Abstract
Objective
The goals of surgical intervention for metastatic spinal cord compression (MSCC) are prolonging survival and improving quality of life. Non-ambulatory paraplegic patients, either at presentation or after treatment, have a much shorter life expectancy than ambulatory patients. We therefore analyzed prognostic factors for survival and postoperative ambulation in patients surgically treated for MSCC.
Methods
We assessed 103 patients with surgically treated MSCC who presented with lower extremity weakness between January 2001 and December 2008. Factors prognostic for overall survival (OS) and postoperative ambulation, including surgical method, age, sex, primary tumor site, metastatic spinal site, surgical levels, Tokuhashi score, and treatment with chemo- or radiation therapy, were analyzed retrospectively.
Results
Median OS was significantly longer in the postoperatively ambulatory group [11.0 months; 95% confidence interval (CI), 9.29-12.71 months] than in the non-ambulatory group (5.0 months; 95% CI, 1.80-8.20 months) (p=0.035). When we compared median OS in patients with high (9-11) and low (0-8) Tokuhashi scores, they were significantly longer in the former (15.0 months; 95% CI, 9.29-20.71 months vs. 9.0 months; 95% CI, 7.48-10.52 months; p=0.003). Multivariate logistic regression analysis showed that preoperative ambulation with or without aid [odds ratio (OR) 5.35; 95% CI 1.57-18.17; p=0.007] and hip flexion power greater than grade III (OR 6.23; 95% CI, 1.29-7.35; p=0.038) were prognostic of postoperative ambulation.
Conclusion
We found that postoperative ambulation and preoperative high Tokuhashi score were significantly associated with longer patient survival. In addition, preoperative hip flexion power greater than grade III was critical for postoperative ambulation.
Keywords: Spinal metastasis, Survival, Ambulation, Cord compression, Hip flexion, Prognostic factor
INTRODUCTION
Metastatic spinal cord compression (MSCC) eventually affects 5-10% of patients with cancer, and spinal instrumentation with direct decompressive surgery has become the standard treatment for metastatic lesions1,2,5,7,9,17). The goals of surgical intervention are to prolong patient survival and improve quality of life4). Non-ambulatory paraplegic patients, either at presentation or after treatment, have a much shorter life expectancy than ambulatory patients8,12,14,18,21,28). Thus, proper patient selection and careful surgical planning are prerequisites for low complication rates, acceptable function, and improved overall prognosis15,18).
Although technically demanding, en bloc surgical resections reduce the risk of tumor recurrence; however, they do not necessarily increase survival in patients with metastatic spinal tumors and are associated with higher complication rates4). Previous studies, however, did not determine the ideal extent of palliative surgery or the proper surgical method, such as circumferential removal versus posterior decompression. We therefore analyzed the factors prognostic for postoperative ambulation in patients who undergo surgery for MSCC. We also compared patient survival period and functional outcomes (pain and Barthel Index) relative to preoperative characteristics and surgical methods.
MATERIALS AND METHODS
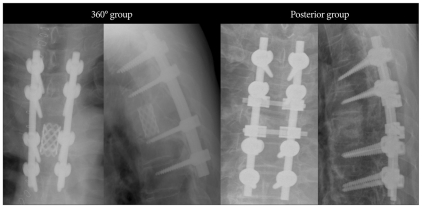
We evaluated 103 consecutive patients (64 men, 39 women; mean age, 54.6 years; range, 25-75 years) with MSCC who presented with lower extremity weakness, with or without back pain, and underwent surgery between January 2001 and December 2008. The mean follow-up duration was 25.8 months (range, 1 to 129 months). Patients were classified by sex, primary tumor site, metastatic site, surgical level, Tokuhashi score, postoperative radiation therapy, postoperative chemotherapy, pre- and post-operative ambulation status, preoperative motor power of hip flexion and surgical method (posterior vs. 360° group). The posterior group was defined by maximal removal of tumor around the spinal cord, including pediculectomy, and fixation in only one direction without anterior support, whereas the 360° group was defined by gross total tumor removal with anterior support and fixation (Fig. 1). The selection of surgery was determined mainly by the different surgical policies of two surgeons. All the cases had vertebral body involvement which compressed the spinal cord with or without posterior column involvement. One surgeon tried to choose only one direction, mainly posterior, but the other surgeon chose the posterior or 360° approach selectively considering the vertebral involvement of metastasis and the general condition of patients. The baseline demographic and clinical characteristics of these groups are shown in Table 1 and detailed primary tumor origins are shown in Table 2.
