Abstract
There are many disorders that may involve the left ventricular (LV) apex; however, they are sometimes difficult to differentiate. In this setting cardiac imaging methods can provide the clue to obtaining the diagnosis. The purpose of this review is to illustrate the spectrum of diseases that most frequently affect the apex of the LV including Tako-Tsubo cardiomyopathy, LV aneurysms and pseudoaneurysms, apical diverticula, apical ventricular remodelling, apical hypertrophic cardiomyopathy, LV non-compaction, arrhythmogenic right ventricular dysplasia with LV involvement and LV false tendons, with an emphasis on the diagnostic criteria and imaging features.
Electronic supplementary material
The online version of this article (doi:10.1007/s13244-011-0091-6) contains supplementary material, which is available to authorized users.
Keywords: Cardiac aneurysm, Left ventricular remodelling, Left ventricular hypertrophy, Tako-Tsubo cardiomyopathy, Isolated non-compaction of the ventricular myocardium
Introduction
The left ventricle (LV) is affected by many diseases with different clinical and morphological features. Within this broad spectrum, a subset of heterogeneous diseases is characterised as preferentially affecting the LV apex. Generally LV assessment begins with echocardiography because of its wide availability and because it is fast, low-priced, portable and robust in experienced hands. However, because of intrinsic or technical limitations (limited field of vision, inadequate acoustic window, inability to definitively depict the endocardial border, especially the anterolateral free wall of the left ventricle in the parasternal short-axis view and the apex) [1, 2] and technical artefacts (reverberations, near-field artefacts), in such cases further investigation, usually with magnetic resonance (MR) imaging, which is the method of choice for the diagnosis of myocardial diseases, may be necessary. MR imaging is less dependent on the operator and is not subject to acoustic window limitations [3]. Due to their high spatial and temporal resolution, MR and CT can clearly visualise the apex of the LV, but CT is not yet recommended as the method of primary choice in the case of suspected left ventricular apical disease. Cine MR imaging with the steady-state free precession pulse sequence can offer the advantages of multiplanar imaging, complete coverage of the entire myocardium without obliquity, and excellent soft-tissue contrast between the myocardial border and the blood pool [4, 5]. In addition, the status of myocardial blood flow can be assessed by using adenosine stress cardiac MR imaging. Delayed enhancement MR imaging techniques can provide unique information for tissue characterisation, specifically for the identification of myocardial fibrosis or scarring [6, 7]. CT has also emerged as a novel technique for evaluating cardiac morphology and function, as well as the coronary artery. Compared with the spatial resolution of MR imaging, that of multidetector CT is usually higher [8]. Therefore, cardiac multidetector CT can offer anatomical and functional information about the cardiac chambers and a high-quality non-invasive coronary angiography [9, 10]. Multidetector CT would be more appropriate in those cases for which there are specific requests to exclude coronary artery disease and in those with contraindications for MR imaging, such as the patient having a pacemaker [11].
Therefore, radiologists play an increasing role in the evaluation of LV apical abnormalities, thus requiring a greater awareness and familiarity with this diseases.
The aim of this paper is to illustrate the spectrum of diseases that most frequently affect the apex of the LV, with an emphasis on the diagnostic criteria and imaging features.
Normal findings
Myocardial thinning (one of the principal signs of myocardial injury) is an important observation in patients suspected of having had ischaemic insults to the heart. However, thinning of the LV apex has been described by anatomists as a normal feature. With the advent of motion-gated cross-sectional cardiac imaging, normal apical thinning can be quite noticeable, especially on computed tomography (CT) in which the spatial resolution is submillimetre [12, 13].
Ischaemia-related diseases
Magnetic resonance imaging has increasing potential in the non-invasive evaluation of patients with myocardial infarction and subsequent complications. The low interobserver variability and the high interstudy reproducibility of MR imaging for quantifying LV volume, wall thickening and mass make it an attractive imaging technique for follow-up studies in patients with ischaemic coronary artery disease and LV remodelling after infarction [14–16] . Moreover, MR imaging can clearly localise the site of the aneurysm, pseudoaneurysm or diverticula, is capable of distinguishing among pericardium, thrombus and myocardium, and delayed enhancement MR imaging helps in the differentiation between a true aneurysm and a pseudoaneurysm [17].
Apical aneurysm and pseudoaneurysm
Pseudoaneurysms or false aneurysms are defined as a rupture of the myocardium that is contained by pericardial adhesions. A pseudoaneurysm usually represents a rare complication of myocardial infarction (Fig. 1, Video 1 and 2), but it may also occur after cardiac surgery, chest trauma and endocarditis [18]. They can occur in different locations, but the purpose of this paper is to describe those arising from the apex of the LV. The wall of a false aneurysm is composed of organised haematoma and pericardium (Table 1). Pathological examination shows fibrous tissue and lacks the myocardial elements that are usually seen in the wall of true aneurysms [18].
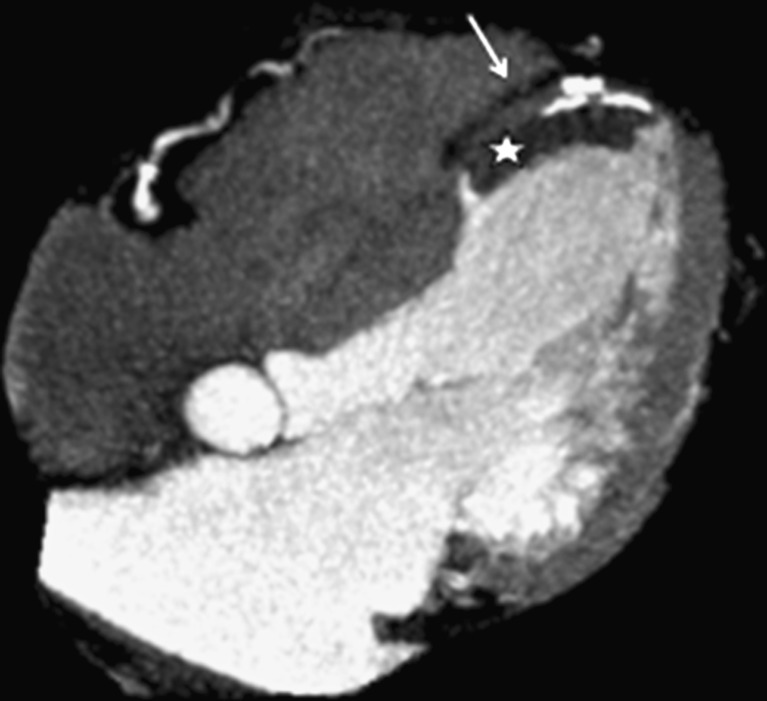
Fig. 1.
Patient with antecedent of myocardial infarction. Contrast-enhanced CT (CECT) shows a small amount of contrast medium outside the ventricular wall due to a pseudoaneurysm (white arrow). Note that the calcifications formed as a result of necrosis define the ventricular wall
Table 1.
Differences among aneurysms, pseudoaneurysms and congenital diverticula
| Aneurysms | Pseudoaneurysms | Congenital diverticula | |
|---|---|---|---|
| Layers | All layers of the ventricular myocardium | Organized hematoma and pericardium | All layers of the ventricular myocardium |
| Cine | Akinetic dyskinetic segment | Akinetic or dyskinetic segment | Shows contraction during systole |
| Myocardial late enhancement | Yes | No, only the border of the pseudoaneurysm will show enhancement | No |
| Pericardial late enhancement | No or faint | Marked | No |
Conversely, a true aneurysm, which occurs in 5–10% of patients with acute myocardial infarction, contains the endocardium, epicardium and a predominantly fibrous tissue that has replaced myocardium in its wall [17]. An important difference is the lower rupture potential of a true aneurysm compared with a pseudoaneurysm. Surgical repair is the treatment of choice for pseudoaneurysms, while true aneurysms, in the absence of other surgical indications (i.e. refractory angina pectoris, congestive heart failure, systemic embolisation or refractory arrhythmia) are treated medically [18, 19]. Cine MR and cine CT imaging reveal, in both aneurysms and pseudoaneurysms, an akinetic/dyskinetic segment in the LV wall [18, 19]. A typical feature of pseudoaneurysm is that it has a narrow ostium connecting them to the ventricle. In most cases, the maximal width of the ostium is less than the maximal parallel internal diameter [18].
