Abstract
Objective
The aim of this study was to evaluate the impact of sodium hyaluronate-sodium carboxymethyl cellulose (HA-CMC), an anti-adhesive material for spinal surgery, on bone fusion by applying it to rat spinal models after lumbar posterolateral fusion.
Methods
Lumbar posterolateral fusion was performed at L4-5 using bone graft substitutes in 30 rats. HA-CMC was injected in 15 rats at a dose of 0.2 cc (HA-CMC group) and a saline solution of 0.2 cc in the other 15 rats (control group). Simple radiographs were taken until postoperative 9 weeks with an interval of one week. At postoperative 4 and 9 weeks, three dimensional computed tomography (3D CT) scanning was performed to observe the process of bone fusion. At 9 weeks, bone fusion was confirmed by gross examination and manual palpation.
Results
There were no statistically significant differences in bone fusion between the two groups. 3D CT scanning did not reveal significant differences between the groups. The gross examination and manual palpation after autopsy performed at 9 weeks confirmed bone union in 93.3% of both groups.
Conclusion
The anti-adhesive material used for spinal surgery did not have adverse effects on spinal fusion in rats.
Keywords: Sodium hyaluronate-sodium carboxymethyl cellulose, Posterolateral spinal fusion, Rat model, Anti-adhesive agent
INTRODUCTION
Spinal decompression and arthrodesis is the gold standard treatment for degenerative and traumatic spine diseases that are associated with neurologic problems.
Epidural fibrosis, which may cause persistent back and leg pain, may develop after spine decompression surgery. Several experimental and clinical studies have focused on the prevention of scar tissue formation, based on the principle that a mechanical barrier could limit the migration of fibroblasts from the superficial layers and the subsequent collagen deposition1,9,19).
To our knowledge, however, no studies have reported on the impact of several types of anti-adhesive agents on spinal arthrodesis. Despite the lack of sufficient knowledge about possible effects of these materials on the bone graft healing process, they are being used after various spinal surgeries including spinal arthrodesis.
The purpose of our study was to analyze the impact of sodium hyaluronate-sodium carboxymethyl cellulose (HA-CMC, GUARDIX-S®, Biorane, Seoul, Korea), the combination of sodium hyaluronate (HA)11,14,17,25) and sodium carboxymethyl cellulose7,8) which is being clinically applied due to its superior anti-adhesive effects, on lumbar posterolateral fusion in rat spinal models.
MATERIALS AND METHODS
Animal models for spinal fusion
The posterior-lateral lumbar spine fusion technique was carried out in 30 male Sprague-Dawley rats [Sam : TacN(SD)BR, Samtako, Seoul, Korea] from L4 to L5 levels. The animals were 6 to 8 weeks in age and weighed from 410 to 510 grams. They were raised in the laboratory under the same temperature and humidity from 2 weeks before. A single orthopedic surgeon performed all procedures, and worked in conjunction with an animal laboratory supervisor for intraoperative and perioperative monitoring of administered anesthesia.
Surgical procedure
An abdominal injection of tiletamine HCl/zolazepam HCl (Zoletil® Virbac, Carros, France) was done at a dose of 1 mg/kg into the rats for general anesthesia. For subanesthetic cases, an additional injection of 0.5 mg/kg was given. After anesthesia, the surgical site was shaved and disinfected with a solution of 10% betadine. Surface landmarks on the dorsum were palpated to identify spinal levels. The iliac crest provided a rough estimate of the interspace between the L5 and L6 spinous processes. A 20 mm dorsal midline skin incision was made centered over the L4-5 spinous process. The transverse processes of L4 and L5 vertebrae were then exposed. Once exposed, the transverse processes and the lateral gutters of the L4 and L5 vertebrae were decorticated by manual excoriation with a microcuret until a blush of cancellous bone was observed. The intended bone graft substitute (PolyBone® KyungWon Medical Co., Seoul, Korea) 0.2 g was placed over the entire span of the fusion bed on each side of the spine. The intended bone graft substitute is prepared from a mixture composed β-tricalcium phosphate, monocalcium phosphate, calcium sulfate hemihydrates, and polyphosphate. A saline solution of 0.2 cc was injected into the 15 rats in the control group and a combination of HA-CMC of 0.2 cc, into the other 15 rats. 0.2 cc was enough to entirely cover the experiment site and did not leak out after closure. Closure was carried out in layers : the dorsolumbar fascia was closed using 5-0 nylon continuous sutures, and the skin was closed with interrupted 5-0 nylon sutures. For 3 postoperative days, antibiotics were given subcutaneously (gentamicin, 0.5 mg/kg).