Fig. 1.
Two different surgical methods are shown. The amount of anterior column removal is larger and anterior support is done in 360° group.
Table 1.
Demographic and clinical characteristics of patient groups
No differences of preoperative characteristics between two group were observed (p>0.05)
Table 2.
Patient survivals relative to primary tumor origin
CI: confidence interval
Although all patients were recommended to undergo radiotherapy (30 gray in 10 fractions) and chemotherapy after surgery, some did not.
Age and interval between weakness and surgery were assessed as continuous variables. Patients were classified into two groups based on primary tumor origin : those with good prognosis (patients with multiple myeloma, thyroid cancer, kidney cancer, breast cancer, prostate cancer, and lymphoma) and those with poor prognosis (patients with colon cancer, stomach cancer, hepatocellular carcinoma, lung cancer, hepatobiliary cancer, thymic cancer, uterine cancer, bladder cancer, and cancer of unknown origin)4,19,22,23,26,27). Metastatic sites were classified into the cervical, thoracic, and lumbar areas. Tokuhashi score was classified into three groups : 0-8, 9-11, and 12-1518,23). Ambulation status was checked at the maximally improved state and classified as ambulatory, including patients with normal ambulation and ambulation with and without aid, and nonambulatory, including patients who could only move in a wheelchair19,20). In addition, we also assessed preoperative motor power of hip flexion as a prognostic factor for postoperative ambulation. The ability of these factors to predict patient survival was analyzed by uni- and multivariate Cox proportional hazard factors analysis (Table 3, 4), and their ability to predict post-operative ambulation status was analyzed by uni- and multivariate logistic regression (Table 5, 6).
Table 3.
Univariate Cox proportional hazard models for survival
Good prognosis: patients with multiple myeloma, thyroid, kidney, breast, prostate cancer, and lymphoma. Poor prognosis: patients with colon, stomach, liver, lung, hepatobiliary, thymus, uterus, bladder cancer, and cancer of unknown origin. CI: confidence interval
Table 4.
Multivariate Cox proportional hazard models for survival
*Means statistically significant factor (p≤0.05) . CI: confidence interval
Table 5.
Univariate logistic regression for post-operative ambulation with or without aid
CI: confidence interval
Table 6.
Multivariate logistic regression for post-operative ambulation with or without aid
*means statistically significant factor (p≤0.05). CI: confidence interval
Overall survival (OS) of patients and subgroups was analyzed by the Kaplan-Meier method and compared by the log rank test.
Back pain, assessed using a visual analogue scale (VAS), and functional status, assessed using the Barthel index, were determined pre- and post-operatively and compared relative to different surgical methods. Postoperative back pain and Barthel index was assessed at the maximally improved state23).
RESULTS
Survival analysis
The median OS of all patients was 10.0 months (95% CI, 8.21-11.80 months) (Fig. 2). When we compared median OS in postoperatively ambulatory and non-ambulatory patients, we found that it was significantly higher in the ambulatory group (11.0 months; 95% CI, 9.29-12.71 months) than in the non-ambulatory group (5.0 months; 95% CI, 1.80-8.20 months) (p=0.035) (Fig. 3). When we compared median OS in patients with high (9-11) and low (0-8) Tokuhashi scores, we found they were significantly longer in the former (15.0 months; 95% CI, 9.29-20.71 months vs. 9.0 months; 95% CI, 7.48-10.52 months; p=0.003) (Fig. 4).
Fig. 2.
This graph shows Kaplan-Meier overall survival curve of surgically treated MSCC patients. Table shows 10-month median survival. metastatic spinal cord compression (MSCC)
Fig. 3.
These two graphs show Kaplan-Meier survival curves of post-operative ambulation group (blue colored graph) and non-ambulation group (green colored graph). Median survival of post-operative ambulation group is longer than that of non-ambulation group. Table of this figure shows 11-month and 5-month median survival in the post-operative ambulation group (blue colored character) and the non-ambulation group (green colored character). Log rank test also shows statistically significant difference between two groups (p=0.035).
Fig. 4.
