Abstract
Magnetic resonance imaging (MRI) is the best imaging modality for preoperative assessment of patients with anal fistula. MRI helps to accurately demonstrate disease extension and predict prognosis. This in turn helps make therapy decisions and monitor therapy. The pertinent anatomy, fistula classification and MRI findings will be discussed.
Keywords: Anus, MRI, Fistula, Crohn’s disease
Background
Etiology and epidemiology
A fistula is defined as a pathologic tract connecting two hollow organs, or one hollow organ and the skin. Sinuses are defined when only one hollow organ or skin in involved. Anal fistula is a somewhat uncommon condition. It affects approximately ten individuals in 100,000. It usually affects men, in their fourth decade [1]. Men are affected two- to four-times more commonly; the reason is thought to be partially due to the higher abundance of anal glands [2]. Infection of the anal glands and crypts is thought to be the cause of later fistula formation. The disease usually begins as an abscess and in chronic stages develops into a fistula in 60% of cases [3]. There are, of course, other etiologies as well, such as trauma during childbirth, Crohn’s disease (see below) and malignancies. The cryptoglandular form of the fistula disease usually manifests itself in the form of chronic discharge and pain. Traditionally, treatment has been surgical, with recurrence happening in up to a quarter of cases [4].
Cryptoglandular fistulas are usually distinguished from fistula due to Crohn’s disease. This distinction is due to the fistula being more complex in the latter group. This distinction is not always clear, however, since at least half of fistulas in patients with Cohn’s disease are simple. Crohn’s fistulas result from transmural spread of chronic granulomatous inflammation. More than one-third of Crohn’s patients have perirectal disease, which could lead to fistula disease [5].
Role of imaging
The role of imaging is, therefore, to outline all hidden tracts and define the relationship of the fistula to the anal sphincter. Inadvertent damage to the anal sphincter can lead to anal incontinence; hence the importance of knowledge of the relation between the fistula tract and the anal sphincter. There are, however, other indications for imaging in anal fistula. Occasionally, general physicians or gastroenterologists wish to know if there are any fistulas present at all. For these physicians, knowledge of exact extension of fistula is not required and a more simple magnetic resonance imaging (MRI) protocol could be sufficient. Also, with the advent of new nonsurgical treatment modalities, monitoring therapy response is becoming more frequently performed.
MRI performed adequately should be regarded as the “gold standard” for preoperative assessment, replacing surgical examination under anesthetic (EUA) in this regard [6, 7]. However, endoanal ultrasonography is used by many surgeons in the preoperative workup of anal fistulas. Although there are some conflicting results, hydrogen peroxide-enhanced endoanal ultrasonography may be comparable with MRI [8]. Endoanal ultrasound alone is sufficient in more simple cases; however, MRI is generally is superior to endoanal ultrasonograhy [9, 10].
MRI helps not only to accurately demonstrate disease extension but also to predict prognosis, make therapy decisions, and monitor therapy [11, 12]. Missed extensions at surgery are usually the cause of recurrence, and adequate surgery is warranted in more extensive disease [9]. MRI has been shown to reduce recurrent disease and, therefore, reoperation. In patients with Crohn’s disease, the recurrence could be due to inadequate medical treatment. MRI can be used for monitoring therapy and predicting prognosis even in patients with Crohn’s disease [13].
Pertinent radiologic anatomy
Boundaries of the anus
The anal canal begins at the anal verge which corresponds to the lowermost portion of the external sphincter. The upper part of puborectalis muscle forms the radiologic upper boundary of the anal canal. Thus, the anus is the infralevator portion of the gastrointestinal tract, surrounded by the ischioanal fossa to each side. The ischioanal fossa is sometimes incorrectly termed the ischiorectal fossa.
Muscular wall
The external sphincter muscle is a voluntary striated muscle, which continues 1.5–2 cm upward until it ends and the fibers of the puborectalis muscle continue as part of the pelvic floor [14]. For radiologic purposes, the levator ani muscle is the muscle that forms the pelvic floor.
The muscular propria layer, which covers the rectum, like most of the gastrointestinal tract, has two layers: the inner circular and the outer longitudinal layer. The circular is continuous with the internal sphincter muscle. This involuntary muscle provides 85% of the resting tone of the anus, the remainder is provided by the external sphincter. However, this muscle when contracted can prohibit defecation. Likewise, damage to this muscle can lead to fecal incontinence [15].
Dentate line
Almost mid-portion in the anal canal there is the dentate line. Here the anal glands extend to the intersphincteric plane. Three-fourths of the fistulas formed from this area extend through the intersphincteric plane to the skin. The dentate line is also where squamous epithelium meets columnar epithelium.