Fusion assessment
Simple radiographs (Digital diagnost TH, Philips, Eindhoven, the Netherlands) were taken on the posteroanterior radiographs of the thoracolumbosacral spine at an interval of one week from immediately after operation to 9 weeks under the same condition (distance between the tube and the cassette was 60 cm, 2.5 mA, 12 milliseconds). On the radiographs, resorption of the bone graft substitute, new bone formation, and degree of bone fusion were observed. The images were graded for fusion bone quantity and evidence of fusion using a numerical radiologic grading system described by O'Loughlin et al.18) (Table 1). Scoring was made by two examiners unaware of experimental details. At postoperative 4 and 9 weeks, three dimensional computed tomography (3D CT) scanning in the axial, sagittal, coronal plane was performed using a CT scanner (Brilliance 64-slice CT, Philips, Eindhoven, the Netherlands) to evaluate a calcified fusion mass at the intertransverse and interspinous process region where bone had not existed. The scans were initiated from the lower endplate of the L4 vertebral body cranially in 3-mm sections, for a total of 100 slices per scan. Through the scans, the presence of trabecular bridging was observed in the transverse and spinous processes at L4 and L5. The images were also graded using the radiologic grading system described by O'Loughlin et al.18) and compared between the control group and the HA-CMC group. At postoperative 9 weeks, all the animals under the experiment were sacrificed by air embolism. Immediately after death, the lumbar spinal segment L4-5 was removed en-bloc from all of the animals, and the majority of soft tissue was removed without disturbing the fusion mass. The harvested lumbar spine was gently palpated, and lateral side-bending motion at the L4-5 level was compared with the motion at the adjacent levels above (L3-4) and below (L5-6). Each spine was assessed for fusion by manual palpation by 2 observers who were blinded to the type of treatment that the animal had received. Manual palpation has been reported to be the most sensitive and specific method of assessing fusion in this model3,4,28). Specimens were assessed in both coronal and sagittal planes and were scored as having "motion" or "no motion" at the L4-5 segment by each examiner. Specimens were considered fused when 2 observers scored the spine as having "no motion". The numerical radiologic grading system, described by O'Loughlin et al.18) was used to evaluate the simple radiographs and 3D CT and this data was statistically analyzed through a Mann-Whitney test. We decided a p-value of less than 0.05 to be considered significant.
Table 1.
Radiologic grading system by O'Loughlin et al.
RESULTS
There were no complications including wound infection or hind limb paresis.
Radiographic analysis
Post-experiment simple radiographs showed compact radio-opaque bone graft substitutes at the L4-5 region and in between these bone graft substitutes a radiolucent space was observed. This radiolucent space between the bone graft substitutes was seen to increase from the 3-4 weeks to the 5-6 weeks after the experiment was preformed. After the 6-7 weeks this space was substituted by a homogenous radio-opaque zone. The authors believe that the increase of the radiolucent space represents the resorption of the bone graft substitutes and the new homogenous radio-opaque zone in the radiolucent space to be the shadow of newly fused bone mass.