True aneurysms will show late enhancement, which indicates that their wall is formed by scar tissue secondary to infarcted myocardial muscle. Owing to the fact that their wall is composed only of pericardium, pseudoaneurysm sacs do not show late enhancement; however, their border will show enhancement, indicating a perianeurysmal infarcted area [17, 18]. There is often marked delayed enhancement of the pericardium in pseudoaneurysms, and it is uncommon or faint in true aneurysm, with a sensitivity and specificity of 100% and 83%, respectively [18]. Associated mural thrombus is common in both aneurysms and pseudoaneurysms (Fig. 2 and Video 3) [17, 18].
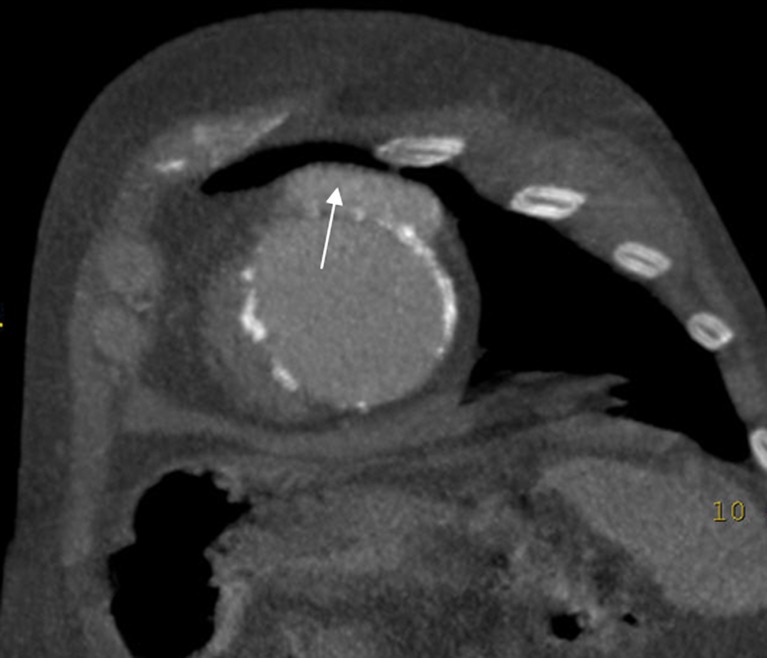
Fig. 2.
True aneurysm in a patient with myocardial infarction. CECT in the four-chamber axis demonstrates a large and thinning area of akinesia in the anteroseptal-apical wall (arrow). A mural thrombus is also observed in this aneurysm (asterisk)
Apical diverticula
Apical diverticulum is a finger-like contractile pouch with connection to the ventricle [19]. It is important to differentiate apical diverticula from aneurysm and pseudoaneurysm. Ventricular diverticula are congenital, often asymptomatic and are usually incidentally found while performing diagnostic imaging procedures for other reasons [20]. The wall of the diverticulum contains all layers of the ventricular myocardium with preserved myocardial architecture [20]. Contrary to a LV aneurysm, this diverticulum shows contraction during systole, because it contains a muscular wall [19, 20]. Delayed enhancement is not seen.
In children, apical diverticula can be associated with Cantrell’s syndrome, which is a rare syndrome characterised by a partial sternal cleft, anterior abdominal wall defects, anterior diaphragmatic defect and intracardiac defects of which the ventricular septal defect is the most common and is invariably present. LV diverticulum is present in 20% to 50% of these cases [19].
Apical ventricular remodelling
Cardiac remodelling refers to the change in size, shape and function of the heart after injury to the ventricles [16, 21, 22]. The injury is typically due to large acute myocardial infarction depending on anterior descending coronary artery occlusion. However, there are other non-ischaemic causes (i.e. chronic pressure or chronic volume overload and genetically determined cardiomyopathy) [21]. Myocardial infarction promotes transformation of both infarcted and non-infarcted regions, which leads to global LV dilation, referred to as “ventricular remodelling”. It is a dynamic process, starting in the acute phase with myocardial thinning and lengthening of the infarcted area, progressing to LV dilatation (Fig. 3) and hypertrophy of the non-infarcted area [21, 22]. Dysfunction with increased sphericity is common, as is the deposition of myocardial fat [23] and the development of apical thrombus (Fig. 4 and Video 4) [21].
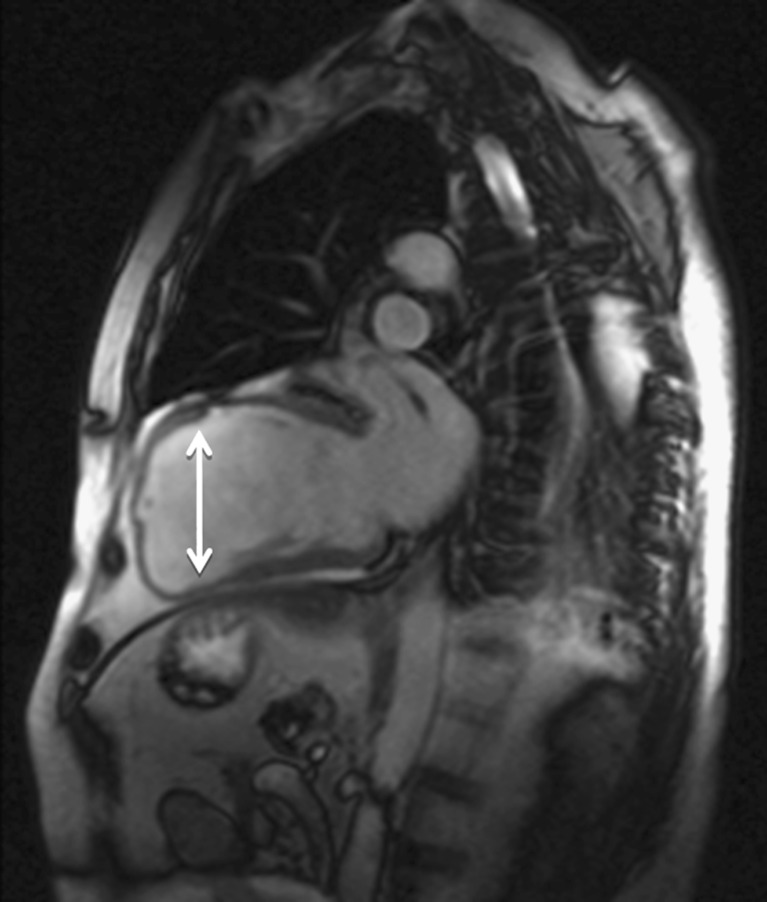
Fig. 3.
