Summary
Mycobacterium tuberculosis (M.tb), which causes tuberculosis, is a host-adapted intracellular pathogen of macrophages. Intracellular pattern recognition receptors in macrophages such as nucleotide-binding oligomerization domain (NOD) proteins regulate pro-inflammatory cytokine production. NOD2-mediated signalling pathways in response to M.tb have been studied primarily in mouse models and cell lines but not in primary human macrophages. Thus we sought to determine the role of NOD2 in regulating cytokine production and growth of virulent M.tb and attenuated Mycobacterium bovis BCG (BCG) in human macrophages. We examined NOD2 expression during monocyte differentiation and observed a marked increase in NOD2 transcript and protein following 2–3 days in culture. Pre-treatment of human monocyte-derived and alveolar macrophages with the NOD2 ligand muramyl dipeptide enhanced production of TNF-α and IL-1β in response to M.tb and BCG in a RIP2-dependent fashion. The NOD2-mediated cytokine response was significantly reduced following knock-down of NOD2 expression by using small interfering RNA (siRNA) in human macrophages. Finally, NOD2 controlled the growth of both M.tb and BCG in human macrophages, whereas controlling only BCG growth in murine macrophages. Together, our results provide evidence that NOD2 is an important intracellular receptor in regulating the host response to M.tb and BCG infection in human macrophages.
Introduction
Tuberculosis (TB) is currently the leading cause of death in HIV-infected persons (WHO, 2006). Multi-drug-resistant Mycobacterium tuberculosis (M.tb) strains have limited our ability to use the current antibiotic supply, and the development of new antibiotics requires a better understanding of the biological mechanisms underlying M.tb growth and survival within the host. Humans are the only known natural host for M.tb and alveolar macrophages (AMs) in the lung microenvironment are the first immune cells that encounter inhaled mycobacteria (Fenton et al., 2005). Thus, knowledge about pathogenic events for M.tb in primary human macrophages is particularly important.
Macrophages are equipped with an array of defence mechanisms to recognize and clear invading pathogens. Several studies demonstrate that M.tb interacts with macrophage membrane receptors such as the mannose receptor (MR), complement receptors, Toll-like receptors (TLR) and scavenger receptors (Fenton et al., 2005). Engagement of specific receptor pathways during phagocytosis of mycobacteria regulates the early host response through defined signalling pathways (Dao et al., 2004; Kang et al., 2005; Torrelles et al., 2008). Among these are the group of cytosolic receptors called nucleotide-binding oligomerization domain (NOD)-like receptors (NLRs) (Franchi et al., 2008) which function as intracellular pattern recognition receptors (PRRs) that recognize pathogens or pathogen secreted molecules (Ting et al., 2010).
There are 23 NLRs which can be divided into four subfamilies depending on the composition of their N-terminal effector domain. For example, those containing a caspase recruitment domain (CARD) as their effector domain are known as the NLRC subfamily and consist of proteins that initiate a pro-inflammatory response by signalling through their CARD domain. NOD2, a member of this subfamily, is found in epithelial cells and antigen presenting cells such as macrophages (Ogura et al., 2001a; Gutierrez et al., 2002; Inohara and Nunez, 2003). NOD2 regulates the production of inflammatory mediators in response to muramyl dipeptide (MDP) which is found in Gram-negative and Gram-positive bacteria and mycobacteria (Girardin et al., 2003; Coulombe et al., 2009). Many studies have found that NOD2 is able to recognize and generate a response to a variety of microorganisms (Opitz et al., 2004; Ferwerda et al., 2005; Kobayashi et al., 2005; Herskovits et al., 2007; Kapetanovic et al., 2007; Hsu et al., 2008; Kim et al., 2008; Till et al., 2008; Deshmukh et al., 2009; Hruz et al., 2009; Loving et al., 2009). NOD2 senses bacteria through its leucine-rich repeats (LRR), then oligomerizes through its NOD domain and is able to recruit receptor interacting protein 2 (RIP2) through an electrostatic interaction of the CARD domain in each protein. This leads to a signalling cascade in which activation and translocation of nuclear factor-κB (NF-κB) into the nucleus occurs in order to transcribe pro-inflammatory cytokines (Inohara and Nunez, 2003; Strober et al., 2006; Abbott et al., 2007; Kufer, 2008; Hu et al., 2010).
Recent studies provide evidence that NOD2 is involved in the host response and pathogenesis of mycobacterial infections. For example, a genome analysis of patients with leprosy found a single-nucleotide polymorphism in the NOD2 gene(Zhang et al., 2009) which is linked to Mycobacterium leprae susceptibility. Furthermore, African American patients with TB have variants in their NOD2 gene rendering them more susceptible to M.tb infection (Austin et al., 2008). In addition, polymorphisms in CARD15, which encodes NOD2, have been linked to inflammatory diseases, including Crohn’s disease (Hugot et al., 2001; Le et al., 2007), whose cause is speculated to be of bacterial origin (Sartor, 2006). The association of Crohn’s disease with Mycobacterium avium subsp. paratuberculosis continues to be reported (Hermon-Taylor, 2009).
The majority of studies to examine the role of NOD2 in the host response to M.tb infection have been performed in mice, murine macrophages or in transfected cell lines (Ferwerda et al., 2005; Gandotra et al., 2007; Yang et al., 2007; Divangahi et al., 2008; Pandey et al., 2009). There are limited reports using either normal peripheral blood mononuclear cells (PBMCs), PBMCs from patients with the NOD2 mutation associated with Crohn’s disease, mixed bronchoalveolar lavage (BAL) leucocytes, and none using human monocyte-derived macrophages (MDMs) (Ferwerda et al., 2005; Lala et al., 2007). A recent report indicates that NOD2 and TLR pathways are non-redundant in the recognition of M.tb, but can synergize to induce a robust pro-inflammatory response (Ferwerda et al., 2005). In addition, it has been shown in bone marrow-derived murine macrophages (BMMs) that RIP2 polyubiquitination occurs during M.tb incubation in a NOD2-dependent manner (Yang et al., 2007). Other studies in mouse macrophages have shown that NOD2 does not have a significant role in controlling M.tb growth during early infection (Gandotra et al., 2007) but may have during late infection (Divangahi et al., 2008). A decrease in pro-inflammatory cytokine production was observed in mouse NOD2 knockout BMMs and naive murine AMs in response to M.tb without affecting intracellular bacterial growth (Gandotra et al., 2007; Divangahi et al., 2008).
Interest in the potential interactions between M.tb and cytosolic PRRs such as NOD2 continues to grow based on recent reports that M.tb secretes virulence factors and immune activators or suppressors through a type VII secretion system (Abdallah et al., 2007; Ganguly et al., 2008; Simeone et al., 2009). Other reports provide evidence that M.tb may be able to escape the phagosome to the cytosol under specific conditions (McDonough et al., 1993; van der Wel et al., 2007).
Based on the known differences between human and mouse macrophages in receptor expression, signalling pathways and innate immune function (Schneemann and Schoedon, 2002; Mestas and Hughes, 2004; Powlesland et al., 2006; Schneemann and Schoeden, 2007), we decided to study NOD2 in human macrophages and in response to M.tb by optimizing an antibody to NOD2 and by accomplishing effective small interfering RNA (siRNA)-mediated knock-down of NOD2 in these cells. We show that NOD2 expression increases during monocyte differentiation. In contrast to previously published work using mouse macrophages, we report that NOD2 is important in controlling both pro-inflammatory cytokine production and the growth of M.tb in human macrophages; whereas NOD2 controls the growth of the related attenuated Mycobacterium bovis BCG (BCG) strain in both human and mouse macrophages.
Results
NOD2 mRNA and protein expression increase as monocytes differentiate into macrophages
The expression of NOD2 in human macrophages has not been studied and there are few reports on NOD2 mRNA expression in human monocytes (Ogura et al., 2001a; Lala et al., 2007). We began our studies by determining the expression of NOD2 during monocyte differentiation to MDMs in an established model by quantitative real-time PCR (qRT-PCR) and Western blot (Henning et al., 2008). Results from qRT-PCR provide evidence that NOD2 transcript levels are increased during the differentiation of monocytes to macrophages. While we saw production of mRNA in day 1 monocytes, the results in Fig. 1A show a significant increase in mRNA at day 3 of differentiation which remains elevated as the cells differentiate further into mature macrophages. Next, we compared the mRNA levels with the amount of protein produced during cell differentiation by Western blotting. Due to the sensitivity and specificity issues in using antibodies specific for human NOD2, we optimized an available commercial antibody (ProSci) with human macrophage lysates. The specificity of this antibody was confirmed by a peptide competition assay (Fig. 1B), an immunoprecipitation (IP) experiment (Fig. 1C) and in siRNA experiments shown later. For IP experiments, proteins in cell lysates from HEK293T cells expressing human NOD2 or HEK293T cells containing the empty vector were immunoprecipitated with NOD2 antibody (Cayman Chemical) and then probed with NOD2 antibody from ProSci. As shown in Fig. 1C, NOD2 antibody specifically binds to the human NOD2 protein expressed in the HEK293T cell line. We were also able to detect NOD2 in human lysates using the antibody available from eBioscience, although with more background (data not shown). Thus, in all subsequent experiments we used the antibody from ProSci. In accordance with the mRNA studies, NOD2 protein levels in cell lysates increased markedly at day 3 of differentiation and remained high on day 5 MDMs (Fig. 1D, upper panel). The densitometric analysis of Western blots from three independent experiments shows that NOD2 protein expression levels increased during cell differentiation (Fig. 1D, lower panel). Finally, our results were confirmed using confocal microscopy where NOD2 protein was easily detected throughout MDMs in a punctuate appearance (Fig. 1E).
Fig. 1.
NOD2 expression increases during monocyte differentiation to macrophages. PBMCs were harvested from Teflon wells each day from day 1 through day 5 and monocytes/MDMs plated on tissue culture plates, and incubated at 37°C/5% CO2 for 2 h. The resultant monocyte/MDM monolayers were either lysed with TRIzol for extraction of total RNA or TN-1 lysis buffer for cell lysate preparation.
A. NOD2 steady-state mRNA levels were measured during monocyte to macrophage differentiation by real-time PCR. Data were normalized to the β actin gene and RCN was determined. Shown are cumulative data obtained from three independent experiments each performed in triplicate (mean ± SEM, *P < 0.05).