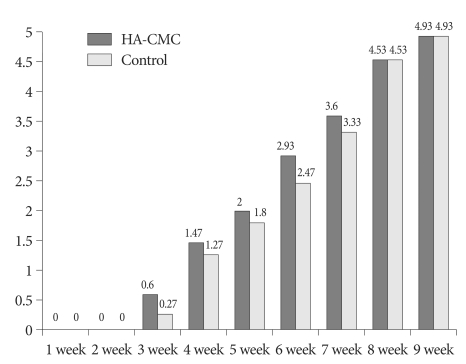
In the control group, a shadow of the radiolucent space between the implanted bone graft substitutes started to increase overall from postoperative 4 weeks (Fig. 1B), forming a shadow of a bone fusion mass with a relatively even density at around 7 weeks (Fig. 1C). However, the HA-CMC group showed absorption between the bone graft substitutes from postoperative 3 weeks (Fig. 2B) and demonstrated a shadow of a bone fusion mass from 6 weeks, one week earlier than the control group (Fig. 2C). On the radiologic grading system by O'Loughlin et al.18), the two groups did not have remarkable changes on simple radiographs until 2 weeks, and started to reveal poor formation of new bone from 3 weeks. Until 5 weeks, no statistically significant differences were found between the two groups, but the HA-CMC group experienced faster bone fusion. At 6 weeks, in particular, the HA-CMC group (Fig. 3D) demonstrated significant progression of bone fusion compared with the control group (p=0.029) (Fig. 3B). However from 7 weeks, no significant differences were found between the two groups and, at 9 weeks, both groups had a bone fusion scored 4.93 (Table 2) (Fig. 4, 5).
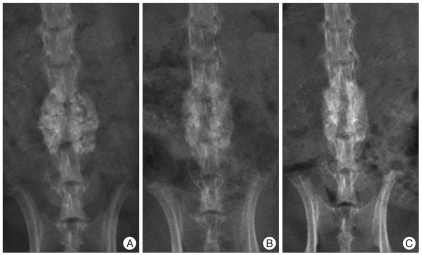
Fig. 1.
In the control group, immediately after operation (A), a shadow of the radiolucent space between the implanted bone graft substitutes started to increase overall from postoperative 4 weeks (B), forming a shadow of a bone fusion mass with a relatively even density at around 7 weeks (C).
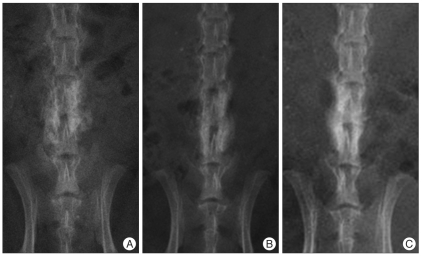
Fig. 2.
Sodium hyaluronate-sodium carboxymethyl cellulose group, immediately after operation (A), absorption between the bone graft substitutes from postoperative 3 weeks (B) and demonstrates a shadow of a bone fusion mass from 6 weeks (C), one week earlier than the control group.
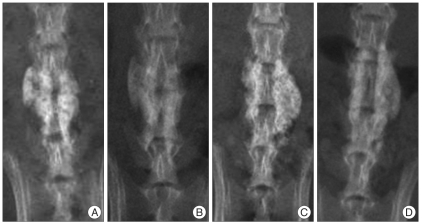
Fig. 3.
At 6 weeks, in particular, the HA-CMC group (C and D) demonstrates significant progression of bone fusion compared with the control group (A and B) : A and C : Immediately after operation, B and D : 6 weeks after operation. HA-CMC : hyaluronate-sodium carboxymethyl cellulose.
Table 2.
Comparison of the radiologic grading system by simple X-ray
HA-CMC : hyaluronate-sodium carboxymethyl cellulose.
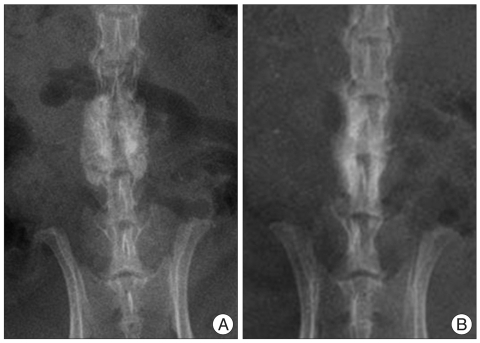
Fig. 4.
