Abstract
Chronic hepatitis B (CHB) is a global health problem affecting more than 350 million people worldwide. Chronic carriage of HBV is related to the age when the infection occurs; the younger the age the higher the chronicity rate. Knowledge of the natural history of CHB is important for the management of the disease. The goal of hepatitis B treatment is to prevent cirrhosis, liver decompensation and hepatocellular carcinoma. In clinical practice, treatment response is determined by the suppression of serum HBV DNA levels. However, current antiviral therapies are usually unable to achieve sustained off-treatment responses and eradicate the infection. Impairment of immune responses including defective innate non-cytolytic antiviral function together with exhausted T cells and the tolerogenic liver environment may all contribute to the poor clinical response. A more comprehensive understanding of the immunological phases of CHB, potential triggers of liver flares and molecular mechanisms underlying viral persistence and immunopathology will help to tailor future therapeutic strategies. A synergistic approach of boosting the immune response of the host by specific immunotherapeutic interventions and effective viral load suppression will be needed to promote sustained viral clearance in chronic infection.
Epidemiology and public health significance
The World Health Organization estimates that 2 billion people have been infected with hepatitis B virus (HBV), and more than 350 million have chronic infection. The carrier rate varies from low (0.1–2%) in USA and western Europe, to intermediate (2–8%) in Mediterranean countries and Japan and high (8–20%) in sub-Saharan Africa and parts of Asia.1 In highly endemic areas, the majority of individuals become infected either perinatally or in early childhood, whereas in low prevalence areas the infection is acquired primarily in adulthood. HBV infection persists in ∼1–5% of infected adults, 20–30% of young children and up to 90% of perinatally infected subjects.1
The virus and natural history of the infection
HBV is a partially double-stranded DNA virus whose genome encodes four overlapping reading frames. During the replication cycle of the virus in hepatocytes, two key events take place. One is the formation of replicative intermediates termed ‘covalently closed circular DNA’ (cccDNA) in the nucleus of cell, which are maintained for the lifetime of the hepatocyte and function as an HBV reservoir responsible for persistence.2 The challenge of current antiviral therapy is to clear and/or prevent the formation of cccDNA. The second key event is the reverse transcription step. The reverse transcriptase, with its inherent error-prone activity, is responsible for the emergence of a highly heterogenous viral population (quasispecies) including those containing mutations affecting the production of e-Antigen (eAg).2
Following an incubation period of 1–4 months, symptomatic hepatitis occurs in about one-third of adults, in ∼10% of children <5 years and only rarely in children <1 year. A fulminant course with high mortality has been reported only occasionally in the paediatric population and in ∼1% of infected adults. Resolution of the infection, which is characterized by the disappereance of hepatitis B surface antigen (HBsAg) from the blood within 6 months of infection, occurs in the majority of infected adults, but only in a minority of infected neonates. Resolution of the infection generally results in lifelong protection from the disease. However, it is important to note that HBV is controlled by an efficient immune response rather than completely eliminated;3 it can therefore reactivate when there is significant immunosuppression, such as in the setting of human immunodeficiency virus or bone marrow transplantion.
The natural course of chronic hepatitis B (CHB) virus infection has been divided into four phases that have been arbitrarily defined as: immune-tolerant, immune-elimination (or immune-clearance), inactive and reactivation.4 The ‘immunotolerant phase’ is characterized by a high level of HBV replication, eAg positivity, and a normal or minimally elevated alanine transaminase (ALT). This period can last up to 20–30 years following perinatal acquisition, but is short or absent in those who acquire infection as an adult. During the ‘immune clearance phase’, there is a reduction in HBV DNA levels and increased liver inflammation. During this phase, which can last for years, the disease activity fluctuates and progressive liver damage occurs. The occurrence of seroconversion from eAg positive to anti-e antibody (20–30%/year) is usually followed by a decrease in viral replication and ALT. At this stage, the patient enters the inactive phase. HBV replication <2000 IU/ml is associated in most patients with biochemical and histological regression of inflammatory activity.5
As a result of the persistence of cccDNA in hepatocyes, some inactive carriers can develop HBV reactivation with either the wild-type and reversion to eAg positivity (1–4%) or with HBV variants with mutations limiting eAg production.6 Recent work has shown that the likelihood of HBeAg-negative CHB is related to the duration of infection, with a cumulative incidence of 24% at 16 years of follow-up associated with a high risk of accelerated progression to fibrosis and cirrhosis.7
The major complications of CHB are cirrhosis and hepatocellular carcinoma (HCC). The overall risk of developing cirrhosis during the lifetime of patients with CHB is 15–40%. The risk of HCC in patients with cirrhosis is estimated to be 2–5%; however, it varies according to the geographical area and may be influenced by the presence of HBsAg, eAg, certain mutations and levels of HBV DNA >20 000 IU/ml.8
Limitations of current treatment
The goal of therapy for CHB is to prevent the progression of the disease. The two main strategies currently used are antiviral therapy with nucleoside/nucleotide analogues (NA) or pegylated interferon α (PEG-IFN-α). NA can effectively suppress HBV DNA to undetectable levels and lead to clinical and histological improvement.9 Although the newer NAs, entecavir and tenofovir, have a high genetic barrier to resistance, they rarely achieve virological control [HBV surface antigen (HBsAg) seroconversion] or sustained off-treatment responses.10 A finite duration of treatment with PEG-IFN-α has a better chance of achieving sustained virological responses. However, side effects, the need for subcutaneous injections and the contraindication profile, including individuals with cirrhosis, remain major limitations to its use.
