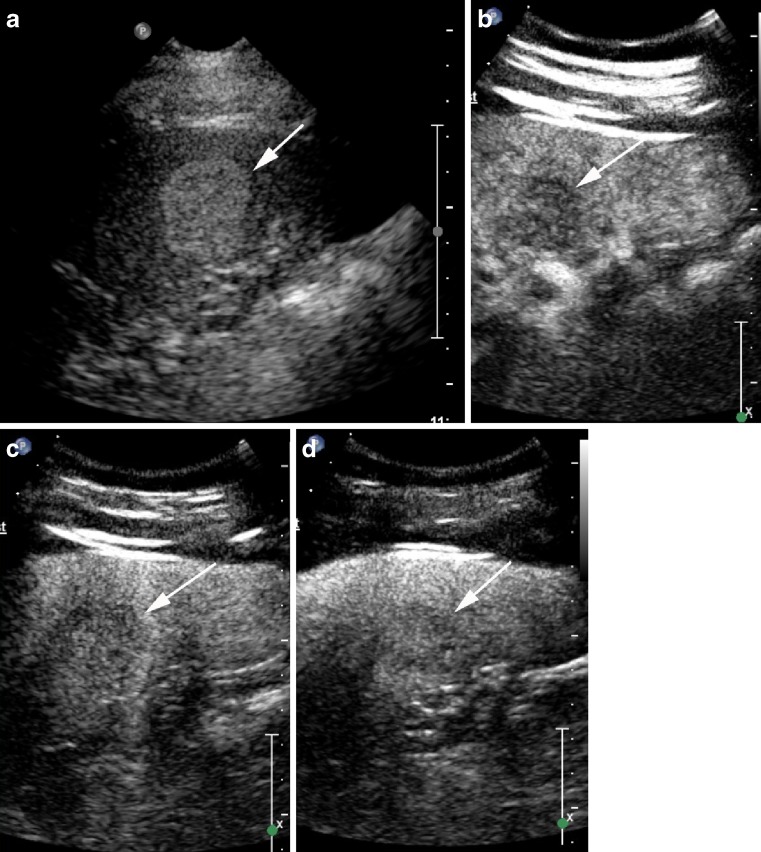
Fig. 4.
Hypoechoic heterogenous focal splenic mass (arrows) on B mode (a) is biopsy-proven diffuse B cell lymphoma. It has homogenous enhancement at 10 s (b), begins to washout by 30 s (c) and has clearly washed out by 60 s (d)
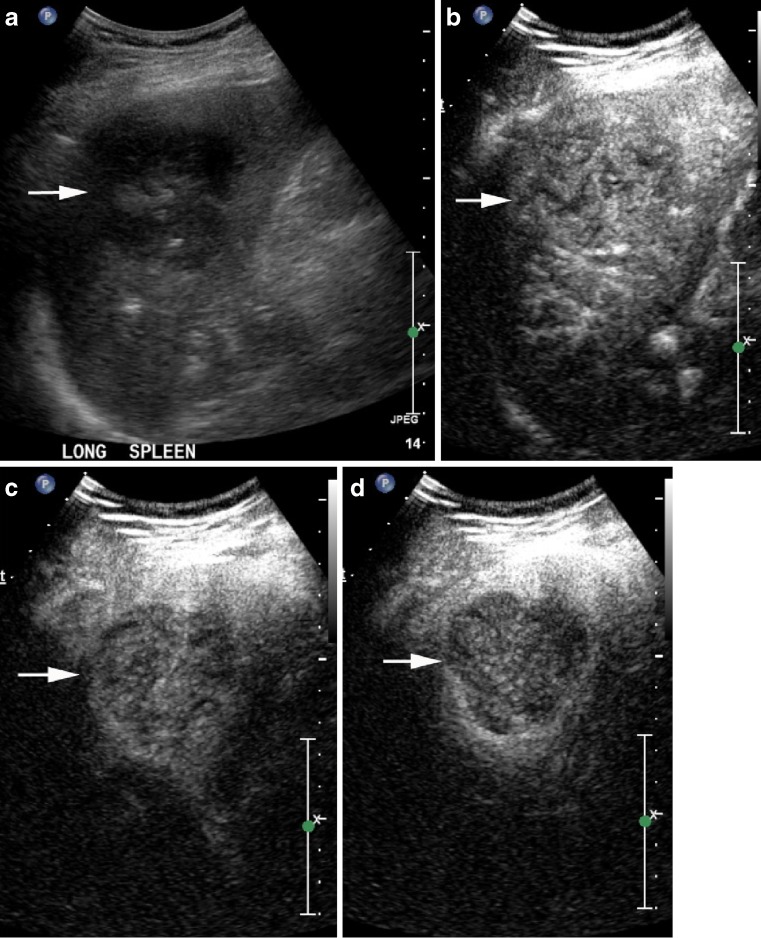
Fig. 5.