Apical ventricular remodelling in a patient with myocardial infarction antecedent. Magnetic resonance imaging demonstrates left ventricular (LV) dilation (white arrow) with distortion of its geometry resulting in impairment in systolic function (EF: 28%)
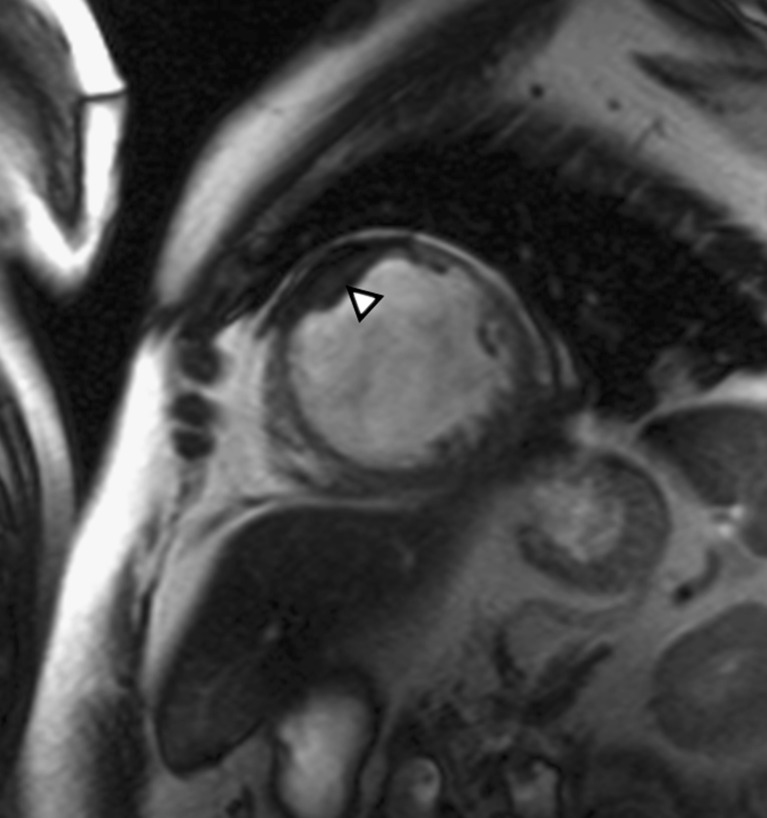
Fig. 4.
Apical ventricular remodelling. MRI in the short axis shows the dilation of the ventricular apex and associated mural thrombus (arrowhead)
Apical hypertrophic cardiomyopathy
Hypertrophic cardiomyopathy (HCM) is a primary and genetic myocardial disease characterised by a hypertrophied, non-dilated LV in the absence of another systemic or cardiac disease (e.g. systemic hypertension, aortic valve stenosis) capable of producing LV hypertrophy [1, 24].
There are several distinctive involvement patterns, one variant being the apical hypertrophic cardiomyopathy (AHC). Unlike usual HCM, AHC possesses typical clinical features and has a relatively good prognosis. However, it seems that the clinical course is less benign in western countries than in Japan [9].
Cardiac MR imaging and multidetector CT are capable of providing clinically useful information for the accurate diagnosis of HCM, as well as for the determination of clinical management strategies. However, MR imaging can allow better characterisation of the pattern and distribution of LV hypertrophy in HCM than in CT. Cardiac MR imaging should be considered as the reference standard for establishing a diagnosis of HCM when the results from echocardiography are inconclusive or are suspected of being false-negative findings. Moreover, cardiac MR imaging is strongly recommended as a powerful imaging technique for differentiating HCM from other cardiomyopathies in the differential diagnosis (Table 2) [3].
Table 2.
Relative merits of each non-invasive imaging technique for the assessment of hypertrophic cardiomyopathy (HCM)
| Factor assessed | Echocar-diographya | Multi-detector CT | MR imaging |
|---|---|---|---|
| LV volume | +++ | ++ | ++++ |
| Ejection fraction | +++ | +++ | ++++ |
| LV filling pressure | +++ | - | ++ |
| Dynamic obstruction | +++ | + | +++ |
| IschemialCFR | + | - | ++ |
| Tissue characterisation | ++ | + | ++++ |
The diagnosis is primarily based on the demonstration of localised apical hypertrophy, defined as an end-diastolic LV apical wall thickness greater than 15 mm or a ratio comparing apical LV and basal LV wall thicknesses of ≥1.3–1.5 [25].
More subjective criteria for the diagnosis of AHC include: obliteration of the LV apical cavity in systole, failure to identify a normal progressive reduction in LV wall thickness towards the apex [25] and apical aneurysm formation with delayed enhancement [25, 26]. The formation of apical aneurysm is thought to be due to ischaemia, which results from reduced capillary density, hyperplasia of the arterial media, increased perivascular fibrosis and myocardial bridging. This process usually occurs in the presence of normal epicardial coronary arteries [25].
Patchy delayed enhancement in a mid-wall location is present in most patients with HCM (Fig. 5) [25].
Fig. 5.
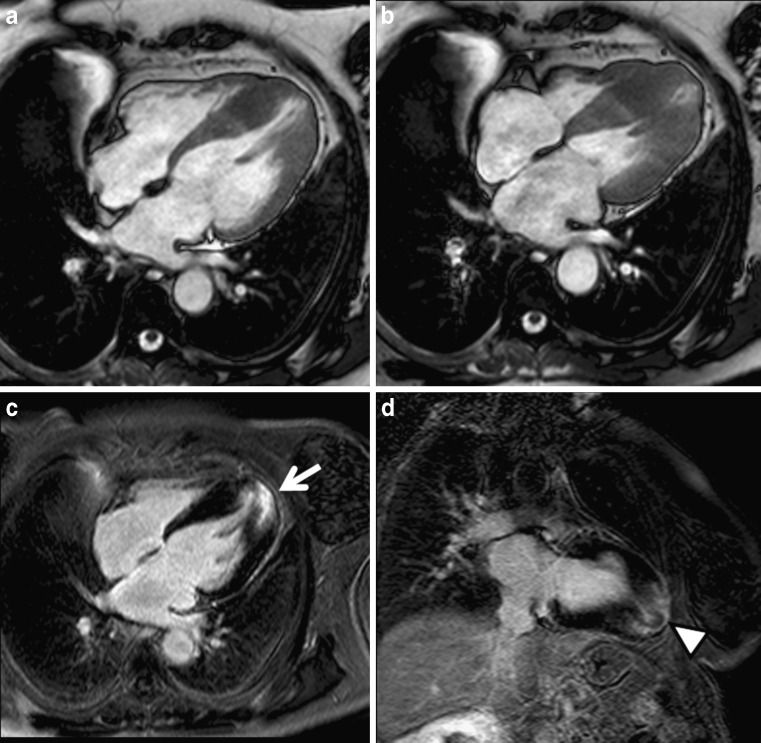
Apical form of hypertrophic cardiomyopathy. Cine MRI in the four-chamber view shows progressive thickening of the ventricular walls towards the apex in diastole (a) and systole (b). Late enhancement in a four-chamber view (c) and vertical long axis (d) demonstrates strong enhancement in the hypertrophied myocardium (arrow). Note the presence of “pseudo diverticulum” in the most apical myocardium (arrowhead)
There are three types of apical HCM: (1) true apical form (“spade-like” configuration), (2) involvement of the apex and symmetric hypertrophy of the four ventricular wall segments, and (3) involvement of the apex with asymmetric involvement of the wall segments (“non-spade” apical HCM) [27].
Tako-Tsubo cardiomyopathy
Transient LV apical ballooning syndrome or Tako-Tsubo cardiomyopathy (TSC) is a primary (Table 3) [24], acquired and reversible cardiomyopathy that usually affects postmenopausal women and is often provoked by a stressful event or physical stress. This disorder shows clinical features practically indistinguishable from acute myocardial infarction in the absence of significant coronary artery stenosis (which can be demonstrated by a normal CT coronarography) with systolic dysfunction that is completely reversible within a few days or weeks [24, 28].
Table 3.
