Abstract
Objective
The Histoculture Drug Response Assay (HDRA), which measures chemosensitivity using minced tumor tissue on drug-soaked gelfoam, has been expected to overcome the limitations of in vitro chemosensitivity test in part. We analyzed interim results of HDRA in malignant gliomas to see if the test can deserve further clinical trials.
Methods
Thirty-three patients with malignant gliomas were operated and their tumor samples were examined for the chemosensitivity to 10 chosen drugs by HDRA. The most sensitive chemotherapy regimen among those pre-established was chosen based on the number of sensitive drugs or total inhibition rate (IR) of the regimen. The response was evaluated by 3 month magnetic resonance image.
Results
Among 13 patients who underwent total resection of the tumor, 12 showed no evidence of disease and one patient revealed progression. The response rate in 20 patients with residual tumors was 55% (3 complete and 8 partial responses). HDRA sensitivity at the cut-off value of more than one sensitive drug in the applied regimen showed a sensitivity of 100%, specificity of 60% and predictability of 70%. Another cut-off value of >80% of total IR revealed a sensitivity of 100%, specificity of 69%, and predictability of 80%. For 12 newly diagnosed glioblastoma patients, median progression-free survival of the HDRA sensitive group was 21 months, while that of the non-sensitive group was 6 months (p=0.07).
Conclusion
HDRA for malignant glioma was inferred as a feasible method to predict the chemotherapy response. We are encouraged to launch phase 2 clinical trial with chemosensitivity on HDRA.
Keywords: Chemotherapy, Drug sensitivity tests, Malignant glioma
INTRODUCTION
Chemotherapy is assuming an increasingly important role in treatment of malignant glioma. Early trials using a nitrosourea-based chemotherapy regimen showed acceptable tumor response rates of 30-50% in postoperative adjuvant or recurrent tumors12,24,34,49). While radiation has been recognized as a standard therapy for malignant glioma, phase II or III studies of radiation therapy with or without chemotherapy showed only a marginal survival benefit at the cost of significant bone marrow suppression4,8-10) except recent temozolomide concomitant chemoradiotherapy for glioblastoma39,44).
Trials such as the opening of a blood-brain barrier, intra-arterial delivery of chemotherapeutic drugs or a high-dose chemotherapy with autologous bone marrow transplantation have been tried to enhance the efficacy of chemotherapy for malignant gliomas16,21,33). However, these methods have hardly proved to be more effective than conventional chemotherapy.
Since early 1990s, investigators tried to define chemosensitivity by various methods to predict tumor response1,7,11,41,46). Later, the American Society of Clinical Oncology (ASCO) did not recommend the chemosensitivity test to be used in clinical practice outside of the clinical trial setting after the review of literature review35). Although the in vitro analysis for tailoring chemotherapy to individual patients remains a priority, they claimed that several technological limitations were still 'not-solved' at the time of reviewing process. Among those chemosensitivity tests, Histoculture Drug Response Assay (HDRA), which supports three-dimensional growth of minced tumor tissue on a gel soaked in the designated drug, has theoretical advantages, which can solve those limitations in part by both eliminating the bias from culture passage and reflecting tumor environment including drug diffusion into the tumor pieces. The studies using HDRA showed 80-100% evaluability and the results varied among individual patients with the same histological tumor6,15,18,22,23,27,29,30,36,40). In patients with postoperative adjuvant chemotherapy, the HDRA exsensitive group showed improved overall/progression-free survival (PFS) compared to the non-sensitive group in gastric cancer6,23), ovarian cancer29), endometrial cancer18), and head and neck cancer36). In studies, at which the sensitive chemotherapeutic drugs were chosen based on the sensitivity results of HDRA and applied to the patients, increased response or improved survival rate was observed in patients whose tumors were sensitive to HDRA6,15).
We retrospectively analyzed the HDRA results of malignant glioma patients for evaluability and predictability of tumor response. In addition, we evaluated the PFS of patients, to whom chemotherapy regimens applied based on HDRA sensitivity results, to evaluate if it is feasible to launch a phase 2 clinical trial.
MATERIALS AND METHODS
The clinical protocol was approved by the Institutional Review Board of the Korea Cancer Center Hospital (former name of Korea Institute of Radiological and Medical Science) in 2001. Informed consent was obtained from all patients.
Eligibility criteria
Patients with histologically proven malignant gliomas (WHO grade III or IV) whose fresh surgical specimens had been sent to undergo HDRA were considered for enrollment in our study. All patients enrolled met the following criteria : 1) age 18 to 65 years; 2) had received a chemotherapy regimen based on HDRA result; 3) Karnofsky Performance Score (KPS) equal or over 50; 4) bone marrow function compatible to undergo chemotherapy on complete blood count (CBC) profile (hemoglobin over 10 g/dL, leukocyte over 4000/µL, platelet over 100000/µL); 5) normal hepatic and renal function.