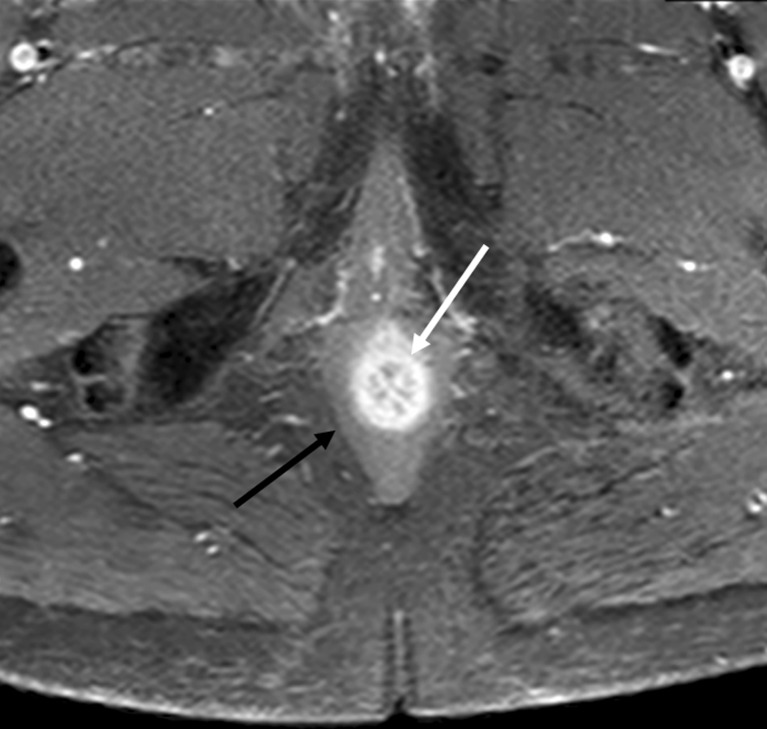
Anal clock
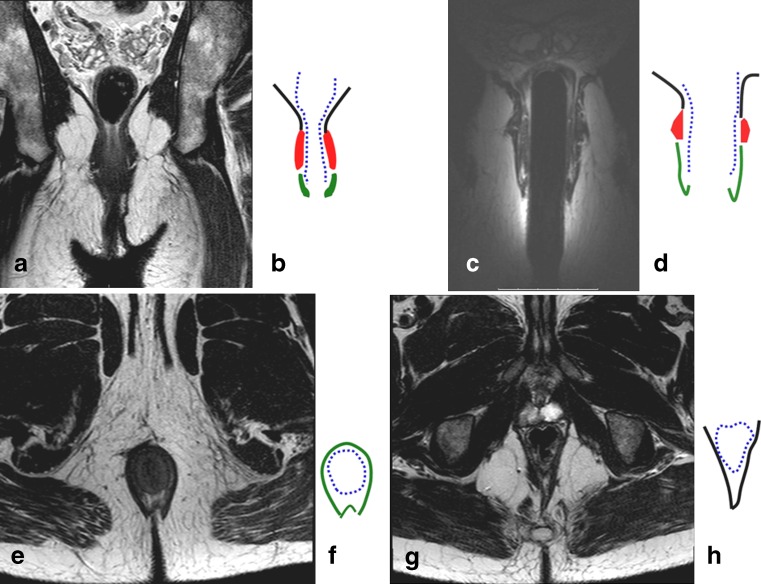
The anal clock is a transversal view of the canal, which corresponds to the radiologic view as well. At 12 o’clock, therefore, is the ventral portion of the anal canal, and at 3 o’clock is the left lateral part, and so on (Fig. 1).
Fig. 1.
Coronal T2-weighted image of the pelvic floor obtained by surface coil (a), and schematic representation (b). Coronal T2-weighted image obtain by endoanal coil (c) and its schematic representation (d). Axial T2-weigted image at the level of sphincters (e) and the corresponding schematic image (f). Axial T2-weighted image at the level higher than the dentate line (g) and corresponding schematic image (h). On all schematic images the black lines represent levator ani muscle; the blue dotted line represents muscular propria and internal sphincter; the red areas represent external sphincter; and the green areas represent the external sphincter
Fistula classification
Primary tracts
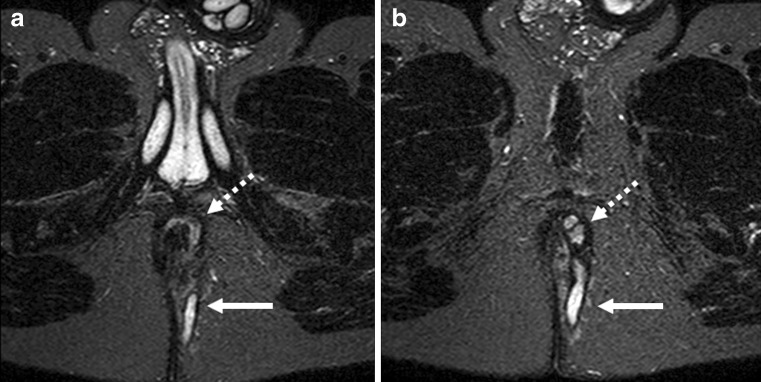
The most common classification is that of Parks (Fig. 2), which is based on extensive study of 400 consecutive cases, many of which were more complex and severe than those seen at the practice of a general surgeon [16]. Parks described the primary tracts as following four patterns. The most common group is the intersphincteric type, where the primary track reaches the perianal skin through the intersphincteric plane (Fig. 3).
Fig. 2.
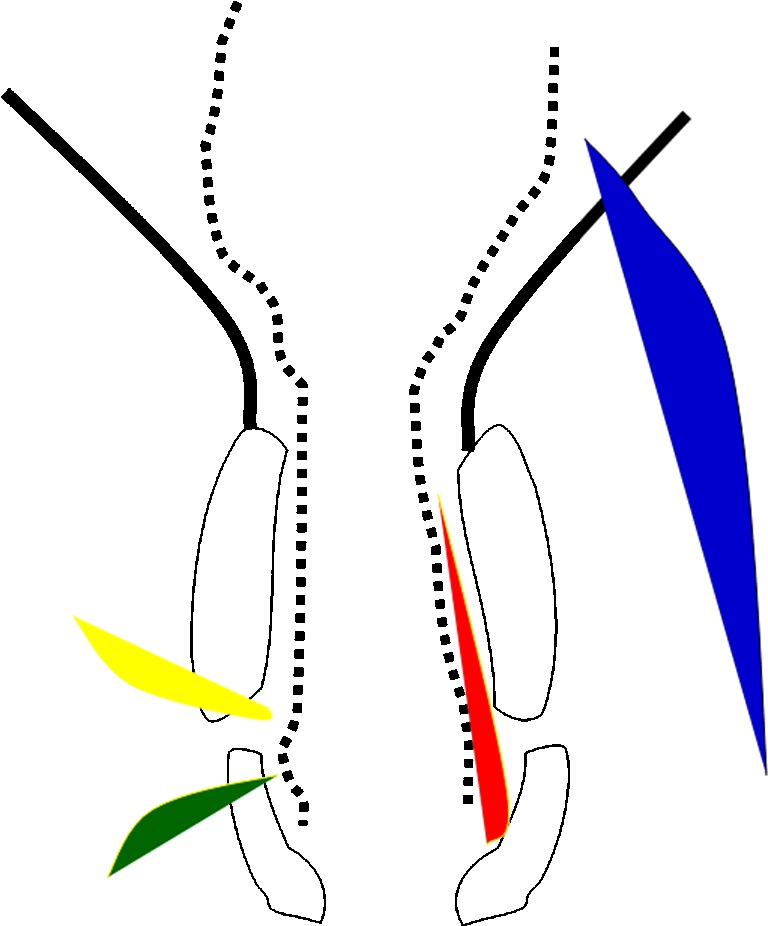
Schematic representation of the anal canal and pelvic musculature in black corresponding to Fig. 1b. The fistulas, as described by Parks, are represented by differently colored shaded areas: red for inter-sphincteric, green for trans-sphincteric, yellow for supra-sphincteric, and blue for extra-sphincteric
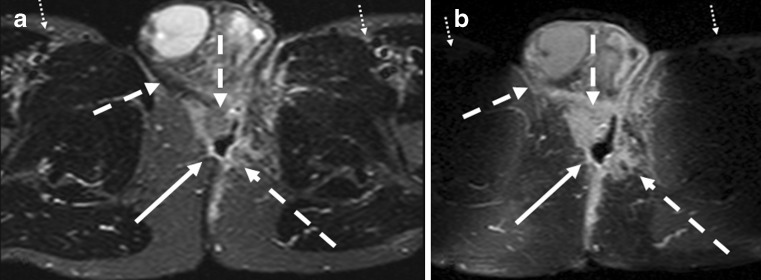
Fig. 3.