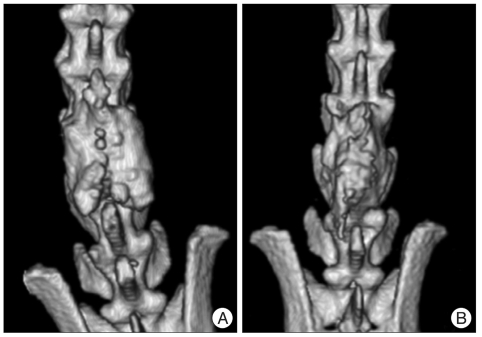
At 9 weeks, no significant differences are found between the two groups, and both groups had a bone fusion (A) control group, (B) HA-CMC group. HA-CMC : hyaluronate-sodium carboxymethyl cellulose.
Fig. 5.
Comparison of the radiologic grading system by simple X-ray.
3D CT scanning
The 3D CT scanning performed at postoperative 4 and 9 weeks showed the result same as the radiographic analysis during the same period. No statistically significant differences were found between the two groups (Table 3) (Fig. 6).
Table 3.
Comparison of the radiologic grading system by three dimensional computed tomography scanning
HA-CMC : hyaluronate-sodium carboxymethyl cellulose.
Fig. 6.
The three dimensional computed tomography scanning performed at postoperative 9 weeks shows the result same as the radiographic analysis during the same period, and both groups had a bone fusion (A) control group, (B) HA-CMC group. HA-CMC : hyaluronate-sodium carboxymethyl cellulose.
Manual palpation
One rat from each group showed "motion", suggesting nonunion. As the other 14 rats of each group did not have the motion, the fusion rate was 93.3%. There were no significant differences between the two groups.
DISCUSSION
Postlaminectomy epidural fibrosis after laminectomy significantly increases the hazards of revision spine surgery and may contribute to the occurrence of failed back syndrome20). Modifications of surgical technique, anti-inflammatory medication, and the use of biologic and synthetic interposing materials, serving as a mechanical barrier between dura and overlying tissue, have been employed in an attempt to prevent or limit the formation of fibrous tissue. A variety of biological, pharmacological, and synthetic materials have been evaluated to address the issue of scar formation in the posterior spine after laminectomy23).
On the other hand, spinal arthrodesis is one of the important success factors for spinal surgery, as it can prevent spinal instability that may occur after extensive postlaminectomy. For this purpose, many surgical techniques and materials facilitating bone fusion have been developed and employed.
To date, however, studies have focused only on the efficacy of anti-adhesive materials and their impact on complications such as infection. No study has reported the effects of anti-adhesive materials on bone fusion. Thus, the purpose of our study was to analyze the impact of the commonly applied anti-adhesive material made of hyaluronate on spinal arthrodesis using rat spinal models. We performed simple serial radiographic examinations from postoperative 1 week to 9 weeks to compare the process and progression of bone fusion between the control group and the HA-CMC group. The 3D CT was performed at postoperative 4 and 9 weeks for a more accurate comparison of bone fusion between the two groups. At 9 weeks, manual palpation was made through autopsy to confirm bone fusion. The intended bone graft substitute is prepared from a mixture composed of β-tricalcium phosphate, monocalcium phosphate, calcium sulfate hemihydrates, and polyphosphate and has the characteristics of a radio-opaque material. Therefore, bone graft substitute that is not resorbed is hard to differentiate with actual bone fusion states on postoperative simple radiographs. Actually, there is no report on when the bone graft substitutes used for this experiment are resorbed and neither any study on the simple radiographic studies of resorption and bone formation. This made it hard to assess the state of the bone fusion. To overcome this difficulty the authors used the grading systems cited earlier to assess the state of bone fusion on the simple radiographs and in order to decrease any bias caused by this method, 3D CT and assessment by manual palpation were also preformed and used for evaluation. On the simple radiographs taken at 6 weeks, the HA-CMC group revealed statistically faster bone resorption and formation of new bone than the control group did. Although statistically not significant, the radiologic grading score for bone fusion was better in the HA-CMC group than in the control group until 7 weeks. The results were also confirmed by 3D CT. By manual palpation, the most accurate method to check bone fusion, no significant differences were found, with both groups demonstrating bone fusion in 93.5%.