Patient characteristics
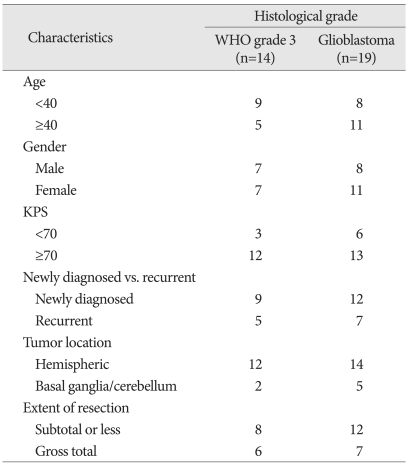
Thirty-five malignant glioma patients agreed to get HDRA. Histological diagnosis included 21 of glioblastoma multiforme, 11 of anaplastic astrocytoma, 2 of anaplastic oligodendroglioma and one of anaplastic pleomorphic xanthoastrocytoma. As two newly diagnosed glioblastoma patients preferred to receive temozolomide concomitant chemoradiotherapy (TMZ CCRT), 33 of them underwent a chemotherapy protocol according the sensitivity result between 2002 and 2009. Mean age was 40.9 years (range 22-65). The number of female patients was 18 and that of male patients was 15. Preoperative KPS was ranged from 50 to 100 (median 80). Tumor location was 'hemispheric' in 26 patients. In six patients, the tumor was extended to basal ganglia and one patient had tumor at cerebellum. Extent of resection was evaluated on postoperative magnetic resonance image (MRI) within 48 hours. Thirteen patients had gross total removal, and another 20 patients were proved to have residual tumors. Twenty-one patients were newly diagnosed malignant glioma and all of them received conventional radiation therapy (5000 to 7020 cGy) one to three months after the completion of chemotherapy protocol. Twelve patients presented with recurrent tumor at the entry of this study. There was no standard protocol after completion of the postoperative chemotherapy for these recurrent patients. These demographic and clinical data according to histopathology was summarized in Table 1.
Table 1.
Clinical characteristics of patients according to histological grade (n=33)
KPS : karnofsky performance score
Histoculture drug response assay
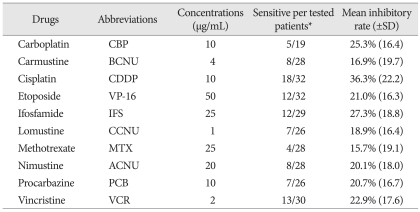
HDRA technique was the same as previously described in the literature20,42). Briefly, tumor specimen (approximately 1 cm in diameter) excluding non-viable portions was freshly harvested from surgical field and contained aseptically to Dulbecco's Modified Eagle Medium (DMEM, Sigma) in 50 mL Falcon tube. After pure mechanical mincing into pieces of approximately 1 mm in diameter, tumor samples were placed onto collagen gels (Gel Foam, Pharmacia & Upjohn, Kalamazoo) immersed in 1 mL of DMEM supplemented with 10% fetal bovine serum and anti-cancer drugs of designated concentration on a 24 well plate. The drugs used are listed in Table 2. The designated in vitro testing concentrations were referenced to literatures and adapted to HDRA culture condition to obtain inhibitory concentration curvature in glioma samples through the evaluability test6,14,19,37,42). After incubation for at least 5 days at 37℃ with 5% CO2, cell viability was tested by MTT [3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide, Sigma] assay28). 100 uL of 0.06% collagenase type I (Sigma) in DMEM and 0.2% MTT in phosphate buffered saline containing 50 mM sodium succinate (Wako Ind.) were added to each well. Plates were incubated for another 4 hours, the medium was removed, and 0.5 mL dimethyl sulfoxide was added to each well to extract the resultant MTT-formazan. Extracts from each well (100 uL) were transferred to a 96-well plate and absorbance measured at 540 nm using a microplate reader (VersaMax). Samples with contamination or absorbance values of less than 15/g of control tumor tissue were classified as "inappropriate". The inhibition rate (IR) of tumor growth was calculated using the following equation : IR (%)=(1-mean absorbance of treated wells per gram of tumor/mean absorbance of control wells per gram of tumor)×100.
Table 2.
The list of drugs tested with their in vitro concentrations
*Sensitive result is defined to be equal or more than 30% of inhibition of tumor growth (see detail in materials & methods)
In our study, the IR cut-off value for chemosensitivity was selected as equal to or greater than 30% (IR30) according to preliminary study result19).
Applied chemotherapy protocol
Candidate chemotherapy protocols were selected from established regimen for malignant glioma or other malignant brain tumors and applied at 2 weeks postoperatively. nimustine (ACNU)-CDDP regimen12), carmustine (BCNU)-cisplatin (CDDP) regimen3), ICE regimen34), and PCV regimen25) were applied as original dose and schedule. High-dose mehtotrexate (MTX) regimen32) was given without other drugs. Each regimen was selected according to the HDRA result as the most number of sensitive drugs included for individual patient. TMZ CCRT regimen, which has methylation status of methyl guanine methyl transferase promoter as an indicator predicting the benefit of chemotherapy, was not included during the period of this study. In case of the same number of sensitive drugs among the regimens, total IR (the sum of inhibition rate of individual drugs in the designated regimen) was used to determine the chemotherapy of choice.
Toxicity evaluation and care
Common Terminology Criteria for Adverse Effect (CTCAE) was referenced to evaluate the toxicities (version 2.0 until 2005 and version 3.0 thereafter). A CBC was checked 10 days after chemotherapy and then twice per week until the bone marrow function recovered. Granulocyte colony stimulating factor was given for patients with grade III or IV myelosuppression (leucocytes <2000/µL, granulocytes <1000/µL). Platelet concentrations were transfused to patients with grade III of CTCAE version 2.0 (<50000/µL) era and grade IV of CTCAE version 3.0 (<25000/µL) era thrombocytopenia or patients with a bleeding episode. If the patients showed grade IV leucopenia, febrile illness or symptomatic bleeding, we recommended hospitalization and neutropenic precautions were taken. Chest X-ray, liver function test, blood urea-nitrogen/creatinine were performed every time before the next cycle of chemotherapy.