Axial T2-weighted images with fat suppression (STIR) at two different levels (a, b). The dotted arrows demonstrate the inter-sphincteric fistula and its extension to the skin (solid arrow)
The next common type, or trans-sphincteric type, occurs when the track courses through the external sphincter muscle, usually involving the ischioanal fossa (Fig. 4). The external opening can be further away from the anus, meaning that the most distal part of the ischioanal fossa is involved. This should not necessarily mean that that the fistula has traversed the external sphincter. The level at which the external sphincter complex is traversed is most often at the mid-anal canal level and the internal opening is usually at around 6 o’clock, at the level of dentate line. This is, however, not invariable.
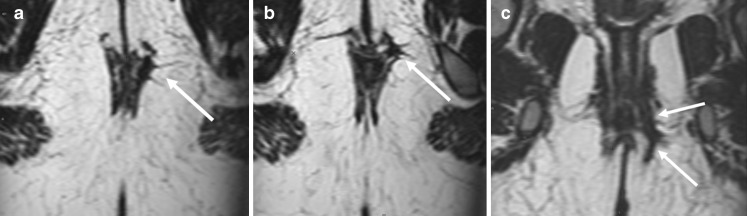
Fig. 4.
Complex fistula with both inter-sphincteric and trans-sphincteric components. An axial T2-weighted and three consecutive thin slice (1 mm) T1-weighted images with fat-saturation after gadolinium contrast. Images show inter-sphincteric fistula (white arrows). There is a thin communicating fistula stretching in the inter-sphincteric plane (thin hatched arrow), going through the external sphincter to reach the fistula lying outside the external sphincter (dotted arrow)
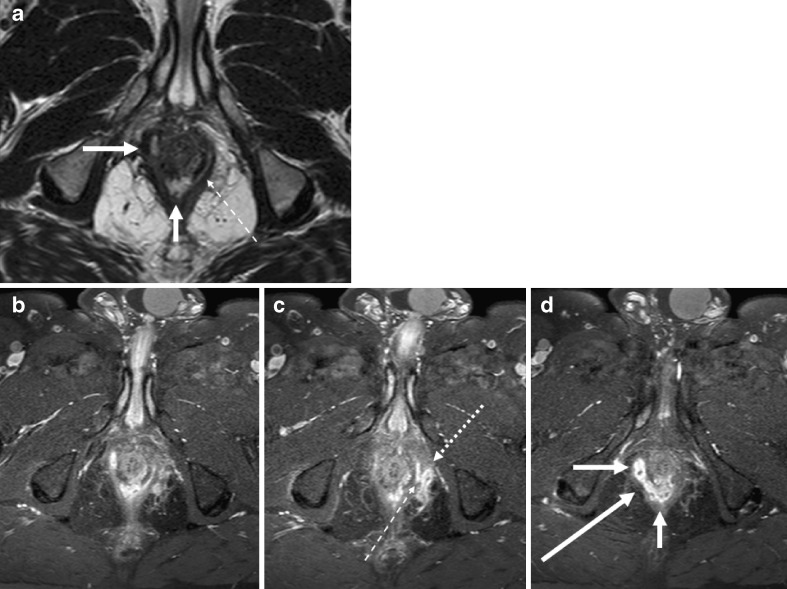
In the suprasphincteric fistula, in contrast to intersphincteric fistula, the fistula courses initially upward above the sphincter muscles (Fig. 5), and then coursing down to the perianal skin (Fig. 6).
Fig. 5.
Two axial T2-weighted images (a, b) demonstrate thick fistula tracks (black arrows) lying between rectal muscular wall and the pelvic floor just above the puborectalis muscle. The internal opening is seen as a large opening into the dorsal aspect of anorectal junction (white arrow)
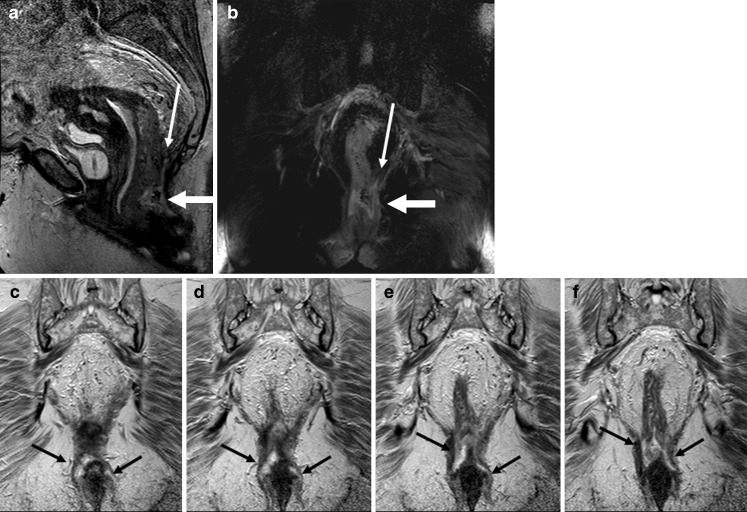
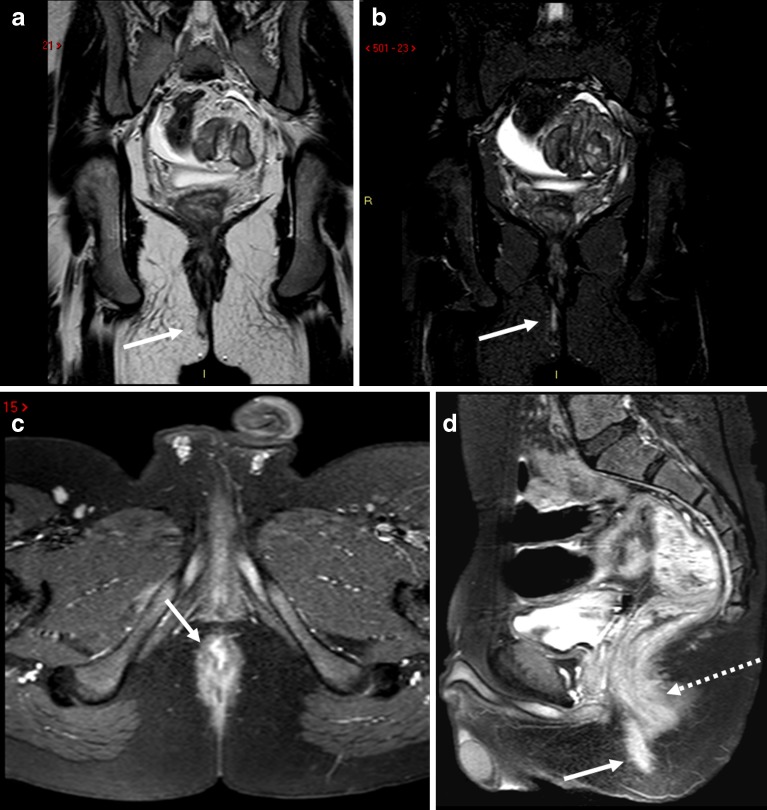
Fig. 6.
