Abstract
This review article discusses technical aspects of computed tomography (CT) imaging of the spine. Patient positioning, and its influence on image quality and movement artefact, is discussed. Particular emphasis is placed on the choice of scan parameters and their relation to image quality and radiation burden to the patient. Strategies to reduce radiation burden and artefact from metal implants are outlined. Data acquisition, processing, image display and steps to reduce artefact are reviewed. CT imaging of the spine is put into context with other imaging modalities for specific clinical indications or problems. This review aims to review underlying principles for image acquisition and to provide a rough guide for clinical problems without being prescriptive. Individual practice will always vary and reflect differences in local experience, technical provisions and clinical requirements.
Keywords: Computed tomography, CT, Spine, Data processing, Techniques, Complications
Introduction
Computed tomography (CT) is the examination of choice for the assessment of the bony structures of the spine. Assessment of the soft tissue structures of the spine is often limited and augmentation with contrast medium administration can be indicated. CT is fast and well-tolerated by almost every patient.
The perceived image quality and the diagnostic performance depend on the choice of imaging parameters and also the post-processing, in particular the reconstruction algorithm and the reformatting parameters, as well as the mode of display.
While generally a robust imaging method, artefacts can occur in CT imaging. In particular, movement artefact and streak artefact due to very high attenuation materials can cause a problem for image interpretation.
CT of the spine can be associated with high radiation doses and radiation protection has to be considered. Depending on the clinical question, the imaging parameters should be adjusted to adhere to the ALARA (as low as reasonably achievable) principle.
Usually a common sense approach is all that is needed to make CT imaging of the spine a relatively quick, simple and highly reliable examination of high diagnostic value.
Patient positioning, scan parameters and radiation burden
The patient position of choice for CT imaging of the spine is supine, i.e. the patient is lying on his/her back. This ensures minimal respiratory movement of the spine and usually good patient comfort, reducing patient movement. If other positions are required, care should be taken to stabilise and secure the patient to prevent movement on the CT table, or even a fall from it. Pressure points must be avoided, especially during interventional procedures by using padding. Respiratory compromise must be avoided. If sedated or anaesthetised, the vital parameters have to be monitored, in particular the airways have to be protected.
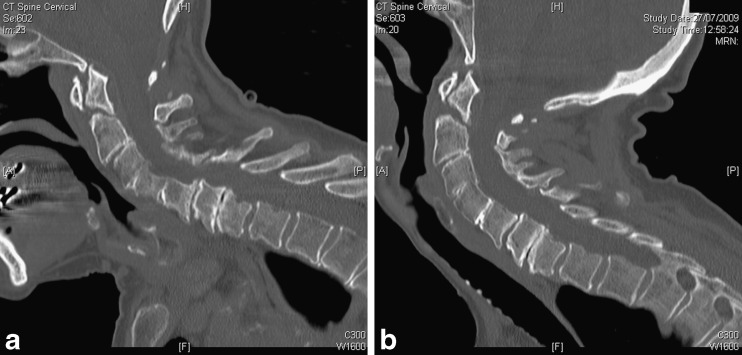
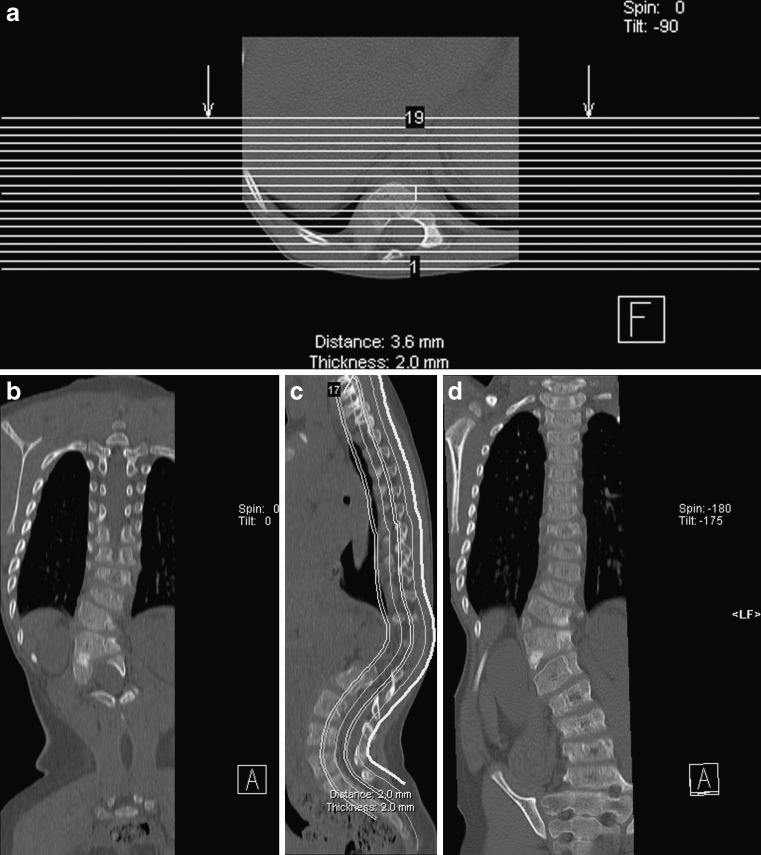
Usually the spine of the patient is aligned along the z-axis of the scanner, the head/neck should be in neutral position. CT does not lend itself to true dynamic imaging especially outside the imaging plane, but, for example, flexion extension imaging or imaging in different degrees of rotation of the spine (especially in the cervical spine) can be useful to address specific clinical questions about stability and alignment (Figs. 1 and 2).
Fig. 1.