Most frequent primary and secondary cardiomyopathies [4]
| Primary cardiomyopathies (mostly confined to the heart) | Secondary cardiomyopathies (cardiac involvement is a part of systemic disease) |
|---|---|
| Genetic: | Infiltrative: |
| Hypertrophic | Amyloidosis |
| Arrhythmogenic | |
| Non-compaction | |
| Mixed: | Storage: |
| Dilated | Hemochromatosis |
| Restricted | |
| Acquired: | Toxicity: |
| Inflammatory | Drugs |
| Tako-Tsubo | Chemical agents |
| Perip artum | |
| Endocrine: | |
| Diabetes mellitus | |
| Hyperthyroidism | |
| Hypothyroidism | |
| Hyperparathyroidism | |
| Pheochromocytoma | |
| Acromegaly | |
| Autoimmune/collagen: | |
| Systemic lupus erythematosis | |
| Dermatomyositis | |
| Rheumatoid arthritis | |
| Scleroderma | |
| Polyarteritis nodosa |
The MR findings are characterised by LV myocardial oedema with a diffuse and transmural distribution better visualised on the STIR T2-weighted sequences as high signal intensity (Figs. 6, 7) that matches with wall-motion abnormalities seen on cine-MRI. Hypokinesis or akinesis in the mid- and apical segments (although it can affect the basal segments alone) of the LV causes “apical ballooning” with compensatory basal segment hyperkinesis, taking the name of Tako-Tsubo from the morphology of LV in the ventriculography, which is similar to the Japanese pot used to capture octopuses. These wall motion abnormalities can produce a dynamic obstruction in the LV outflow (LVOT) seen as a “jet” in the LVOT plane and also mitral regurgitation [28].
Fig. 6.
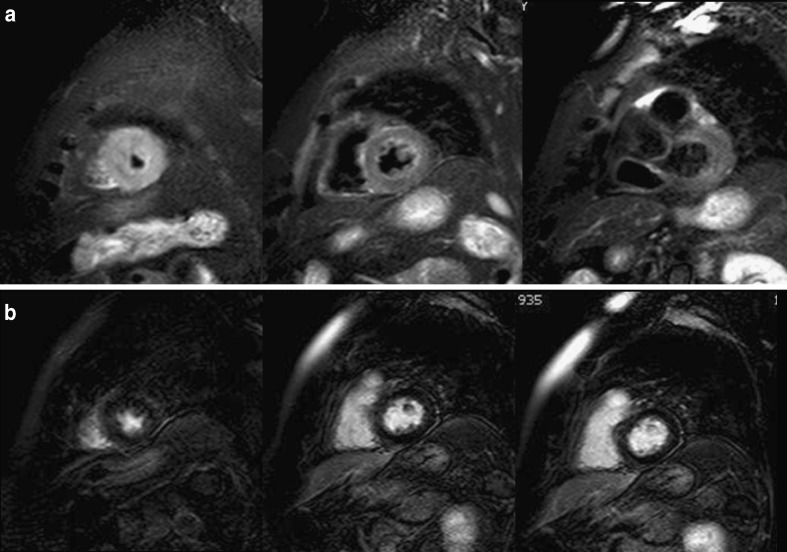
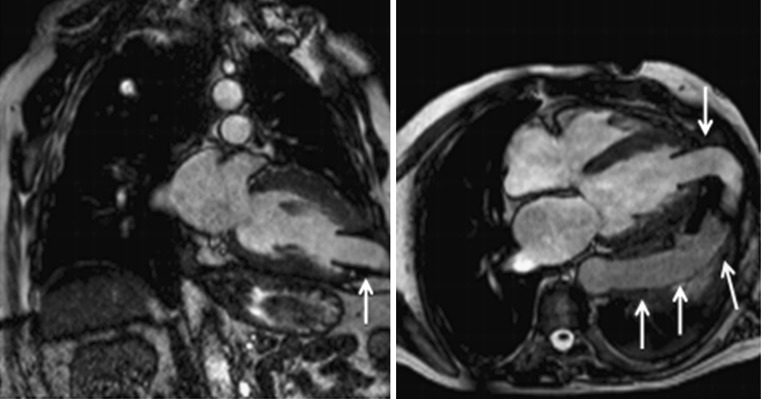
A 72-year-old woman with Tako-Tsubo cardiomyopathy who was seen in the emergency room for acute chest pain, raised cardiac enzymes and ST elevation segments. Conventional coronarography did not show significant epicardial stenosis. a STIR T2-weighted images in the short-axis view show diffuse oedema in apical segments with transmural location. Note how the mid and especially the basal segments are spared. b Delayed enhancement sequence shows normal and viable myocardium ruling out necrosis in the LV wall
Fig. 7.
Tako-Tsubo cardiomyopathy in a 62-year-old woman. STIR T2-weighted sequence in the vertical long-axis view shows the characteristic location of oedema fully involving the apical segments and sparing the basal segments
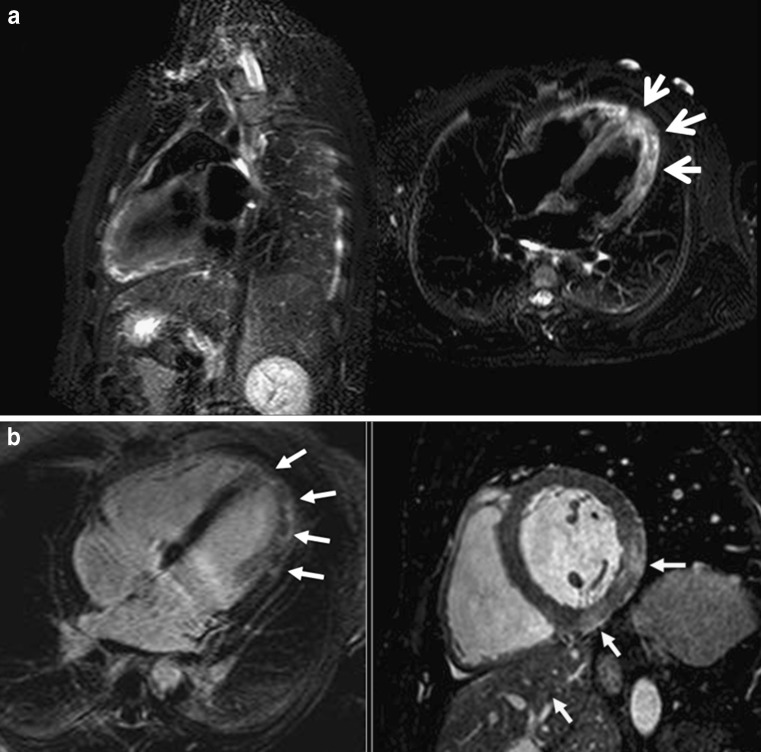
Tako-Tsubo cardiomyopathy and acute myocardial infarction (Fig. 8) can be clinically indistinguishable. Nevertheless, the differential diagnosis of the disorders can be suspected by the oedema location in the apical and mid-segments of LV that does not correlate with a vascular territory (Table 4) [29], and TSC has rarely been reported to cause late enhancement. Wall-motion abnormalities extend beyond the region supplied by one coronary artery. This feature is characteristic of Tako-Tsubo cardiomyopathy, whereas wall-motion abnormalities observed in acute myocardial infarction are often more localised. Acute myocarditis can also be clinically similar to TSC (acute chest pain, elevated cardiac enzymes levels and ECG changes), but has a more diffuse, heterogeneous distribution of the oedema (Fig. 9), often with sub-epicardial affectation that may be accompanied by pericardial enhancement [28].
Fig. 8.
A 68-year-old man with acute myocardial infarction. STIR T2-weighted images show high signal intensity (white arrow) due to ventricular oedema that has a vascular distribution involving segments irrigated by the left anterior descending artery
Table 4.
Differences among Tako-Tsubo, acute myocardial infarction (AMI) and myocarditis
| Tako-Tsubo | AMI | Myocarditis | |
|---|---|---|---|
| Hyperintensity distribution in T2-STIR | Transmural or diffuse | Transmural or subendocardial | Medium or subepicardic |
| Location | Apical and mid ventricular segments without vascular distribution | Vascular distribution | Patchy, without vascular distribution |
| Hyperintensity durability in T2-STTR | 2 weeks | More than 2 weeks | |
Fig. 9.