Hyaluronic acid is a high-molecular-weight polysaccharide that is richly found in the extracellular matrix of soft connective tissues and synovial fluid5,10,24). Other preliminary investigations suggest that hyaluronic acid is an effective and safe antiscar material that can be applied in vivo without causing significant adverse effects in postlaminectomy animal models16,21,22). Also, hyaluronate has been reported to decrease adhesion formation in areas such as around traumatized tendons11) in the intraperitoneal cavity2,6,13,26,29).
From our experiment with rat models, we found that the HA-CMC did not, at least, cause adverse effects to spinal arthrodesis and rather facilitated the initial bone union process. The key cells involved in bone union are osteocytes, osteoblasts, and osteoclasts. A cell surface receptor expressed by these cells and fibroblasts, CD44, is known to bind hyaluronic acid as well as osteopontin, a secreted noncollagenous phosphoprotein found in bone matrix. In vitro studies have suggested that the interaction between this receptor and matrix elements may enhance cell migration27). Jecob et al.15) suggested that osteoprogenitor cells may migrate in the adjacent hyaluronic acid matrix and deposit bone. On the other hand, when used in tenorrhaphy, hyaluronate is known to prevent adhesion of the ligament and increase expression of vascular endothelial growth factor and type IV collagen, facilitating angiogenesis for revascularization and healing of the ligament12). Based on the results of in vitro studies, we concluded that HA facilitated osteogenic and osteoconductive effects as well as angiogenesis in the bone graft site, leading to union of the bone graft substitute and new bone formation. However, we used radiographic analysis, not histologic and molecular biological analyses, to assess the impact of HA-CMC on bone fusion. Thus, our study could not provide an accurate analysis on the mechanism of HA-CMC's impact on bone fusion. Further studies on this area are considered necessary.
Our experiment was initiated to answer the question "how HA-CMC would affect bone fusion if it were used in spinal decompression and fusion to prevent adhesion?" The results of the experiment indicated that the HA-CMC used in spinal surgery did not have an impact on the final bone fusion in rat spinal models. However, as our results are based on animal models, the risk of additional complications should be considered when applying HA-CMC clinically.
Acknowledgements
This work was supported by a grant from the Kyung Hee university in 2008 (KHU-20080610).
References
- 1.Alkalay RN, Kim DH, Urry DW, Xu J, Parker TM, Glazer PA. Prevention of postlaminectomy epidural fibrosis using bioelastic materials. Spine (Phila Pa 1976) 2003;28:1659–1665. doi: 10.1097/01.BRS.0000083161.67605.40. [DOI] [PubMed] [Google Scholar]
- 2.Belluco C, Meggiolaro F, Pressato D, Pavesio A, Bigon E, Donà M, et al. Prevention of postsurgical adhesions with an autocrosslinked hyaluronan derivative gel. J Surg Res. 2001;100:217–221. doi: 10.1006/jsre.2001.6248. [DOI] [PubMed] [Google Scholar]
- 3.Boden SD, Martin GJ, Jr, Morone M, Ugbo JL, Titus L, Hutton WC. The use of coralline hydroxyapatite with bone marrow, autogenous bone graft, or osteoinductive bone protein extract for posterolateral lumbar spine fusion. Spine (Phila Pa 1976) 1999;24:320–327. doi: 10.1097/00007632-199902150-00003. [DOI] [PubMed] [Google Scholar]