Flexion (a) and extension (b) CT in a patient with a dens fracture. Radiographs were inconclusive, CT allows for fast and accurate assessment of bone alignment at the endpoints of flexion and extension
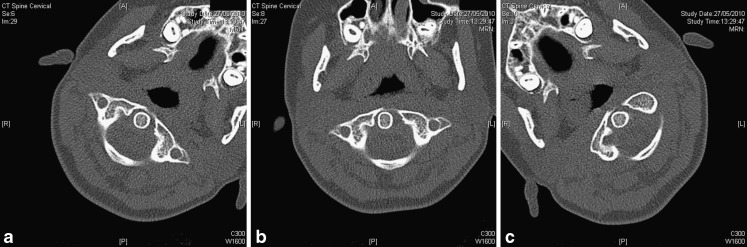
Fig. 2.
a–c An 11-year-old girl with torticollis since a trampolining accident. CT was performed to exclude a fixed rotation anomaly. CT with the patient’s head turned to the left (a), in neutral position (b) and turned to the right (c) demonstrates free movement in the atlantoaxial joint. The examination was performed in low-dose technique, kVp 100, reference mAs 50. Despite these low settings, the examination results in good image quality. CT is not well-suited for true dynamic imaging, but “snapshot” imaging at various stages of a continuous movement is possible
External high attenuation material (especially metal) should be removed if possible to reduce artefact.
The choice of imaging parameters determines the image quality and the radiation dose. Generally, examinations with high kV and mAs settings, thin collimation and low pitch result in the best image quality. The downside is a relatively high radiation exposure of the patient and increased examination time and tube loading. Tube loading is no longer relevant for the newer multidetector-row CTs (MD-CT). For older generation scanners, the technical ability of the scanner will determine the practically achievable collimation, pitch and exposure factors. The examination time can be a relevant factor for image quality. Breathing and especially movement artefact can significantly affect image quality. In particular, movement artefact can render examinations non-diagnostic. In confused or otherwise uncooperative patients, exam speed rather than top resolution might be the main priority.
For orthopaedic imaging, scanning with thin-slice collimation is preferable, ideally 1.5 mm or less. The pitch has to be less than 2, otherwise the volume is not completely imaged. Assuming the patient keeps still, lower pitch means better image quality. For higher order multislice CTs, the pitch is often chosen significantly lower than this, around 0.3-0.5. For modern scanners with pitch-corrected mAs, this does not result in a dose penalty but does increase the imaging time (which can lead to image degradation due to movement).
On scanners with fixed tube currents, the dose is strongly dependent on the pitch.
On modern scanners with adaptive tube current modulation, the patient dose is largely determined by the selection of a desired image quality and the tube current is modulated to achieve this. The main manufacturers employ similar methods to achieve this but use different terminology for this (Smart mA, GE; Z-DOM, Philips; CareDose 4D, Siemens; Sure Exposure, Toshiba). The tube current is adjusted according to the patient’s size and attenuation along the z-axis (longitudinal tube current modulation) and also during the rotation in the xy-axis (angular tube current modulation) depending on the patient attenuation [1]. The user chooses a desired image quality directly (Philips, Siemens, Toshiba) or determines a desired noise level (GE, Toshiba). The automatic exposure control (AEC) of the CT then adjusts the necessary tube current. The scout view gives an indication of the patient attenuation and, while planning the examination on the scout view, the system is able to calculate the patient dose for the diagnostic scan. The system indicates the CTDIvol (CT dose index per volume) and the DLP (dose length product) prior to the exam. These are generic markers of the incurred dose and indicate the physical radiation dose to the patient. The conversion into an effective dose, simplified the biological impact, is not straightforward and depends on the age, anatomy and sex of the patient. The younger the patient the more radiosensitive, and tissues with high metabolic and proliferative activity are more radiosensitive than those with low activity. Glandular and gonadal tissue and red marrow are highly radiosensitive, leading to a relatively high effective dose for radiation to the neck and trunk, while extremities are less radiosensitive resulting in low effective doses [2–6].
The estimates about the received effective dose when performing CT of the whole spine vary. A recent publication put it as high as 41.5 mSv (though the exact imaging protocol was not provided) [2]. The cervical spine was found to be exposed to an effective dose of about 4.4 mSv, the thoracic spine 18.0 mSv and the lumbosacral spine to 19.2 mSv. The estimated effective doses in this study were higher than in most other publications. Most studies use the calculated exposure factors as obtained from the scan parameters for dose assessment; in the study of Biswas et al. [2] the dose was actually measured. The reason for the discrepancy is not clear and unfortunately a comparison with the dose as calculated by the imaging parameters was not done. It has been suggested to use low-dose CT protocols for trauma imaging of the cervical spine to reduce the patient dose [7]. The authors of this study have assessed the impact of dose reduction by the reduction of the kV (130 kV to 110 kV and 120 kV to 100 kV) and mAs (tube current modulation off versus on) and found low-dose protocols still to be satisfactory despite reduced image quality. For specialist application, such as imaging of spinal scoliosis, special low-dose protocols with a reduced area of coverage compared with the routine protocol can result in dramatic dose savings up to factor 40 [8]!
For newer generation MD-CT, the main challenge is to achieve images allowing accurate and confident diagnosis while avoiding excessive radiation exposure. Most radiologists will prefer good image quality, resulting in high diagnostic confidence, even though the clinical question might be answered with a lower-dose exam. Radiologists have a tendency to prefer “good looking” high-dose imaging over still diagnostic but noisier and contrast-reduced imaging. It is very difficult to establish exactly the minimal necessary dose for any given examination [1]. Judicious use of audit can help to reduce the radiation burden to the patient without affecting subjective image quality [4]. The use of phantoms can help to establish the minimal acceptable kV and mAs to obtain acceptable images [9].
On modern pitch-corrected multislice scanners, the impact of detector collimation and pitch on the patient dose is surprisingly small. The patient dose will rise significantly with the kVp (kVpeak) and the patient dose will rise linearly with the mAs. For this reason, the main strategies for controlling the radiation dose are as follows.
Tight control of the scanned area, the smaller the exposed area the smaller the dose.
Choosing an appropriate kVp and reference mAs or image quality for the AEC; this requires considered input by the radiologist.
An easy way to gain a better understanding of the impact of the exposure factors on dose is to use a phantom and to observe the change of CTDI and DLP with varying imaging parameters.