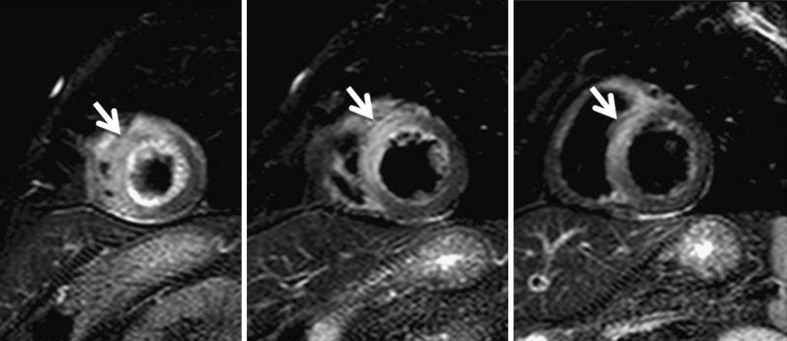
A 25-year-old man with acute myocarditis. a STIR T2-weighted sequence planned in the vertical long axis and four-chamber view showing oedema with a different form of Tako-Tsubo disease. The location of the oedema is diffuse but affecting all LV planes; also, the appearance of the oedema is patched. b Delayed enhancement sequence in the four-chamber view and post-contrast balance sequence in the short-axis view showing typical enhancement in the mid- and subepicardial LV wall (arrows)
Another characteristic feature of TSC is the lack of hypoperfusion on the first-pass imaging and at rest and of late enhancement on the delayed contrast sequence (Fig. 6) in the LV segments where there is STIR spotted oedema, which allows the differentiation from anterior ST elevation myocardial infarction. A rare but serious complication is the thrombus formation in the LV. The LV wall thickness is normal [28].
In combination with clinical history and catheterisation findings, cardiac MR imaging can be useful in supporting the diagnosis and monitoring functional recovery [30]. MRI is also useful in differentiating Tako-Tsubo cardiomyopathy from myocardial infarction and myocarditis. Although coronary CT angiography is not indicated in the initial evaluation of patients with Tako-Tsubo cardiomyopathy, reports are emerging of the use of coronary CT angiography in the subsequent evaluation of patients with TSC [31].
Left ventricular non-compaction
LV non-compaction (LVNC) may be an isolated finding (isolated left ventricular non-compaction) or may be associated with other congenital heart anomalies (left ventricular non-compaction). It is a congenital cardiomyopathy [24] characterised by a thickened wall with multiple prominent ventricular trabeculations and deep intertrabecular recesses (sinusoids) in communication with the ventricular cavity, resulting in systolic and diastolic ventricular dysfunction. Non-compaction involves predominantly the apical portion of the LV chamber, with or without right ventricular involvement, due to an arrest in the normal embryogenesis [32–34].
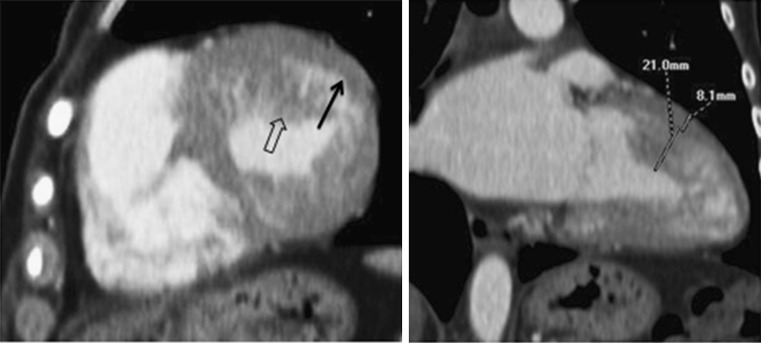
Because both the left and right ventricles normally have trabeculations, there is always the risk of over-diagnosis of this disease. It is therefore essential to be thorough while establishing the relationship between compacted and non-compacted myocardium when the diagnosis of this entity is being made. Using MRI, the most appropriate criteria would be a ratio between non-compacted and compacted areas greater than 2.3 (Fig. 10) in a diastolic long-axis view [35].
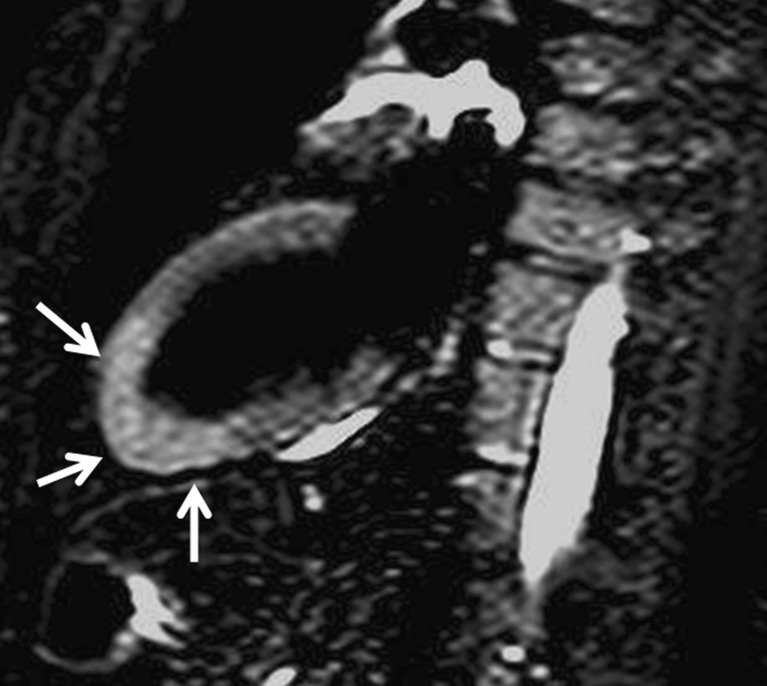
Fig. 10.
Contrast-enhanced CT in a patient with LV non-compaction showing deep trabeculations in the apex of the LV wall that define recesses communicating with the main ventricular chamber (arrows). In this patient the ratio between non-compacted and compacted areas is 2.6 (diagnosis >2.3)
Although ventricular non-compaction can appear similar to ventricular diverticula or prominent trabeculations (while virtually always <3 in number in normal variants), diagnosis of ventricular non-compaction usually requires the finding of more than three deep intertrabecular recesses in one imaging plane apically from the insertion of the papillary muscles [20, 32, 36]. False tendons, dilated cardiomyopathy, endocardial fibroelastosis and cardiac metastases are other important differential diagnostic considerations [34, 36].
Although echocardiography has been the diagnostic test of choice for non-compaction, other techniques have been used for the diagnosis, including CT and MRI [34]. Echocardiography may not visualise the apical region optimally, leading to underestimation of the degree of left ventricular non-compaction [37]. MRI provides good correlation with echo for the localisation and extent of non-compaction, and is useful in cases of poor echocardiographic image quality. In addition, the demonstration of differences in MRI signal intensity in non-compacted myocardium may help identify substrates for potentially lethal arrhythmias [34].
Arrhythmogenic right ventricular dysplasia
Arrhythmogenic right ventricular dysplasia (ARVD) is an uncommon form of inheritable cardiomyopathy [24] and is characterised by progressive loss of myocytes and fibrofatty replacement of the right ventricular myocardium [24, 38–41].
The diagnosis of ARVD is based on established criteria determined by a task force comprising the European Society of Cardiology and the International Society and Federation of Cardiology [42].
The diagnosis of ARVD is based on the presence of major and minor criteria (Table 5) [39, 42]. These criteria include, in addition to personal and family history, 12-lead ECG, echocardiography, right ventricular angiography, cardiac magnetic resonance imaging and computerised tomography [24, 39]. Although these criteria are specific, they lack sensitivity and have never been validated, in part because there is no single definitive means of making the diagnosis [39]. Endomyocardial biopsy from the right ventricular free wall is a sensitive diagnostic marker when fibrofatty infiltration is associated with surviving strands of myocytes [24] and is the preferred method for making the diagnosis [39]. Experience with CT in the diagnosis of ARVD is limited, but it has been used in the detection of morphological abnormalities [38, 43, 44] and wall motion abnormalities, particularly in patients with implantable defibrillators [43]. MRI is established as the imaging technique of choice in the assessment of ARVD [39, 44].
