Abstract
Purpose
Rapamycin inhibits vascular endothelial growth factor (VEGF) expression. VEGF is a tumor-elaborated protein that stimulates neovascularization. This inhibition can cause transient “normalization” of the generally dysfunctional tumor vasculature, resulting in improved tumor perfusion and oxygenation. We hypothesized that this may potentiate the antitumor effects of adjuvant ionizing radiation.
Methods
Mice bearing orthotopic Rh30 alveolar rhabdomyosarcomas were treated with rapamycin (5 mg/kg IP daily ×5). Tumors were then evaluated for changes in intratumoral oxygenation, perfusion, vessel permeability, and microvessel density. Additional tumor-bearing mice were treated with 5 doses of rapamycin, irradiation (4Gy), or 5 doses of rapamycin with irradiation administered on the 1st or 6th day of rapamycin treatment.
Results
Although tumor vessel permeability changed only minimally, microvessel density decreased (3,153±932 vs. 20,477±3,717.9 pixels/HPF) while intratumoral oxygenation increased significantly (0.0385±0.0141 vs. 0.0043±0.0023 mmHg/mm3) after 5 doses of rapamycin. Contrast-enhanced ultrasound demonstrated a significantly increased rate of change of signal intensity after 5 days of rapamycin, suggesting improved intratumoral perfusion. Tumor volume 14 days after treatment was smallest in mice treated with the combination of rapamycin given before irradiation.
Conclusion
Combination therapy with rapamycin given prior to irradiation to normalize the tumor vasculature, thereby improving tumor oxygenation, increased the sensitivity of alveolar rhabdomyosarcoma xenografts to adjuvant irradiation.
Keywords: Rhabdomyosarcoma, Rapamycin, VEGF inhibition, Ionizing Radiation
Rhabdomyosarcoma (RMS) is the most common soft tissue sarcoma in children, accounting for nearly fifty percent of pediatric soft tissue sarcomas, with alveolar RMS (ARMS) being the most aggressive histologic subtype [1]. The current treatment for ARMS includes 3 modalities: surgical resection, radiation therapy, and systemic chemotherapy. Ionizing radiation (IR) is an essential component of multimodal therapy for ARMS, although the efficacy of IR depends on the presence of oxygen in the target tumor tissue for the generation of free radicals. This creates DNA injury, leading to tumor cell apoptosis and decreased tumor size. Yet tumor tissue is relatively hypoxic and thus higher doses of radiation are required which increases the chance of complications due to irradiation. These complications include growth arrest of normal tissues and the potential for a second malignancy later in life [2]. Thus, ways to improve the efficacy of ionizing radiation by augmenting target cell oxygenation would be advantageous. Rapamycin is a macrolide antibiotic that inhibits the mammalian target of rapamycin (mTOR). mTOR plays a significant role in regulating tumor cell growth and metabolism; its inhibition causes suppression of tumor cell invasion, proliferation, and metastasis and causes apoptosis of tumor cells. In addition, rapamycin also has strong anti-angiogenic properties related to suppression of vascular endothelial growth factor (VEGF) [3].
Tumor growth depends on angiogenesis, the process of new blood vessel formation. However, tumor neovessels are typically tortuous with defective basement membranes and abnormal pericyte lining, making them leaky and resulting in heterogeneous tumor perfusion with areas of hypoxia. However, we and others have shown that inhibition of VEGF can lead to pruning of immature vessels, leaving the remaining vessels more “normal” in structure and function [4,5]. This process of vascular “normalization” can result in improved intratumoral perfusion and oxygenation [6].
We hypothesized that altering the tumor microenvironment using rapamycin to increase oxygenation, and therefore radiosensitivity, would lead to decreased tumor growth in RMS when combined with IR.