B. NOD2 antibody specificity was confirmed by peptide blocking using twofold excess NOD2 peptide (used to generate the NOD2 antibody) and Western blotting using anti-NOD2 antibody. Shown is a representative blot from two independent experiments.
C. HEK293T vector (lane 1) and HEK293T cells expressing NOD2 (lane 2) were lysed and 100 µg of total protein in each case was immunoprecipitated with NOD2 antibody (Cayman Chemicals) or isotype control (IgG1 kappa) and subsequent Western blots of the immunoprecipitants probed with NOD2 antibody (ProSci). Shown is a representative Western blot from two independent experiments.
D. Monocyte/macrophage cell lysates were analysed for NOD2 protein expression by Western blot using NOD2 antibody. The same membrane was re-probed with β actin to verify equal loading of lysate proteins. Shown is a representative Western blot and the bar graph shows the cumulative densitometric analysis of Western blots from three different donors (mean ± SEM, *P < 0.05, **P < 0.005).
E. Day 5 MDM monolayers on coverslips were fixed, permeabilized and stained using either NOD2 antibody or isotype control antibody followed by Alexa Flour 488-conjugated secondary antibody. Cells were then examined by confocal microscopy (Olympus FV1000). Shown is a representative section (63× magnification) of a Z series image from one of three independent experiments performed on duplicate coverslips.
Activation of NOD2 enhances the M.tb- and BCG-mediated pro-inflammatory cytokine response through the NF-κB pathway
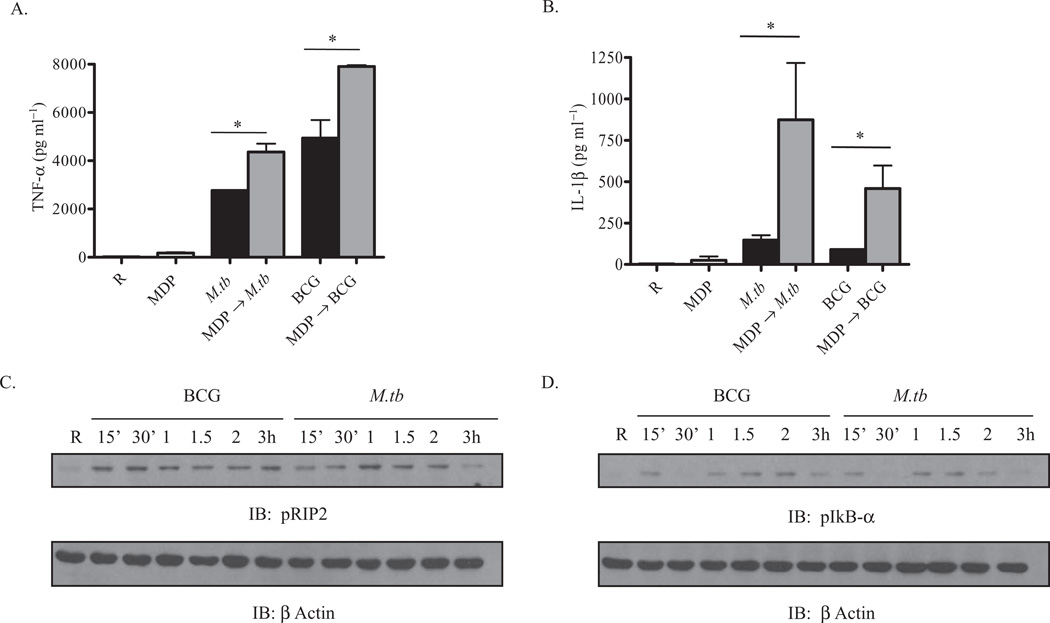
We next investigated whether activation of NOD2 leads to an increase in the M.tb- and BCG-mediated pro-inflammatory cytokine response in macrophages. Incubation of macrophages with MDP, a NOD2 agonist, is known to induce the production of pro-inflammatory cytokines such as IL-1β (Li et al., 2004). A recent report indicates that in some TB patients, NOD2 mRNA is enhanced in PBMCs; furthermore, patients on anti-tuberculosis treatment have enhanced NOD2 levels in leucocytes (Lala et al., 2007). MDMs were pre-incubated with the NOD2 ligand MDP for 90 min followed by incubation with M.tb or BCG as described in Experimental procedures in the presence of MDP. Cell-free culture supernatants were analysed for TNF-α and IL-1β secretion at 24 h. The data show an additive (TNF-α) or synergistic (IL-1β) effect on cytokine production in cells pre-treated with MDP compared with cells incubated with mycobacteria alone (Fig. 2A and B). Thus, our results indicate that pre-engagement of NOD2 by its ligand significantly enhances M.tb- or BCG-mediated pro-inflammatory cytokine production. This effect is likely due to the activation of NF-κB through the phosphorylation of IκBα and its subsequent degradation (Karin and Ben-Neriah, 2000; Yang et al., 2007).
Fig. 2.
The NOD2 ligand MDP enhances M.tb- and BCG-mediated cytokine production in human macrophages, a process involving activation of RIP2 and IkBα.
A and B. MDM monolayers in a 24-well tissue culture plates were pre-incubated with or without MDP (5 µg ml−1) for 90 min and then incubated with M.tb or BCG (moi 5:1) for 2 h, washed three times and repleted with MDP (5 µg ml−1) in RPMI with 1% autologous serum for 24 h. Supernatants were collected and the amounts of TNF-α (A) and IL-1β (B) were measured by ELISA. Shown is the mean ± SD of a representative experiment performed in triplicate (n = 5, *P < 0.05).
C and D. In parallel, MDMs were incubated with M.tb or BCG (moi 5:1) for different time points and cells were lysed with TN-1 lysis buffer for RIP2 (C) and IkBα (D) phosphorylation, and re-probed with β actin as a loading control. Shown is a representative Western blot from three independent experiments.
To further explore the signalling pathway(s) involved, we examined whether M.tb- or BCG-mediated signalling events include those known for the NOD2 pathway. NOD2 engagement leads to recruitment of a serine threonine kinase adapter protein called RIP2 by an electrostatic interaction through their CARD domains, which in turn causes autophosphorylation of RIP2 at serine 176 (Nembrini et al., 2009) in cell lines and murine macrophages. Activated RIP2 phosphorylates inhibitor of kBα (IkBα) resulting in the activation and translocation of NF-κB. MDMs were incubated with M.tb or BCG for different time points and the cell lysates were analysed for activation of RIP2 and IkBα by Western blotting of the phosphorylated proteins. The data show that both M.tb and BCG phosphorylate RIP2 during incubation but with different kinetics and pattern. BCG- and M.tb-mediated phosphorylation of RIP2 peaks at 30 min and 1 h, respectively, and the phosphorylation decreased at 3 h during M.tb incubation (Fig. 2C). M.tb and BCG infection also leads to phosphorylation of serine 32 of IkBα (Fig. 2D) which is known to result in its degradation and subsequent activation of NF-κB. We found that phosphorylation occurs as early as 15 min for both mycobacteria and continues through 2 h for BCG but not M.tb. In addition, there is a decrease at 30 min which can be explained by the degradation of IκBα and recruitment of newly re-synthesized pools of IκBα from the cytoplasm, as previously described (Karin and Ben-Neriah, 2000).
siRNA-mediated knock-down of NOD2 activity in human macrophages leads to loss of its activity
In order to more definitively examine the causal role of NOD2 in controlling the nature of the inflammatory response and growth of mycobacteria in human macrophages, we developed an effective and reliable protocol for knock-down of NOD2 in human macrophages by using NOD2-specific siRNA and Amaxa nucleofector reagent. MDMs were transfected with scramble siRNA or NOD2-specific siRNA under optimized conditions, plated in tissue culture plates, and lysed after 72 h and 96 h. The cell lysates were analysed for NOD2 protein levels by Western blotting. Results shown in Fig. 3A demonstrate the loss of NOD2 protein in MDMs transfected with NOD2-specific siRNA at both time points. These results were confirmed by confocal microscopy (Fig. 3B). Cell enumeration of scramble siRNA- and NOD2 siRNA-treated MDMs evaluated at 72 h post nucleofection by napthol blue black nuclear staining of cells demonstrated no loss of cells on the monolayer in either experimental group (Fig. 3C). There was also no difference in monolayer cell viability as seen by trypan blue exclusion staining (95% viable in both groups, data not shown). An assay was performed in order to verify that NOD2 protein knock-down led to loss of its function. Scramble siRNA- and NOD2 siRNA-transfected macrophages were treated with MDP and the cell-free culture supernatants were analysed for TNF-α production (Fig. 3D). NOD2 knock-down led to a significant reduction in TNF-α production in response to MDP. We also tested the specificity of NOD2 knock-down by stimulating the NOD2 siRNA-transfected MDMs with the NOD1 ligand H-Ala-d-γ-Glu-diaminopimelic acid (iE-DAP) and measured TNF-α levels in the supernatant. There was no significant change in NOD1-mediated TNF-α production between scramble siRNA- and NOD2 siRNA-transfected cells providing evidence that NOD2 knock-down by siRNA did not affect the activity of NOD1, another NLR family member (Fig. 3D). We also found that expression of the effector proteins TLR2, Syk and total RIP2 is unaffected in scramble siRNA- and NOD2 siRNA-transfected MDMs during M.tb and BCG infection (Fig. 3E).
Fig. 3.
Loss of NOD2 activity in human macrophages by siRNA knock-down. MDMs were transfected with either 368 nM scramble siRNA or NOD2 siRNA using the Amaxa nucleofector.
A. The transfected macrophages were plated in RPMI containing 20% autologous serum and incubated for the indicated time points (72 h and 96 h). At each time point macrophages were lysed and NOD2 or the loading control β actin protein levels were examined by Western blot (representative blot from n = 3).
B. NOD2 or scramble siRNA-transfected MDM monolayers on coverslips were incubated for 72 h, washed, fixed, permeabilized and stained using either NOD2 antibody or isotype control antibody followed by Alexa Flour 488-conjugated secondary antibody. Cells were then examined by confocal microscopy. Shown is a representative micrograph from the Z series (63× magnification for isotype; 126× magnification for scramble/NOD2 siRNA, n = 3).