How can the immune response be harnessed to treat HBV?
The immune system has the inherent capacity to control HBV infection, as evidenced by the majority of infected adults who resolve acute infection, and by the resolution of CHB in recipients following bone marrow transplantation from an immune donor.11 Thus, a synergistic approach of boosting the immune response of the host along with specific immunotherapeutic interventions and effective viral load suppression is needed to promote sustained viral clearance in chronic infection.
The role of innate immunity in HBV infections has remained the subject of controversy. Despite the lack of induction of type I IFN response and attenuated innate immunity during acute infection, resolution is achieved.12 Evasion or suppression of innate responses may be more relevant in established chronic infection, perpetuating viral replication. Natural killer cells are emerging as innate effectors in viral infections, but in CHB they have defective non-cytolytic antiviral functions and are biased to cytotoxicity and expression of the death ligand tumour necrosis factor (TNF)-related apoptosis inducing ligand, a functional profile that may exacerbate liver injury.13,14
The role of T cells in HBV infection is better established.15 In contrast to the robust and multi-specific T-cell responses observed in patients during self-limiting HBV infection, chronic infection is characterized by weak and transient responses.16 When considering the nature of T-cell exhaustion in HBV, important considerations are the tolerizing liver environment and the high levels of viral replication, along with a large excess of circulating sAg and HBeAg that are likely to all contribute to the progressive functional decline of virus-specific T cells.17
Recent research has highlighted a number of T-cell intrinsic defects induced or compounded by defective antigen presentation in the liver,18 such as the up-regulation of a pro-apoptotic molecule called BCl2-interacting mediator (BIM), which mediates deletion of HBV-specific CD8 T cells.19 The residual T-cell responses are driven to exhaustion by a number of co-inhibitory molecules including PD-1 and CTLA-4.20 Although therapeutic blockade of these receptors may constitute a promising approach, co-expression of additional inhibitory molecules on T cell populations illustrates the complexity of co-operation between the numerous co-inhibitory pathways.
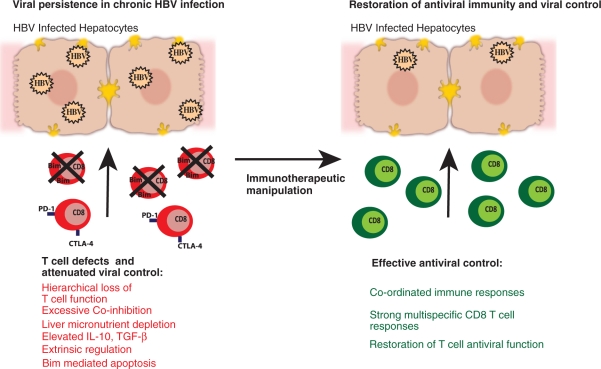
These intrinsic defects can be perpetuated by extrinsic factors present in the liver microenvironment. The liver nutrient milieu,21 regulatory populations and high levels of the immunosuppressive cytokines IL-10 and tumour growth factor (TGF)-β can hamper the ability of T cells to expand and survive, thereby attenuating viral control.12 Immunotherapeutic targeting of IL-10 and TGF-β in CHB may constitute an additional therapeutic intervention, with the caveat that these cytokines govern a critical balance between impeding pathogen clearance and restraining immunopathology (Figure 1).
Figure 1.
Chronic HBV infection is characterized by a number of T-cell defects leading to viral persistence. HBV-specific T cells are tolerized or deleted in the HBV-infected liver. The aim of immunotherapeutic manipulation is to target selected pathways restoring T-cell antiviral function and recovering successful immunity.