There is a bewildering array of scan protocols for the spine in the literature, which makes it difficult to give firm recommendations. The scanner manufacturers provide sample protocols and there are dedicated websites discussing CT protocols. A small collection of resources are provided in List 1.
The author of this article is not aware of an authoritative source providing encompassing study data for the choice of particular CT protocols. This onerous task remains with the users. One way of reducing patient dose is to make moderate adjustments to the imaging protocols while auditing the image quality [3, 8].
Sample CT protocols for spine imagine are provided in Table 1. These can only be seen as a rough reference for an average patient of 70-80 kg. Slimmer and younger patients can be imaged with lower kV and mA, larger patients with higher. The mA changes required to subjectively achieve good or satisfactory image quality are related to but not directly proportional to the patient size/weight. The dose reduction for small patients and children is less than might be expected and the dose increase for large/obese patients is less than might be expected [1].
Table 1.
Example technical factor settings for spinal CT. There is large variability in the literature and authoritative suggestions are not possible. For obese patients the mA (and possibly kV) has to be increased, often to the maximal setting. For targeted limited examinations of the lumbar and thoracic spine the cervical presents can be used
| kV | mAs | Detector collimation [mm] | Pitch | Reconstructed slice thickness [mm] | |
|---|---|---|---|---|---|
| Single-slice CT | |||||
| Cervical spine | 120 | 240 | 3 | 1-1.5 | 3 |
| Thoracic & lumbar spine | 140 | 280 | (3-)5 | 1-1.5 | 3 |
| Four-slice CT | |||||
| Cervical spine | 140 | 200 | 1 | 1 | 1.25-2 |
| Thoracic & lumbar spine | 140 | 300 | 1-2.5 | 1-1.5 | 1.25-2 |
| 16-slice CT | |||||
| Cervical spine | 120 | 330 | 0.75 | 0.5 | 1 |
| Thoracic & lumbar spine | 120 | 380 | 0.75–1.5 | 0.5 | 1–1.5 |
| 64-slice CT | |||||
| Cervical spine | 120 | 330 | 0.6 | 0.9 | 1 |
| Thoracic & lumbar spine | 120 | 380 | 0.6–1.25 | 0.9 | 1–1.5 |
Data processing: image reconstruction, reformatting, windowing and other display options
The terms “reconstruction” and “reformatting” are sometimes used synonymously but describe very different processes. Because they are sometimes confused, they are briefly reviewed.
Image reconstruction is the term describing the calculation of images from the raw data obtained from the detector modules of the CT scanner. This is a process that cannot be performed in real time. The reconstruction of image data with a soft tissue or bone algorithm can only be performed from the raw data. Once the raw data is lost or deleted image reconstructions are no longer possible. Reformatting or other three-dimensional (3D) image manipulation of the image data is still possible.
Often the first reconstruction is performed in relatively thick slices to ensure a quick workflow. If no obvious problem is identified, the patient can be taken out of the scanner while thinner reconstructions are calculated. While theoretically any imaging plane can be chosen as reconstruction plane, usually reconstructions are performed in the axial plane. In orthopaedic imaging reconstructions should routinely be performed with soft tissue and bone kernels. The maximal spatial resolution along the z-axis is determined by the detector collimation used at image acquisition and for technical reasons is slightly thicker than this. Choosing a thinner slice thickness for the reconstruction does not improve the z-axis resolution. Choosing a reconstruction slice thickness larger than the detector collimation results in decreased z-axis resolution. In orthopaedic imaging on higher order MD-CTs the minimal reconstruction thickness is often chosen as 1 mm, even though thinner detector collimations are possible. The reason is that the images are usually displayed with a slice thickness of ≥1 mm, thinner slices lead to noisy images (unless very high mA settings are chosen). The reconstruction algorithm should contain an overlap in z-axis to improve the visualisation of lesions mainly located in the xy-plane, for example a fracture of the dens axis with minimal displacement. Typically, each reconstructed slice shares half of its raw data with the slice below and half with the slice above [7, 10–16].
The image quality is best when viewing images in the window setting appropriate for the chosen algorithm. Soft tissue algorithms typically are performed with a kernel setting of 20-40 and bone algorithms typically use a kernel setting of 60 or 70. The kernel number is an indicator for the sharpness of edges and the smoothness of images. It is highly advisable to routinely reconstruct images in high and low kernel settings, again, once the raw data has been deleted image reconstruction is no longer possible.
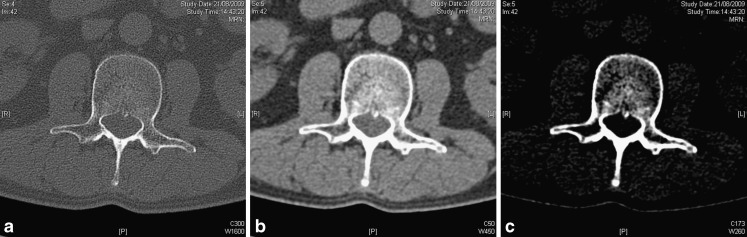
Once image data has been reconstructed from the raw data, the image data can be reformatted in any thickness and plane in real time (Fig. 3). Generally, a soft tissue kernel reconstruction should be used to view the soft tissues and a bone kernel reconstruction to view the bony structures (Fig. 4). Often the images are reformatted in 3-mm thickness, resulting in a less noisy appearance than thinner reformats, which allows better assessment of the soft tissues (Fig. 3). The diagnostic accuracy varies with the reformatting parameters and it has been shown that with both 1-mm and 3-mm thickness reformats some spine fractures are being missed [7, 12, 13].