Lymphomatous mass bulging from the spleen (arrows) on B mode (a) contains islands of enhancement at 13 s (b) and more diffuse enhancement at 3 min 15 s (c) that is markedly less than normal spleen
Metastases
Splenic metastases from visceral tumours occur most frequently secondary to lung cancer, malignant melanoma and breast cancer [21]. Isolated splenic metastases are rare but have been described [22, 23]. Splenic metastases typically enhance slightly less than or to a similar degree to normal spleen and are often complex lesions followed by rapid washout (Fig. 6). Similar to lymphoma, late phase imaging can depict microcirculation in areas of viable tumour that may have otherwise been mistaken for necrosis, which is particularly important when assessing response to chemotherapy. It is impossible to differentiate lymphoma from metastases on the splenic CEUS appearance alone, although necrosis is more commonly seen in metastatic disease.
Fig. 6.
Melanoma metastasis (arrows) is hypoenhancing at 33 s (a), with washout at 1 min 36 s (b) and markedly hypoenhancing at 3 min 23 s (c)
Abscess
Splenic abscess may relate to Gram-negative or Gram-positive bacteria with fungal abscesses virtually confined to heavily immunosuppressed populations. A typical bacterial abscess does not enhance during any phase while a fungal or tuberculous abscess is hypoenhancing during the early and the late phases and with no enhancement or microcirculation depicted within areas of pus [10]. However other authors describe an enhancing rim and occasional internal septations [20] (Fig. 7). In our experience it is impossible to differentiate an abscess with an enhancing capsule from a tumour with central necrosis but viable peripheral tumour rind. In these cases clinical correlation is vital, and histologic/microbiological sampling may need to be considered. This is similar to the experience in the liver with CEUS alone struggling to differentiate many malignant and inflammatory lesions [17, 24].
Fig. 7.
A tuberculous abscess (arrows) appears solid on B mode (a) and has a subtle hyperenhancing capsule (arrowheads) at 7 s (b) with no central enhancement
Infarct
Splenic infarcts can have a variable appearance on B mode ultrasound but are typically hypoechoic and wedge shaped with the echogenicity increasing with time. However they can also demonstrate parenchymal heterogeneity and occasionally appear mass like. With ultrasound contrast they do not enhance [13], are well defined and often the wedge shaped nature of the lesion is better appreciated (Fig. 8). In our experience it is not uncommon to see internal vessels within an infarct representing clot lysis with reperfusion of large vessels (Fig. 9). These vessels are typically smooth, regular and well formed making them easily distinguishable from the irregular chaotic vessels seen with neoplasia.
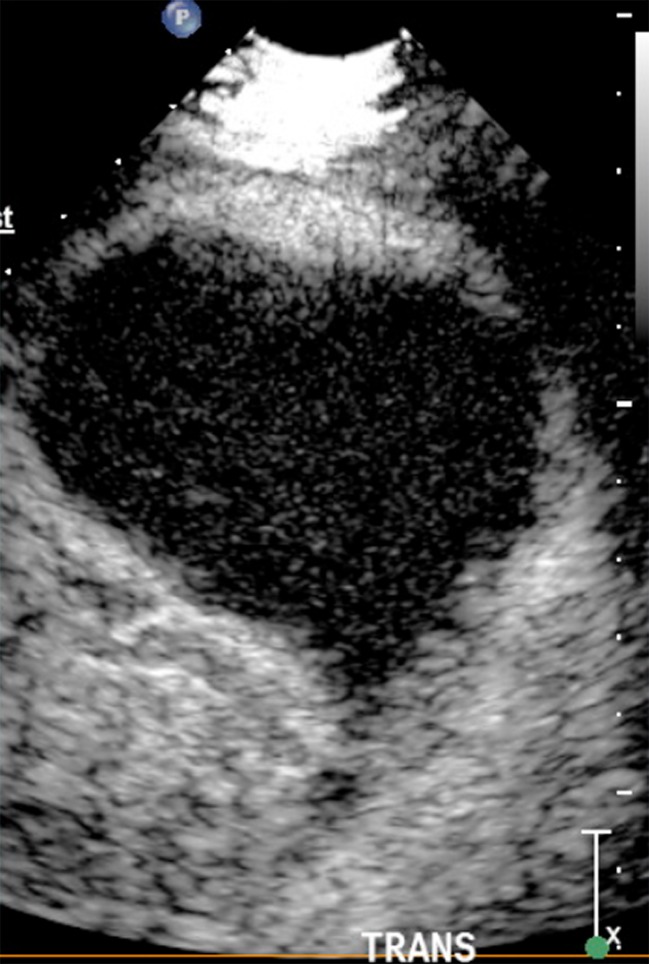
Fig. 8.