C. Cell numbers were assessed 72 h post transfection to ensure equal numbers of cells between scramble siRNA- and NOD2 siRNA-transfected cells. Nuclei in cell lysates were counted on a haemacytometer after nuclei were stained with Napthol blue black. Shown are cumulative data obtained from three independent experiments each performed in triplicate (mean ± SEM).
D. Scramble siRNA- or NOD2 siRNA-transfected MDM monolayers in culture for 72 h were treated with MDP (5 µg ml−1) or DAP (10 µg ml−1) for 24 h. Cell supernatants were analysed for TNF-α by ELISA. Shown is a representative experiment performed in triplicate (mean ± SD, n = 2, **P < 0.005; NS P = 0.1519).
E. Scramble siRNA- or NOD2 siRNA-transfected MDM monolayers were incubated with M.tb or BCG (moi 5:1) for the indicated time points (15 min and 2 h) and cells were lysed with TN-1 buffer. TLR2, Syk and RIP2 were analysed by Western blot. Shown is a representative experiment of two independent experiments.
NOD2 regulates the macrophage TNF-α and IL-1β cytokine response to M.tb and BCG
We next investigated whether NOD2 regulates the production of pro-inflammatory cytokines during mycobacterial infection which can control the host defence response through autocrine and paracrine functions (Flesch and Kaufmann, 1993). Results shown in Fig. 2 had already indicated a potential link between NOD2 and the cytokine response to mycobacteria. Scramble and NOD2 siRNA-transfected MDM monolayers were incubated for 72 h to achieve the maximum NOD2 knock-down and subsequently incubated with M.tb or BCG (moi 5:1) for 24 h. The cell-free culture supernatants were analysed for cytokine levels by ELISA. We found that NOD2 deficiency leads to a significant decrease in the production of TNF-α and IL-1β in response to M.tb and BCG (Fig. 4A and B). Next, we examined RIP2 activation in NOD2 knock-down MDMs in response to M.tb and BCG infection. NOD2-deficient MDM monolayers were incubated with M.tb or BCG for short time periods and cell lysates were analysed for RIP2 phosphorylation by Western blot. Our results show that NOD2 siRNA-transfected MDMs have a partial but reproducible decrease in RIP2 phosphorylation (Fig. 4C: upper panel, Western blot; lower panel, band densitometry), particularly at 2 h, compared with scramble siRNA-transfected MDMs in the case of both M.tb and BCG. The partial reduction of RIP2 phosphorylation may be explained by the fact that the mycobacteria also produce NOD1 agonists that activate RIP2 independently of NOD2. The smaller reduction of RIP2 phosphorylation in NOD2 knock-down cells incubated with M.tb compared with BCG may relate to the amount of the NOD1 agonist DAP in the former (Phiet et al., 1976).
Fig. 4.
NOD2 controls M.tb- and BCG-mediated cytokine production and RIP2 activation in human macrophages.
A and B. Scramble or NOD2 siRNA-transfected MDM monolayers were incubated with M.tb or BCG (moi 5:1) for 2 h, washed three times with warm RPMI and repleted in 1% autologous serum for 24 h. Cell culture supernatants were analysed for TNF-α (A) and IL-1β (B) production by ELISA. The graphs shown are from a representative experiment of n = 3. *P < 0.05 or **P < 0.005.
C. Scramble- or NOD2 siRNA-transfected MDM monolayers were incubated with M.tb or BCG (moi 5:1) for the indicated time points (15 min and 2 h) and cells were lysed with TN-1 buffer. NOD2-dependent RIP2 phosphorylation was analysed by Western blot of cell lysates from scramble- and NOD2-deficient MDMs (upper panel). Shown is a representative experiment of three independent experiments. Densitometry of the band intensities in this experiment is shown as a bar graph (lower panel).
Expression of NOD2 in human AMs and its role in regulating the production of TNF-α and IL-1β in response to M.tb
A recent report showed the presence of NOD2 transcripts in human alveolar lavage cells, but to date there is no evidence for the presence of NOD2 protein in AMs (Lala et al., 2007). Therefore we examined whether NOD2 protein is expressed in human AMs. Cell lysates were prepared from MDMs and AMs, the latter were isolated from healthy donors and both cell types were analysed for NOD2 expression by Western blot. Results shown in Fig. 5A demonstrate that AMs express a detectable amount of NOD2 protein but the level is less than that in an equivalent number of MDMs. To further examine the potential involvement of NOD2 in the production of inflammatory cytokines in response to M.tb, we incubated AMs with or without MDP and then with M.tb (moi 1:1) for 24 h. The cell culture supernatants were analysed for TNF-α and IL-1β production. We found that MDP or M.tb treatment induces the production of TNF-α whereas only M.tb induces the production of IL-1β, and that the levels of both cytokines were further enhanced by the addition of both MDP and M.tb (Fig. 5B and C). The magnitude of the responses was relatively low when compared with the response seen with MDMs (Fig. 2). Together, these results provide evidence that AMs express functional NOD2 protein in the lung microenvironment, albeit with lower activity than in MDMs.
Fig. 5.
M.tb-mediated cytokine production in human AMs treated with MDP.
A. The NOD2 expression level in healthy donor AMs was compared with day 5 MDMs using protein-matched cell lysates by Western blot with a NOD2-specific antibody and β actin as a loading control. Shown is a representative blot of two independent experiments.
B and C. AMs were pre-incubated with MDP (5 µg ml−1) or media alone for 90 min and then incubated with M.tb (moi 1:1) for 2 h, washed three times and repleted with MDP in 1% autologous serum for 24 h. Culture supernatants were measured for TNF-α (B) and IL-1β (C) production by ELISA. Shown is a representative experiment of the mean ± SD of triplicate wells in each test group (n = 2, *P < 0.05).
NOD2 controls the intracellular growth of M.tb and BCG in human macrophages
We next focused our studies on determining the role of NOD2 in controlling the growth of mycobacteria in human macrophages. MDMs were transfected with scramble or NOD2 siRNA and after 72 h of transfection, macrophages were incubated with M.tb or BCG (moi 5:1) for 24 h. Experiments were performed for 24 h due to the fact that NOD2 protein levels in macrophages began to reappear after 120 h of knock-down. Intracellular growth of M.tb and BCG was determined by a colony-forming unit (cfu) assay (Olakanmi et al., 2000). The results in Fig. 6A show that silencing of NOD2 in macrophages significantly enhances the growth of M.tb (84.6 ± 8.0%, n = 3). A similar result was observed for BCG growth in Fig. 6B (108.4 ± 30.4%, n = 5). Western blotting confirmed NOD2 knock-down in each experiment (Fig. 6C). We next determined whether the increase in mycobacterial growth at 24 h was the result of increased cell association (both bacterial attachment and phagocytosis). We compared the association of bacteria with scramble and NOD2 siRNA-transfected MDMs by phase-contrast and fluorescence microscopy after 2 h infection. Our results show that bacterial association is not altered by NOD2 siRNA transfection (Fig. 6D and E). Thus, these results indicate that NOD2 plays a role in regulating the early host defence response during infection of both M.tb and BCG.
Fig. 6.
NOD2 controls the intracellular growth of M.tb and BCG in human macrophages.
A and B. Scramble or NOD2 siRNA-transfected MDMs in monolayer culture for 72 h were incubated with M.tb or BCG (moi 5:1) for 2 h, washed three times with warm RPMI, repleted in 1% autologous serum for 24 h and lysed to analyse cfu for (A) M.tb growth and (B) BCG growth. Shown is a representative experiment (mean ± SD of triplicate wells in each test group) for each bacterium (for M.tb n = 3 and for BCG n = 5; *P < 0.05).
C. Western blot in the experiment shown confirming NOD2 knock-down.
D and E. Mycobacterial association with macrophages was assessed by phase-contrast and fluorescent microscopy. Scramble or NOD2 siRNA-transfected MDMs were plated on coverslips for 72 h and then incubated with M.tb (D) or BCG (E) for 2 h (moi 10:1). Cells were then washed, fixed in 10% formalin, mycobacteria were stained with auramine-rhodamine and enumerated as bacteria per macrophage. Cumulative data from three independent experiments, each performed in triplicate, are shown as fold change relative to the scramble siRNA condition (mean ± SEM).
Effects of NOD2 on M.tb and BCG growth in mouse macrophages and on the cytokine response to infection
Previous studies have reported that NOD2 is not critical for controlling the growth of M.tb and BCG in mouse macrophages (Gandotra et al., 2007; Divangahi et al., 2008). Based on our results in human macrophages, we elected to examine the host response to these mycobacteria in mouse macrophage experiments. We reproduced the published finding that NOD2 does not control the growth of M.tb in mouse macrophages using wild-type (WT) and NOD2−/− BMMs (Fig. 7A). In contrast to the published work with BCG that analysed growth in naive murine AMs at 48 h (Divangahi et al., 2008), our data show that NOD2 is important in controlling the growth of BCG at the later time points of 72 h and 168 h in WT and NOD2−/− BMMs (Fig. 7B). We also examined the cell association of bacteria with WT and NOD2−/− BMMs by microscopy and found no difference with M.tb and only a small difference with BCG (Fig. 7C and D). Finally, consistent with the literature, we found a reduction in the pro-inflammatory cytokine IL-1β in response to M.tb and BCG at 24 h, 72 h and 168 h in NOD2−/− BMMs (Fig. 7E).
Fig. 7.
Effect of NOD2 on M.tb and BCG growth and cytokine response in mouse WT and NOD2−/− BMMs.
A and B. BMMs from WT and NOD2−/− mice were plated in 24-well tissue culture plates and incubated with M.tb (1:1) or BCG (2.5:1) for 2 h, washed three times, and immediately lysed or incubated for different time points (2 h, 24 h, 72 h and 168 h) prior to lysis for assessment of cfu for M.tb (A) and BCG (B). Shown is a representative experiment for each bacterium (mean ± variance of duplicate wells in each test group, n = 2).
C and D. Cell association of M.tb (C) and BCG (D) with WT and NOD2−/− BMMs was analysed at 2 h post infection by microscopy. Cumulative data from three independent experiments, each performed in triplicate, are shown as fold change relative to WT condition (mean ± SEM, *P < 0.05).