Response evaluation
The mass response was evaluated by MRI taken at 3 months after completion of 2-3 cycles of chemotherapy (2 cycles for 6 weeks interval regimen and 3 cycles for 4 weeks interval regimen) as compared to the baseline MRI, which was taken within 48 hours of surgery. MacDonald criteria for malignant glioma was adopted26) as the product of the maximal cross-sectional enhancing diameters are used to access tumor response. Complete response (CR) was defined as disappearance of enhancing tumor and non-measurable disease sustained for at least 4 weeks. Partial response (PR) was defined by a decrease in tumor volume of 50% or more. Progressive disease (PD) was defined as an appearance of any new lesions or estimated increase of more than 25% in the tumor volume. The other status was classified as stable disease (SD). The time-to-progression was defined as the time when the patients showed clinical deterioration with the evidence of radiological recurrence.
Statistical methods
The Kaplan-Meier method was used to obtain progression-free survival using SPSS software (version 12.0, Chicago, IL, USA). Above end-point results were compared between patient groups for HDRA results by Log-rank test. Various factors from HDRA results were also verified their significance for the tumor response using t-test and Fisher's exact test.
RESULTS
Evaluability of malignant glioma samples for HDRA
We counted a number of 'appropriate' results from 10 tested drugs per given amount of samples (1 cm3) and define the results to be 'evaluable' if the test give sensitivity of more than five of tested drugs. Average 7.9 drugs (range 2-10) were tested per sample with appropriate sensitivity results. In two patients, the results were not 'evaluable' as only 2 and 4 appropriate sensitivity results came out, respectively. Thus, apparent evaluable rate was 31 out of 33 tumors (94%). The main reason for failure was inadequate viability of samples, especially necrotic portion of tumor. After careful elimination of necrotic portion, the number appropriate sensitivity results per sample was raised to mean 9.6 drugs (range 8-10) since 2004.
Chemosensitivity based on HDRA
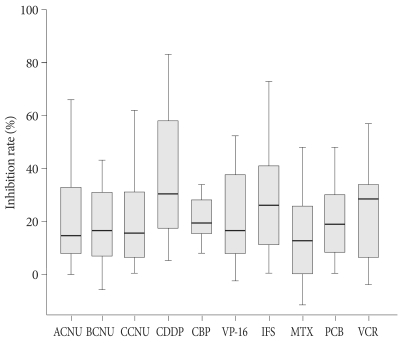
The inhibition rates of tested drugs from each patient are plotted in Fig. 1. Not only the spectrum of sensitive drug among individual patient, but also the inhibition rate of each drug was varied and ranged widely. As an average, the most sensitive drug was CDDP at a IR of 36.3%±22.2% (range 5-83) followed by ifosfamide (IFS) of 27.3%±18.8% (0-73), carboplatin (CBP) of 25.3%±16.4% (8-63), vincristine (VCR) of 22.9%±17.6% (-4-57), etoposide (VP-16) of 21.0%±16.3% (-3-52), procarbazine (PCB) of 20.7%±16.7% (0-70), ACNU of 20.1%±18.0% (0-66), CCNU of 18.9±16.4% (0-62), BCNU of 16.9%±19.7% (-13-43), and MTX of 15.7%±19.1% (-12-79). We adopted IR of more than 30% as a cut-off value for sensitivity of each drug and the number of sensitive drugs in established regimen was one of the most valuable factors for selecting proper chemotherapy regimen. The frequency of sensitivity above the IR threshold was also listed in Table 2. CDDP was the most 'evaluated-as-sensitive' drug in 18 out of 32 tests followed by VCR (13/30), IFS (12/29), VP-16 (12/32), ACNU and BCNU (8/28), CCNU and PCB (7/26), CBP (5/19), and MTX (4/28).
Fig. 1.
The inhibition rates of each drug obtained by HDRA are plotted in histogram. Solid horizontal bars represent median value. Rectangular box is 95% confidence interval and I shape bar indicates range of the observed value, respectively. HDRA : Histoculture Drug Response Assay, ACNU : nimustine, BCNU : carmustine, CCNU : lomustine, CDDP : cisplatin, CBP : carboplatin, VP-16 : etoposide, IFS : ifosfamide, MTX : methotrexate, PCB : procarbazine, VCR : vincristine.
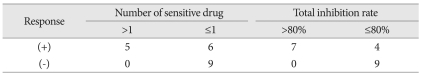
The number of sensitive drugs ranged from 0 to 8, and averaged at 2.8 drugs per patient. As we adopted pre-established chemotherapy protocol, the number of sensitive drugs in a chosen regimen was 1.3 drugs (range 0-3) per the regimen. Four patients had 3 sensitive drugs in the selected regimen, 9 patients had 2 sensitive drugs, 13 patients had one sensitive drug and 7 patients had no sensitive drug in their regimen. For these 'no-sensitive-drug' patients, total inhibition rate (a sum of inhibition rate of each drug in the regimen) was used as selecting criteria for chemotherapy regimen. The most frequently applied regimen was ACNU-CDDP chemotherapy in 13 patients followed by PCV chemotherapy in 9 patients, ICE chemotherapy in 8 patients, 2 cases of BCUN-CDDP chemotherapy and one case of high-dose MTX chemotherapy.
Tumor response rate
Tumor response was evaluated on MRI at 3 months after the chemotherapy. Among 13 patients, whose tumors were totally resected, 12 patients evaluated as 'no evidence of disease' and one patient showed progression (PD). Among other 20 patients with residual tumor, 3 patients showed CR, and 8 patients showed PR. The tumors progressed in 6 patients (PD) and were remained as the range of SD in 3 patients. Thus, the response rate was 55% in patients with residual tumors.