Wedge shaped splenic infarct with no enhancement
Fig. 9.
A subacute splenic infarct mimics an almost spherical mass on B mode (arrows) (a) with its wedge shaped nature better appreciated at 65 s with a well defined smooth and regular recanalised vessel at its apex (b)
Cysts
A simple cyst is easily diagnosed on B mode ultrasound, however cysts complicated by haemorrhage or infection may be more challenging. CEUS shows cysts to be non-enhancing structures that are well defined and lack the enhancing periphery seen with necrotic tumours and abscesses (Fig. 10) enabling confident diagnosis.
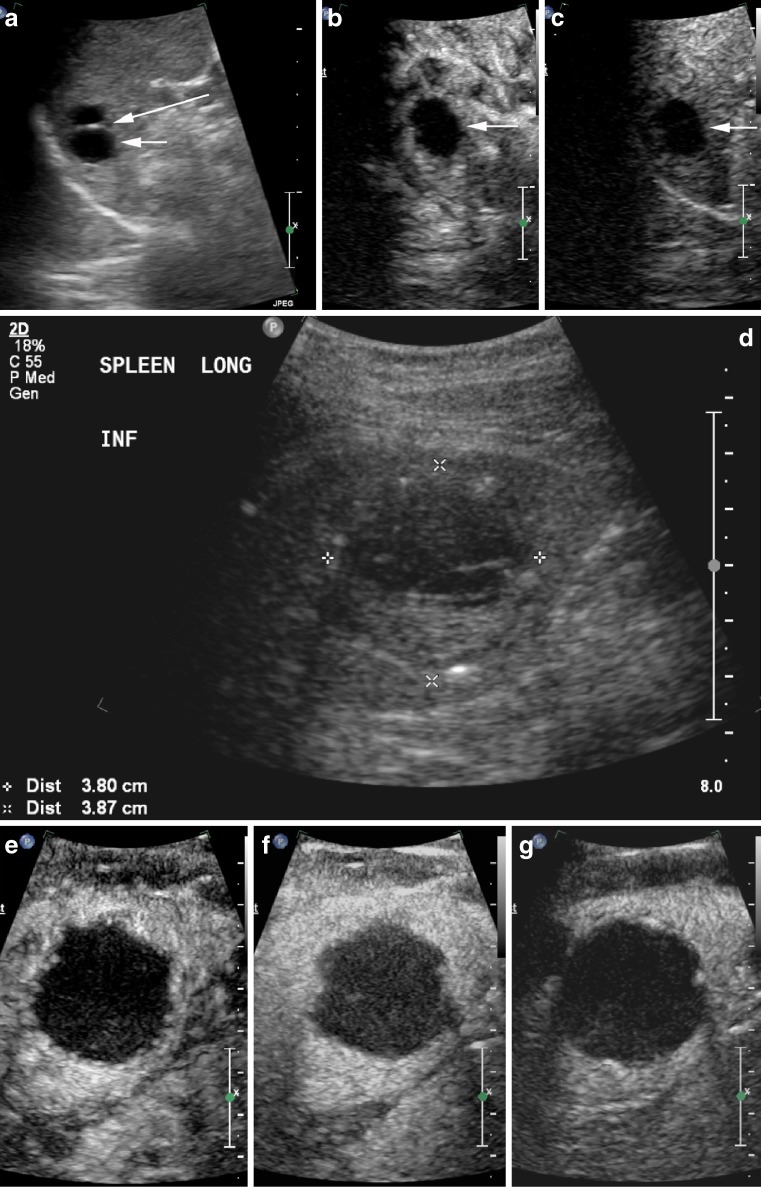
Fig. 10.