E. The cytokine response IL-1β was analysed by ELISA in culture supernatants harvested from M.tb- and BCG-infected WT and NOD2−/− BMMs. The data shown are from a representative experiment (mean ± SD, n = 2, with four test results in each group, *P < 0.05; **P < 0.005, ***P < 0.0005).
Discussion
Macrophages play a critical role in the recognition and removal of pathogens through various PRRs, including intracellular receptors such as NLR family CARD-containing domain 4 (NLRC4), baculoviral IAP repeat-containing protein 1 (NAIP), NOD1 and NOD2. Studies on host recognition of pathogens by human macrophages have demonstrated the importance of transmembrane receptors; however, the field is relatively lacking in defining the role of intracellular receptors in these cells. NOD2 is an intracellular PRR that senses bacteria through its LRR domain and induces a pro-inflammatory response. While this inflammatory response can activate the macrophage, it also allows for the recruitment and activation of other immune cells to the site of infection. NOD2 has been shown to serve as a microbial sensor and activator of the pro-inflammatory response to several pathogens, including M.tb (Opitz et al., 2004; Ferwerda et al., 2005; Kobayashi et al., 2005; Gandotra et al., 2007; Herskovits et al., 2007; Kapetanovic et al., 2007; Divangahi et al., 2008). In the case of Helicobacter pylori, it is thought that the bacterium is able to use a secretion system to secrete peptidoglycan fragments that are then recognized by NOD2 in the cytosol (Franchi et al., 2008). Since M.tb is an intracellular pathogen, and known to secrete virulence factors, it is necessary to understand the role of intracellular receptors and immune functions in human macrophages with regards to this pathogen. Demonstrating a role for NOD2 can lead to the development of new methods for targeting this host defence molecule/pathway for therapy in the case of M.tb.
The presence of NOD2 mRNA has only been reported in monocytes (Ogura et al., 2001a). Thus, we initiated studies of NOD2 mRNA transcript levels as monocytes differentiate into macrophages using an established in vitro model. Our results indicate that NOD2 steady-state mRNA transcripts increase as monocytes differentiate into macrophages, specifically on day 3 of differentiation. This result is similar to our previously published data on a membrane PRR that recognizes M.tb, the MR, whose mRNA transcript levels are also enhanced in macrophages when compared with monocytes. On the other hand, TLR2 and TLR4 expression follows a different pattern on these cells (Henning et al., 2008).
Since mRNA levels do not always correlate with protein expression, we elected to explore NOD2 protein expression in human macrophages compared with monocytes. Studies in the field have been limited to assaying for mRNA levels in primary human cells; whereas protein expression has been studied in cell lines by flag tagging NOD2 (Barnich et al., 2005; Legrand-Poels et al., 2007). Thus, a major contribution to the field by the current work is the optimization of available antibodies for characterization of NOD2 protein expression in primary human monocytes/macrophages by Western blot and confocal microscopy. Our findings demonstrate that NOD2 protein is expressed in human macrophages and levels increase during monocyte to macrophage differentiation, suggesting a particularly important role for NOD2 in human macrophages. Our microscopy results show NOD2 protein in a punctuate pattern in cells, suggesting the possibility that it resides within organelles or in complexes present in the cytoplasm.
We also detected NOD2 protein in human AMs from healthy donors. An interesting finding in our work is that AMs appear to express less of NOD2 protein than MDMs. It is possible that reduced NOD2 levels in AMs represent another phenotypic marker of alternatively activated macrophages of which AMs are a prototype and consequently lead to less effective killing of M.tb on first contact during the innate immune response. On the other hand, later during TB disease, NOD2 may play an important role in host defence since NOD2 mRNA levels are increased in patients with TB disease (Lala et al., 2007).
NOD2 is engaged in response to pathogens which leads to the production of pro-inflammatory cytokines. In cell lines expressing NOD2, incubation with M.tb enhances NF-κB activation (Ferwerda et al., 2005; Coulombe et al., 2009). We found that pre-stimulation of human macrophages (both MDMs and AMs) with the NOD2 ligand MDP followed by M.tb or BCG incubation caused a significant increase in TNF-α and IL-1β production. Although the addition of MDP is not the paradigm for human infection, the result provided evidence for the potential involvement of NOD2 in augmenting the cytokine response to M.tb and BCG. This result raised the question as to what are the major downstream signalling kinases activated in response to M.tb or BCG. RIP2 is a kinase that autophosphorylates at serine 176, and its kinase activity is necessary for NOD2-mediated pro-inflammatory cytokine production (Nembrini et al., 2009). We found that M.tb and BCG both activate RIP2 by phosphorylation at serine 176 in a NOD2-dependent manner; however, the kinetics and pattern of activation were different for these two bacteria. RIP2 phosphorylation peaked earlier for BCG (30 min) and continued throughout the time-course; whereas RIP2 phosphorylation peaked at 1 h for M.tb and the level of phosphorylation decreased by 3 h. Consistent with this we observed that BCG continues to activate downstream IκBα [thereby enabling the transcriptional activity of NF-κB and the resultant upregulation of expression of macrophage pro-inflammatory response genes (Karin and Ben-Neriah, 2000)] through 2 h whereas activation of IκBα decreases for M.tb at this time point. Thus, it appears that M.tb but not BCG can downregulate RIP2 and IκBα activation perhaps as a mechanism to dampen the innate immune response. One possibility for this downregulation is that M.tb possesses virulence factors that affect this pathway that are not present in BCG such as those present in the Region of Difference-1 (RD1) which is responsible for the production of several virulence-associated proteins in M.tb (Abdallah et al., 2007). For example, culture filtrate protein-10 kDa (CFP-10) and early secreted antigenic target-6 kDa (ESAT-6) in M.tb but not in BCG have been shown to inhibit lipopolysaccharide-induced NF-κB transactivation by inhibiting reactive oxygen species production (Ganguly et al., 2008). Apart from RIP2, recent work from our laboratory has identified a PPARγ-mediated pathway for M.tb that is not operative for BCG in human macrophages (Rajaram et al., 2010).
To more directly assess a causal role for NOD2 in regulating the inflammatory response to mycobacteria in primary human macrophages, we developed a reliable knock-down protocol for NOD2 that is specific and does not appreciably affect the viability of the cells on the monolayer. To date, studies on the role of NOD2 in response to different pathogens in primary human cells have relied on either expressing the NOD2 allele with the Crohn’s disease mutation in cell lines (Ogura et al., 2001b) or by obtaining cells from individuals homozygous for the NOD2 mutation, which was shown to render this protein reduced in activity or non-functional (Inohara et al., 2003). Obtaining cells from Crohn’s disease patients with this mutation is difficult and the results may not be consistent among patients. Also, since these patients have a mutation in this protein, their cells may be primed in other ways that may alter the immunological response. The accomplished knock-down of NOD2 in human macrophages is an important advance enabling the study of NOD2 more comprehensively in primary monocytes/macrophages.
Our results are consistent with those found with mouse macrophages with regards to the role of NOD2 in controlling the inflammatory response to mycobacteria. Gandotra et al. found that TNF-α and IL-12 production is decreased in NOD2 knockout BMMs in response to M.tb (Gandotra et al., 2007) and Divangahi et al. found that these cytokines are decreased in NOD2 knockout naive alveolar mouse macrophages in response to BCG (Divangahi et al., 2008). We observed that both TNF-α and IL-1β production are significantly reduced in NOD2-deficient human macrophages and found a decrease in RIP2 phosphorylation in response to M.tb and BCG infection. It is noteworthy that RIP2 activity is only partially reduced rather than abolished by the knock-down of NOD2, which may be due to the activation of RIP2 by other intracellular receptors. For example, NOD1 is involved in the activation of RIP2 through the CARD–CARD interaction (Nembrini et al., 2009).
The role of NOD2 in IL-1β production during M.tb and BCG infection is of interest. A recent publication reports that PBMCs from Crohn’s disease patients that are homozygous for the 3020insC NOD2 mutation exhibit decreased production of IL-1β in response to M.tb (Kleinnijenhuis et al., 2009). The mechanism for the production of mature IL-1β in response to M.tb involved the activation of ERK, p38 and RIP2 following recognition by TLR2/TLR6 and NOD2 in PBMCs; however, the mechanism was not explored in human macrophages. In PBMCs, NOD2 is reported to have a dual effect on pro-IL-1β mRNA transcription and secretion of mature IL-1β (Ferwerda et al., 2008). Our preliminary observations suggest that during M.tb infection, NOD2 knock-down macrophages demonstrate either no effect or an increase in pro-IL-1β transcription. On the other hand NOD2 does appear to play a role in caspase-1 activation during M.tb infection (M. Brooks et al., unpubl. data). In our results in which we added MDP to untransfected macrophages followed by M.tb infection, we observed an additive effect on TNF-α secretion, but a synergistic effect on IL-1β secretion (Figs 2 and 5). We speculate that the differential response of TNF-α and IL-1β may be explained by NOD2 being relatively more important in the release of mature IL-1β due to a role in processing pro-IL-1β through caspase-1. The relationship between NOD2 and caspase-1 has been reported (Pan et al., 2007; Hsu et al., 2008).
Previous studies have reported that NOD2 knockout in mouse macrophages has no effect on controlling M.tb growth (Gandotra et al., 2007; Divangahi et al., 2008). In contrast, we demonstrate here for the first time that NOD2 plays a role in controlling intramacrophage growth of M.tb and BCG in human macrophages. The difference in the role of NOD2 in controlling mycobacterial growth is validated by doing a direct side by side comparison of mycobacterial growth in NOD2-deficient human macrophages and macrophages from NOD2 knockout mice. This difference can be generally explained by the growing list of differences seen in the innate immune response of human versus mouse macrophages (Bogdan, 2001; Mestas and Hughes, 2004; Boot et al., 2005; Reginato and Olsen, 2007; Rigamonti et al., 2008; Gavrilin et al., 2009; Amer, 2010). It is relevant that the NOD2 knockout mouse does not show any signs of abnormalities in the intestinal tract, which is inconsistent with the NOD2 mutations leading to Crohn’s disease and inflammatory bowel disease in humans (Pauleau and Murray, 2003). In addition, differences in promoter structure between humans and mice indicate divergent mechanisms in NOD2 regulation (Hu et al., 2010).