Table 5.
Diagnostic criteria for arrhythmogenic right ventricular dysplasia (ARVD)
| Diagnostic criteria | |
|---|---|
| I. Global and/or regional dysfunction and structural alterations | • Major |
| —Severe dilatation and reduction of right ventricular ejection fraction with no (or only mild) left and ventricular impairment | |
| —Localized right ventricular aneurysms (akinetic or dyskinetic areas with diastolic bulging) | |
| —Severe segmental dilatation of the right ventricle | |
| • Minor | |
| —Mild global right ventricular dilatation and/or ejection fraction reduction with normal left ventricle | |
| —Mild segmental dilatation of the right ventricle | |
| —Regional right ventricular hypokinesia | |
| II. Tissue characterisation of walls | • Major |
| —Fibrofatty replacement of myocardium at endomyocardial biopsy | |
| III. Repolarisation abnormalities | • Minor |
| —Inverted T waves in right precordial leads (V2 and V3) (people >12 years old; in absence of right bundle branch block) | |
| IV. Depolarisation of conduction abnormalities | • Major |
| —Epsilon waves or localised prolongation (>110 ms) of the QRS complex in right precordial leads (V1—V3) | |
| • Minor | |
| —Late potentials (signal-averaged ECG) | |
| V. Arrhythmias | • Minor |
| —Left bundle branch block type ventricular tachycardia (sustained or nonsustained; ECO, Holter, exercise testing) | |
| —Frequent ventricular extrasystoles on Holter (>1,000/24 h) | |
| VI. Family history | • Major |
| —Familial disease confirmed at necropsy or surgery | |
| • Minor | |
| —Familial history of premature sudden death (at <35 years) due to suspected right ventricular cardiomyopathy | |
| —Familial history (clinical diagnosis based on present criteria) | |
—Diagnosis is made if two major, or one major and two minor, or four minor criteria are satisfied. List is adapted from [42]
Reports of left ventricular involvement have led to the recognition of ARVD as a diffuse disease of the heart muscle affecting both ventricles [40, 41, 45, 46]. There is evidence of LV involvement with fibrofatty replacement, chamber enlargement and myocarditis in up to 75% of patients [24]. Focal left ventricular dyskinesia can also be present in the setting of fatty infiltration within the left ventricle [39]. Late left ventricular enhancement has also been described in patients with ARVD [39, 47]. In addition, these changes can definitely affect the LV apex.
Left ventricular false tendons
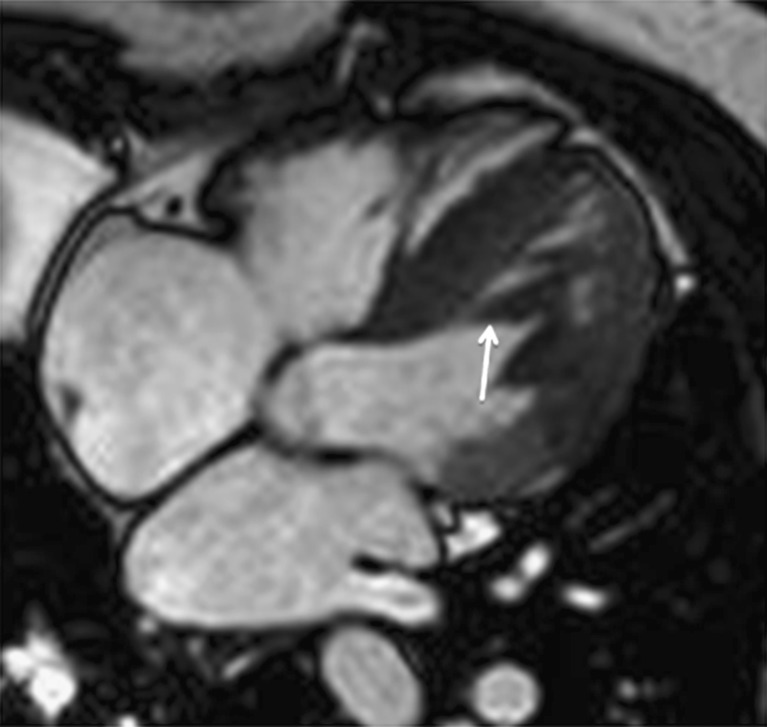
Besides the normal papillary muscles and chordae tendinae, in a minority of the population, false tendons or false chordae occur as normal variants in the LV [48, 49]. They might be single or multiple, usually thin, but sometimes very prominent, leading to confusing echocardiograms. They extend from the interventricular septum to the LV free wall (Fig. 11), or rarely, from the septum to the papillary muscle [36, 48, 50]. The LV false tendons are seen with considerable frequency in the echocardiographic evaluation of patients referred for having clinically innocent heart murmurs. They have a fibrous or fibromuscular structure (the thinner the tendon is, the higher fibrous component they have, and at greater thickness, a greater fibromuscular component) [48].
Fig. 11.
MRI in the four-chamber view showing a fibromuscular band that stretches across the LV from the septum to a papillary muscle (false tendon). This finding represents an anatomic variant that should not be mistaken for abnormalities such as tumours, subaortic membranes, thrombus borders or septal hypertrophy
Apico-aortic conduit
As an infrequent finding radiologists could discover left apical devices secondary to iatrogenic manoeuvres that involve the ventricular apex; one of them is the implantation of an LV apico-aortic conduit (AAC) (Fig. 12), which is an alternative approach for patients with aortic stenosis and conditions that either preclude a median sternotomy or present a high risk of aortic cross-clamping and aortotomy [51, 52]. It has three components: an outflow graft to the descending aorta, a valved conduit and an elbow apical connector. An apico-aortic conduit decreases the LV aortic pressure gradient, preserves or improves ventricular function, and maintains normally distributed blood flow through the systemic and coronary circulation [53].
Fig. 12.
Apico-aortic bypass is an alternative surgical option to aortic valve replacement for patients with a porcelain aorta or LV outflow tract obstruction. It is formed by a graft positioned in the ventricular apex with a valve into the conduit and anastomosed to the thoracic aorta (arrows)
Conclusions
The diagnosis of diseases affecting the apex of the LV can be challenging. This is mainly caused by the technical limitations of echocardiography. CT and MRI are non-invasive techniques that, because of their high temporal and spatial resolution, are valuable in assessing the apex of the LV, overcoming the technical limitations of echocardiography and contributing to the differential diagnosis (i.e. apical diverticula, aneurysms and pseudoaneurysms).
Electronic supplementary material
True aneurysm. CECT in the four-chamber view shows a dyskinetic area during systole leading to thrombus formation (AVI 1362 kb)
Apical ventricular remodelling. MRI in the short axis shows the dilation of the ventricular apex with associated mural thrombus (AVI 1712 kb)
Contributor Information
Silvia Cisneros, Phone: +351-349-44006000, FAX: +351-944-006180, Email: silviacisneros@gmail.com.
Ricardo Duarte, Email: guerra.duarte@gmail.com.
Gabriel C. Fernandez-Perez, Email: gabriel.fdez.perez@gmail.com
Daniel Castellon, Email: danielcastellon@gmail.com.
Julia Calatayud, Email: julimuse@yahoo.es.
Iñigo Lecumberri, Email: ilecumbe@seram.org.
Eneritz Larrazabal, Email: eneritzlarrazabal@gmail.com.
Berta Irene Ruiz, Email: bertaruiz82@hotmail.com.