Complications of chemotherapy
Varying degrees of myelosuppression occurred in all patients 2-3 weeks after the chemotherapy. Grade III or IV myelosuppression was observed in 21 (64%) patients. However, at the end of the chemotherapy, all these patients recovered to WHO grade I or II on follow-up CBC. Three out of five patients with grade IV leucopenia were noted to have a fever of unknown origin. Empirical antibiotics could prevent patients from further toxicities. Grade IV thrombocytopenia was observed in 9 (27%) patients and these patients received platelet transfusions. No symptomatic bleeding episodes were happened in all patients. Other toxicities such as renal, hepatic and otologic side effects were not observed. No treatment related mortality occurred in our series.
HDRA results predicting tumor response
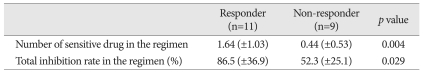
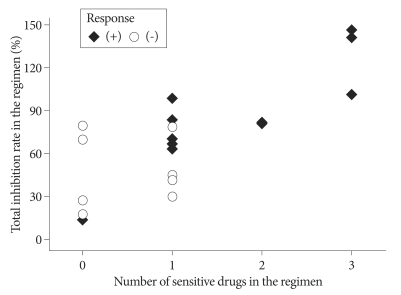
Following the recent recommendation from Response Assessment in Neuro-Oncology working group45), 13 patients without measureable tumor at the baseline MRI were excluded from response analysis. For the response group, the number of sensitive drug included in the chosen chemotherapy regimen was a mean of 1.64±1.03 while that number in the non-response group was 0.44±0.53. The difference was statistically significant (p=0.004, t-test). The total IR of the response group was averaged 86.5%±36.9% while that of non-response group was 52.3%±25.1%. The difference of total IR between the response group and the non-response group was also statistically significant (p=0.029, t-test) (Table 3). To determine the cut-off value of predictability of tumor response, above two indices were coplotted as tumor response remarked (Fig. 2). All five patients having 2 or more sensitive drugs in their regimen showed the tumor response. Five out of 9 patients with one sensitive drug revealed the tumor response and only one patient among 6 patients without sensitive drug in their regimen showed response (Table 4). Thus, apparent sensitivity of more than 1 sensitive drug in the regimen was 100%. Specificity and predictability of this cut-off value were 60% and 70%, respectively. When the cut-off value of equal or more than 1 sensitive drug was applied, the HDRA results showed sensitivity of 73%, specificity of 83% and predictability of 80%. As for another HDRA result of total IR, all 7 patients having more than 80% of total IR showed the tumor response while 4 out of 13 patients of equal or less than 80% got the tumor response after chemotherapy. Thus, the cut-off value of total IR of >80% revealed sensitivity of 100%, specificity of 69%, and predictability of 80% (Table 4).
Table 3.
Sensitivity Indices from HDRA according to the tumor response (n=20)*
*Patients with post-operative non-measurable disease were excluded from the response analysis
HDRA : Histoculture Drug Response Assay
Fig. 2.
Scatter plot representing the number of HDRA sensitive drugs (X axis) and total inhibition rate (the sum of inhibition rate of each drug, Y axis) in the chosen chemotherapy regimen. Filled lozenge is individual who showed tumor response and hallow circle is non-responder. HDRA : Histoculture Drug Response Assay.
Table 4.
Predictability of HDRA sensitivity according to various cut-off values (n=20)*
*Patients with post-operative non-measurable disease were excluded from the response analysis
HDRA : Histoculture Drug Response Assay
Progression-free survival after chemotherapy and overall survival
For patients, who were newly diagnosed and underwent radiation therapy after the chemotherapy, PFS and overall survival (OS) according to histological grade was obtained (7 anaplastic astrocytomas and 12 glioblastomas, separately). For the mean follow-up of 70 months (range 18 to 116), 3 out of 7 anaplastic astrocytoma patients showed progression. Mean PFS of these patients was 75 months. Two patients progressed at 8 and 37 months respectively, received salvage radiosurgery (one patient underwent 2nd operation later on). The other patient, revealed recurrence on 13 months, deferred any re-treatment and died at 18 months. Thus apparent 2, 3 and 5-year survival rate was the same 86%.
For 12 newly diagnosed glioblastoma patients, median PFS was 9 months and the 6-months and one-year PFS was 67% and 38%, respectively. As 9 out of 12 glioblastoma patients were expired during observation period, median OS was 17 months. The 2-year and 3-year survival rates were 46% and 23%, respectively. Two patients recurred at 4 months and 12 months underwent 2nd operation followed by chemotherapy. One patient still alive at 12 months after salvage therapy and the other patients died at 29 months. Another patient recurred at 14 months received radiosurgery but died 3 months after. For 7 recurrent glioblastoma patients, median PFS was 6 months and the 6-months PFS was 38%. Among these, 2 patients showed response to chosen chemotherapy regimen, and recurrence was not observed during the observation period of 4 and 17 months, respectively.
HDRA results affecting progression-free survival
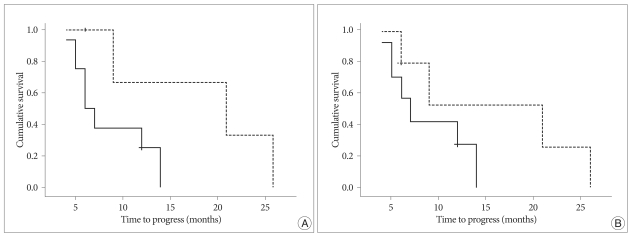
To evaluate if HDRA results affected PFS, Log-rank test was performed in 12 glioblastoma patients, who were newly diagnosed and underwent radiation therapy after the chemotherapy based on HDRA. When the cut-off value of more than one sensitive drug in the regimen was adopted, the sensitive group showed median PFS of 21 months and the non-sensitive group revealed median PFS of 6 months. Although the difference failed to show statistical difference (p=0.067), there's a tendency of increasing PFS in the HDRA sensitive group (Fig. 3A). Another cut-off value of total IR more than 80% was also analyzed to see if the value affects patients PFS. Although the median PFS of HDRA sensitive group in terms of total IR was apparently increased compared to that of HDRA non-sensitive group (21 months versus 7 months), the difference was not statistically significant (p=0.17) (Fig. 3B).