Sagittal T2-weighted image (a) and T2-weighted coronal image with fat-saturation (b) show an abscess (thick white arrows on a and b) at the level of anorectal junction. There is blind sinus (thin white arrow) extending upward above the pelvic floor. Consecutive coronal T2-weighted images (c–f) show extension of the abscess in the inter-sphincteric planes bilaterally down
All the above-mentioned types have an intersphincteric portion and communication to the anal canal. The fourth type according to Parks, or extrasphincteric, does not behave in this way. Extrasphincteric fistulas are recognized by the absence of any MR signal, suggesting sepsis within the intersphincteric space because, by definition, there is no communication with the anal canal. Here, conditions such as Crohn’s disease, adenocarcinoma or diverticular disease should be sought.
Secondary tracts
The primary tracks described above can be complicated by secondary tracks. Supralevator, ischioanal and horseshoe extensions are common. Horseshoe extensions are circular extensions to both sides of the internal opening (Fig. 7). Any type of fistula may show a circumferential spread, but a typical horseshoe-shaped fistula has two tracts and one internal opening, often in the midline posteriorly at the level of the inferior border of the puborectalis muscle. Horseshoe fistulas can extend in the intersphincteric, ischioanal, or supralevator directions. A fistula medial to the levator plate or puborectalis muscle is supralevator, and a fistula lateral to these muscles is infralevator.
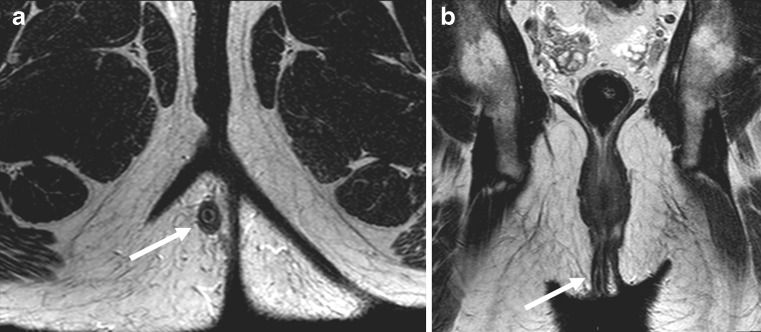
Fig. 7.
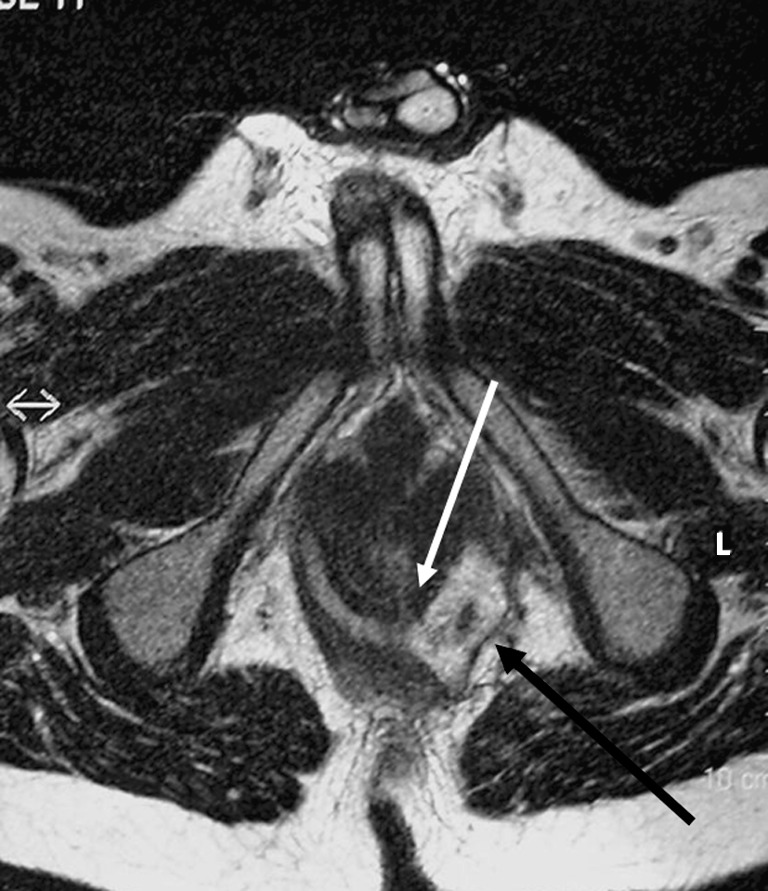
Horseshoe fistula in the intersphincteric plane on an axial T2-weighted image. The internal opening is located at 5–6 o’ clock as a thin white extension (white arrow). The left fistula is more an abscess with debris and extension beyond the external sphincter (black arrow)
MRI classification
MRI has in several studies been shown to be highly predictive of patient outcome [17–20]. At St James’s University Hospital the following MRI grading classification is used: 0, normal appearance; 1, simple linear intersphincteric fistula; 2, intersphincteric fistula with intersphincteric abscess or secondary fistulous track; 3, trans-sphincteric fistula; 4, trans-sphincteric fistula with abscess or secondary track within the ischioanal or ischiorectal fossa; 5, supralevator and translevator disease. This MR grading has been shown to correlate with outcome: grades 1 and 2 are associated with favorable outcome (i.e., no recurrences and therefore no need for reoperations), while grades 3–5 are associated with less favorable outcome (leading to recurrences needing reoperations).