MATERIAL AND METHODS
Animal Model
All murine experiments were performed in accordance with a protocol approved by the Institutional Animal Care and Use Committee at St. Jude Children’s Research Hospital. An orthotopic model of rhabdomyosarcoma was established by injecting 2 million Rh30 human alveolar rhabdomyosarcoma cells into the right calf muscle of approximately 6-week-old male CB-17 SCID mice (Taconic, Germantown, NY). Intramuscular tumor size was estimated by measuring the size of the tumor-injected right calf (width2 × length × 0.5) and subtracting the volume of the normal left calf. Approximately three weeks after injection the tumors measured ~250mm3 and were sized matched into appropriate groups.
Rapamycin (LKT Laboratories, Inc., St. Paul, MN) was given at a dose of 5 mg/kg intraperitoneally (IP) for 5 consecutive days. Control and treatment groups were evaluated at the end of 5 days of rapamycin, and then 5 days later after discontinuation of rapamycin. To analyze the effect of rapamycin on the efficacy of IR, treatment groups were administered vehicle only [phosphate buffered saline, (PBS)] (Cellgro, Manassas, VA), 5 days of rapamycin alone, IR alone, or 5 days of rapamycin with IR on the 1st, 6th, or 10th day. A single dose of 4 Gy IR was administered using the Orthovoltage D3000 X-ray tube (Gulmay Medical Ltd., Buford, GA). Tumors were measured at the beginning of treatment and 14 days later.
In Vitro Methods
Western blot analysis was performed using mTOR (Cell Signaling 4517), phospho-mTOR (Cell Signaling 5538), and GAPDH antibodies (Ambion 4300). VEGF levels in protein extracted from Rh-30 cells were measured using R&D Systems VEGF ELISA kit (DVE00).
Immunohistochemistry
Formalin-fixed, paraffin-embedded 4um thick tumor sections were stained for CD34 (Santa Cruz, sc-18917, Santa Cruz, CA) to identify endothelial cells, as previously described [7]. Three fields per tumor sample were digitally photographed at 200X, focusing on endothelial cell “hotspots” in areas without tumor necrosis. Positive staining was quantified using ImageJ (NIH analysis software) and reported as the number of pixels per high power field (HPF). In order to assess intratumoral hypoxia, mice were injected with IP Hypoxyprobe-1 (pimonidazole) (hpi, Burlington, MA) approximately 30 min prior to sacrifice. Paraffin-embedded tumor sections were then stained using the Hypoxyprobe-1 Plus Kit (hpi). Hypoxia was quantified using Adobe Photoshop and ImageJ as described above. Hypoxia was then analyzed as ratio of hypoxic staining areas to tumor volume to account for the effect increased tumor volume has on tumor necrosis and central hypoxia. Apoptosis was measured using the DeadEnd Colorimetric TUNEL System (Promega, PRG7130) that end-labels the fragmented DNA of apoptotic cells using a modified TUNEL assay. Slides were also stained with rabbit anti-human Ki67 antibody (Vector labs, VP-K451) to measure proliferation and reported as the percentage of positive cells per high power field.
Evans Blue Dye Assay
Tumor vessel permeability was assessed using an Evans blue dye assay as described previously [8]. Samples were quantified in duplicate by spectrophotometry at 620 nm.
In Vivo Oxygen Tension Measurement
Intratumoral oxygen tension was measured using the OxyLab fiber optic probe (Oxford Optronics, Oxford, UK). Mice were anesthetized with ketamine/xylazine and maintained on continuous oxygen via nose cone. A small incision (< 2 mm) was made in the skin at the base the tumor and the oxygen probe was placed into the base of the tumor and positioned 3/4 of the way into the tumor. The probe was allowed to equilibrate for approximately 20 min before the measurement was recorded.
Contrast-enhanced Ultrasonography
Optison ultrasound contrast agent (GE Healthcare, Princeton, NJ) was used to perform contrast-enhanced ultrasonography, on a Vevo 770 (Visual Sonics, Toronto, Ontario) small animal ultrasound machine using a 40 MHz linear transducer. One hundred microliters of Optison was injected into the venous system by retro-orbital injection. Images were recorded on a cine-clip beginning immediately before the contrast injection and continued for approximately 60 seconds, at a frame rate of 14 to 18 Hz. A region of interest was drawn to encompass a portion of the tumor close to the transducer and the cine clip was evaluated for changes in tumor signal intensity from pre-contrast-enhanced baseline to initial peak enhancement (ΔSI in decibels, dB) and rate of signal intensity increase from baseline to peak (RSI in decibels per second).