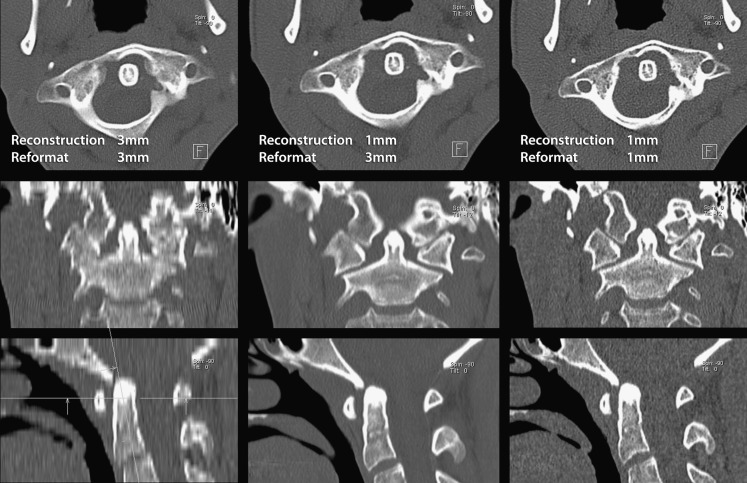
Fig. 3.
A patient with a healed atlas fracture, demonstrating the influence of the reconstruction and reformatting parameters on the image quality. A reconstruction thickness of 3 mm in the axial plane results in good image quality in the axial plane (3-mm thickness); however, reformatting in 3-mm thickness in the sagittal and coronal plane results in poor image quality. After reconstruction in 1-mm thickness and reformatting in 3-mm thickness in all three planes, there is a much better image quality in the sagittal and coronal plane. When reformatting the images in 1-mm thickness, the spatial resolution is increased best appreciated on the coronal image in the occipitoatlantal articulation. The images are also much noisier than after reformatting in 3-mm thickness. The images should always be reconstructed in relatively thin slice thickness. Reformatting in thicker slices then still results in good quality images
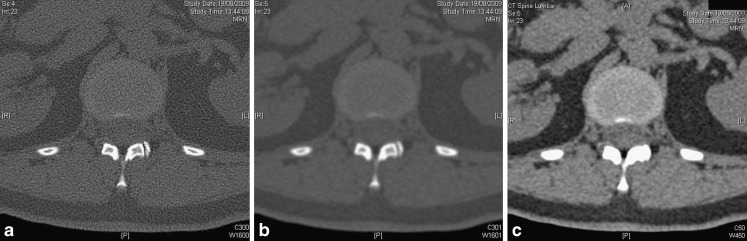
Fig. 4.
a–c A patient with an accessory facet joint ossicle. In the axial plane this is easily seen using a bone algorithm and bone windows for display (a). Using a soft tissue algorithm for reconstruction and bone windows for display, the lesion is hard to visualise (b). Using a soft tissue reconstruction algorithm and soft tissue windows for display, the lesion is invisible (c). Images should routinely be reconstructed with a sharp (for bone and lung) and soft (for soft tissues) algorithm and be displayed with appropriate window settings
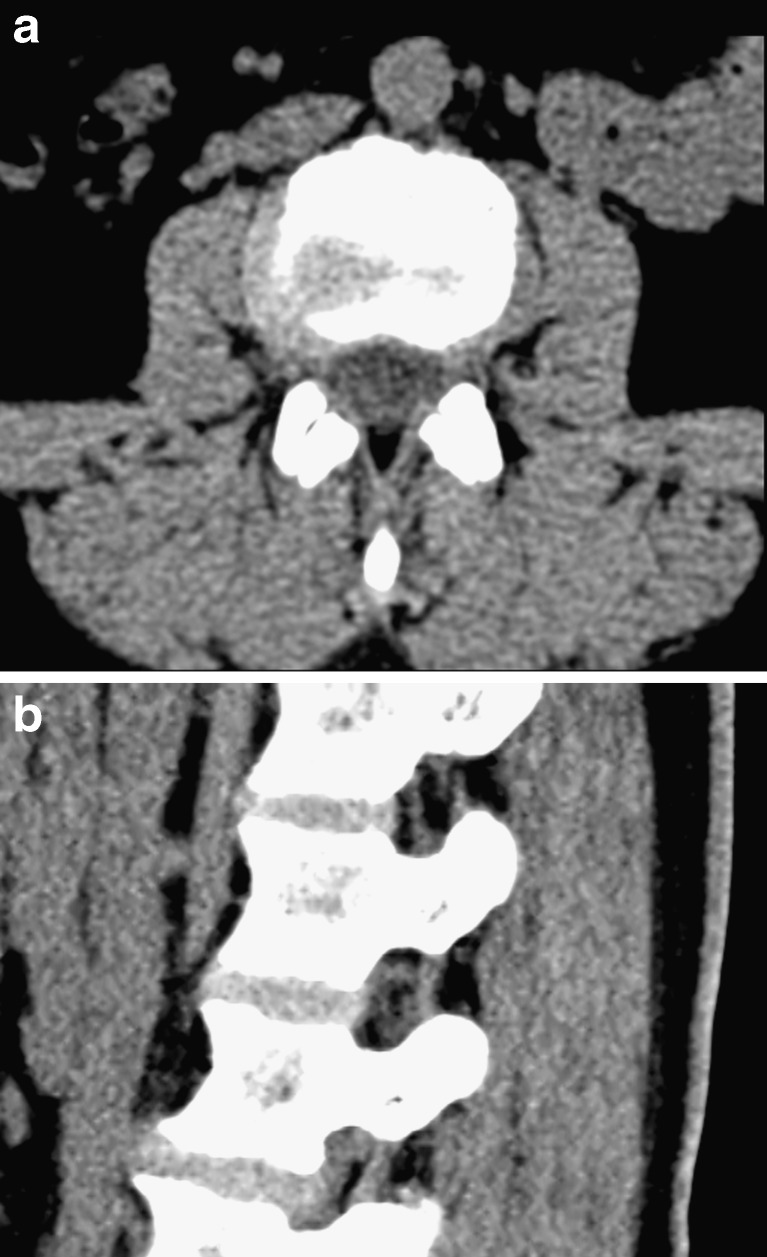
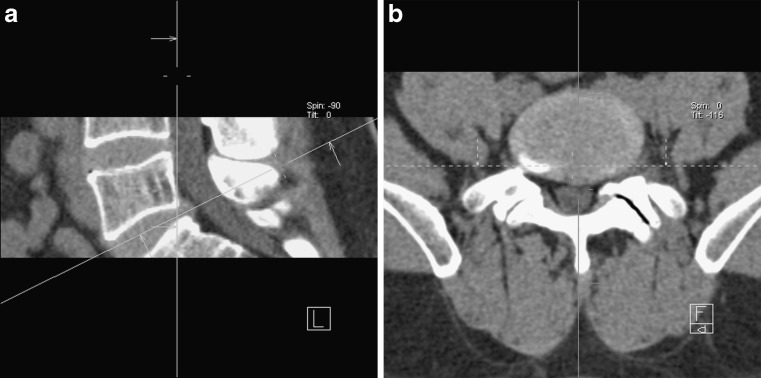
Curved structures like the spine are easiest viewed in a 3D viewer with real-time reformatting. If only 2D viewers are available curved reformats can be helpful (Fig. 5). Curved reformats are obtained by projecting a 3D structure onto a curved plane. This technique is well established in vascular imaging and will be familiar to most radiologists. It can be of help in assessing complex bony abnormalities of the spine but should always be viewed in conjunction with conventional slices/projections.