Remaining challenges
Implementation of universal vaccination against HBV in newborns has already reduced the incidence of HBV-related HCC in countries like Taiwan.22 More widespread uptake of the vaccine and other prevention measures should dramatically reduce the number of new cases of HBV in the coming decades. In the meantime, there is a pressing need to develop more effective treatment approaches and better biomarkers for disease monitoring for the huge burden of infected individuals. Quantification of sAg has recently been shown to help to distinguish inactive from active eAg negative disease and to identify those patients with a sustained response to IFN-α.23
Future potential therapeutic approaches targeting innate immunity include directing IFN-α towards infected hepatocytes with TCR-like monoclonal antibodies,24 substituting IFN-α with IFN-λ or stimulating innate recognition with agonists for pattern recognition receptors.
Chimeric antigen receptors and T-cell receptor gene transfer are new exciting strategies attempting to re-direct the specificity of existing T cells against HBV.24 The remaining concern is whether recovery of virus-specific T cells will result in increased liver injury, disease flares and decompensated liver disease. Previous work has demonstrated that in patients with active disease and liver inflammation it is the influx of non-antigen-specific infiltrate that is largely responsible for liver damage.25 These data lend support to the idea that future immunotherapeutic startegies should aim to restore virus-specific T-cell defects, while blocking potential pathways of liver damage.
Funding
Wellcome Trust Starter Grant to G.N.; Medical Research Council Clinical Research Training Fellowship to D.P.; MRC Grant (G0801213 to M.K.M.).
Conflict of interest: MKM has provided consultancy/advisory boards for Roche, Transgene and ITS and received an unrestricted educational grant from BMS.
Acknowledgements
We thank members of our laboratory and collaborators for inspiring discussions and apologize to the many authors whose work we could not quote because of space constraints.
References
- 1.Liaw YF, Chu CM. Hepatitis B virus infection. Lancet. 2009;373:582–92. doi: 10.1016/S0140-6736(09)60207-5. [DOI] [PubMed] [Google Scholar]
- 2.Locarnini S, Zoulim F. Molecular genetics of HBV infection. Antiviral Therapy. 2010;3(15 Suppl.):3–14. doi: 10.3851/IMP1619. [DOI] [PubMed] [Google Scholar]
- 3.Rehermann B, Ferrari C, Pasquinelli C, Chisari FV. The hepatitis B virus persists for decades after patients' recovery from acute viral hepatitis despite active maintenance of a cytotoxic T-lymphocyte response. Nat Med. 1996;2:1104–8. doi: 10.1038/nm1096-1104. [DOI] [PubMed] [Google Scholar]
- 4.Villa E, Fattovich G, Mauro A, Pasino M. Natural history of chronic HBV infection: special emphasis on the prognostic implications of the inactive carrier state versus chronic hepatitis. Dig Liver Dis. 2011;43(Suppl. 1):S8–14. doi: 10.1016/S1590-8658(10)60686-X. [DOI] [PubMed] [Google Scholar]
- 5.Chen CJ, Yang HI, Iloeje UH. Hepatitis B virus DNA levels and outcomes in chronic hepatitis B. Hepatology. 2009;49:S72–84. doi: 10.1002/hep.22884. [DOI] [PubMed] [Google Scholar]
- 6.Fattovich G, Olivari N, Pasino M, D'Onofrio M, Martone E, Donato F. Long-term outcome of chronic hepatitis B in Caucasian patients: mortality after 25 years. Gut. 2008;57:84–90. doi: 10.1136/gut.2007.128496. [DOI] [PubMed] [Google Scholar]
- 7.Hsu YS, Chien RN, Yeh CT, Sheen IS, Chiou HY, Chu CM, et al. Long-term outcome after spontaneous HBeAg seroconversion in patients with chronic hepatitis B. Hepatol. 2002;35:1522–7. doi: 10.1053/jhep.2002.33638. [DOI] [PubMed] [Google Scholar]
- 8.Chen CJ, Yang HI, Su J, Jen CL, You SL, Lu SN, et al. Risk of hepatocellular carcinoma across a biological gradient of serum hepatitis B virus DNA level. JAMA. 2006;295:65–73. doi: 10.1001/jama.295.1.65. [DOI] [PubMed] [Google Scholar]
- 9.Dienstag JL. Benefits and risks of nucleoside analog therapy for hepatitis B. Hepatology. 2009;49:S112–21. doi: 10.1002/hep.22920. [DOI] [PubMed] [Google Scholar]
- 10.Papatheodoridis GV, Manolakopoulos S, Dusheiko G, Archimandritis AJ. Therapeutic strategies in the management of patients with chronic hepatitis B virus infection. Lancet Infect Dis. 2008;8:167–78. doi: 10.1016/S1473-3099(07)70264-5. [DOI] [PubMed] [Google Scholar]