Anechoic cyst (short arrow) with a septation (long arrow) (a) in a patient with previous colorectal carcinoma. There is no enhancement within the cyst or the avascular septation at 12 s (b) or 2 min 30 s (c). A different patient with a heterogenous complex cyst (callipers on B mode, d). There is no enhancement within this cyst or its dependent debris and no enhancing capsule (e 32 s, f 66 s, g 2 min 37 s)
Sarcoid
Sarcoid is an idiopathic non-caseating granulomatous disease most commonly involving the pulmonary parenchyma and mediastinal lymph nodes, although disease may involve any organ. Splenic sarcoid may manifest as splenomegaly and/or multiple splenic parenchymal nodules and is seen in approximately 15% of patients with sarcoid [25]. The nodules are typically small, multiple and frequently associated with intra-abdominal adenopathy [26]. We have been able to find only a single case report describing the CEUS appearance of splenic sarcoid [27], and this article describes the lesions as being non-enhancing during all phases. In our experience we have found that the granulomas do enhance in the arterial phase but less than normal splenic parenchyma, and they remain hypoenhancing throughout the parenchymal and late phases (Fig. 11). The degree of hypoenhancement compared to normal spleen was relatively constant with no definite comparative washout. In this case clinical correlation and angiotensin-converting enzyme (ACE) assay were critical to establishing a confident diagnosis.
Fig. 11.
Innumerable hypoechoic granulomas (a) in a mildly enlarged spleen in an 18-year-old female with sarcoid. The granulomas are hypoenhancing in the arterial phase (b: 23 s), and remain hypoenhancing in the parenchymal phase (c: 1 min 45 s) and in the late phase (d: 3 min) with no definite washout compared to the background spleen
Conclusion
Contrast-enhanced ultrasound has been shown to be a safe and accurate method to differentiate benign from malignant focal splenic lesions particularly when coupled with appropriate clinical information. There is enough evidence to support its use as a first line test, particularly for focal splenic lesions detected at B mode ultrasound when it will often provide a diagnosis. The small proportion of lesions that remain indeterminate following CEUS can subsequently be investigated with CT, MRI or nuclear medicine where appropriate.
References
- 1.Bolondi L, Correas JM, Lencioni R, Weskott HP, Piscaglia F. New perspectives for the use of contrast-enhanced liver ultrasound in clinical practice. Dig Liver Dis. 2007;39:187–195. doi: 10.1016/j.dld.2006.08.008. [DOI] [PubMed] [Google Scholar]
- 2.EFSUMB Study Group et al. Guidelines and good clinical practice recommendations for Contrast Enhanced Ultrasound (CEUS) – update 2008. Ultraschall Med. 2008;29:28–44. doi: 10.1055/s-2007-963785. [DOI] [PubMed] [Google Scholar]
- 3.Correas JM, Bridal L, Lesavre A, et al. Ultrasound contrast agents: properties, principles of action, tolerance, and artifacts. Eur Radiol. 2001;11:1316–1328. doi: 10.1007/s003300100940. [DOI] [PubMed] [Google Scholar]
- 4.Quaia E, D’Onofrio M, Palumbo A, Rossi S, Bruni S, Cova M. Comparison of contrast-enhanced ultrasonography versus baseline ultrasound and contrast-enhanced computed tomography in metastatic disease of the liver: diagnostic performance and confidence. Eur Radiol. 2006;16:1599–1609. doi: 10.1007/s00330-006-0192-7. [DOI] [PubMed] [Google Scholar]
- 5.Lam KY, Tang V. Metastatic tumors to the spleen. Arch Pathol Lab Med. 2000;124(4):526–530. doi: 10.5858/2000-124-0526-MTTTS. [DOI] [PubMed] [Google Scholar]
- 6.Castellino RA. Hodgkin disease: practical concepts for the diagnostic radiologist. Radiology. 1986;159:305–310. doi: 10.1148/radiology.159.2.3515414. [DOI] [PubMed] [Google Scholar]
- 7.Castellino RA. The non-Hodgkin lymphomas: practical concepts for the diagnostic radiologist. Radiology. 1991;178:315–321. doi: 10.1148/radiology.178.2.1987584. [DOI] [PubMed] [Google Scholar]
- 8.Picardi M, Soricelli A, Pane F, et al. Contrast-enhanced harmonic compound US of the spleen to increase staging accuracy in patients with hodgkin lymphoma: a prospective study. Radiology. 2009;251(2):574–582. doi: 10.1148/radiol.2512081293. [DOI] [PubMed] [Google Scholar]