References
- 1.Maron BJ. Hypertrophic cardiomyopathy: a systematic review. JAMA. 2002;287(10):1308–1320. doi: 10.1001/jama.287.10.1308. [DOI] [PubMed] [Google Scholar]
- 2.Prasad K, Atherton J, Smith GC, McKenna WJ, Frenneaux MP, Nihoyannopoulos P. Echocardiographic pitfalls in the diagnosis of hypertrophic cardiomyopathy. Heart. 1999;82(suppl 3):III8–III15. doi: 10.1136/hrt.82.2008.iii8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chun EJ, Choi SI, Jin KN, Kwag HJ, Kim YJ, Choi BW, Lee W, Park JH. Hypertrophic cardiomyopathy: assessment with MR imaging and multidetector CT. Radiographics. 2010;30(5):1309–1328. doi: 10.1148/rg.305095074. [DOI] [PubMed] [Google Scholar]
- 4.Pennell DJ, Sechtem UP, Higgins CB, et al. Clinical indications for cardiovascular magnetic resonance (CMR): consensus panel report. Eur Heart J. 2004;25(21):1940–1965. doi: 10.1016/j.ehj.2004.06.040. [DOI] [PubMed] [Google Scholar]
- 5.Lima JA, Desai MY. Cardiovascular magnetic resonance imaging: current and emerging applications. J Am Coll Cardiol. 2004;44(6):1164–1171. doi: 10.1016/j.jacc.2004.06.033. [DOI] [PubMed] [Google Scholar]
- 6.Maron MS, Maron BJ, Harrigan C, et al. Hypertrophic cardiomyopathy phenotype revisited after 50 years with cardiovascular magnetic resonance. J Am Coll Cardiol. 2009;54(3):220–228. doi: 10.1016/j.jacc.2009.05.006. [DOI] [PubMed] [Google Scholar]
- 7.Nagueh SF, Mahmarian JJ. Noninvasive cardiac imaging in patients with hypertrophic cardiomyopathy. J Am Coll Cardiol. 2006;48(12):2410–2422. doi: 10.1016/j.jacc.2006.07.065. [DOI] [PubMed] [Google Scholar]
- 8.Yoshida M, Takamoto T. Left ventricular hypertrophic patterns and wall motion dynamics in hypertrophic cardiomyopathy: an electron beam computed tomographic study. Intern Med. 1997;36(4):263–269. doi: 10.2169/internalmedicine.36.263. [DOI] [PubMed] [Google Scholar]
- 9.Ghersin E, Lessick J, Litmanovich D, Engel A, Reisner S. Comprehensive multidetector CT assessment of apical hypertrophic cardiomyopathy. Br J Radiol. 2006;79:e200–e204. doi: 10.1259/bjr/53601277. [DOI] [PubMed] [Google Scholar]
- 10.Juergens KU, Wessling J, Fallenberg EM, Mönnig G, Wichter T, Fischbach R. Multislice cardiac spiral CT evaluation of atypical hypertrophic cardiomyopathy with a calcified left ventricular thrombus. J Comput Assist Tomogr. 2000;24(5):688–690. doi: 10.1097/00004728-200009000-00004. [DOI] [PubMed] [Google Scholar]
- 11.Williams TJ, Manghat NE, McKay-Ferguson A, Ring NJ, Morgan-Hughes GJ, Roobottom CA. Cardiomyopathy: appearances on ECG-gated 64-detector row computed tomography. Clin Radiol. 2008;63(4):464–474. doi: 10.1016/j.crad.2007.07.024. [DOI] [PubMed] [Google Scholar]
- 12.Ferencik M, Abbara S, Hoffmann U, Cury RC, Brady TJ, Achenbach S. Left ventricular thin-point detection using multidetector spiral computed tomography. Am J Cardiol. 2004;93(7):949–951. doi: 10.1016/j.amjcard.2003.12.033. [DOI] [PubMed] [Google Scholar]
- 13.Johnson KM, Johnson HE, Dowe DA. Left ventricular apical thinning as normal anatomy. J Comput Assist Tomogr. 2009;33(3):334–337. doi: 10.1097/RCT.0b013e3181870356. [DOI] [PubMed] [Google Scholar]
- 14.Globits S, Marco T, Schwitter J, et al. Assessment of early left ventricular remodelling in orthotopic heart transplant recipients with cine magnetic resonance imaging: potential mechanisms. J Heart Lung Transplant. 1997;16:504–510. [PubMed] [Google Scholar]
- 15.Semelka RC, Tomei E, Wagner S, et al. Inter-study reproducibility of dimensional and functional measurements between cine magnetic resonance studies in the morphologically abnormal left ventricle. Am Heart J. 1990;119:1367–1373. doi: 10.1016/S0002-8703(05)80187-5. [DOI] [PubMed] [Google Scholar]
- 16.Sabed M, Watzinger N, Krombach GA, et al. Left ventricular remodeling after infarction: sequential MR imaging with oral nicorandil therapy in rat model. Radiology. 2002;224:830–837. doi: 10.1148/radiol.2243011372. [DOI] [PubMed] [Google Scholar]
- 17.Kumbasar B, Wu KC, Kamel IR, Lima JAC, Bluemke DA. Left ventricular true aneurysm: diagnosis of myocardial viability shown on MR imaging. AJR Am J Roentgenol. 2002;179:472–474. doi: 10.2214/ajr.179.2.1790472. [DOI] [PubMed] [Google Scholar]
- 18.Konen E, Merchant N, Gutierrez C, et al. True versus false left ventricular aneurysm: differentiation with MR imaging-initial experience. Radiology. 2005;236:65–70. doi: 10.1148/radiol.2361031699. [DOI] [PubMed] [Google Scholar]
- 19.Marijon E, Ou P, Fermont L. Diagnosis and outcome in congenital ventricular diverticulum and aneurism. J Thorac Cardiovasc Surg. 2006;131:433–437. doi: 10.1016/j.jtcvs.2005.09.046. [DOI] [PubMed] [Google Scholar]
- 20.Srichai MB, Hecht EM, Kim DC, Jacobs JE. Ventricular diverticula on cardiac CT: more common than previously thought. AJR Am J Roentgenol. 2007;189:204–208. doi: 10.2214/AJR.06.1223. [DOI] [PubMed] [Google Scholar]
- 21.Sutton MGJ, Sharpe N. Left ventricular remodeling after myocardial infarction: pathophysiology and therapy. Circulation. 2000;101:2981–2988. doi: 10.1161/01.cir.101.25.2981. [DOI] [PubMed] [Google Scholar]
- 22.Visser CA. Left ventricular remodelling after myocardial infarction: importance of residual myocardial viability and ischaemia. Heart. 2003;89:1121–1122. doi: 10.1136/heart.89.10.1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Goldfarb JW, Roth M, Han J. Myocardial fat deposition after left ventricular myocardial infarction: assessment by using MR Water-Fat separation imaging. Radiology. 2009;253:65–73. doi: 10.1148/radiol.2532082290. [DOI] [PubMed] [Google Scholar]
- 24.Maron B, Towbin J, Thiene G, et al. Contemporary definitions and classification of the cardiomyopathies: an American Heart Association scientific statement from the Council on Clinical Cardiology, Heart Failure and Transplantation Committee; quality of care and outcomes research and functional genomics and translational biology interdisciplinary working groups; and council on epidemiology and prevention. Circulation. 2006;113:1807–1816. doi: 10.1161/CIRCULATIONAHA.106.174287. [DOI] [PubMed] [Google Scholar]
- 25.Hansen MW, Merchant N. MRI of hypertrophic cardiomyopathy: part I, MRI appearances. AJR Am J Roentgenol. 2007;189:1335–1343. doi: 10.2214/AJR.07.2286. [DOI] [PubMed] [Google Scholar]
- 26.Zenovich AG, Lesser JR, Casey SA, Maron BJ. Hypertrophic cardiomyopathy with apical aneurysm. Circulation. 2004;110:e450. doi: 10.1161/01.CIR.0000145174.74063.C1. [DOI] [PubMed] [Google Scholar]
- 27.Bogaert J, Taylor AM. Nonischemic myocardial disease. In: Bogaert J, Dymarkowski S, Taylor AM, editors. Clinical cardiac MRI. Berlin: Springer; 2005. pp. 217–263. [Google Scholar]