Statistical Analysis
SigmaPlot (SPSS, Inc., Chicago, IL) was used to perform unpaired Student’s t-tests and to graphically present data. A p value of less than 0.05 was considered significant. All results are given as mean ± SE. Bliss’s independent joint action principle was used to define the synergy index (SI), with 95% confidence intervals being used to assess drug interaction. A SI less than 1 with a 95% confidence interval that does not cross zero suggests synergy of two therapies. A p value of less than 0.05 was considered significant [9].
RESULTS
Effect of Rapamycin on tumor cells in vitro
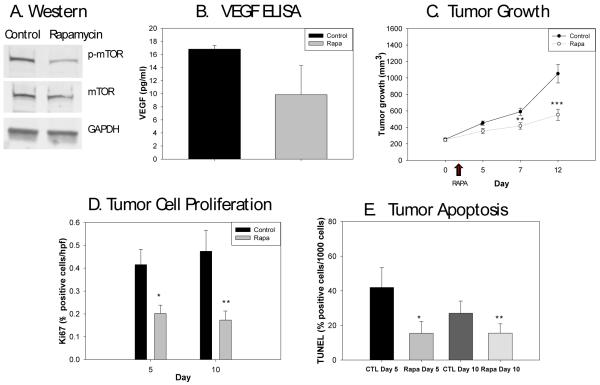
The levels of mTOR phosphorylation and VEGF expression in Rh-30 cells treated with rapamycin were assessed to confirm the known activities of rapamycin. Western blot analysis on protein extracted from Rh-30 cells treated with 100 nM rapamycin for 48 hours confirmed inhibition of mTOR phosphorylation (Fig. 1A). Human VEGF levels were also analyzed by ELISA and found to decrease with rapamycin treatment (9.85±2.55 vs. 16.82±0.33 pg/ml, p=0.05) (Fig. 1B).
Figure 1.
Effects of rapamycin treatment on Rh30 cells. 1A) Western blot analysis shows decreased phosphorylation of mTOR in the tumor cells treated with rapamycin compared to control cells. GAPDH was used as a control for comparison. 1B) VEGF levels were lower in rapamycin treated mice compared to controls (p=0.05). 1C) Rapamycin was administered on days 2-7 of tumor growth measurements (arrow indicating administration of rapamycin). Rapamycin treated tumors were significantly smaller compared to control tumors at day 5 (*p=0.02), day 7 (p=**0.008), and day 12 (***p=0.001). 1D) The percentage of cells actively proliferating was significantly less in the rapamycin treated tumors on days 5 (*p=0.0000003) and 10 (**p=0.0000008) after the start of therapy compared to controls. 1E) Apoptosis, as determined by TUNEL, was significantly decreased after 5 days of rapamycin compared to controls (*p=0.00002), and this difference persisted 5 days later (**p=0.003).
Effect of Rapamycin on Tumor Growth
Mice bearing intramuscular Rh-30 tumors were size-matched into control and treatment groups (n=20/group). Rapamycin was administered for 5 consecutive days to the treated group and vehicle (PBS) was administered to the control group. Tumors were measured prior to and on days 3, 5, and 10 after the start of therapy. Rapamycin monotherapy restricted Rh-30 tumor growth; treated tumors were significantly smaller compared to control tumors immediately after the five-day course of rapamycin (417.6±38.4 vs. 590.4±43.4 mm3, p=0.008), and remained so 5 days later (553.6±67.2 vs. 1,051.8±111.9 mm3, p=0.001) (Fig. 1C).