- 9.Görg C, et al. Contrast enhanced ultrasound of splenic lymphoma involvement. Eur J Radiol. 2009 doi: 10.1016/j.ejrad.2009.11.012. [DOI] [PubMed] [Google Scholar]
- 10.Stang A, Keles H, Hentschke S, et al. Differentiation of benign from malignant focal splenic lesions using sulphur hexafluoride-filled microbubble contrast-enhanced pulse-inversion sonography. AJR Am J Roengenol. 2009;193:709–721. doi: 10.2214/AJR.07.3988. [DOI] [PubMed] [Google Scholar]
- 11.Bachmann C, Gorg C. Color Doppler sonographic findings in focal spleen lesions. Eur J Radiol. 2005;56:386–390. doi: 10.1016/j.ejrad.2004.10.016. [DOI] [PubMed] [Google Scholar]
- 12.Metser U, Miller E, Kessler A, et al. Solid splenic masses: evaluation with 18F-FDG PET/CT. J Nucl Med. 2005;46:52–59. [PubMed] [Google Scholar]
- 13.Yu X, Yu J, Liang P, Liu F. Real-time contrast-enhanced ultrasound in diagnosing of focal spleen lesions. Eur J Radiol. 2011 doi: 10.1016/j.ejrad.2010.12.052. [DOI] [PubMed] [Google Scholar]
- 14.Gorg C. The forgotten organ: contrast enhanced sonography of the spleen. Eur J Radiol. 2007;64:189–201. doi: 10.1016/j.ejrad.2007.06.036. [DOI] [PubMed] [Google Scholar]
- 15.Herbay A, Barreiros AP, Ignee A, et al. Contrast-enhanced ultrasonography with sonovue: differentiation between benign and malignant lesions of the spleen. J Ultrasound Med. 2009;28:421–434. doi: 10.7863/jum.2009.28.4.421. [DOI] [PubMed] [Google Scholar]
- 16.Chami L, Lassau N, Malka D, Ducreux M, Bidault S, Roche A, Elias D. Benefits of contrast-enhanced sonography for the detection of liver lesions: comparison with histologic findings. AJR Am J Roentgenol. 2008;190:683–690. doi: 10.2214/AJR.07.2295. [DOI] [PubMed] [Google Scholar]
- 17.Bhayana D, Kim TK, Jang HJ. Hypervascular liver masses on contrast-enhanced ultrasound: the importance of washout. AJR Am J Roentgenol. 2010;194:977–983. doi: 10.2214/AJR.09.3375. [DOI] [PubMed] [Google Scholar]
- 18.Benedetti E, Proietti A, Miccoli P, et al. Contrast-enhanced ultrasonography in nodular splenomegaly associated with type B Niemann-Pick disease: an atypical haemangioma enhancement pattern. J Ultrasound. 2009;12:85–92. doi: 10.1016/j.jus.2009.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gorg C, Weide R, Schwerk WB. Malignant splenic lymphoma: sonographic patterns, diagnosis and follow-up. Clin Radiol. 1997;52(7):535–540. doi: 10.1016/S0009-9260(97)80331-5. [DOI] [PubMed] [Google Scholar]
- 20.Catalano O, et al. Contrast-enhanced sonography of the spleen. AJR Am J Roentgenol. 2005;184:1150–1156. doi: 10.2214/ajr.184.4.01841150. [DOI] [PubMed] [Google Scholar]
- 21.Schon CA, et al. Splenic metastases in a large unselected autopsy series. Pathol Res Pract. 2006;202(5):351–356. doi: 10.1016/j.prp.2005.12.008. [DOI] [PubMed] [Google Scholar]
- 22.Okuyama T, et al. Isolated splenic metastasis of sigmoid colon cancer: a case report. Jpn J Clin Oncol. 2001;31(7):341–345. doi: 10.1093/jjco/hye065. [DOI] [PubMed] [Google Scholar]
- 23.Tang H, et al. Isolated splenic metastasis from lung cancer: ring leader of continuous fever. Eur Respir Rev. 2010;19(117):253–256. doi: 10.1183/09059180.00004110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu GJ, Lu MD, Xie XY, Xu HX, Xu ZF, Zheng YL. Real-time contrast-enhanced ultrasound imaging of infected focal liver lesions. J Ultrasound Med. 2008;27:657–666. doi: 10.7863/jum.2008.27.4.657. [DOI] [PubMed] [Google Scholar]
- 25.Warshauer DM, Dumbleton SA, Molina PL, et al. Abdominal CT findings in sarcoidosis: radiologic and clinical correlation. Radiology. 1994;192:93–98. doi: 10.1148/radiology.192.1.8208972. [DOI] [PubMed] [Google Scholar]
- 26.Warshauer DM, Molina PL, Hamman SM, et al. Nodular sarcoidosis of the liver and spleen: analysis of 32 cases. Radiology. 1995;195:757–762. doi: 10.1148/radiology.195.3.7754007. [DOI] [PubMed] [Google Scholar]
- 27.Perez-Grueso MJ, Repiso A, Gomez R, et al. Splenic focal lesions as manifestation of sarcoidosis: characterization with contrast-enhanced sonography. J Clin Ultrasound. 2007;35(7):405–408. doi: 10.1002/jcu.20322. [DOI] [PubMed] [Google Scholar]