Fig. 3.
Comparison of progression-free survival of patients with newly diagnosed glioblastoma according to the HDRA sensitivity (n=12). A cut-off value of equal or more than 2 sensitive drugs (A) shows a tendency of prolonged progression-free survival in sensitive group (p=0.067), while that of total inhibition rate of more than 80% (B) fails to show statistical significance (p=0.17). Dotted line represents patients with HDRA sensitive result and solid line is for non-sensitive patients at each cut-off value. HDRA : Histoculture Drug Response Assay.
DISCUSSION
Rationale of HDRA
Two meta-analysis of adjuvant chemotherapy in malignant glioma concluded small but clear survival benefit and suggested the need to define the subgroups of patients who would benefit from the chemotherapy5,38). Malignant gliomas are a heterogeneous group of tumors and even in the same histopathology, their response to cytotoxic drugs may differ26). Studies to predict the response from chemotherapy in malignant glioma based on in vitro chemosensitivity has been tried since early 1980's2,17). However, in vitro chemosensitivity test are still not to be considered as standard tool for either predicting response of established chemotherapy protocol or selecting appropriate chemotherapy for individual patient35). In 1989, Yung pointed out two major problems of in vitro chemosensitivity testing and its clinical application in human gliomas; 1) cellular heterogeneity within a single tumor as well as between tumors, 2) artificial selective pressure according to tissue culture system48). At that time, most of chemosensitivity system adopted early-passaged cell lines established from patient's tumor sample, which on a controversy of losing the character of original tumor. The HDRA system using directly minced tumor tissues without culture passage can be free of these selection problems. Even more, three-dimensional tissue culture system of approximately 1 mm diameter can preserve interactions between tumor composing cells and maintain intact cytoarchitecture, possibly compromising drug accessibility, in a state close to the in vivo condition. Regional heterogeneity in human solid tumor histoculture was studied by Weaver et al.43). They measured the labeling index of tumor cells by automated image analysis and compared drug sensitivity parameters between the region with the highest proliferative activity and entire tumor tissue. In spite of definite difference of labeling index, pharmacodynamic parameters including inhibitory concentration of 30, 50 and 70% were not significantly different. Our procedure of selecting viable portion for tissue culture especially in case of glioblastoma, which have necrotic portion in nature, can drive toward more proliferative region. Although it is still on debate whether the most proliferative region of tumor can represent drug resistance, according to the result of Weaver et al., this selection can reflect the drug sensitivity characteristics of entire tumor.
As ASCO working group suggested, the assay-guided choice of chemotherapeutic agent is of clinical importance but still yet to have problems to be solved such as various results across different disease sites, time frame affecting clinical decision and giving the same choice of chemotherapy that would have been chosen in the absence of assay result. Studies in other solid tumors observed different sensitivity according to the HDRA results with multiple chemotherapy regimens and revealed correlation between HDRA sensitivity and clinical response15,31,47). However, the results should be accepted in caution due to relatively small number of patients or non-randomized, 'not-controlled' nature of the trials. Iwadate et al.17) performed clinical trial to chose chemotherapeutic agents in glioblastoma patients. They prepared tumor cell suspension by mincing and exposed to the drug for 8 hours, and then cultured in drug-free medium for 72 hours. Drug sensitivity was measured by flow cytometric detection of apoptosis. They treated patients with two or three drugs showing the highest sensitivity on unrevealed 'dose and schedule' modified by their own criteria in conjunction with conventional radiation. In spite of the above shortcomings, median survival of 20.5 months and 3-year survival rate of 10% can be considered as improving result compared to historical data and gave a possibility of providing tailored chemotherapy regimen based on in vitro drug sensitivity.
In our study, the established chemotherapy regimen, which has been used for malignant gliomas, was applied without any modification of dose and schedule. Our strategy had an advantage that side-effects can be expected from previous studies and experiences. The overall incidence of hematologic toxicity was not different from the historical data and all toxicities could be managed without fatality in our series. Although Iwadate et al. described that there were no significant side effects, we would like to suggest that new combination of sensitive drugs should be approached cautiously at an aware of unexpected side-effects or drug interactions. For more direct comparison of the choice based on the assay, we should have two controlled groups of one following HDRA and the other neglecting HDRA in the future study.
Drug concentration and cut-off values
Chemosensitivity mainly depends on the cut-off values for IR and drug concentration of in vitro setting. It is obvious that high concentration or low cut-off value leads to more of false positive result and vice versa if low concentration or high cut-off value was adopted. Hasegawa et al. studied the relationship between these factors and clinical response in induction chemotherapy of head and neck cancers13). They concluded that optimal drug concentration can be obtained from dose-response curve of different concentration of tested drug and the cut-off value should be confirmed by its predictability for clinical response. In our study, we were unable to obtain a sophisticated dose-response curve of each drug used for malignant glioma. However, we adjusted the drug concentration from previous histoculture studies and determined final concentration through preliminary study with malignant brain tumor samples to obtain acceptable range of inhibition rate19). Our average inhibition rate of tested drugs in the regimen ranged from 16 to 36% and the distribution of IR in each drug could validate our drug concentration and 30% IR as a cut-off value. However, to obtain more solid proof of optimal concentration, it should be confirmed that the combination of a drug concentration and cut-off value correlate with clinical response through a study with large number of patients.