Tumor cell proliferation and apoptosis were measured with Ki-67 and TUNEL staining at the end of 5 days of rapamycin and again 5 days later. Immediately after 5 days of rapamycin, treated tumors had a significantly lower percentage of proliferating cells than control tumors (0.202±0.012 vs. 0.416±0.022 % positive cells/HPF, p=0.0000003). This effect persisted 5 days after the end of treatment (0.173±0.013 vs. 0.475±0.037 % positive cells/HPF, p=0.0000008) (Fig. 1D). Interestingly, apoptosis was also significantly decreased after 5 days of rapamycin compared to controls (15.4±2.3 vs. 42.0±3.8, p=0.00002), and remained lower 5 days later (15.5±1.8 vs. 27.1±2.9, p=0.003) (Fig. 1E).
Effect of Rapamycin on Tumor Vessel Phenotype
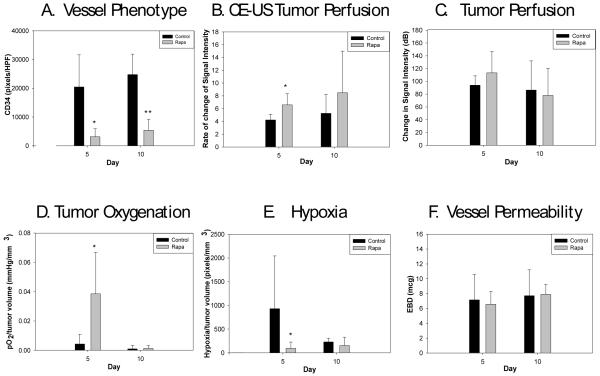
Additional Rh-30 tumors were size-matched into treatment and control groups (n=20/group). Rapamycin was administered for 5 consecutive days to the treated group. Five mice from each of the rapamycin-treated and control cohorts were sacrificed and tumor tissue harvested for immunohistochemical analysis after 5 daily doses of rapamycin and again 5 days after the completion of the five-day course. CD34 staining for endothelial cells was performed to evaluate microvessel density, which was significantly lower than controls after 5 doses of rapamycin (3,152.8±931.7 vs. 20,476.6±3,717.9 pixels/HPF, p=0.0003) and remained so 5 days after the discontinuation of rapamycin (5,426.9±1,271.7 vs. 24,794.2±2,884.1 pixels/HPF, p=0.00001) compared to untreated tumors (Fig. 2A).
Figure 2.
Effects of rapamycin treatment in vivo. 2A) Microvessel density was significantly lower in rapamycin treated tumors at day 5 (*p=0.0003) and 5 days after the completion of rapamycin therapy (**p=0.00001). 2B) RSI, the rate of change in signal intensity on contrast-enhanced ultrasound, was significantly higher after 5 doses of rapamycin treatment (*p=0.03). 2C) Delta SI, the change in signal intensity, was not significantly different between the treated and untreated tumors. 2D) After 5 doses of rapamycin therapy the ratio of intratumoral oxygenation to tumor volume (pO2/mm3) was significantly higher in the treated group (n=8) compared to controls (n=7) (*p=0.007 on day 5); this effect abated 5 days later. 2E) Hypoxia was analyzed as ratio of hypoxic staining areas to tumor volume. After 5 doses of rapamycin therapy, the ratio of hypoxia to tumor volume was significantly lower than control tumors (*p=0.04), this difference abated by 5 days after the end of therapy. 2F) An Evans blue dye assay was used to measure vascular permeability. At the end of 5 doses of rapamycin and 5 days after completion of rapamycin there was no difference observed in tumor vascular permeability.
We have previously demonstrated that contrast-enhanced ultrasound can be used to evaluate changes in tumor perfusion [10]. RSI, the rate of change in signal intensity, was significantly higher after 5 doses of rapamycin (6.6±0.8 vs. 4.2±0.4 dB/sec, p=0.03), indicating improved and more efficient tumor perfusion (Fig. 2B). Delta SI, the change in signal intensity on contrast-enhanced ultrasound, a measure of tumor perfusion, was not significantly increased in the treated tumors at the end of 5 days of rapamycin therapy compared to control tumors (113.4±14.9 vs. 94.0±6.6 dB, p=0.27) (Fig. 2C).