There have been reports over time providing evidence that M.tb can translocate into the cytosol of macrophages under defined conditions (Leake et al., 1984; Myrvik et al., 1984; McDonough et al., 1993; van der Wel et al., 2007) and also that mycobacterial phagosomes are porous (Teitelbaum et al., 1999). Nonetheless, the weight of the evidence to date is that mycobacteria replicate in phagosomes in human and mouse macrophages. In the context of the current work, we speculate that M.tb interacts with NOD2 by either secreting virulence factors from the phagosome into the cytosol via a secretion system(s) (Abdallah et al., 2007; Pandey et al., 2009), or possibly translocates proteins across the phagosome through the action of a yet to be defined flippase. Alternatively, a NOD2–RIP2 complex has been reported to signal from the cell membrane along with the need for NOD2 to be present at the membrane of intestinal epithelial cells for signalling to occur (Barnich et al., 2005; Lecine et al., 2007). These reports raise the possibility that NOD2 may localize with the phagosome of M.tb leading to engagement of this cytosolic PRR during residence of the bacterium within the phagosome.
The mechanism(s) involved in the recognition of NOD2 by M.tb and BCG may differ since BCG lacks the ESX-1 secretion system and is reported to be unable to stimulate NOD2-dependent RIP2 polyubiquitination in murine macrophages (Pandey et al., 2009). Nonetheless, BCG does generate TNF-α in a NOD2-dependent manner (this report and Coulombe et al., 2009). Of relevance, mycobacteria contain the N-acetyl muramic acid hydroxylase, NamH, which glycosylates MDP causing it to be a more potent stimulator of the immune response via NOD2 suggesting that NOD2 may be more tuned at sensing mycobacterial infections in generating an immune response (Coulombe et al., 2009).
In summary, here we report that NOD2 is present and functional in human macrophages, including AMs. We provide evidence that NOD2 plays a role in controlling the growth of M.tb and BCG in human macrophages along with regulating the nature of the inflammatory response. The role for NOD2 differs with respect to growth of M.tb and BCG in human and mouse macrophages. Finally, the assays established and described in this report now enable a more thorough evaluation of the role of NOD2 in regulating the innate immune response of human macrophages to mycobacteria and other infectious agents.
Experimental procedures
Buffers and reagents
Dulbecco’s PBS with and without Ca2+ and Mg2+ ions, Iscoves, FBS, TRIzol and RPMI 1640 medium with l-glutamine were purchased from Invitrogen (Invitrogen Life Technologies). RHH medium [RPMI 1640 plus 10 mM HEPES (Invitrogen) plus 0.4% human serum albumin (CSL Behring LLC)] was used for cell culture experiments. N-Acetylmuramyl-l-alanyl-d-isoglutamine hydrate (MDP; Sigma-Aldrich) and H-Ala-d-γ-Glu-diaminopimelic acid (iE-DAP; AnaSpec) were used for functional assays involving ELISAs. 7H11 agar was prepared with Bacto Middlelebrook 7H11 agar, oleic acid-albumin-dextrose-catalase enrichment medium and glycerol (Difco Laboratories).
Antibodies
Unconjugated rabbit anti-human NOD2 was purchased from ProSci and another anti-human NOD2 antibody was obtained from Dr Núñez’s laboratory and available by eBioscience. Syk (N-19), TLR2, β actin, goat anti-rabbit IgG-HRP and donkey anti-goat IgG-HRP antibodies were purchased from Santa Cruz Biotechnology. The aforementioned antibodies were used for Western blot. The NOD2 antibody from ProSci, Alexa Fluor 488-conjugated goat anti-rabbit IgG (Molecular Probes and Invitrogen) and normal rabbit IgG (Santa Cruz Biotechnology) were used for confocal microscopy experiments. Monoclonal anti-human NOD2 (Cayman Chemical) and anti-mouse IgG1 kappa (Abcam) were used for IP experiments. Antibodies specific for phospho-RIP2 (Ser176) and phospho-IκBα (Ser32) were purchased from Cell Signaling Technology.
Bacterial strains and culture
Lyophilized M.tb H37Rv (ATCC #25618) and BCG (ATCC #35734) were obtained from American Tissue Culture Collection, reconstituted and used as described (Schlesinger et al., 1990). The concentration of bacteria (1–2 × 108 bacteria ml−1) and the degree of clumping (≤ 10%) were determined by counting in a Petroff–Hausser chamber. Bacteria prepared in this fashion are ≥ 90% viable by cfu assay.
Isolation and culture of human monocytes and macrophages
Blood obtained from healthy, Purified Protein Derivative negative human volunteers using an approved protocol by The Ohio State University Institutional Review Board (IRB) was processed as described (Horwitz and Silverstein, 1980). Briefly, PBMCs were isolated from heparinized blood on a Ficoll-Paque cushion (Amersham Biosciences/GE Healthcare) and cultured in Teflon wells (Savillex) for 1 (monocytes) through 5 (MDMs) days in the presence of RPMI 1640 medium containing 20% autologous serum (2.0 × 106 PBMCs ml−1) at 37°C/5% CO2 (Beharka et al., 2002). Human AMs were isolated from BALs of healthy human donors as previously described (Gaynor et al., 1995) using an approved protocol by The Ohio State University IRB. Briefly, the BAL was centrifuged (200 g, 4°C, 10 min), supernatant was removed, and the pellet resuspended in RPMI and washed two more times with RPMI. AMs were plated in tissue culture plates with 10% human serum supplemented with penicillin (1000 U ml−1), incubated in CO2 incubator at 37°C for 2 h, washed with warm RPMI to remove non-adherent cells and penicillin, and used for experiments.
Mice and mouse macrophages
Wild-type C57BL/6 and NOD2−/− mice were backcrossed to C57BL/6 background for 10 generations (Kobayashi et al., 2005). BMMs were prepared from femurs of 5- to 8-week-old mice as previously described (Brieland et al., 1994; Amer et al., 2006).
RNA isolation
One- to 5-day-old PBMCs in Teflon wells were harvested and mononuclear phagocytes adhered to a six-well tissue culture plate with 10% autologous serum (8 × 106 PBMCs ml−1). After washing away lymphocytes, the monocytes or MDMs (8 × 105 cells) were lysed in TRIzol and total RNA was isolated by using the Qiagen RNAeasy column method. The Experion (Bio-Rad) was used to determine the quality and quantity of RNA samples.
Gene expression studies by qRT-PCR
RNA (100 ng) was reverse transcribed to cDNA by reverse transcriptase enzyme (SuperScript II, Invitrogen) and qRT-PCR was performed using a human NOD2 TaqMan gene expression kit (Applied Biosystems). NOD2 amplification was normalized to β actin as a housekeeping gene (ΔCt). Relative copy number (RCN) and fold change were determined. RCN was calculated as follows: RCN = E−ΔCt × 100, where E is the efficiency (2 = 100% efficiency) and Ct = Ct (target) − Ct (reference) (Gavrilin et al., 2006). Fold change was calculated from RCN compared with day 1. Triplicate samples were analysed in duplicate wells in each experiment.
Confocal microscopy
Untransfected MDMs or transfected (scramble siRNA or NOD2 siRNA) MDMs (2 × 105) were adhered to a glass coverslip in a 24-well tissue culture plate for 2 h at 37°C and washed to remove lymphocytes. The cells were washed, fixed with 2% paraformaldehyde (10 min at room temperature), permeabilized by treatment with 100% methanol for 4 min, washed and then incubated overnight in 10% non-fat milk in PBS as blocking reagent. The cells were then incubated with NOD2 (2.5 µg ml−1) or the appropriate isotype (2.5 µg ml−1) control antibody for 1 h at room temperature, washed with blocking reagent, and counterstained with an Alexa Fluor 488-conjugated secondary antibody (1:500 dilution) for 1 h at room temperature. Nuclei were labelled with 0.1 µg ml−1 of the DNA stain 4,6-diamidino-2-phenylindole (DAPI; Invitrogen/Molecular Probes) in PBS for 5 min at room temperature. The cells were next washed with PBS and coverslips were mounted on glass slides. Slides were viewed using an Olympus Flowview 1000 Laser Scanning Confocal microscope.
Transfection of MDMs
PBMCs were transfected with Accell scramble siRNA or NOD2 siRNA purchased from Thermo Scientific Dharmacon RNAi technologies that targeted the sequence CUUUAGGAUGUACAGUUA (368 nM) using the Amaxa Nucleofector (Lonza Group). Briefly, PBMCs (1 × 107) were resuspended in 100 µl of nucleofector solution followed by the addition of 5 µg ml−1 scramble siRNA or NOD2 siRNA, incubated at room temperature for 5 min and nucleofected according to the manufacturer’s instructions. PBMCs were then seeded on 12-well plates containing 1.0 ml of RPMI supplemented with 10% autologous serum and incubated for 2 h at 37°C and 5% CO2. After 2 h, adhered transfected cells (MDMs) were washed and repleted with warm RPMI containing 20% autologous serum for 72 h. Transfected cells were used for subsequent experiments, such as cfu assays, cytokine assays, confocal microscopy, cell enumeration assays and Western blotting. Assessment of monolayer density in experiments was performed as described below.