CONCLUSION
This is a pilot study to evaluate technical feasibility of HDRA in malignant glioma if it can yield high proportion of interpretable results and the difference between individual patients were varied enough to affect the choice of pre-established chemotherapy regimen. Although our interim results from small number of patients are not enough to provide any decisive information, high evaluability and sensitivity of the assay with an observed tendency of prolonged PFS in the sensitive group deserve further prospective clinical trials using HDRA sensitivity result as a predictor of chemotherapy response in malignant gliomas.
References
- 1.Andreotti PE, Cree IA, Kurbacher CM, Hartmann DM, Linder D, Harel G, et al. Chemosensitivity testing of human tumors using a microplate adenosine triphosphate luminescence assay : clinical correlation for cisplatin resistance of ovarian carcinoma. Cancer Res. 1995;55:5276–5282. [PubMed] [Google Scholar]
- 2.Bogdahn U. Chemosensitivity of malignant human brain tumors. Preliminary results. J Neurooncol. 1983;1:149–166. doi: 10.1007/BF00182961. [DOI] [PubMed] [Google Scholar]
- 3.Boiardi A, Eoli M, Salmaggi A, Pollo B, Milanesi I, Broggi G, et al. Cisplatin and BCNU chemotherapy for anaplastic oligoastrocytomas. J Neurooncol. 2000;49:71–75. doi: 10.1023/a:1006489919811. [DOI] [PubMed] [Google Scholar]
- 4.Choi IS, Lee SH, Kim TY, Bang JS, Paek SH, Kim S, et al. Phase II study of chemotherapy with ACNU plus cisplatin followed by cranial irradiation in patients with newly diagnosed glioblastoma multiforme. J Neurooncol. 2002;60:171–176. doi: 10.1023/a:1020605617452. [DOI] [PubMed] [Google Scholar]
- 5.Fine HA, Dear KB, Loeffler JS, Black PM, Canellos GP. Meta-analysis of radiation therapy with and without adjuvant chemotherapy for malignant gliomas in adults. Cancer. 1993;71:2585–2597. doi: 10.1002/1097-0142(19930415)71:8<2585::aid-cncr2820710825>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 6.Furukawa T, Kubota T, Hoffman RM. Clinical applications of the histoculture drug response assay. Clin Cancer Res. 1995;1:305–311. [PubMed] [Google Scholar]
- 7.Furukawa T, Kubota T, Watanabe M, Takahara T, Yamaguchi H, Takeuchi T, et al. High in vitro-in vivo correlation of drug response using sponge-gel-supported three-dimensional histoculture and the MTT end point. Int J Cancer. 1992;51:489–498. doi: 10.1002/ijc.2910510325. [DOI] [PubMed] [Google Scholar]
- 8.Gilbert M, O'Neill A, Grossman S, Grunnet M, Mehta M, Jubelirer S, et al. A phase II study of preradiation chemotherapy followed by external beam radiotherapy for the treatment of patients with newly diagnosed glioblastoma multiforme : an Eastern Cooperative Oncology Group study (E2393) J Neurooncol. 2000;47:145–152. doi: 10.1023/a:1006402123397. [DOI] [PubMed] [Google Scholar]
- 9.Grossman SA, O'Neill A, Grunnet M, Mehta M, Pearlman JL, Wagner H, et al. Phase III study comparing three cycles of infusional carmustine and cisplatin followed by radiation therapy with radiation therapy and concurrent carmustine in patients with newly diagnosed supratentorial glioblastoma multiforme : Eastern Cooperative Oncology Group Trial 2394. J Clin Oncol. 2003;21:1485–1491. doi: 10.1200/JCO.2003.10.035. [DOI] [PubMed] [Google Scholar]
- 10.Grossman SA, Wharam M, Sheidler V, Kleinberg L, Zeltzman M, Yue N, et al. Phase II study of continuous infusion carmustine and cisplatin followed by cranial irradiation in adults with newly diagnosed high-grade astrocytoma. J Clin Oncol. 1997;15:2596–2603. doi: 10.1200/JCO.1997.15.7.2596. [DOI] [PubMed] [Google Scholar]
- 11.Guo HY, Colangelo D, Li L, Connors KM, Kubota T, Hoffman RM. In vitro histoculture of human tumors with fluorescent dye end-points measured by confocal microscopy : high correlation of in vitro and in vivo chemosensitivity. Anticancer Res. 1992;12:1055–1061. [PubMed] [Google Scholar]
- 12.Gwak HS, Youn SM, Kwon AH, Lee SH, Kim JH, Rhee CH. ACNU-cisplatin continuous infusion chemotherapy as salvage therapy for recurrent glioblastomas : phase II study. J Neurooncol. 2005;75:173–180. doi: 10.1007/s11060-005-1858-8. [DOI] [PubMed] [Google Scholar]
- 13.Hasegawa Y, Goto M, Hanai N, Ijichi K, Adachi M, Terada A, et al. Evaluation of optimal drug concentration in histoculture drug response assay in association with clinical efficacy for head and neck cancer. Oral Oncol. 2007;43:749–756. doi: 10.1016/j.oraloncology.2006.09.003. [DOI] [PubMed] [Google Scholar]
- 14.Haselsberger K, Peterson DC, Thomas DG, Darling JL. Assay of anticancer drugs in tissue culture : comparison of a tetrazolium-based assay and a protein binding dye assay in short-term cultures derived from human malignant glioma. Anticancer Drugs. 1996;7:331–338. [PubMed] [Google Scholar]