Fig. 5.
a–d A patient with scoliosis due to developmental anomalies in the thoracolumbar junction. On conventional coronal reformats the vertebral morphology and alignment is not easily assessed (a, b). Curved coronal reformats (c, d) have been chosen to “remove” kyphosis and lordosis, the spine is projected onto a curved plane, making it easier to assess the scoliosis and morphology
Volume rendering is another display technique that can help to visualise and understand abnormalities with complex anatomy. They are easily obtained on a 3D workstation and can be saved in a 2D format.
In special clinical circumstances the use of non-standard window settings can be of help. For example, disc pathology of the spine is better assessed with narrow window settings (Figs. 6 and 7). Streak artefact due to metal implants can be reduced by using very wide window settings.
Fig. 6.
a, bA patient with foraminal narrowing due to disc bulging especially on the right (a). Narrowing of the window width compared with normal soft tissue windows results in improved visualisation of the foraminal narrowing on sagittal reformats (b)
Fig. 7.
a–c A patient with a notochordal rest. On conventional bone window settings (a) (bone algorithm) the sclerosis is only faintly visible. On soft tissue window settings (b) (soft tissue algorithm) the lesion is better visualised. The best visualisation is achieved with individually adjusted narrow window settings (c)
Not all workstations or viewing stations perform equally well and this can affect diagnostic performance. This is best appreciated by viewing and manipulating the same set of DICOM images on different workstations.
Clinical considerations and techniques
To image the whole spine in an elective setting, magnetic resonance imaging (MRI) is generally preferred if possible. However, MRI is not possible in every patient due to a variety of reasons, such as severe claustrophobia, incompatible implants, critical medical condition, etc. Whole-spine imaging with CT is more commonly part of a trauma or tumour-staging protocol. In tumour imaging large parts of the spine are frequently imaged, typically the thoracic, lumbar and sacral spine as part of a CT chest/abdomen/pelvis. In PET-CT imaging, often the whole body is imaged, including the whole spine.
CT can show lytic or sclerotic bone lesions but marrow infiltration without bone change is difficult or impossible to identify [17].
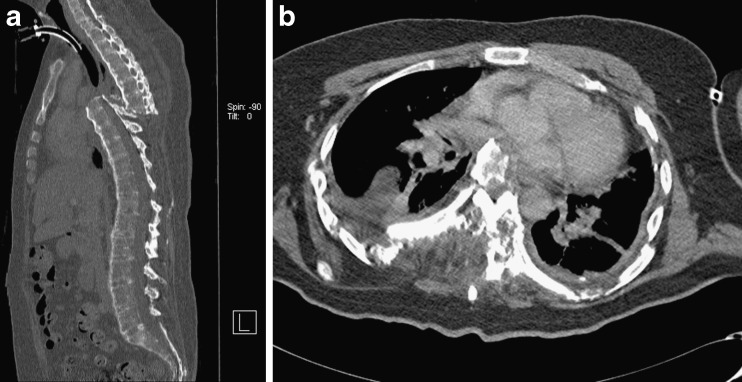
The strength of CT is that it can be performed in the vast majority of patients. It is quick and provides information not only about the spine but also of the adjacent soft tissues and organs (Fig. 8). This is particularly valuable in trauma imaging.
Fig. 8.
a, b Patient with ankylosing spondylitis run over by a car. CT not only demonstrates the grossly displaced overriding spinal fracture (a) but also the impingement of cardiovascular structures by the spine fracture (b). CT is superior to MRI in ease and speed of examination in trauma cases
In imaging of spinal infection, MRI is generally more useful as it allows to visualise bone marrow and soft tissue changes. CT is, however, more sensitive for reactive bone changes in infection [18].
CT is in principle is well suited to image bony abnormalities in developmental abnormalities; however, the associated radiation dose should lead to the use of MRI where possible. In addition, soft tissue abnormalities of the spine—including cord, meningeal and neural abnormalities—are much better imaged with MRI.
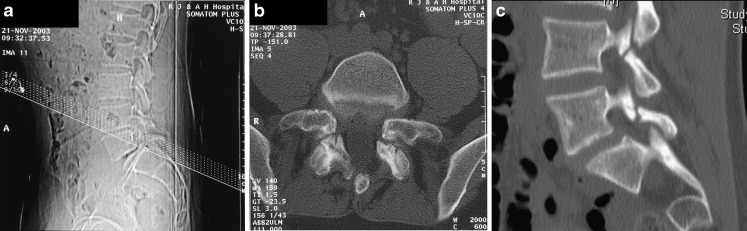
CT is well suited to answer specific questions in focal areas of the spine. Examples are imaging of spondylolysis (size of bony gap?, evidence of healing?), focal developmental abnormalities of the bony spine (dysraphism?, scoliosis due to underlying bony abnormality?), characterisation of soft tissue lesions (calcified?, containing gas?, etc.) and areas of abnormal bone on MRI. If imaging large areas, dose-reduction protocols should be applied if appropriate [8]. Reverse gantry CT for imaging of spondylolysis can no longer be advocated on MD-CT systems (Fig. 9). The reason for gantry tilt was the insufficient spatial resolution outside the image plane for single-slice CT systems. This no longer holds true for MD-CT performed with thin detector collimation and appropriate reconstruction and reformatting. It should also be noted that with a tilted gantry spiral acquisition is not possible, which increases the imaging time and decreases the quality of reformats.