Macrophage stimulation/infection, cell lysis, immunoprecipitation and Western blotting
Day 5 MDMs or MDMs transfected with scramble siRNA or NOD2 siRNA (2.0 × 105) were incubated with M.tb or BCG (moi 5:1; triplicate wells) in RHH for 30 min at 37°C in 5% CO2 on a platform shaker for equal dispersion of bacteria followed by an additional incubation for 90 min without shaking. The cells were then washed three times with warm RPMI, repleted with RPMI containing 1% human autologous serum and incubated for 24 h at which time supernatants were collected for ELISAs (see below). For experiments using the NOD2 ligand MDP, 2.0 × 105 day 5 MDMs were adhered to a 24-well plate for 2 h at 37°C in 5% CO2, washed to remove lymphocytes and then incubated with MDP (5 µg ml−1) for 90 min. Cells were then incubated with bacteria as described above (2 h total), washed and repleted with MDP in 1% autologous serum for 24 h. Cell culture supernatants were harvested and used for ELISAs. The monolayers were washed once with PBS and lysed in TN-1 lysis buffer [50 mM Tris (pH 8.0), 10 mM EDTA, 10 mM Na4PO7, 10 mM NaF, 1% Triton X-100, 125 mM NaCl, 10 mM Na3VO4, 10 µg ml−1 aprotinin and 10 µg ml−1 leupeptin], incubated on ice for 10 min and then centrifuged at 17 949 g for 10 min at 4°C to remove cell debris (Rajaram et al., 2006). For infections, MDM monolayers were incubated with M.tb or BCG in RHH for 15 min, 30 min, 1 h, 1.5 h, 2 h and 3 h (moi 5:1). At each time point, cells were washed with PBS and lysed as above. For NOD2 knock-down assessment, lysates were collected as above at 72 h and 96 h post knock-down. Protein concentrations of the cleared lysates were measured using the BCA-protein assay kit (Pierce). Proteins were separated by SDS-PAGE and analysed by Western blot by probing with the primary and secondary antibody of interest and development using ECL (Amersham Biosciences). For the IP experiment whole-cell lysates were collected from HEK293T (empty vector) and NOD2-expressing HEK293T cells. Equal amounts of protein were incubated overnight with NOD2 antibody (Cayman Chemicals) or control IgG1 kappa. Then cells were incubated for 2 h with Protein G agarose beads (Invitrogen), washed, pellets boiled in Laemmli buffer and loaded on a 7.5% SDS-PAGE gel, and Western blot was performed using a NOD2 antibody (ProSci). The protein band intensities were measured using the online ImageJ software provided by the NIH. Background intensity was subtracted from each sample and then normalized to β-actin. Fold change was determined as follows: (treated sample band intensity)/(untreated sample band intensity).
ELISAs
For MDM experiments, cell-free culture supernatants were collected at 24 h and used to measure TNF-α and IL-1β by ELISA (R&D Systems). For mouse macrophage studies, WT and NOD2−/− macrophage monolayers were incubated with serum-opsonized mycobacteria (M.tb moi 1:1 and BCG moi 2.5:1) in the same manner as described for MDMs. Cell-free culture supernatants were collected at 24 h, 72 h and 168 h and measured for IL-1β by ELISA (BD Biosciences).
M.tb and BCG intracellular growth assays in macrophages
A cfu assay using infected macrophage lysates was performed as described (Olakanmi et al., 2000). Briefly, scramble siRNA- or NOD2 siRNA-transfected MDM monolayers were incubated with M.tb or BCG (moi 5:1; triplicate wells) in RHH for 30 min at 37°C in 5% CO2 on a platform shaker for equal dispersion of bacteria and for an additional 90 min stationary. The cells were then washed three times with warm RPMI and either lysed or repleted with RPMI containing 1% human autologous serum and incubated for 2 h or 24 h. The supernatants and MDM monolayers were then processed for ELISA or enumeration of cfu respectively (triplicate samples in each test group). The number of colonies was counted after 4 weeks and data analysed. For mouse macrophage experiments, WT and NOD2−/− macrophages were incubated with serum-opsonized mycobacteria (M.tb moi 1:1 and BCG moi 2.5:1) in Iscoves plus 10% FBS using the same incubation protocol as for MDMs and then lysed at 2 h, 24 h, 72 h and 168 h.
M.tb and BCG cell association assay with macrophages
Scramble siRNA- or NOD2 siRNA-transfected MDM monolayers (2.0 × 105), or WT or NOD2−/− mouse macrophages monolayers (2.5 × 105) were incubated with M.tb or BCG (moi 10:1; triplicate wells) in RHH for 30 min at 37°C in 5% CO2 on a platform shaker for equal dispersion of bacteria and then for an additional 90 min without shaking. The cells were then washed three times with warm RPMI and fixed with 10% formalin for 10 min at room temperature.
Coverslips were then washed three times with PBS, dried and cell-associated M.tb or BCG was stained with auraminerhodamine (BD Biosciences). Three hundred consecutive macrophages/coverslip/test group were counted using phase-contrast and fluorescence microscopy (Schlesinger, 1993).
Cell enumeration of NOD2 siRNA-transfected MDMs in monolayer culture
Scramble siRNA- and NOD2 siRNA-transfected MDMs were plated in 24-well plates and after 2 h, washed and repleted with 20% autologous serum for 72 h. Cells were then washed one time with PBS and treated with 1% Cetavlon in 0.1 M citric acid with 0.05% Napthol blue black (Sigma-Aldrich), pH 2.2, for 15 min at room temperature. Cell lysates were then loaded on a haemacytometer and stained nuclei enumerated using phase-contrast microscopy (Nakagawara and Nathan, 1983).
NOD2 antibody peptide competition assay
The NOD2 antibody and the NOD2 peptide (ProSci) used to produce the antibody were incubated in a ratio of 2:1 (peptide : antibody) at 37°C for 30 min in a 5% milk solution in low-retention tubes. Day 5 MDM lysates were run in parallel on a 7.5% SDS-PAGE gel, transferred to a nitrocellulose membrane and the membrane cut so that each lane could be probed with either the antibody–peptide mixture or antibody alone overnight at 4°C. Western blotting was performed as described above. Membranes were then developed using ECL.
Statistics
A paired one-tailed Student’s t-test was used to analyse the differences between two groups in figures showing one representative figure of three independent experiments. An unpaired one-tailed Student’s t-test was used to analyse differences between two groups in figures showing cumulative data from three independent experiments. Significance was P < 0.05.
Acknowledgements
This work was supported by National Institutes of Health Grant AI059639 (to L.S.S.) and an NSF Graduate Research Fellowship Program (to M.N.B.). We give special thanks to Dr Mark Wewers for performing bronchoscopies and his helpful thoughts, and Dr Neil Warner in Dr Núñez’s laboratory for providing NOD2-expressing HEK293T cells. We also would like to acknowledge the Campus Microscopy and Imaging Facility at The Ohio State University (Columbus, OH).
References
- Abbott DW, Yang Y, Hutti JE, Madhavarapu S, Kelliher MA, Cantley LC. Coordinated regulation of Toll-like receptor and NOD2 signaling by K63-linked polyubiquitin chains. Mol Cell Biol. 2007;27:6012–6025. doi: 10.1128/MCB.00270-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abdallah AM, Gey van Pittius NC, Champion PA, Cox J, Luirink J, Vandenbroucke-Grauls CM, et al. Type VII secretion – mycobacteria show the way. Nat Rev Microbiol. 2007;5:883–891. doi: 10.1038/nrmicro1773. [DOI] [PubMed] [Google Scholar]
- Amer A, Franchi L, Kanneganti TD, Body-Malapel M, Ozoren N, Brady G, et al. Regulation of Legionella phagosome maturation and infection through flagellin and host Ipaf. J Biol Chem. 2006;281:35217–35223. doi: 10.1074/jbc.M604933200. [DOI] [PubMed] [Google Scholar]
- Amer AO. Modulation of caspases and their non-apoptotic functions by Legionella pneumophila. Cell Microbiol. 2010;12:140–147. doi: 10.1111/j.1462-5822.2009.01401.x. [DOI] [PubMed] [Google Scholar]
- Austin CM, Ma X, Graviss EA. Common nonsynonymous polymorphisms in the NOD2 gene are associated with resistance or susceptibility to tuberculosis disease in African Americans. J Infect Dis. 2008;197:1713–1716. doi: 10.1086/588384. [DOI] [PubMed] [Google Scholar]
- Barnich N, Aguirre JE, Reinecker HC, Xavier R, Podolsky DK. Membrane recruitment of NOD2 in intestinal epithelial cells is essential for nuclear factor- {kappa}B activation in muramyl dipeptide recognition. J Cell Biol. 2005;170:21–26. doi: 10.1083/jcb.200502153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beharka AA, Gaynor CD, Kang BK, Voelker DR, McCormack FX, Schlesinger LS. Pulmonary surfactant protein A up-regulates activity of the mannose receptor, a pattern recognition receptor expressed on human macrophages. J Immunol. 2002;169:3565–3573. doi: 10.4049/jimmunol.169.7.3565. [DOI] [PubMed] [Google Scholar]
- Bogdan C. Nitric oxide and the immune response. Nat Immunol. 2001;2:907–916. doi: 10.1038/ni1001-907. [DOI] [PubMed] [Google Scholar]
- Boot RG, Bussink AP, Verhoek M, de Boer PA, Moorman AF, Aerts JM. Marked differences in tissue-specific expression of chitinases in mouse and man. J Histochem Cytochem. 2005;53:1283–1292. doi: 10.1369/jhc.4A6547.2005. [DOI] [PubMed] [Google Scholar]
- Brieland J, Freeman P, Kunkel R, Chrisp C, Hurley M, Fantone J, et al. Replicative Legionella pneumophila lung infection in intratracheally inoculated A/J mice. A murine model of human Legionnaires’ disease. Am J Pathol. 1994;145:1537–1546. [PMC free article] [PubMed] [Google Scholar]