Intratumoral oxygenation was significantly increased in tumors treated with 5 doses of rapamycin (16.38±4.52 vs. 2.50±1.56 mmHg, p=0.004) when compared to controls. This effect abated by 5 days after the completion of rapamycin treatment (0.63±0.38 vs. 0.66±0.62 mmHg, p=0.97, treated vs. control), indicating the increase in tumor oxygenation was transient. When tumor volume was accounted for in order to eliminate the effect tumor size has on oxygenation and perfusion, oxygenation was still significantly improved with 5 doses of rapamycin compared to controls (0.0385±0.0141 vs. 0.0043±0.0023 pO2/mm3, p=0.007) (Fig. 2D).
Tumor sections were also stained with pimonidazole (hypoxyprobe) to detect areas of hypoxia. Hypoxia was analyzed as ratio of hypoxic staining areas to tumor volume to account for the effect increased tumor volume has on tumor necrosis and central hypoxia. After 5 doses of rapamycin, the ratio of hypoxia to tumor volume was significantly lower than control tumors (94.6±43.9 vs. 932±370.6 pixels/mm3, p=0.04); however, this difference resolved by 5 days after the end of therapy (Fig. 2E). We also assessed the effect of rapamycin inhibition of mTOR and VEGF on tumor vessel leakiness using an Evans blue dye assay. However, tumor vessel permeability changed only minimally in the treated group compared to controls (6.6±0.97 vs.7.2±1.3 mcg, p=0.75) (Fig. 2F).
Effect of Combined Treatment with Rapamycin and Ionizing Radiation on ARMS Xenograft Growth and Oxygenation
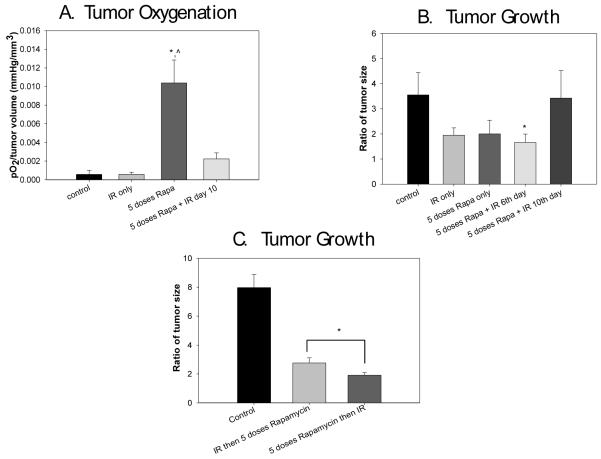
The changes we observed in tumor oxygenation and perfusion after rapamycin treatment led us to hypothesize that it would also enhance the effect of ionizing irradiation. In an initial set of experiments, five treatment groups (vehicle only, 5 days of rapamycin only, IR only, and 5 days of rapamycin with IR on the 6th or 10th day) were used to evaluate the effect rapamycin administration before IR would have on Rh30 ARMS xenograft growth. The effect tumor volume might have on oxygenation was again accounted for by dividing tumor oxygenation by volume (pO2/mm3). Intratumoral oxygen tension for all groups was measured just prior to the administration of IR. Pre-treatment of tumors with rapamycin 5 days before the administration of IR resulted in higher intratumoral oxygenation when controlled for tumor size compared to control tumors (0.0104±0.0007 vs. 0.0005±0.0002, p=0.0000009). The ratio of oxygenation to tumor size was also significantly higher in the groups given 5 doses of rapamycin compared to IR only (0.0104±0.0008 vs. 0.0005±0.0001 mmHg/mm3, p=0.000005) (Fig. 3A).
Figure 3.