- 15.Hirano Y, Ushiyama T, Suzuki K, Fujita K. Clinical application of an in vitro chemosensitivity test, the Histoculture Drug Response Assay, to urological cancers : wide distribution of inhibition rates in bladder cancer and renal cell cancer. Urol Res. 1999;27:483–488. doi: 10.1007/s002400050139. [DOI] [PubMed] [Google Scholar]
- 16.Hochberg FH, Parker LM, Takvorian T, Canellos GP, Zervas NT. High-dose BCNU with autologous bone marrow rescue for recurrent glioblastoma multiforme. J Neurosurg. 1981;54:455–460. doi: 10.3171/jns.1981.54.4.0455. [DOI] [PubMed] [Google Scholar]
- 17.Iwadate Y, Fujimoto S, Namba H, Yamaura A. Promising survival for patients with glioblastoma multiforme treated with individualised chemotherapy based on in vitro drug sensitivity testing. Br J Cancer. 2003;89:1896–1900. doi: 10.1038/sj.bjc.6601376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kanasugi M, Aoki D, Suzuki N, Susumu N, Nakata S, Horiuchi M, et al. Sensitivity to cisplatin determined by the histoculture drug response assay and clinical response of endometrial cancer. Int J Gynecol Cancer. 2006;16:409–415. doi: 10.1111/j.1525-1438.2006.00409.x. [DOI] [PubMed] [Google Scholar]
- 19.Kim IK, Yoo H, Shin SH, Youn SM, Rhee CH, Lee SH, et al. Histoculture drug response assay for brain tumors : a preliminary study for evaluability and compatibility. J Korean Brain Tumor Soc. 2010;9:67–73. [Google Scholar]
- 20.Kim JC, Kim DD, Lee YM, Kim TW, Cho DH, Kim MB, et al. Evaluation of novel histone deacetylase inhibitors as therapeutic agents for colorectal adenocarcinomas compared to established regimens with the histoculture drug response assay. Int J Colorectal Dis. 2009;24:209–218. doi: 10.1007/s00384-008-0590-1. [DOI] [PubMed] [Google Scholar]
- 21.Kochii M, Kitamura I, Goto T, Nishi T, Takeshima H, Saito Y, et al. Randomized comparison of intra-arterial versus intravenous infusion of ACNU for newly diagnosed patients with glioblastoma. J Neurooncol. 2000;49:63–70. doi: 10.1023/a:1006457502972. [DOI] [PubMed] [Google Scholar]
- 22.Kodera Y, Ito S, Fujiwara M, Mochizuki Y, Ohashi N, Ito Y, et al. In vitro chemosensitivity test to predict chemosensitivity for paclitaxel, using human gastric carcinoma tissues. Int J Clin Oncol. 2006;11:449–453. doi: 10.1007/s10147-006-0618-x. [DOI] [PubMed] [Google Scholar]
- 23.Kubota T, Sasano N, Abe O, Nakao I, Kawamura E, Saito T, et al. Potential of the histoculture drug-response assay to contribute to cancer patient survival. Clin Cancer Res. 1995;1:1537–1543. [PubMed] [Google Scholar]
- 24.Lassen U, Kristjansen PE, Wagner A, Kosteljanetz M, Poulsen HS. Treatment of newly diagnosed glioblastoma multiforme with carmustine, cisplatin and etoposide followed by radiotherapy. A phase II study. J Neurooncol. 1999;43:161–166. doi: 10.1023/a:1006254716877. [DOI] [PubMed] [Google Scholar]
- 25.Levin VA, Edwards MS, Wright DC, Seager ML, Schimberg TP, Townsend JJ, et al. Modified procarbazine, CCNU, and vincristine (PCV 3) combination chemotherapy in the treatment of malignant brain tumors. Cancer Treat Rep. 1980;64:237–244. [PubMed] [Google Scholar]
- 26.Macdonald DR, Cascino TL, Schold SC, Jr, Cairncross JG. Response criteria for phase II studies of supratentorial malignant glioma. J Clin Oncol. 1990;8:1277–1280. doi: 10.1200/JCO.1990.8.7.1277. [DOI] [PubMed] [Google Scholar]
- 27.Morioka H, Yabe H, Morii T, Yamada R, Kato S, Yuasa S, et al. In vitro chemosensitivity of human soft tissue sarcoma. Anticancer Res. 2001;21:4147–4151. [PubMed] [Google Scholar]
- 28.Mosmann T. Rapid colorimetric assay for cellular growth and survival : application to proliferation and cytotoxicity assays. J Immunol Methods. 1983;65:55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- 29.Nakada S, Aoki D, Ohie S, Horiuchi M, Suzuki N, Kanasugi M, et al. Chemosensitivity testing of ovarian cancer using the histoculture drug response assay : sensitivity to cisplatin and clinical response. Int J Gynecol Cancer. 2005;15:445–452. doi: 10.1111/j.1525-1438.2005.15307.x. [DOI] [PubMed] [Google Scholar]
- 30.Ohie S, Udagawa Y, Kozu A, Komuro Y, Aoki D, Nozawa S, et al. Cisplatin sensitivity of ovarian cancer in the histoculture drug response assay correlates to clinical response to combination chemotherapy with cisplatin, doxorubicin and cyclophosphamide. Anticancer Res. 2000;20:2049–2054. [PubMed] [Google Scholar]
- 31.Pathak KA, Juvekar AS, Radhakrishnan DK, Deshpande MS, Pai VR, Chaturvedi P, et al. In vitro chemosensitivity profile of oral squamous cell cancer and its correlation with clinical response to chemotherapy. Indian J Cancer. 2007;44:142–146. doi: 10.4103/0019-509x.39376. [DOI] [PubMed] [Google Scholar]