Abscess
Any widening of the primary or the secondary tract could be considered an abscess. There is no clear-cut definition of when a fistula is large enough to be called an abscess; the arbitrary limit of 1 cm can be used.
Crohn’s disease
Complex Crohn’s fistulas can consist of multiple tracts and abscesses [21]. A well-defined primary tract is often not recognizable. The tracts tend to be relatively large, often with extensions. The anal canal, the rectum, sigmoid colon, small bowel loops, and other pelvic organs maybe involved. The fistulas and abscesses can be located below, above or within the levator ani muscle. Unilateral thickening of the levator muscle may be reactive and is not always due to an intramuscular abscess. Proctitis and thickened perirectal fascia are commonly seen [22].
MRI protocol and findings
The indication for imaging determines to a large extent the imaging protocol.
Occasionally, nonsurgical specialists, e.g., gastroenterologists and general physicians wish to know if there are any fistulas present. Occasionally the external opening may heal but with a deeply located abscess or fistula tract remaining, making diagnosis of fistula difficult clinically. This has become relevant since treatment of perianal Crohn’s disease with anti-TNF-alpha drugs is contraindicated in the presence of an abscess [23]. For this indication, the pelvic anatomy is not important and therefore a simpler protocol might be sufficient (Fig. 8).
Another indication for imaging could be follow-up of fistulas (Fig. 9) treated with nonsurgical methods, especially in Crohn’s disease [13]. For the above-mentioned indications, perhaps a simpler protocol would be enough. Understandably, disappearance of areas with high signal on T2-weighted imaging (Fig. 10) and normalization of enhancement on postgadolinium T1-weighted imaging are signs of fistula healing.
Most radiology authorities work at centers with surgical teams specializing in surgical treatment of fistulas. At these locations, the indication for imaging is surgical planning. The MRI protocol at these centers must depict the fistula tract with or without fluid content, and also the pelvic anatomy and its musculature [24].
Fig. 8.
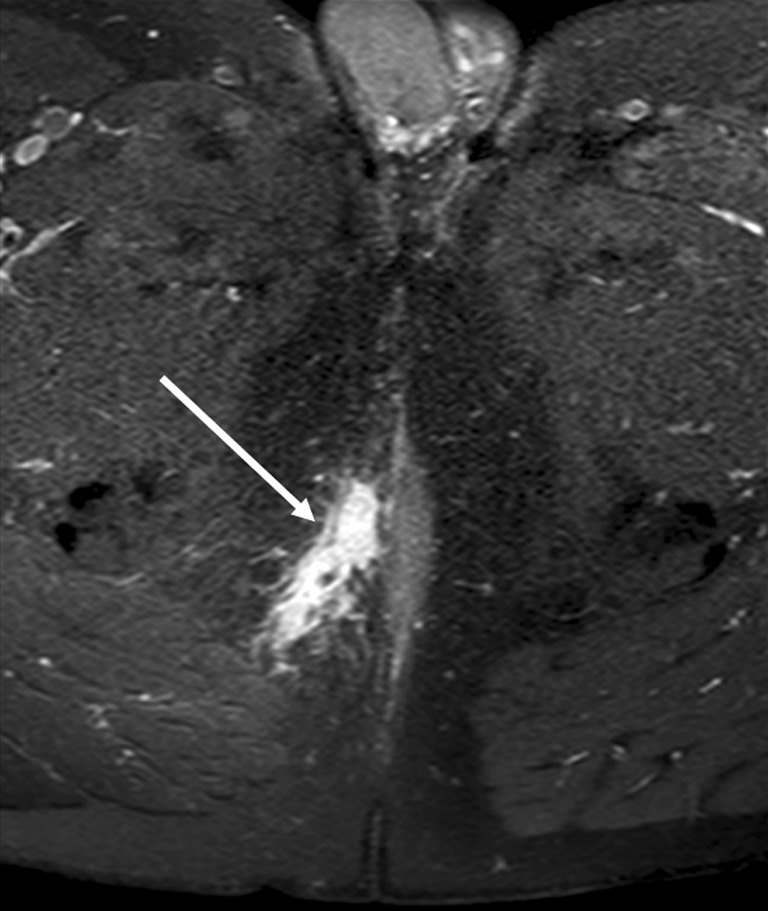
T1-weighted image with fat-saturation after gadolinium contrast enhancement. Inflammation around a fistula is depicted by avid contrast enhancement (white arrow). The entire abdomen and pelvis of patient had been imaged, and therefore a simpler protocol had been applied without TSE T2-weigted images over the pelvic area
Fig. 9.
Sagittal (a) and coronal (b) T2-weighted images of a patient treated with seton at follow-up. The seton is seen as a dark inner structure in the middle of the fistula (white arrows)
Fig. 10.
Reformatted axial (a, b) and coronal (c) T2-weighted images based on three-dimensional (3D) volume-based imaging. The fistula tract does not show any high signal intensity (white arrows) and this is indicative of fibrotic rest
Sequences
The most common sequences are T2-weighted images with or without different forms of fat saturation, and T1-weighted images before and after gadolinium enhancement and with fat saturation. At T2-weighted images, fistulas have a central high-signal-intensity tract that is surrounded by a relatively low-signal-intensity wall. The inner high-signal intensity is the true lumen and granulation tissue, and the outer low-signal intensity part is comprised of fibrotic tissue. With progressive fibrosis the high-signal intensity of the lumen decreases, denoting chronic phase of a fistula.
Fat-suppression should be employed if using turbo-spin-echo (TSE) T2-sequences, which could cause confusion as the high signal of the fistula could easily be missed due to high signal of surrounding fat. Therefore, most authors favor STIR (short-inversion-time inversion recovery) imaging since it combines fat-suppression and structural delineation [25]. With experience and time, however, TSE images are quite adequate, with the advantage of higher structural delineation. Therefore, we prefer, in contrast, TSE images for the delineation of fistulas. We have seen inexperienced radiologists interpret other high-signal findings due to fat for fistulas (Fig. 11).
Fig. 11.