Fig. 9.
a–c CT for the assessment of spondylolysis: for the reverse gantry technique as performed on single slice scanners the imaging plane is chosen in the plane of the posterior elements (a). The high in plane resolution allows for good assessment of bony continuity (b). However once the gantry is tilted spiral acquisition is no longer possible. This technique is obsolete for MD-CT where real-time 3D reformatting allows to chose the image plane to optimally display the lesion, in this case spondylolysis of L4 and L5 (c)
CT is not the optimal “screening” examination for degenerative disease due to the radiation dose penalty and inferior soft tissue contrast compared with MRI. However, CT is excellent at demonstrating bony degenerative change. In elderly patients the disadvantages of CT for imaging degenerative disease of the spine are ameliorated. Usually there is a larger amount of epidural and paravertebral fat in older patients, especially in the lumbar spine, allowing for good or at least adequate assessment of intervertebral formina and any compromise of the intraspinal epidural space. This can be aided by adapting the window settings, especially choosing a narrow window width (Fig. 10). The differentiation of intra- and paradiscal gas and calcification is obvious with CT. The differentiation of dehydrated disc material and osteophytes is also immediate. In addition, for patients with a limited life expectancy, radiation protection issues lose in importance.
Fig. 10.
CT of the spine can often accurately depict disc disease and neural compromise. Especially the presence of epidural fat can help depict disc disease
If plain CT is non-diagnostic, it can be enhanced by the use of iodinated contrast medium. Intravenous contrast medium injection can enhance the diagnosis of disc herniations by providing improved soft tissue delineation by outlining the epidural venous plexus [19, 20].
CT myelography is also suited for this and in addition demonstrates the outline of nerve roots, the cauda equina and the spinal cord. Delayed imaging can demonstrate syringohydromyelia formation. In the cervical spine, CT myelography can aid the diagnosis of nerve root injuries by demonstrating nerve avulsions directly and by showing leakage of contrast-enhanced cerebrospinal fluid (CSF). Reformatting in the coronal and coronal oblique plane aid the diagnosis [21]. However, myelography is more invasive than an i.v. injection and delayed imaging adds further radiation [20, 22].
Discography can be enhanced by post-procedure CT to better demonstrate any disc changes and the internal disc architecture [20, 22, 23].
Quantitative CT (QCT) of the spine was initially established as dedicated examination of the bone mineral density (BMD) of the lumbar spine using single-slice CT scanners. Usually only a single slice through the mid vertebral body L1-L3 was obtained. The BMD of the cortical and trabecular bone can then be established using a calibration phantom, usually placed under the patient’s spine. With time it was realised that QCT can also be performed on volume acquisitions of the spine. It is now possible to perform QCT assessment of the spine even after CT of the abdomen with i.v. administration of iodinated contrast medium as long as the calibration phantom has been included in the exam. If i.v. contrast medium has been given, correction factors have to be applied to allow for the increased attenuation of the bone marrow [24–27].
CT can be used to guide interventional procedures of the spine. Examples are biopsy, nerve root and facet joint blocks or epidural injections, radiofrequency ablations, kypho- and vertebroplasties and, increasingly, screw placement in spinal surgery. The CT scanner can be used in the traditional biopsy mode, taking images and displaying them slightly delayed or in fluoroscopy mode. In fluoroscopy mode there is real-time imaging of the area in the gantry. The patient position can be directly steered by the radiologist or radiographer operating the equipment. The radiation dose to the patient and operator is increased compared with the conventional technique. The benefit of a potentially quicker procedure due to real-time imaging must be balanced against this [28].
Imaging of patients with metal implants in the spine can be difficult with MRI; especially, ferromagnetic implants cause significant artefact. Many implants used in spinal surgery are made from titanium, which is fortuitous as it causes relatively minor artefact in CT imaging and also is often but not always well imaged with MRI (Fig. 11).
Fig. 11.
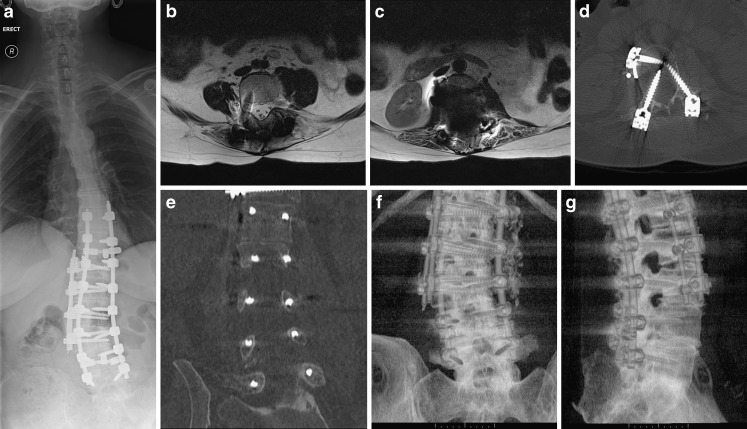
a–g Metal implants in the spine are frequently made of titanium. This does usually allow for satisfactory MRI. However, if ferrous metals or multiple implants are used MRI can become non-diagnostic. CT is usually still able to provide diagnostic images. In this example, two posterior and one lateral rod with corresponding anchoring screws have been used (a). Above the level of the lateral rod MRI results in diagnostic images (b), at the level of the lateral rod the artefact becomes too marked (c). CT imaging allows for excellent visualisation of the spine and implants and demonstrates misplacement of several pedicle screws (d, e). The coronal reformat in particular allows for a quick and accurate assessment of screw misplacement. Volume rendering allows for easy visualisation of implant placement in relation to the spine (f, g)
For patients with metal implants, the routine CT protocols for the spine are usually sufficient to obtain good quality images. To image ferromagnetic metal implants high kV (140), high mAs (≥350), thin collimation (1 mm) and low pitch (≤1) are advised. Apart from the high kV, this is usually already the case for CT protocols for the spine. The CT imaging of metal implants of the spine is therefore no different from that of metal implants in other parts of the body [16, 29]. When only titanium implants were used such as in titanium screw placement in the spine, low-dose protocols can be employed with the kV set as low as 80 and the mAs as low as 25 [30]. In the presence of metal-related artefact, image reconstruction using a soft tissue algorithm results in a better image quality than the use of a sharper bone algorithm. Using very wide window settings can further reduce the impact of streak artefact [16]. However, even with adapted imaging protocols artefact can remain; radiographs, despite their limitations, should not be dismissed as useful tools for the imaging of metal implants (Fig. 12).