- Coulombe F, Divangahi M, Veyrier F, de Leseleuc L, Gleason JL, Yang Y, et al. Increased NOD2-mediated recognition of N-glycolyl muramyl dipeptide. J Exp Med. 2009;206:1709–1716. doi: 10.1084/jem.20081779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dao DN, Kremer L, Guerardel Y, Molano A, Jacobs WR, Jr, Porcelli SA, et al. Mycobacterium tuberculosis lipomannan induces apoptosis and interleukin-12 production in macrophages. Infect Immun. 2004;72:2067–2074. doi: 10.1128/IAI.72.4.2067-2074.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deshmukh HS, Hamburger JB, Ahn SH, McCafferty DG, Yang SR, Fowler VG., Jr Critical role of NOD2 in regulating the immune response to Staphylococcus aureus. Infect Immun. 2009;77:1376–1382. doi: 10.1128/IAI.00940-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Divangahi M, Mostowy S, Coulombe F, Kozak R, Guillot L, Veyrier F, et al. NOD2-deficient mice have impaired resistance to Mycobacterium tuberculosis infection through defective innate and adaptive immunity. J Immunol. 2008;181:7157–7165. doi: 10.4049/jimmunol.181.10.7157. [DOI] [PubMed] [Google Scholar]
- Fenton MJ, Riley LW, Schlesinger LS. Receptor-mediated recognition of Mycobacterium tuberculosis by host cells. In: Cole ST, Eisenach KD, McMurray DN, Jacobs WR Jr, editors. Tuberculosis and the Tubercle Bacillus. New York: ASM Press; 2005. pp. 405–426. [Google Scholar]
- Ferwerda G, Girardin SE, Kullberg BJ, Le Bourhis L, de Jong DJ, Langenberg DM, et al. NOD2 and toll-like receptors are nonredundant recognition systems of Mycobacterium tuberculosis. PLoS Pathog. 2005;1:279–285. doi: 10.1371/journal.ppat.0010034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferwerda G, Kramer M, de Jong D, Piccini A, Joosten LA, Devesaginer I, et al. Engagement of NOD2 has a dual effect on proIL-1beta mRNA transcription and secretion of bioactive IL-1beta. Eur J Immunol. 2008;38:184–191. doi: 10.1002/eji.200737103. [DOI] [PubMed] [Google Scholar]
- Flesch IEA, Kaufmann SHE. Role of cytokines in tuberculosis. Immunobiology. 1993;189:316–339. doi: 10.1016/S0171-2985(11)80364-5. [DOI] [PubMed] [Google Scholar]
- Franchi L, Park JH, Shaw MH, Marina-Garcia N, Chen G, Kim YG, et al. Intracellular NOD-like receptors in innate immunity, infection and disease. Cell Microbiol. 2008;10:1–8. doi: 10.1111/j.1462-5822.2007.01059.x. [DOI] [PubMed] [Google Scholar]
- Gandotra S, Jang S, Murray PJ, Salgame P, Ehrt S. Nucleotide-binding oligomerization domain protein 2-deficient mice control infection with Mycobacterium tuberculosis. Infect Immun. 2007;75:5127–5134. doi: 10.1128/IAI.00458-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganguly N, Giang PH, Gupta C, Basu SK, Siddiqui I, Salunke DM, et al. Mycobacterium tuberculosis secretory proteins CFP-10, ESAT-6 and the CFP10:ESAT6 complex inhibit lipopolysaccharide-induced NF-kappaB transactivation by downregulation of reactive oxidative species (ROS) production. Immunol Cell Biol. 2008;86:98–106. doi: 10.1038/sj.icb.7100117. [DOI] [PubMed] [Google Scholar]
- Gavrilin MA, Bouakl IJ, Knatz NL, Duncan MD, Hall MW, Gunn JS, et al. Internalization and phago-some escape required for Francisella to induce human monocyte IL-1beta processing and release. Proc Natl Acad Sci USA. 2006;103:141–146. doi: 10.1073/pnas.0504271103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gavrilin MA, Mitra S, Seshadri S, Nateri J, Berhe F, Hall MW, et al. Pyrin critical to macrophage IL-1beta response to Francisella challenge. J Immunol. 2009;182:7982–7989. doi: 10.4049/jimmunol.0803073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaynor CD, McCormack FX, Voelker DR, McGowan SE, Schlesinger LS. Pulmonary surfactant protein A mediates enhanced phagocytosis of Mycobacterium tuberculosis by a direct interaction with human macrophages. J Immunol. 1995;155:5343–5351. [PubMed] [Google Scholar]
- Girardin SE, Boneca IG, Viala J, Chamaillard M, Labigne A, Thomas G, et al. Nod2 is a general sensor of peptidoglycan through muramyl dipeptide (MDP) detection. J Biol Chem. 2003;278:8869–8872. doi: 10.1074/jbc.C200651200. [DOI] [PubMed] [Google Scholar]
- Gutierrez O, Pipaon C, Inohara N, Fontalba A, Ogura Y, Prosper F, et al. Induction of Nod2 in myelomonocytic and intestinal epithelial cells via nuclear factor-kappa B activation. J Biol Chem. 2002;277:41701–41705. doi: 10.1074/jbc.M206473200. [DOI] [PubMed] [Google Scholar]
- Henning LN, Azad AK, Parsa KV, Crowther JE, Tridandapani S, Schlesinger LS. Pulmonary surfactant protein A regulates TLR expression and activity in human macrophages. J Immunol. 2008;180:7847–7858. doi: 10.4049/jimmunol.180.12.7847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermon-Taylor J. Mycobacterium avium subspecies paratuberculosis, Crohn’s disease and the Doomsday scenario. Gut Pathog. 2009;1:15. doi: 10.1186/1757-4749-1-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herskovits AA, Auerbuch V, Portnoy DA. Bacterial ligands generated in a phagosome are targets of the cytosolic innate immune system. PLoS Pathog. 2007;3:e51. doi: 10.1371/journal.ppat.0030051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horwitz MA, Silverstein SC. Legionnaries’ disease bacterium (Legionella pneumophila) multiplies intracellularly in human monocytes. J Clin Invest. 1980;66:441–450. doi: 10.1172/JCI109874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hruz P, Zinkernagel AS, Jenikova G, Botwin GJ, Hugot JP, Karin M, et al. NOD2 contributes to cutaneous defense against Staphylococcus aureus through alpha-toxin-dependent innate immune activation. Proc Natl Acad Sci USA. 2009;106:12873–12878. doi: 10.1073/pnas.0904958106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu LC, Ali SR, McGillivray S, Tseng PH, Mariathasan S, Humke EW, et al. A NOD2–NALP1 complex mediates caspase-1-dependent IL-1beta secretion in response to Bacillus anthracis infection and muramyl dipeptide. Proc Natl Acad Sci USA. 2008;105:7803–7808. doi: 10.1073/pnas.0802726105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu C, Sun L, Hu Y, Lu D, Wang H, Tang S. Functional characterization of the NF-kappaB binding site in the human NOD2 promoter. Cell Mol Immunol. 2010;7:288–295. doi: 10.1038/cmi.2010.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hugot JP, Chamaillard M, Zouali H, Lesage S, Cezard JP, Belaiche J, et al. Association of NOD2 leucine-rich repeat variants with susceptibility to Crohn’s disease. Nature. 2001;411:599–603. doi: 10.1038/35079107. [DOI] [PubMed] [Google Scholar]
- Inohara N, Nunez G. NODs: intracellular proteins involved in inflammation and apoptosis. Nat Rev Immunol. 2003;3:371–382. doi: 10.1038/nri1086. [DOI] [PubMed] [Google Scholar]
- Inohara N, Ogura Y, Fontalba A, Gutierrez O, Pons F, Crespo J, et al. Host recognition of bacterial muramyl dipeptide mediated through NOD2. Implications for Crohn’s disease. J Biol Chem. 2003;278:5509–5512. doi: 10.1074/jbc.C200673200. [DOI] [PubMed] [Google Scholar]
- Kang PB, Azad AK, Torrelles JB, Kaufman TM, Beharka A, Tibesar E, et al. The human macrophage mannose receptor directs Mycobacterium tuberculosis lipoarabinomannan-mediated phagosome biogenesis. J Exp Med. 2005;202:987–999. doi: 10.1084/jem.20051239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapetanovic R, Nahori MA, Balloy V, Fitting C, Philpott DJ, Cavaillon JM, et al. Contribution of phagocytosis and intracellular sensing for cytokine production by Staphylococcus aureus-activated macrophages. Infect Immun. 2007;75:830–837. doi: 10.1128/IAI.01199-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karin M, Ben-Neriah Y. Phosphorylation meets ubiquitination: the control of NF-[kappa]B activity. Annu Rev Immunol. 2000;18:621–663. doi: 10.1146/annurev.immunol.18.1.621. [DOI] [PubMed] [Google Scholar]
- Kim YG, Park JH, Shaw MH, Franchi L, Inohara N, Nunez G. The cytosolic sensors Nod1 and Nod2 are critical for bacterial recognition and host defense after exposure to Toll-like receptor ligands. Immunity. 2008;28:246–257. doi: 10.1016/j.immuni.2007.12.012. [DOI] [PubMed] [Google Scholar]
- Kleinnijenhuis J, Joosten LA, van de Veerdonk FL, Savage N, van Crevel R, Kullberg BJ, et al. Transcriptional and inflammasome-mediated pathways for the induction of IL-1beta production by Mycobacterium tuberculosis. Eur J Immunol. 2009;39:1914–1922. doi: 10.1002/eji.200839115. [DOI] [PubMed] [Google Scholar]
- Kobayashi KS, Chamaillard M, Ogura Y, Henegariu O, Inohara N, Nunez G, et al. Nod2-dependent regulation of innate and adaptive immunity in the intestinal tract. Science. 2005;307:731–734. doi: 10.1126/science.1104911. [DOI] [PubMed] [Google Scholar]
- Kufer TA. Signal transduction pathways used by NLR-type innate immune receptors. Mol Biosyst. 2008;4:380–386. doi: 10.1039/b718948f. [DOI] [PubMed] [Google Scholar]
- Lala S, Dheda K, Chang JS, Huggett JF, Kim LU, Johnson MA, et al. The pathogen recognition sensor, NOD2, is variably expressed in patients with pulmonary tuberculosis. BMC Infect Dis. 2007;7:96. doi: 10.1186/1471-2334-7-96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le BL, Benko S, Girardin SE. Nod1 and Nod2 in innate immunity and human inflammatory disorders. Biochem Soc Trans. 2007;35:1479–1484. doi: 10.1042/BST0351479. [DOI] [PubMed] [Google Scholar]