Sagittal T2-weighted image with fat-saturation. The ischioanal abscess (arrowheads) is communicating via a fistula (dotted arrow) to another fistula (hatched arrow). The peri-prostatic vessels and other structures with high-signal intensity on T2-weighted images (solid arrows) can cause confusion if mistaken for fistulas
Some normal pelvic structures have relatively high signal on STIR, such as the periprostatic or vaginal vessels and the internal sphincter. Asymmetry helps to distinguish between fistulas and normal higher signal intensity sometimes but not always. We use STIR in one plane only to demonstrate fluid channel more efficiently, but use TSE in three planes for adequate assessment of fistulas. These planes are the sagittal, semi-coronal (parallel to anal canal or perpendicular to pelvic floor) and perpendicular to anal canal. At least for one plane, we recommend imaging with a higher echo-time, which delineates fistulas very clearly. Another sequence that we have used recently is T2-weighted volume imaging with the capability of performing reformatted images. Though our studies are not finished yet, we believe that this single sequence can replace all T2-weighted imaging.
T1-weighted imaging may not always be necessary. It has, however, been sometimes beneficial to have these images [26]. Unenhanced T1-weighted imaging may help diagnose postoperative hemorrhage or fat-containing grafts. The tracks enhance vividly after gadolinium administration. Some normal structures (Fig. 12) and structures close to a fistula (Fig. 13) can cause confusion since they can have avid contrast enhancement. This could be at least partially explained by the timing of imaging (Fig. 14). We have not found any reports questioning the usefulness of postcontrast imaging, but only publications about more time-consuming measurements showing intensity curves correlating with degree of inflammation or, more commonly, patient’s symptoms. Those who have demonstrated the positive effect of contrast enhancement have usually used dynamic imaging looking at time intensity curves. There is a possibility that the timing of the contrast enhancement plays a role in the assessment of degree of inflammation (Fig. 14). Moreover, we are not aware of any studies evaluating the additive value of contrast enhancement to other sequences. Some radiologists, therefore, do not use contrast enhancement; whenever only surgery is planned, T2-weighted imaging is adequate. The parameters for T1-weighted imaging are very similar to other pelvic imaging protocols, with TR 9 ms, TE 4–5 ms, FOV 20–26 cm, matrix 256, flip angle 10, slice thickness 1 mm and number of slices 100. We use 3D-volume T1-weighted imaging with fat suppression (THRIVE in Philips, VIBE in Siemens and FAME in GE). Using volume imaging enables us to image in only one plane. Not all the authors use the same parameters, and there is no clear advantage of any of these. However, it seems prudent to use the same sequence before and after contrast enhancement for most reliable comparisons.
Fig. 12.
The internal sphincter and anal mucosa (white arrow) normally show marked contrast enhancement (as shown on this axial T1-weighted image with fat-saturation) and higher signal on T2-weighted images. The external sphincter does not demonstrate the same amount of contrast enhancement
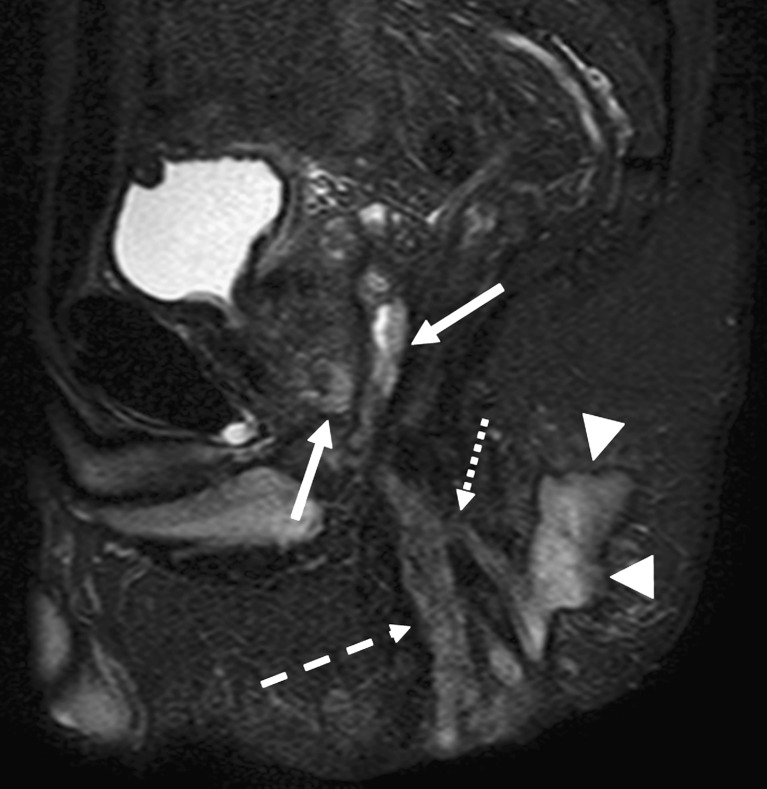
Fig. 13.
Patient with abscess behind the root of the scrotum. Axial STIR (a) and T1-weighted with contrast-enhancement (b) show an abscess (white arrows) with central cavity. The extension of the inflammation seems larger on contrast-enhanced images compared with STIR (hatched arrows). Also note the nonuniform fat saturation on b compared with a (small dotted arrows)
Fig. 14.
Simple fistula (white arrows) shown on TSE T2-weighted (a) and STIR (b) images. The axial T1-weighted image after gadolinium contrast (c) shows avid contrast enhancement of the fistula (white arrow) but to lesser degree the anal sphincter. The sagittal T1-weighted image (d) a few minutes later (note the filling of the urinary bladder) demonstrates decreased distinction in degree of contrast enhancement between the fistula (white arrow) and the anal sphincter (hatched arrow), consistent with time intensity curve characteristics described by Horsthuis et al. [13]
For T2-weighted imaging, most radiologists use a TSE or FSE (fast-spin-echo) T2 sequence. The parameters for this sequence are TR 3,000–4,000 ms, TE 120–150 ms, FOV 20–26 cm, slice thickness 3–5 mm, matrix 512, flip angle 90, number of slices 24 (for 4-mm slice thickness), and fold-over direction. Please note that the field of view does not need to be too large. Very seldom do fistulas extend beyond the true pelvis.