Fig. 12.
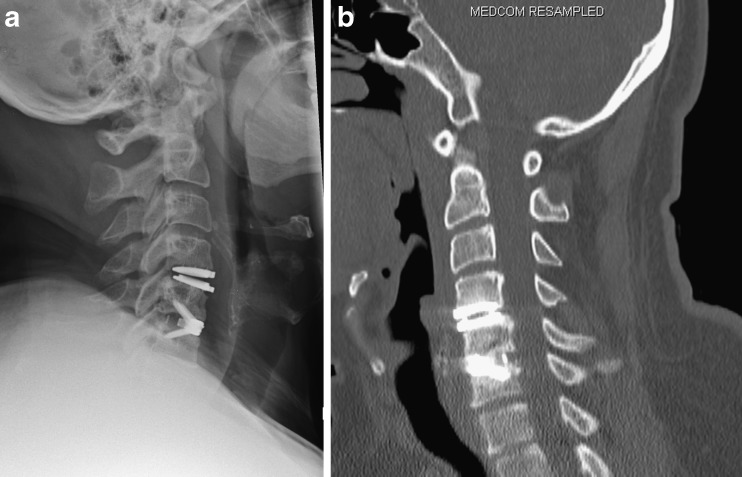
a, b Patient who had previously undergone disc replacement at C4/5 and fusion at C5/6. Lateral radiographs show a radiolucent zone in C4 vertebra adjacent to the disc implant (a). This is not visualised on CT (b). The depiction of bony lysis adjacent to metal implants can be difficult. If lysis is seen in either CT imaging or radiographs this is likely to be a true finding
Conclusion
MD-CT imaging of the spine allows for excellent bone and often acceptable soft tissue imaging. The highest image quality is achieved with thin collimation, high kV, high mAs and low pitch protocols. However, this results in a relatively high radiation burden and the judicious choice of imaging parameters is part of the radiologist’s remit. Image reconstruction in a soft tissue and bone algorithm should be followed by 3D reformatting; if necessary, supplemented by volume rendering or other post-processing techniques. In select cases the use of contrast medium can be helpful.
References
- 1.McCollough CH, Bruesewitz MR, Kofler JM., Jr CT dose reduction and dose management tools: overview of available options. Radiographics. 2006;26(2):503–512. doi: 10.1148/rg.262055138. [DOI] [PubMed] [Google Scholar]
- 2.Biswas D, Bible JE, Bohan M, et al. Radiation exposure from musculoskeletal computerized tomographic scans. J Bone Joint Surg Am. 2009;91(8):1882–1889. doi: 10.2106/JBJS.H.01199. [DOI] [PubMed] [Google Scholar]
- 3.Richards PJ, George J. Diagnostic CT radiation and cancer induction. Skeletal Radiol. 2009;39(5):421–424. doi: 10.1007/s00256-009-0819-2. [DOI] [PubMed] [Google Scholar]
- 4.Richards PJ, George J, Metelko M, et al. Spine computed tomography doses and cancer induction. Spine (Phila Pa 1976) 2010;35(4):430–433. doi: 10.1097/BRS.0b013e3181cdde47. [DOI] [PubMed] [Google Scholar]
- 5.McCollough CH, Christner JA, Kofler JM. How effective is effective dose as a predictor of radiation risk? AJR Am J Roentgenol. 2010;194(4):890–896. doi: 10.2214/AJR.09.4179. [DOI] [PubMed] [Google Scholar]
- 6.Christner JA, Kofler JM, McCollough CH. Estimating effective dose for CT using dose-length product compared with using organ doses: consequences of adopting international commission on radiological protection publication 103 or dual-energy scanning. AJR Am J Roentgenol. 2010;194(4):881–889. doi: 10.2214/AJR.09.3462. [DOI] [PubMed] [Google Scholar]
- 7.Mulkens TH, Marchal P, Daineffe S, et al. Comparison of low-dose with standard-dose multidetector CT in cervical spine trauma. AJNR Am J Neuroradiol. 2007;28(8):1444–1450. doi: 10.3174/ajnr.A0608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Abul-Kasim K, Overgaard A, Maly P, et al. Low-dose helical computed tomography (CT) in the perioperative workup of adolescent idiopathic scoliosis. Eur Radiol. 2009;19(3):610–618. doi: 10.1007/s00330-008-1178-4. [DOI] [PubMed] [Google Scholar]
- 9.Abul-Kasim K, Gunnarsson M, Maly P, et al. Radiation dose optimization in CT planning of corrective scoliosis surgery. Neuroradiol J. 2008;21:374–382. doi: 10.1177/197140090802100313. [DOI] [PubMed] [Google Scholar]
- 10.Hauser CJ, Visvikis G, Hinrichs C, et al. Prospective validation of computed tomographic screening of the thoracolumbar spine in trauma. J Trauma. 2003;55(2):228–234. doi: 10.1097/01.TA.0000076622.19246.CF. [DOI] [PubMed] [Google Scholar]
- 11.Link TM, Meier N, Rummeny EJ, et al. Artificial spine fractures: detection with helical and conventional CT. Radiology. 1996;198(2):515–519. doi: 10.1148/radiology.198.2.8596859. [DOI] [PubMed] [Google Scholar]
- 12.Obenauer S, Alamo L, Herold T, et al. Imaging skeletal anatomy of injured cervical spine specimens: comparison of single-slice vs multi-slice helical CT. Eur Radiol. 2002;12(8):2107–2111. doi: 10.1007/s00330-001-1253-6. [DOI] [PubMed] [Google Scholar]
- 13.Phal PM, Riccelli LP, Wang P, et al. Fracture detection in the cervical spine with multidetector CT: 1-mm versus 3-mm axial images. AJNR Am J Neuroradiol. 2008;29(8):1446–1449. doi: 10.3174/ajnr.A1152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Roos JE, Hilfiker P, Platz A, et al. MDCT in emergency radiology: is a standardized chest or abdominal protocol sufficient for evaluation of thoracic and lumbar spine trauma? AJR Am J Roentgenol. 2004;183(4):959–968. doi: 10.2214/ajr.183.4.1830959. [DOI] [PubMed] [Google Scholar]