- 4.Boden SD, Schimandle JH, Hutton WC. An experimental lumbar intertransverse process spinal fusion model Radiographic, histologic, and biomechanical healing characteristics. Spine (Phila Pa 1976) 1995;20:412–420. doi: 10.1097/00007632-199502001-00003. [DOI] [PubMed] [Google Scholar]
- 5.Davis L, Aries LJ. An experimental study upon the prevention of adhesions about repaired nerves and tendons. Surgery. 1937;2:877–888. [Google Scholar]
- 6.Diamond MP The Sepracoat Adhesion Study Group. Reduction of de novo postsurgical adhesions by intraoperative precoating with Sepracoat (HAL-C) solution : a prospective, randomized, blinded, placebo-controlled multicenter study. Fertil Steril. 1998;69:1067–1074. doi: 10.1016/s0015-0282(98)00057-0. [DOI] [PubMed] [Google Scholar]
- 7.Elkins TE, Ling FW, Ahokas RA, Abdella TN, Homsey CA, Malinak LR. Adhesion prevention by solutions of sodium carboxymethylcellulose in the rat. II. Fertil Steril. 1984;41:929–932. doi: 10.1016/s0015-0282(16)47910-0. [DOI] [PubMed] [Google Scholar]
- 8.Fredericks CM, Kotry I, Holtz G, Askalani AH, Serour GI. Adhesion prevention in the rabbit with sodium carboxymethylcellulose solutions. Am J Obstet Gynecol. 1986;155:667–670. doi: 10.1016/0002-9378(86)90304-2. [DOI] [PubMed] [Google Scholar]
- 9.Ganzer D, Giese K, Völker L, Pietzner U, Follak N, Merk H. Two-year results after lumbar microdiscectomy with and without prophylaxis of a peridural fibrosis using Adcon-L. Arch Orthop Trauma Surg. 2003;123:17–21. doi: 10.1007/s00402-002-0455-y. [DOI] [PubMed] [Google Scholar]
- 10.Gonzalez RI. Experimental tendon repair within the flexor tunnels; use of polyethylene tubes for improvement of functional results in the dog. Surgery. 1949;26:181–198. [PubMed] [Google Scholar]
- 11.Hagberg L, Gerdin B. Sodium hyaluronate as an adjunct in adhesion prevention after flexor tendon surgery in rabbits. J Hand Surg Am. 1992;17:935–941. doi: 10.1016/0363-5023(92)90474-4. [DOI] [PubMed] [Google Scholar]
- 12.Halici M, Karaoglu S, Canoz O, Kabak S, Baktir A. Sodium hyaluronate regulating angiogenesis during Achilles tendon healing. Knee Surg Sports Traumatol Arthrosc. 2004;12:562–567. doi: 10.1007/s00167-004-0536-2. [DOI] [PubMed] [Google Scholar]
- 13.Hooker GD, Taylor BM, Driman DK. Prevention of adhesion formation with use of sodium hyaluronate-based bioresorbable membrane in a rat model of ventral hernia repair with polypropylene mesh--a randomized, controlled study. Surgery. 1999;125:211–216. [PubMed] [Google Scholar]
- 14.Işik S, Oztürk S, Gürses S, Yetmez M, Güler MM, Selmanpakoğlu N, et al. Prevention of restrictive adhesions in primary tendon repair by HA-membrane : experimental research in chickens. Br J Plast Surg. 1999;52:373–379. doi: 10.1054/bjps.1999.3128. [DOI] [PubMed] [Google Scholar]
- 15.Jacob A, Faddis BT, Chole RA. MeroGel hyaluronic acid sinonasal implants: osteogenic implications. Laryngoscope. 2002;112:37–42. doi: 10.1097/00005537-200201000-00007. [DOI] [PubMed] [Google Scholar]
- 16.Massie JB, Schimizzi AL, Huang B, Kim CW, Garfin SR, Akeson WH. Topical high molecular weight hyaluronan reduces radicular pain post laminectomy in a rat model. Spine J. 2005;5:494–502. doi: 10.1016/j.spinee.2004.12.003. [DOI] [PubMed] [Google Scholar]