Fistula openings
The location of external openings is of little importance, since both the patient and the surgeon can find it often easily themselves. Goodsall’s rule states that the external opening of a fistula situated behind the transverse anal line will open into the anal canal in the midline posteriorly. An anterior opening is usually associated with a radial tract, however. The exception to the rule is anterior fistulas lying more than 3 cm from the anus, which may have a curved track (similar to posterior fistulas) [27]. This rule is, however, not without many exceptions [28]. The internal opening will decide the extent of sphincter division during fistulotomy. Since the dentate line cannot be identified on MRI, its position must be inferred as the middle of the anal canal. If no internal opening can be detected, then a sinus should be diagnosed instead of a fistula.
Coils
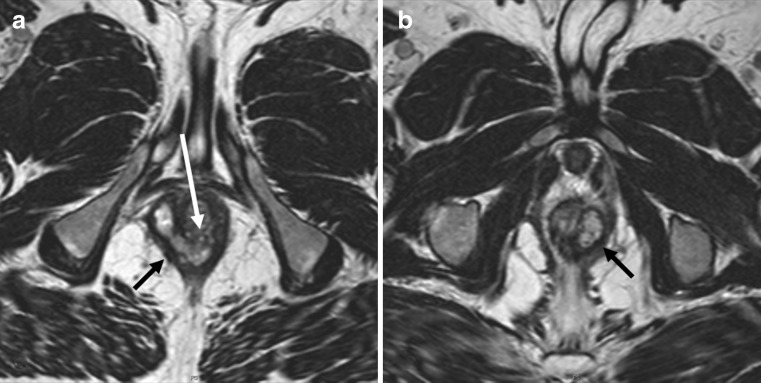
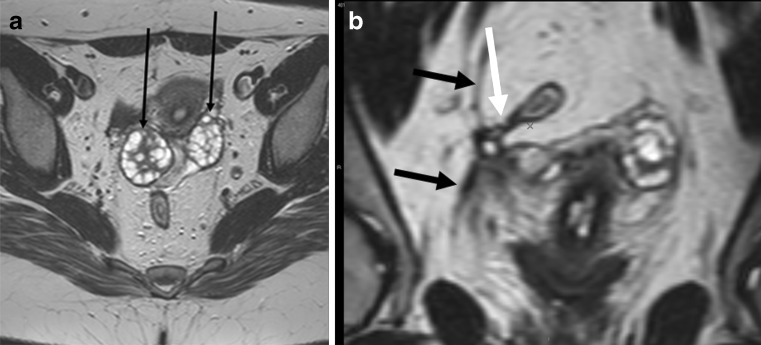
Most authors use pelvic surface coils, the same that is used for almost all pelvic imaging. Endorectal and, more appropriately, endoanal probes are less commonly used. Though endoanal probes provide better depiction of internal opening than surface coils, their limited field of view is a problem (Fig. 15). Also, placement of these probes is not always easy or possible. Combining both coils probably provides the best diagnostic accuracy, yet is cumbersome to employ [29]. In our practice, we do not use anal probes for depiction of fistula anymore.
Fig. 15.
Patient with healed peri-anal fistula shown in Fig. 10. The larger field of view with surface coil enabled even demonstration of pathology in the pelvis. Axial T2-weighted image (a) and reformatted coronal image (b) demonstrate ovaries (small black arrows) drawn medially due to retraction of a healed abscess in the Douglas pouch. There is also fibrosis of right pelvic fascia (black arrows) retracted medially. Finally a small fistula is evident stretching from rectosigmoid junction (white arrow)
Final points:
Some authors have used MR fistulography, though this is not normal practice.
We recommend a short period of fasting (4 h) before imaging. To our knowledge, there are no studies looking into the necessity of using antiperistaltic agents for fistula imaging. Since most fistulas are located below the pelvic floor, bowel peristalsis should not be a problem in the majority of cases. However, in cases where there is suspicion of supralevator extension of the disease, antiperistaltic agents might prove essential.
The scan duration time is variable on the number of sequences used. Each sequence and plane of imaging takes 3–6 min. Thus, a total of 15–30 min could be allocated for pelvic imaging. Using only 3D-volume T2-weighted imaging might reduce this time to around 10 min (6–7 min for imaging and the rest for patient positioning).
We are not aware of any studies comparing different field strengths, but our own experience with both 3 T and 1.5 T has not shown any noticeable difference.
Conclusion
MRI has become the dominant method for the evaluation of fistulas and conveying information to clinicians, especially surgeons. Knowledge of pelvic anatomy is a necessity and T2-weighted imaging with adequate depiction of fistula tracts in relation to the pelvic floor and sphincter is the main imaging sequence.
References
- 1.Read DR, Abcarian H. A prospective survey of 474 patients with anorectal abscess. Dis Colon Rectum. 1979;22(8):566–568. doi: 10.1007/BF02587008. [DOI] [PubMed] [Google Scholar]
- 2.Lunniss PJ, Jenkins PJ, Besser GM, Perry LA, Phillips RK. Gender differences in incidence of idiopathic fistula-in-ano are not explained by circulating sex hormones. Int J Colorectal Dis. 1995;10(1):25–28. doi: 10.1007/BF00337582. [DOI] [PubMed] [Google Scholar]
- 3.Robinson AM, Jr, DeNobile JW. Anorectal abscess and fistula-in-ano. J Natl Med Assoc. 1988;80(11):1209–1213. [PMC free article] [PubMed] [Google Scholar]
- 4.Quah HM, Tang CL, Eu KW, Chan SY, Samuel M. Meta-analysis of randomized clinical trials comparing drainage alone vs primary sphincter-cutting procedures for anorectal abscess-fistula. Int J Colorectal Dis. 2006;21(6):602–609. doi: 10.1007/s00384-005-0060-y. [DOI] [PubMed] [Google Scholar]
- 5.Makowiec F, Jehle EC, Becker HD, Starlinger M. Perianal abscess in Crohn’s disease. Dis Colon Rectum. 1997;40(4):443–450. doi: 10.1007/BF02258390. [DOI] [PubMed] [Google Scholar]
- 6.Saino P. Fistula-in-ano in a defined population: incidence and epidemiological aspects. Ann Chir Gynaecol. 1984;73:219–224. [PubMed] [Google Scholar]
- 7.Lunniss PJ, Armstrong P, Barker PG, Reznek RH, Phillips RK. Magnetic resonance imaging of anal fistulae. Lancet. 1992;340:394–396. doi: 10.1016/0140-6736(92)91472-K. [DOI] [PubMed] [Google Scholar]