Effect of rapamycin combined with irradiation in vivo. Rapamycin was given for 5 days with IR administered either on the 6th or 10th day after the start of therapy. 3A) Intratumoral oxygen tension for all groups was measured just prior to the administration of IR. Treatment of tumors with 5 days of rapamycin just prior IR resulted in higher intratumoral oxygenation compared to control tumors (*p=0.0000009) when controlling for size differences. Oxygenation was also significantly higher in the group given 5 doses of rapamycin compared to IR only (^p=0.000005). 3B) Tumor volumes in the combination therapy groups are represented as the ratio of change in tumor volume from the start of therapy to fourteen days after the end of therapy. The cohort that received combination therapy with rapamycin plus IR on the 6th day had significant tumor growth restriction compared to controls (*p=0.002). 3C) Tumors treated with 5 doses of rapamycin prior to IR had significantly slower tumor growth than tumors treated with IR first followed by 5 days of rapamycin (*p=0.001).
We then tested our hypothesis that improved oxygenation at the time of IR administration in the group pre-treated with rapamycin would lead to improved antitumor efficacy of radiation therapy [11]. Tumors were prospectively divided into treatment groups, with 5 mice per group. Fourteen days following IR, tumor sizes were compared. Tumor volumes were represented as the ratio of tumor volume from the beginning to the end of treatment to account for differences in starting tumor size. The cohort that received combination therapy with rapamycin and IR on the 6th day had significant tumor growth restriction compared to controls (1.66±0.15 vs. 3.55±0.4, p=0.002) (Fig. 3B). This effect on tumor oxygenation had abated by five days after completion of rapamycin and is reflected in the lack of efficacy when radiation was delayed until this point. The group given combination therapy with rapamycin administered for 5 days with IR on the 6th day demonstrated an additive effect at day 14 compared to IR alone when analyzed using Bliss’s independent joint action principle to define the synergy index. The synergy index of rapamycin given for 5 days before IR was 0.412 with a 95% CI of −0.002 to 0.827, because the 95% confidence interval crossed zero it is considered additive, not synergistic. The synergy index of rapamycin started 10 days before IR was 1.137 with a 95% CI of 0.649 to 1.625, which is actually considered antagonistic. These findings emphasize the importance of the timing of rapamycin administration in respect to IR to achieve an additive effect. We also compared tumor growth between mice treated with IR on day zero, then 5 days of rapamycin, to mice treated with 5 days of rapamycin with IR administered on the 6th day to confirm that the timing of IR in combination with rapamycin is more effective at restricting tumor growth when given at the end of rapamycin therapy, at the time of maximal increase in oxygenation. The ratio of tumor growth was significantly less in the mice treated with 5 doses rapamycin before IR compared to mice treated with IR first (1.91±0.09 vs. 2.77±0.16, p=0.001) (Fig. 3C).
DISCUSSION
The additive effects of antiangiogenic therapy and radiation have previously been explained by a “normalization” effect on tumor microvessels, whereby VEGF inhibition eradicates the immature vessels of the dysfunctional tumor vasculature, effectively resulting in an increase in tumor perfusion and oxygenation. In this study we used rapamycin to inhibit mTOR and VEGF as an adjuvant to ionizing radiation in the treatment of ARMS in vivo because of its ability to normalize dysfunctional tumor vasculature. However, this work demonstrates that VEGF inhibition via rapamycin is transient, creating a period of time during which there is improved intratumoral perfusion and oxygenation, thus resulting in improved antitumor efficacy of IR during a specific window.
We demonstrated that rapamycin alters the tumor vessel microenvironment, with the majority of its effects present within a few days of active rapamycin therapy and resolving within 5 days after cessation of rapamycin administration. These temporary changes after rapamycin administration created a window of vascular normalization in which there was increased oxygenation and more efficient tumor perfusion. We then added ionizing irradiation as an adjuvant to rapamycin in order to take advantage of this window of improved tumor perfusion and oxygenation. We observed the greatest improvement in oxygenation after combination treatment with rapamycin given for 5 days before IR. We also observed an additive effect in slowing tumor growth in the group treated with combination therapy, supporting our hypothesis that the antiangiogenic effects of rapamycin would serve to potentiate antitumor effects of ionizing radiation thus enabling an enhanced effect of radiation without increasing dosage. Our cumulative results suggest that combination therapy with rapamycin given prior to IR as an adjuvant may be effective in the treatment of ARMS and improve patient outcomes. Careful consideration of the timing and duration of rapamycin as an adjuvant to IR will be needed to optimize the effectiveness of combination therapy in clinical trials.