- 17.Miller JA, Ferguson RL, Powers DL, Burns JW, Shalaby SW. Efficacy of hyaluronic acid/nonsteroidal anti-inflammatory drug systems in preventing postsurgical tendon adhesions. J Biomed Mater Res. 1997;38:25–33. doi: 10.1002/(sici)1097-4636(199721)38:1<25::aid-jbm4>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 18.O'Loughlin PF, Cunningham ME, Bukata SV, Tomin E, Poynton AR, Doty SB, et al. Parathyroid hormone 1-34 augments spinal fusion, fusion mass volume, and fusion mass quality in a rabbit spinal fusion model. Spine (Phila Pa 1976) 2009;34:121–130. doi: 10.1097/BRS.0b013e318191e687. [DOI] [PubMed] [Google Scholar]
- 19.Quist JJ, Dhert WJ, Meij BP, Visser WJ, Oner FC, Hazewinkel HA, et al. The prevention of peridural adhesions. A comparative long-term histomorphometric study using a biodegradable barrier and a fat graft. J Bone Joint Surg Br. 1998;80:520–526. doi: 10.1302/0301-620x.80b3.8010. [DOI] [PubMed] [Google Scholar]
- 20.Robertson JT. Role of peridural fibrosis in the failed back : a review. Eur Spine J. 1996;5(Suppl 1):S2–S6. doi: 10.1007/BF00298565. [DOI] [PubMed] [Google Scholar]
- 21.Schimizzi AL, Massie JB, Murphy M, Perry A, Kim CW, Garfin SR, et al. High-molecular-weight hyaluronan inhibits macrophage proliferation and cytokine release in the early wound of a preclinical postlaminectomy rat model. Spine J. 2006;6:550–556. doi: 10.1016/j.spinee.2005.12.005. [DOI] [PubMed] [Google Scholar]
- 22.Shih HN, Fang JF, Chen JH, Yang CL, Chen YH, Sung TH, et al. Reduction in experimental peridural adhesion with the use of a crosslinked hyaluronate/collagen membrane. J Biomed Mater Res B Appl Biomater. 2004;71:421–428. doi: 10.1002/jbm.b.30106. [DOI] [PubMed] [Google Scholar]
- 23.Temel SG, Ozturk C, Temiz A, Ersozlu S, Aydinli U. A new material for prevention of epidural fibrosis after laminectomy : oxidized regenerated cellulose (interceed), an absorbable barrier. J Spinal Disord Tech. 2006;19:270–275. doi: 10.1097/01.bsd.0000203946.11546.d9. [DOI] [PubMed] [Google Scholar]
- 24.Thomas SC, Jones LC, Hungerford DS. Hyaluronic acid and its effect on postoperative adhesions in the rabbit flexor tendon. A preliminary look. Clin Orthop Relat Res. 1986:281–289. [PubMed] [Google Scholar]
- 25.Tuncay I, Ozbek H, Atik B, Ozen S, Akpinar F. Effects of hyaluronic acid on postoperative adhesion of tendo calcaneus surgery : an experimental study in rats. J Foot Ankle Surg. 2002;41:104–108. doi: 10.1016/s1067-2516(02)80033-3. [DOI] [PubMed] [Google Scholar]
- 26.Urman B, Gomel V, Jetha N. Effect of hyaluronic acid on postoperative intraperitoneal adhesion formation in the rat model. Fertil Steril. 1991;56:563–567. [PubMed] [Google Scholar]
- 27.Yamazaki M, Nakajima F, Ogasawara A, Moriya H, Majeska RJ, Einhorn TA. Spatial and temporal distribution of CD44 and osteopontin in fracture callus. J Bone Joint Surg Br. 1999;81:508–515. doi: 10.1302/0301-620x.81b3.9398. [DOI] [PubMed] [Google Scholar]
- 28.Yee AJ, Bae HW, Friess D, Robbin M, Johnstone B, Yoo JU. Accuracy and interobserver agreement for determinations of rabbit posterolateral spinal fusion. Spine (Phila Pa 1976) 2004;29:1308–1313. doi: 10.1097/01.brs.0000127184.43765.61. [DOI] [PubMed] [Google Scholar]
- 29.Yoldemir T, Sagol S, Adakan S, Oztekin K, Ozsener S, Karadadas N. Comparison of the reduction of postoperative adhesions by two barriers, one solution, and two pharmacologic agents in the rat uterine model. Fertil Steril. 2002;78:335–339. doi: 10.1016/s0015-0282(02)03224-7. [DOI] [PubMed] [Google Scholar]