- 8.West RL, Zimmerman DD, Dwarkasing S, Hussain SM, Hop WC, Schouten WR, Kuipers EJ, Felt-Bersma RJ. Prospective comparison of hydrogen peroxide-enhanced three-dimensional endoanal ultrasonography and endoanal magnetic resonance imaging of perianal fistulas. Dis Colon Rectum. 2003;46(10):1407–1415. doi: 10.1007/s10350-004-6758-z. [DOI] [PubMed] [Google Scholar]
- 9.Maier AG, Funovics MA, Kreuzer SH, Herbst F, Wunderlich M, Teleky BK, Mittlböck M, Schima W, Lechner GL. Evaluation of perianal sepsis: comparison of anal endosonography and magnetic resonance imaging. J Magn Reson Imaging. 2001;14(3):254–260. doi: 10.1002/jmri.1181. [DOI] [PubMed] [Google Scholar]
- 10.Buchanan GN, Halligan S, Bartram CI, Williams AB, Tarroni D, Cohen CR. Clinical examination, endosonography, and MR imaging in preoperative assessment of fistula in ano: comparison with outcome-based reference standard. Radiology. 2004;233(3):674–681. doi: 10.1148/radiol.2333031724. [DOI] [PubMed] [Google Scholar]
- 11.Ziech M, Felt-Bersma R, Stoker J. Imaging of perianal fistulas. Clin Gastroenterol Hepatol. 2009;7(10):1037–1045. doi: 10.1016/j.cgh.2009.06.030. [DOI] [PubMed] [Google Scholar]
- 12.Morris J, Spencer JA, Ambrose NS. MR imaging classification of perianal fistulas and its implications for patient management. Radiographics. 2000;20(3):623–635. doi: 10.1148/radiographics.20.3.g00mc15623. [DOI] [PubMed] [Google Scholar]
- 13.Horsthuis K, Lavini C, Bipat S, Stokkers PC, Stoker J. Perianal Crohn disease: evaluation of dynamic contrast-enhanced MR imaging as an indicator of disease activity. Radiology. 2009;251(2):380–387. doi: 10.1148/radiol.2512072128. [DOI] [PubMed] [Google Scholar]
- 14.Bennett AE (2008) Correlative anatomy of the anus and rectum. Semin Ultrasound CT MR 29(6):400–408 [DOI] [PubMed]
- 15.Jorge JM, Wexner SD. Anatomy and physiology of the rectum and anus. Eur J Surg. 1997;163(10):723–731. [PubMed] [Google Scholar]
- 16.Parks AG, Gordon PH, Hardcastle JD. A classification of fistula-in-ano. Br J Surg. 1976;63:1–12. doi: 10.1002/bjs.1800630102. [DOI] [PubMed] [Google Scholar]
- 17.Spencer JA, Ward J, Beckingham IJ, Adams C, Ambrose NS. Dynamic contrast-enhanced MR imaging of perianal fistulas. AJR Am J Roentgenol. 1996;167(3):735–741. doi: 10.2214/ajr.167.3.8751692. [DOI] [PubMed] [Google Scholar]
- 18.Spencer JA, Chapple K, Wilson D, Ward J, Windsor AC, Ambrose NS. Outcome after surgery for perianal fistula: predictive value of MR imaging. AJR Am J Roentgenol. 1998;171(2):403–406. doi: 10.2214/ajr.171.2.9694464. [DOI] [PubMed] [Google Scholar]
- 19.Chapple KS, Spencer JA, Windsor AC, Wilson D, Ward J, Ambrose NS. Prognostic value of magnetic resonance imaging in the management of fistula-in-ano. Dis Colon Rectum. 2000;43(4):511–516. doi: 10.1007/BF02237196. [DOI] [PubMed] [Google Scholar]
- 20.Morris J, Spencer JA, Ambrose NS. MR imaging classification of perianal fistulas and its implications for patient management. Radiographics. 2000;20(3):623–635. doi: 10.1148/radiographics.20.3.g00mc15623. [DOI] [PubMed] [Google Scholar]
- 21.Tjandra JJ, Sissons GR. Magnetic resonance imaging facilitates assessment of perianal Crohn’s disease. Aust N Z J Surg. 1994;64(7):470–474. doi: 10.1111/j.1445-2197.1994.tb02258.x. [DOI] [PubMed] [Google Scholar]
- 22.Essary B, Kim J, Anupindi S, Katz JA, Nimkin K. Pelvic MRI in children with Crohn disease and suspected perianal involvement. Pediatr Radiol. 2007;37(2):201–208. doi: 10.1007/s00247-006-0372-2. [DOI] [PubMed] [Google Scholar]
- 23.Osterman MT, Lichtenstein GR. Infliximab in fistulizing Crohn’s disease. Gastroenterol Clin N Am. 2006;35(4):795–820. doi: 10.1016/j.gtc.2006.09.007. [DOI] [PubMed] [Google Scholar]
- 24.Halligan S, Buchanan G. MR imaging of fistula-in-ano. Eur J Radiol. 2003;47(2):98–107. doi: 10.1016/S0720-048X(03)00162-1. [DOI] [PubMed] [Google Scholar]
- 25.Halligan S, Healy JC, Bartram CI. Magnetic resonance imaging of fistula-in-ano: STIR or SPIR? Br J Radiol. 1998;71(842):141–145. doi: 10.1259/bjr.71.842.9579177. [DOI] [PubMed] [Google Scholar]
- 26.Szyszko TA, Bush J, Gishen P, Sellu D, Desouza NM. Endoanal magnetic resonance imaging of fistula-in-ano: a comparison of STIR with gadolinium-enhanced techniques. Acta Radiol. 2005;46(1):3–8. doi: 10.1080/02841850510015947. [DOI] [PubMed] [Google Scholar]
- 27.Goodsall DH, Miles WE. Diseases of the anus and rectum. London: Longmans, Green & Co; 1900. [Google Scholar]
- 28.Cirocco WC, Reilly JC. Challenging the predictive accuracy of Goodsall’s rule for anal fistulas. Dis Colon Rectum. 1992;35(6):537–542. doi: 10.1007/BF02050532. [DOI] [PubMed] [Google Scholar]
- 29.deSouza NM, Gilderdale DJ, Coutts GA, Puni R, Steiner RE. MRI of fistula-in-ano: a comparison of endoanal coil with external phased array coil techniques. J Comput Assist Tomogr. 1998;22(3):357–363. doi: 10.1097/00004728-199805000-00004. [DOI] [PubMed] [Google Scholar]