- Leake ES, Myrvik QN, Wright MJ. Phagosomal membranes of Mycobacterium bovis BCG-immune alveolar macrophages are resistant to disruption by Mycobacterium tuberculosis H37Rv. Infect Immun. 1984;45:443–446. doi: 10.1128/iai.45.2.443-446.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lecine P, Esmiol S, Metais JY, Nicoletti C, Nourry C, McDonald C, et al. The NOD2–RICK complex signals from the plasma membrane. J Biol Chem. 2007;282:15197–15207. doi: 10.1074/jbc.M606242200. [DOI] [PubMed] [Google Scholar]
- Legrand-Poels S, Kustermans G, Bex F, Kremmer E, Kufer TA, Piette J. Modulation of Nod2-dependent NF-kappaB signaling by the actin cytoskeleton. J Cell Sci. 2007;120:1299–1310. doi: 10.1242/jcs.03424. [DOI] [PubMed] [Google Scholar]
- Li J, Moran T, Swanson E, Julian C, Harris J, Bonen DK, et al. Regulation of IL-8 and IL-1beta expression in Crohn’s disease associated NOD2/CARD15 mutations. Hum Mol Genet. 2004;13:1715–1725. doi: 10.1093/hmg/ddh182. [DOI] [PubMed] [Google Scholar]
- Loving CL, Osorio M, Kim YG, Nunez G, Hughes MA, Merkel TJ. Nod1/Nod2-mediated recognition plays a critical role in induction of adaptive immunity to anthrax after aerosol exposure. Infect Immun. 2009;77:4529–4537. doi: 10.1128/IAI.00563-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonough KA, Kress Y, Bloom BR. Pathogenesis of tuberculosis: interaction of Mycobacterium tuberculosis with macrophages. Infect Immun. 1993;61:2763–2773. doi: 10.1128/iai.61.7.2763-2773.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mestas J, Hughes CC. Of mice and NOT MEN: differences between mouse and human immunology. J Immunol. 2004;172:2731–2738. doi: 10.4049/jimmunol.172.5.2731. [DOI] [PubMed] [Google Scholar]
- Myrvik QN, Leake ES, Wright MJ. Disruption of phagosomal membranes of normal alveolar macrophages by the H37Rv strain of Mycobacterium tuberculosis. A correlate of virulence. Am Rev Respir Dis. 1984;129:322–328. [PubMed] [Google Scholar]
- Nakagawara A, Nathan CF. A simple method for counting adherent cells: application to cultured human monocytes, macrophages and multi-nucleated giant cells. J Immunol Methods. 1983;56:261–268. doi: 10.1016/0022-1759(83)90418-0. [DOI] [PubMed] [Google Scholar]
- Nembrini C, Kisielow J, Shamshiev AT, Tortola L, Coyle AJ, Kopf M, et al. The kinase activity of Rip2 determines its stability and consequently Nod1- and Nod2-mediated immune responses. J Biol Chem. 2009;284:19183–19188. doi: 10.1074/jbc.M109.006353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogura Y, Inohara N, Benito A, Chen FF, Yamaoka S, Nunez G. Nod2, a Nod1/Apaf-1 family member that is restricted to monocytes and activates NF-kappaB. J Biol Chem. 2001a;276:4812–4818. doi: 10.1074/jbc.M008072200. [DOI] [PubMed] [Google Scholar]
- Ogura Y, Bonen DK, Inohara N, Nicolae DL, Chen FF, Ramos R, et al. A frameshift mutation in NOD2 associated with susceptibility to Crohn’s disease. Nature. 2001b;411:603–606. doi: 10.1038/35079114. [DOI] [PubMed] [Google Scholar]
- Olakanmi O, Britigan BE, Schlesinger LS. Gallium disrupts iron metabolism of mycobacteria residing within human macrophages. Infect Immun. 2000;68:5619–5627. doi: 10.1128/iai.68.10.5619-5627.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Opitz B, Puschel A, Schmeck B, Hocke AC, Rosseau S, Hammerschmidt S, et al. Nucleotide-binding oligomerization domain proteins are innate immune receptors for internalized Streptococcus pneumoniae. J Biol Chem. 2004;279:36426–36432. doi: 10.1074/jbc.M403861200. [DOI] [PubMed] [Google Scholar]
- Pan Q, Mathison J, Fearns C, Kravchenko VV, da Silva CJ, Hoffman HM, et al. MDP-induced interleukin-1beta processing requires Nod2 and CIAS1/NALP3. J Leukoc Biol. 2007;82:177–183. doi: 10.1189/jlb.1006627. [DOI] [PubMed] [Google Scholar]
- Pandey AK, Yang Y, Jiang Z, Fortune SM, Coulombe F, Behr MA, et al. NOD2, RIP2 and IRF5 play a critical role in the type I interferon response to Mycobacterium tuberculosis. PLoS Pathog. 2009;5:e1000500. doi: 10.1371/journal.ppat.1000500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pauleau AL, Murray PJ. Role of nod2 in the response of macrophages to toll-like receptor agonists. Mol Cell Biol. 2003;23:7531–7539. doi: 10.1128/MCB.23.21.7531-7539.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phiet PH, Wietzerbin J, Zissman E, Petit JF, Lederer E. Analysis of the cell wall of five strains of Myocbacterium tuberculosis BCG and of an attenuated human strain, W 115. Infect Immun. 1976;13:677–681. doi: 10.1128/iai.13.3.677-681.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powlesland AS, Ward EM, Sadhu SK, Guo Y, Taylor ME, Drickamer K. Widely divergent biochemical properties of the complete set of mouse DC-SIGN-related proteins. J Biol Chem. 2006;281:20440–20449. doi: 10.1074/jbc.M601925200. [DOI] [PubMed] [Google Scholar]
- Rajaram MV, Ganesan LP, Parsa KV, Butchar JP, Gunn JS, Tridandapani S. Akt/Protein kinase B modulates macrophage inflammatory response to Francisella infection and confers a survival advantage in mice. J Immunol. 2006;177:6317–6324. doi: 10.4049/jimmunol.177.9.6317. [DOI] [PubMed] [Google Scholar]
- Rajaram MV, Brooks MN, Morris JD, Torrelles JB, Azad AK, Schlesinger LS. Mycobacterium tuberculosis activates human macrophage peroxisome proliferator-activated receptor gamma linking mannose receptor recognition to regulation of immune responses. J Immunol. 2010;185:929–942. doi: 10.4049/jimmunol.1000866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reginato AM, Olsen BR. Genetics and experimental models of crystal-induced arthritis. Lessons learned from mice and men: is it crystal clear? Curr Opin Rheumatol. 2007;19:134–145. doi: 10.1097/BOR.0b013e328040c00b. [DOI] [PubMed] [Google Scholar]
- Rigamonti E, Chinetti-Gbaguidi G, Staels B. Regulation of macrophage functions by PPAR-alpha, PPAR-gamma, and LXRs in mice and men. Arterioscler Thromb Vasc Biol. 2008;28:1050–1059. doi: 10.1161/ATVBAHA.107.158998. [DOI] [PubMed] [Google Scholar]
- Sartor RB. Mechanisms of disease: pathogenesis of Crohn’s disease and ulcerative colitis. Nat Clin Pract Gastroenterol Hepatol. 2006;3:390–407. doi: 10.1038/ncpgasthep0528. [DOI] [PubMed] [Google Scholar]
- Schlesinger LS. Macrophage phagocytosis of virulent but not attenuated strains of Mycobacterium tuberculosis is mediated by mannose receptors in addition to complement receptors. J Immunol. 1993;150:2920–2930. [PubMed] [Google Scholar]
- Schlesinger LS, Bellinger-Kawahara CG, Payne NR, Horwitz MA. Phagocytosis of Mycobacterium tuberculosis is mediated by human monocyte complement receptors and complement component C3. J Immunol. 1990;144:2771–2780. [PubMed] [Google Scholar]
- Schneemann M, Schoeden G. Macrophage biology and immunology: man is not a mouse. J Leukoc Biol. 2007;81:579. doi: 10.1189/jlb.1106702. [DOI] [PubMed] [Google Scholar]
- Schneemann M, Schoedon G. Species differences in macrophage NO production are important. Nat Immunol. 2002;3:102. doi: 10.1038/ni0202-102a. [DOI] [PubMed] [Google Scholar]
- Simeone R, Bottai D, Brosch R. ESX/type VII secretion systems and their role in host–pathogen interaction. Curr Opin Microbiol. 2009;12:4–10. doi: 10.1016/j.mib.2008.11.003. [DOI] [PubMed] [Google Scholar]
- Strober W, Murray PJ, Kitani A, Watanabe T. Signalling pathways and molecular interactions of NOD1 and NOD2. Nat Rev Immunol. 2006;6:9–20. doi: 10.1038/nri1747. [DOI] [PubMed] [Google Scholar]
- Teitelbaum R, Cammer M, Maitland ML, Freitag NE, Condeelis J, Bloom BR. Mycobacterial infection of macrophages results in membrane-permeable phagosomes. Proc Natl Acad Sci USA. 1999;96:15190–15195. doi: 10.1073/pnas.96.26.15190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Till A, Rosenstiel P, Brautigam K, Sina C, Jacobs G, Oberg HH, et al. A role for membrane-bound CD147 in NOD2-mediated recognition of bacterial cytoinvasion. J Cell Sci. 2008;121:487–495. doi: 10.1242/jcs.016980. [DOI] [PubMed] [Google Scholar]
- Ting JP, Duncan JA, Lei Y. How the noninflammasome NLRs function in the innate immune system. Science. 2010;327:286–290. doi: 10.1126/science.1184004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torrelles JB, Azad AK, Henning LN, Carlson TK, Schlesinger LS. Role of C-type lectins in mycobacterial infections. Curr Drug Targets. 2008;9:102–112. doi: 10.2174/138945008783502467. [DOI] [PubMed] [Google Scholar]
- van der Wel N, Hava D, Houben D, Fluitsma D, van Zon M, Pierson J, et al. M. tuberculosis and M. leprae translocate from the phagolysosome to the cytosol in myeloid cells. Cell. 2007;129:1287–1298. doi: 10.1016/j.cell.2007.05.059. [DOI] [PubMed] [Google Scholar]
- WHO. Clinical aspects of HIV/AIDS: pulmonary manifestations. 2006 [WWW document]. URL http://www.searo.who.int/en/Section10/Section18/Section356/Section407_2230.htm.
- Yang Y, Yin C, Pandey A, Abbott D, Sassetti C, Kelliher MA. NOD2 pathway activation by MDP or Mycobacterium tuberculosis infection involves the stable polyubiquitination of Rip2. J Biol Chem. 2007;282:36223–36229. doi: 10.1074/jbc.M703079200. [DOI] [PubMed] [Google Scholar]
- Zhang FR, Huang W, Chen SM, Sun LD, Liu H, Li Y, et al. Genomewide association study of leprosy. N Engl J Med. 2009;361:2609–2618. doi: 10.1056/NEJMoa0903753. [DOI] [PubMed] [Google Scholar]