- 28.Fernandez GC, Aguilar JA, Tardaguila G, et al. Takotsubo cardiomyopathy. Assessment with cardiac MRI. AJR Am J Roentgenol. 2010;195:W139–W145. doi: 10.2214/AJR.09.3369. [DOI] [PubMed] [Google Scholar]
- 29.Assomull RG, Lyne JC, Keenan N, et al. The role of cardiovascular magnetic resonance in patients presenting with chest pain, raised troponin, and unobstructed coronary arteries. Eur Heart J. 2007;28:1242–1249. doi: 10.1093/eurheartj/ehm113. [DOI] [PubMed] [Google Scholar]
- 30.Cummings KW, Bhalla S, Javidan-Nejad C, Bierhals AJ, Gutierrez FR, Woodard PK. A pattern-based approach to assessment of delayed enhancement in nonischemic cardiomyopathy at MR imaging. Radiographics. 2009;29:89–103. doi: 10.1148/rg.291085052. [DOI] [PubMed] [Google Scholar]
- 31.Scheffel H, Stolzmann P, Karlo C, et al. Tako-tsubo phenomenon: dual-source computed tomography and conventional coronary angiography. Cardiovasc Intervent Radiol. 2008;31(1):226–227. doi: 10.1007/s00270-007-9140-4. [DOI] [PubMed] [Google Scholar]
- 32.Monserrat L. Miocardiopatía no compactada: una enfermedad en busca de criterios. Rev Esp Cardiol. 2008;61(2):112–115. [PubMed] [Google Scholar]
- 33.Hughes SE, McKenna WJ. New insights into the pathology of inherited cardiomyopathy. Heart. 2005;91:257–264. doi: 10.1136/hrt.2004.040337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Weiford BC, Subbarao VD, Mulhern KM. Noncompaction of the ventricular myocardium. Circulation. 2004;109:2965–2971. doi: 10.1161/01.CIR.0000132478.60674.D0. [DOI] [PubMed] [Google Scholar]
- 35.Petersen SE, Selvanayagam JB, Wiesmann F, et al. Left ventricular non-compaction: insights from cardiovascular magnetic resonance imaging. J Am Coll Cardiol. 2005;46:101–105. doi: 10.1016/j.jacc.2005.03.045. [DOI] [PubMed] [Google Scholar]
- 36.Stöllberger C, Finsterer J. Pitfalls in the diagnosis of left ventricular hypertrabeculation/non-compaction. Postgrad Med J. 2006;82:679–683. doi: 10.1136/pgmj.2006.046169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Alhabshan F, Smallhorn JF, Golding F, Musewe N, Freedom RM, Yoo SJ. Extent of myocardial noncompaction: comparison between MRI and echocardiographic evaluation. Pediatr Radiol. 2005;35:1147–1151. doi: 10.1007/s00247-005-1551-2. [DOI] [PubMed] [Google Scholar]
- 38.Kimura F, Sakai F, Sakomura Y, Fujimura M, Ueno E, Matsuda N, et al. Helical CT features of arrhythmogenic right ventricular cardiomyopathy. Radiographics. 2002;22:1111–1124. doi: 10.1148/radiographics.22.5.g02se031111. [DOI] [PubMed] [Google Scholar]
- 39.Murphy DT, Shine SC, Cradock A, et al. Cardiac MRI in arrhythmogenic right ventricular cardiomyopathy. AJR Am J Roentgenol. 2010;194(4):W299–W306. doi: 10.2214/AJR.09.3450. [DOI] [PubMed] [Google Scholar]
- 40.Pasquale CG, Heddle WF. Left sided arrhythmogenic ventricular dysplasia in siblings. Heart. 2001;86:128–130. doi: 10.1136/heart.86.2.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Corrado D, Fontaine G, Marcus FI, et al. Arrhythmogenic right ventricular dysplasia/cardiomyopathy: need for an international registry. Study group on arrhythmogenic right ventricular dysplasia/cardiomyopathy of the working groups on myocardial and pericardial disease and arrhythmias of the European Society of Cardiology and of the Scientific Council on Cardiomyopathies of the World Heart Federation. Circulation. 2000;101:E101–E106. doi: 10.1161/01.cir.101.11.e101. [DOI] [PubMed] [Google Scholar]
- 42.McKenna WJ, Thiene G, Nava A, et al. Diagnosis of arrhythmogenic right ventricular dysplasia/cardiomyopathy. Task force of the working group myocardial and pericardial disease of the european society of cardiology and of the scientific council on cardiomyopathies of the International Society and Federation of Cardiology. Br Heart J. 1994;71:215–218. doi: 10.1136/hrt.71.3.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bomma C, Dalal D, Tandri H, et al. Evolving role of multidetector computed tomography in evaluation of arrhythmogenic right ventricular dysplasia/cardiomyopathy. Am J Cardiol. 2007;100:99–105. doi: 10.1016/j.amjcard.2007.02.064. [DOI] [PubMed] [Google Scholar]
- 44.Arda K, Ciledag N, Kacmaz F, Tufekcioglu O, Sereflisan Y. Arrhythmogenic right ventricular dysplasia; radiologic findings of the left ventricle: a case report and review of the literature. Indian J Radiol Imaging. 2006;16(1):131–134. doi: 10.4103/0971-3026.29069. [DOI] [Google Scholar]
- 45.Corrado D, Basso C, Thiene G, et al. Spectrum of clinicopathologic manifestations of arrhythmogenic right ventricular cardiomyopathy/dysplasia: a multicenter study. J Am Coll Cardiol. 1997;30:1512–1520. doi: 10.1016/S0735-1097(97)00332-X. [DOI] [PubMed] [Google Scholar]
- 46.Horimoto M, Akino M, Takenaka T, Igarashi K, Inoue H, Kawakami Y. Evolution of left ventricular involvement in arrhythmogenic right ventricular cardiomyopathy. Cardiology. 2000;93:197–200. doi: 10.1159/000007026. [DOI] [PubMed] [Google Scholar]
- 47.Sen-Chowdhry S, Prasad SK, Syrris P, et al. Cardiovascular magnetic resonance in arrhythmogenic right ventricular cardiomyopathy revisited: comparison with task force criteria and genotype. J Am Coll Cardiol. 2006;48:2132–2140. doi: 10.1016/j.jacc.2006.07.045. [DOI] [PubMed] [Google Scholar]
- 48.Malouf J, Gharzuddine W, Kutayli F. A reappraisal of the prevalence of left ventricular false tendons in children and adults. Br Heart J. 1986;55:587–591. doi: 10.1136/hrt.55.6.587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Casta A, Wolf WJ. Left ventricular bands (false tendons): echocardiographic and angiocardiographic delineation in children. Am Heart J. 1986;111(2):321–324. doi: 10.1016/0002-8703(86)90147-X. [DOI] [PubMed] [Google Scholar]
- 50.Gullace G, Yuste P, Letouzey JP, et al. Aspetti ecocardiografici dei falsi tendini intraventricolari. G Ital Cardiol. 1987;17:318–328. [PubMed] [Google Scholar]
- 51.Vassiliades TA., Jr Off-pump apicoaortic conduit insertion for high-risk patients with aortic stenosis. Eur J Cardiothorac Surg. 2003;23:156–158. doi: 10.1016/S1010-7940(02)00738-8. [DOI] [PubMed] [Google Scholar]
- 52.Crestanello JA, Zehr KJ, Daly RC, et al. Is there a role for the left ventricle apical-aortic conduit for acquired aortic stenosis? J Heart Valve Dis. 2004;13:57–62. [PubMed] [Google Scholar]
- 53.Renzulli A, Gregorio R, Feo M, Ismeno G, Covino FE, Cotrufo M. Long-term results of apico-aortic valved conduit. Tex Heart J. 2000;27:24–28. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
True aneurysm. CECT in the four-chamber view shows a dyskinetic area during systole leading to thrombus formation (AVI 1362 kb)
Apical ventricular remodelling. MRI in the short axis shows the dilation of the ventricular apex with associated mural thrombus (AVI 1712 kb)