- 32.Poortmans PM, Kluin-Nelemans HC, Haaxma-Reiche H, Van't Veer M, Hansen M, Soubeyran P, et al. High-dose methotrexate-based chemotherapy followed by consolidating radiotherapy in non-AIDS-related primary central nervous system lymphoma : European Organization for Research and Treatment of Cancer Lymphoma Group Phase II Trial 20962. J Clin Oncol. 2003;21:4483–4488. doi: 10.1200/JCO.2003.03.108. [DOI] [PubMed] [Google Scholar]
- 33.Qin D, Ou G, Mo H, Song Y, Kang G, Hu Y, et al. Improved efficacy of chemotherapy for glioblastoma by radiation-induced opening of blood-brain barrier : clinical results. Int J Radiat Oncol Biol Phys. 2001;51:959–962. doi: 10.1016/s0360-3016(01)01735-7. [DOI] [PubMed] [Google Scholar]
- 34.Sanson M, Ameri A, Monjour A, Sahmoud T, Ronchin P, Poisson M, et al. Treatment of recurrent malignant supratentorial gliomas with ifosfamide, carboplatin and etoposide : a phase II study. Eur J Cancer. 1996;32A:2229–2235. doi: 10.1016/s0959-8049(96)00299-7. [DOI] [PubMed] [Google Scholar]
- 35.Schrag D, Garewal HS, Burstein HJ, Samson DJ, Von Hoff DD, Somerfield MR. American Society of Clinical Oncology Technology Assessment: chemotherapy sensitivity and resistance assays. J Clin Oncol. 2004;22:3631–3638. doi: 10.1200/JCO.2004.05.065. [DOI] [PubMed] [Google Scholar]
- 36.Singh B, Li R, Xu L, Poluri A, Patel S, Shaha AR, et al. Prediction of survival in patients with head and neck cancer using the histoculture drug response assay. Head Neck. 2002;24:437–442. doi: 10.1002/hed.10066. [DOI] [PubMed] [Google Scholar]
- 37.Skalski V, Rivas J, Panasci L, McQuillan A, Feindel W. The cytotoxicity of sarcosinamide chloroethylnitrosourea (SarCNU) and BCNU in primary gliomas and glioma cell lines : analysis of data in reference to theoretical peak plasma concentrations in man. Cancer Chemother Pharmacol. 1988;22:137–140. doi: 10.1007/BF00257311. [DOI] [PubMed] [Google Scholar]
- 38.Stewart LA. Chemotherapy in adult high-grade glioma : a systematic review and meta-analysis of individual patient data from 12 randomised trials. Lancet. 2002;359:1011–1018. doi: 10.1016/s0140-6736(02)08091-1. [DOI] [PubMed] [Google Scholar]
- 39.Stupp R, Mason WP, van den Bent MJ, Weller M, Fisher B, Taphoorn MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352:987–996. doi: 10.1056/NEJMoa043330. [DOI] [PubMed] [Google Scholar]
- 40.Suda S, Akiyama S, Sekiguchi H, Kasai Y, Ito K, Nakao A. Evaluation of the histoculture drug response assay as a sensitivity test for anticancer agents. Surg Today. 2002;32:477–481. doi: 10.1007/s005950200080. [DOI] [PubMed] [Google Scholar]
- 41.Vescio RA, Connors KM, Kubota T, Hoffman RM. Correlation of histology and drug response of human tumors grown in native-state three-dimensional histoculture and in nude mice. Proc Natl Acad Sci U S A. 1991;88:5163–5166. doi: 10.1073/pnas.88.12.5163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Vescio RA, Redfern CH, Nelson TJ, Ugoretz S, Stern PH, Hoffman RM. In vivo-like drug responses of human tumors growing in three-dimensional gel-supported primary culture. Proc Natl Acad Sci USA. 1987;84:5029–5033. doi: 10.1073/pnas.84.14.5029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Weaver JR, Wientjes MG, Au JL. Regional heterogeneity and pharmacodynamics in human solid tumor histoculture. Cancer Chemother Pharmacol. 1999;44:335–342. doi: 10.1007/s002800050986. [DOI] [PubMed] [Google Scholar]
- 44.Wen PY, Kesari S. Malignant gliomas in adults. N Engl J Med. 2008;359:492–507. doi: 10.1056/NEJMra0708126. [DOI] [PubMed] [Google Scholar]
- 45.Wen PY, Macdonald DR, Reardon DA, Cloughesy TF, Sorensen AG, Galanis E, et al. Updated response assessment criteria for high-grade gliomas : response assessment in neuro-oncology working group. J Clin Oncol. 2010;28:1963–1972. doi: 10.1200/JCO.2009.26.3541. [DOI] [PubMed] [Google Scholar]
- 46.Yamauchi M, Satta T, Ito A, Kondo T, Takagi H. A feasibility study of the SDI test for the evaluation of gastrointestinal cancer sensitivity to anticancer drugs. J Surg Oncol. 1991;47:253–260. doi: 10.1002/jso.2930470410. [DOI] [PubMed] [Google Scholar]
- 47.Yoshimasu T, Oura S, Hirai I, Tamaki T, Kokawa Y, Hata K, et al. Data acquisition for the histoculture drug response assay in lung cancer. J Thorac Cardiovasc Surg. 2007;133:303–308. doi: 10.1016/j.jtcvs.2006.06.030. [DOI] [PubMed] [Google Scholar]
- 48.Yung WK. In vitro chemosensitivity testing and its clinical application in human gliomas. Neurosurg Rev. 1989;12:197–203. doi: 10.1007/BF01743984. [DOI] [PubMed] [Google Scholar]
- 49.Yung WK, Janus TJ, Maor M, Feun LG. Adjuvant chemotherapy with carmustine and cisplatin for patients with malignant gliomas. J Neurooncol. 1992;12:131–135. doi: 10.1007/BF00172662. [DOI] [PubMed] [Google Scholar]