- 11.Hui CK, Lie A, Au WY, Leung YH, Ma SY, Cheung WW, et al. A long-term follow-up study on hepatitis B surface antigen-positive patients undergoing allogeneic hematopoietic stem cell transplantation. Blood. 2005;106:464–9. doi: 10.1182/blood-2005-02-0698. [DOI] [PubMed] [Google Scholar]
- 12.Dunn C, Peppa D, Khanna P, Nebbia G, Jones M, Brendish N, et al. Temporal analysis of early immune responses in patients with acute hepatitis B virus infection. Gastroenterology. 2009;137:1289–300. doi: 10.1053/j.gastro.2009.06.054. [DOI] [PubMed] [Google Scholar]
- 13.Dunn C, Brunetto M, Reynolds G, Christophides T, Kennedy PT, Lampertico P, et al. Cytokines induced during chronic hepatitis B virus infection promote a pathway for NK cell-mediated liver damage. J Exp Med. 2007;204:667–80. doi: 10.1084/jem.20061287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Peppa D, Micco L, Javaid A, Kennedy PT, Schurich A, Dunn C, et al. Blockade of immunosuppressive cytokines restores NK cell antiviral function in chronic hepatitis B virus infection. PLoS Pathogens. 2010;6:e1001227. doi: 10.1371/journal.ppat.1001227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Maini MK, Boni C, Lee CK, Larrubia JR, Reignat S, Ogg GS, et al. The role of virus-specific CD8(+) cells in liver damage and viral control during persistent hepatitis B virus infection. J Exp Med. 2000;191:1269–80. doi: 10.1084/jem.191.8.1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bertoletti A, Maini MK. Protection or damage: a dual role for the virus-specific cytotoxic T lymphocyte response in hepatitis B and C infection? Curr Opin Immunol. 2000;12:403–8. doi: 10.1016/s0952-7915(00)00108-4. [DOI] [PubMed] [Google Scholar]
- 17.Webster GJ, Reignat S, Brown D, Ogg GS, Jones L, Seneviratne SL, et al. Longitudinal analysis of CD8+ T cells specific for structural and nonstructural hepatitis B virus proteins in patients with chronic hepatitis B: implications for immunotherapy. J Virol. 2004;78:5707–19. doi: 10.1128/JVI.78.11.5707-5719.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Maini MK, Schurich A. The molecular basis of the failed immune response in chronic HBV: therapeutic implications. J Hepatol. 2010;52:616–9. doi: 10.1016/j.jhep.2009.12.017. [DOI] [PubMed] [Google Scholar]
- 19.Lopes AR, Kellam P, Das A, Dunn C, Kwan A, Turner J, et al. Bim-mediated deletion of antigen-specific CD8 T cells in patients unable to control HBV infection. J Clin Investig. 2008;118:1835–45. doi: 10.1172/JCI33402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schurich A, Khanna P, Lopes AR, Han KJ, Peppa D, Micco L, et al. Role of the coinhibitory receptor cytotoxic T lymphocyte antigen-4 on apoptosis-Prone CD8 T cells in persistent hepatitis B virus infection. Hepatology. 2011;53:1494–503. doi: 10.1002/hep.24249. [DOI] [PubMed] [Google Scholar]
- 21.Das A, Hoare M, Davies N, Lopes AR, Dunn C, Kennedy PT, et al. Functional skewing of the global CD8 T cell population in chronic hepatitis B virus infection. J Exp Med. 2008;205:2111–24. doi: 10.1084/jem.20072076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chang MH. Hepatitis B vaccination: disease and cancer prevention-a Taiwanese experience. Clin Liver Dis. 2010;14:521–30. doi: 10.1016/j.cld.2010.05.010. [DOI] [PubMed] [Google Scholar]
- 23.Brunetto MR, Moriconi F, Bonino F, Lau GK, Farci P, Yurdaydin C, et al. Hepatitis B virus surface antigen levels: a guide to sustained response to peginterferon alfa-2a in HBeAg-negative chronic hepatitis B. Hepatology. 2009;49:1141–50. doi: 10.1002/hep.22760. [DOI] [PubMed] [Google Scholar]
- 24.Bertoletti A, Gehring A. Therapeutic vaccination and novel strategies to treat chronic HBV infection. Expert Rev Gastroenterol Hepatol. 2009;3:561–9. doi: 10.1586/egh.09.48. [DOI] [PubMed] [Google Scholar]
- 25.Maini MK, Reignat S, Boni C, Ogg GS, King AS, Malacarne F, et al. T cell receptor usage of virus-specific CD8 cells and recognition of viral mutations during acute and persistent hepatitis B virus infection. European J Immunol. 2000;30:3067–78. doi: 10.1002/1521-4141(200011)30:11<3067::AID-IMMU3067>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]