- 15.Wintermark M, Mouhsine E, Theumann N, et al. Thoracolumbar spine fractures in patients who have sustained severe trauma: depiction with multi-detector row CT. Radiology. 2003;227(3):681–689. doi: 10.1148/radiol.2273020592. [DOI] [PubMed] [Google Scholar]
- 16.Douglas-Akinwande AC, Buckwalter KA, Rydberg J, et al. Multichannel CT: evaluating the spine in postoperative patients with orthopedic hardware. Radiographics. 2006;26(Suppl 1):S97–S110. doi: 10.1148/rg.26si065512. [DOI] [PubMed] [Google Scholar]
- 17.Tins BJ, Lalam RK, Cassar-Pullicino VN et al (2009) Bone metastases. 2: Pelvis and appendicular skeleton. In: Davies AM, Sundaram M, James SL (eds) Imaging of bone tumors and tumor-like lesions. Springer, Berlin Heidelberg, pp 346–365.
- 18.Tins BJ, Cassar-Pullicino VN, Lalam RK. Magnetic resonance imaging of spinal infection. Top Magn Reson Imaging. 2007;18(3):213–222. doi: 10.1097/RMR.0b013e3180f60c3f. [DOI] [PubMed] [Google Scholar]
- 19.Russell EJ, D'Angelo CM, Zimmerman RD, et al. Cervical disk herniation: CT demonstration after contrast enhancement. Radiology. 1984;152(3):703–712. doi: 10.1148/radiology.152.3.6463252. [DOI] [PubMed] [Google Scholar]
- 20.Voelker JL, Mealey J, Jr, Eskridge JM, et al. Metrizamide-enhanced computed tomography as an adjunct to metrizamide myelography in the evaluation of lumbar disc herniation and spondylosis. Neurosurgery. 1987;20(3):379–384. doi: 10.1227/00006123-198703000-00004. [DOI] [PubMed] [Google Scholar]
- 21.Yamazaki H, Doi K, Hattori Y, et al. Computerized tomography myelography with coronal and oblique coronal view for diagnosis of nerve root avulsion in brachial plexus injury. J Brachial Plex Peripher Nerve Inj. 2007;2:16. doi: 10.1186/1749-7221-2-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ketonen L, Gyldensted C. Lumbar disc disease evaluated by myelography and postmyelography spinal computed tomography. Neuroradiology. 1986;28(2):144–149. doi: 10.1007/BF00327887. [DOI] [PubMed] [Google Scholar]
- 23.Kluner C, Kivelitz D, Rogalla P, et al. Percutaneous discography: comparison of low-dose CT, fluoroscopy and MRI in the diagnosis of lumbar disc disruption. Eur Spine J. 2006;15(5):620–626. doi: 10.1007/s00586-005-1030-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Adams JE. Quantitative computed tomography. Eur J Radiol. 2009;71(3):415–424. doi: 10.1016/j.ejrad.2009.04.074. [DOI] [PubMed] [Google Scholar]
- 25.Lang T. Quantitative computed tomography. Radiol Clin N Am. 2010;48(3):589–600. doi: 10.1016/j.rcl.2010.03.001. [DOI] [PubMed] [Google Scholar]
- 26.Lenchik L, Shi R, Register TC, et al. Measurement of trabecular bone mineral density in the thoracic spine using cardiac gated quantitative computed tomography. J Comput Assist Tomogr. 2004;28(1):134–139. doi: 10.1097/00004728-200401000-00023. [DOI] [PubMed] [Google Scholar]
- 27.Bauer JS, Henning TD, Mueller D, et al. Volumetric quantitative CT of the spine and hip derived from contrast-enhanced MDCT: conversion factors. AJR Am J Roentgenol. 2007;188(5):1294–1301. doi: 10.2214/AJR.06.1006. [DOI] [PubMed] [Google Scholar]
- 28.Schmid G, Schmitz A, Borchardt D, et al. Effective dose of CT- and fluoroscopy-guided perineural/epidural injections of the lumbar spine: a comparative study. Cardiovasc Interv Radiol. 2006;29(1):84–91. doi: 10.1007/s00270-004-0355-3. [DOI] [PubMed] [Google Scholar]
- 29.Olsen RV, Munk PL, Lee MJ, et al. Metal artifact reduction sequence: early clinical applications. Radiographics. 2000;20(3):699–712. doi: 10.1148/radiographics.20.3.g00ma10699. [DOI] [PubMed] [Google Scholar]
- 30.Abul-Kasim K, Strombeck A, Ohlin A, et al. Reliability of low-radiation dose CT in the assessment of screw placement after posterior scoliosis surgery, evaluated with a new grading system. Spine (Phila Pa 1976) 2009;34(9):941–948. doi: 10.1097/BRS.0b013e31819b22a4. [DOI] [PubMed] [Google Scholar]