ACKNOWLEDGEMENTS
This work was supported by the Assisi Foundation of Memphis, the US Public Health Service Childhood Solid Tumor Program Project Grant No. CA23099, the Cancer Center Support Grant No. 21766 from the National Cancer Institute, and by the American Lebanese Syrian Associated Charities.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- [1].Loeb DM, Thornton K, Shokek O. Pediatric Soft Tissue Sarcomas. Surg Clin N Am. 2008;88:615–627. doi: 10.1016/j.suc.2008.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Raney RB, Maurer HM, Anderson JR, et al. The Intergroup Rhabdomyosarcoma Study Group (IRSG): major lessons from the IRS-I through IRS-IV studies as background for the current IRS-V treatment protocols. Sarcoma. 2001;5:9–15. doi: 10.1080/13577140120048890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Seeliger H, Guba M, Kleespies A, et al. Role of mTOR in solid tumor systems: a therapeutical target against primary tumor growth, metastases, and angiogenesis. Cancer Metastasis Rev. 2007;26:611–621. doi: 10.1007/s10555-007-9077-8. [DOI] [PubMed] [Google Scholar]
- [4].Dickson PV, Hamner JB, Sims TL, et al. Bevacizumab-Induced Transient Remodeling of the Vasculature in Neuroblastoma Xenografts Results in Improved Delivery and Efficacy of Systemically Administered Chemotherapy. Clin Cancer Res. 2007;13:3942–50. doi: 10.1158/1078-0432.CCR-07-0278. [DOI] [PubMed] [Google Scholar]
- [5].Kim KJ, Li B, Winer J, et al. Inhibition of vascular endothelial growth factor-induced angiogenesis suppresses tumour growth in vivo. Nature. 1993;362:841–844. doi: 10.1038/362841a0. [DOI] [PubMed] [Google Scholar]
- [6].Guba M, von Breitenbuch P, Steinbauer M, et al. Rapamycin inhibits primary and metastatic tumor growth by antiangiogenesis: involvement of vascular endothelial growth factor. Nature Medicine. 2002;8:128–135. doi: 10.1038/nm0202-128. [DOI] [PubMed] [Google Scholar]
- [7].Spurbeck WW, Ng CY, Strom TS, et al. Enforced expression of tissue inhibitor of matrix metalloproteinase-3 affects functional capillary morphogenesis and inhibits tumor growth in a murine tumor model. Blood. 2002;100:3361–8. doi: 10.1182/blood.V100.9.3361. [DOI] [PubMed] [Google Scholar]
- [8].Farrell C, Stewart P, Del Maestro R. A new glioma model in rat: the C6 spheroid implantation technique permeability and vascular characterization. J Neuro-oncol. 1987;4:403–15. doi: 10.1007/BF00195612. [DOI] [PubMed] [Google Scholar]
- [9].Plackett RL, Hewlett PS. Statistical aspects of the independent joint action of poisons, particularly insecticides: The toxicity of a mixture of poisons. Ann Appl Biol. 1948;35:347–58. doi: 10.1111/j.1744-7348.1948.tb07379.x. [DOI] [PubMed] [Google Scholar]
- [10].McCarville MB, Streck CJ, Dickson PV, et al. Angiogenesis inhibitors in a murine neuroblastoma model: quantitative assessment of intratumoral blood flow with contrast-enhanced gray-scale US. Radiology. 2006;240:73–81. doi: 10.1148/radiol.2401050709. [DOI] [PubMed] [Google Scholar]
- [11].Manegold PC, Paringer C, Kulka U, et al. Antiangiogenic Therapy with Mammalian Target of Rapamycin Inhibitor RAD001 (Everolimus) Increases Radiosensitivity in Solid Cancer. Clin Cancer Res. 2008;14(3):892–900. doi: 10.1158/1078-0432.CCR-07-0955. [DOI] [PubMed] [Google Scholar]