These two graphs show Kaplan-Meier survival curves of low Tokuhashi score group (blue colored graph) and high Tokuhashi score group (green colored graph). Median survival of low Tokuhashi score group is shorter than that of high Tokuhashi score group. Table of this figure shows 9-month and 15-month median survival in the low Tokuhashi score group (blue colored character) and the high Tokuhashi score group (green colored character). Log rank test also shows statistically significant difference between two groups (p=0.003).
OS in 15 categories of primary tumor origin was analyzed by the Kaplan-Meier method and compared by the log rank test. We found that survival was not related to primary tumor origin (Table 2). However, if we classified these origins into two groups, i.e. good and poor prognosis groups, we found OS of good prognosis group was significantly longer than that of poor prognosis group (12.0 months; 95% CI, 9.32-14.68 months vs. 9.0 months; 95% CI, 7.25-10.75 months; p=0.039) (Fig. 5).
Fig. 5.
These two graphs show Kaplan-Meier survival curves of good prognosis group (blue colored graph) and poor prognosis group (green colored graph). Median survival of poor prognosis is shorter than that of good prognosis group. Table of this figure shows 12-month and 9-month median survival in the good prognosis group (blue colored character) and the poor prognosis group (green colored character). Log rank test also shows statistically significant difference between two groups (p=0.039).
Uni- and multivariate Cox proportional hazard analysis showed that primary origin with good prognosis [hazard ratio (HR) 0.627; 95% CI, 0.479-0.899, p=0.039], high Tokuhashi score (HR 0.524; 95% CI, 0.335-0.820, p=0.005), postoperative ambulation, with or without aid (HR 1.59; 95% CI, 1.021-2.645, p=0.048), were significantly associated with OS (Table 3, 4). None of the other factors, including operative method, age, metastatic site, surgical levels, radiation therapy, and chemotherapy was significant for survival in uni- and multivariate Cox proportional hazard analysis (Table 3, 4).
Postoperative ambulation
Uni- and multivariate logistic regression analysis showed that preoperative ambulation with or without aid [odds ratio (OR) 5.35; 95% CI, 1.57-18.17; p= 0.007] and hip flexion greater than grade III (OR 6.23; 95% CI, 1.29-7.35; p=0.039) were prognostic for postoperative ambulation. None of the other factors, including operative method, age, sex, primary origin, metastatic site, surgical levels, Tokuhashi score, radiation therapy, chemotherapy, and interval between weakness and surgery was significant for survival in uni- and multivariate logistic regression analysis (Table 5, 6).
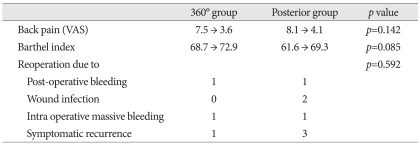
The scores for back pain (VAS) and Barthel index were improved after surgery, but did not differ significantly between patients who underwent posterior and 360° surgery. Ten patients (9.7%) experienced surgical complications requiring second surgery, such as wound infections, extensive bleeding, and symptomatic recurrence. None of these patients, however, showed evidence of instrument failure, such as loosening, displacement or fracture (Table 7).
Table 7.
Pain, functional improvement, and complications
Back pain (VAS) and Barthel index was measured before surgery and at maximal improvement state. VAS: visual analogue scale
DISCUSSION
Recent advances in neuroimaging and surgical techniques have led to direct decompressive surgery with instrumentation becoming the standard treatment for MSCC1,2,10). The goals of surgical intervention are to prolong patient survival and improve quality of life4). The efficacy of palliative surgery for metastatic spinal tumors has been assessed by ambulation status and survival time6,16,27). Other objectives in treatment of spinal metastasis include the prevention of neurological decline, the alleviation of pain, the restoration of lost neurological function, and the stabilization of the spine4).
Survival analysis
Patients with postoperative ambulatory function have a much longer life expectancy than those without ambulatory function18,21,28), suggesting a close relationship between ambulation and survival. Tokuhashi score has also been shown useful in predicting survival18,24). We also found that postoperative ambulation status and Tokuhashi score were significantly related to OS. All of our patients had Tokuhashi scores below 11 because patients who had good preoperative Tokuhashi scores seldom showed neurological deterioration and were not referred for surgical treatment.
Tumor type has been shown to predict survival in patients with MSCC. Patients with myeloma or metastases from thyroid, kidney, breast and prostate cancer have been reported to live significantly longer than those with sarcoma and metastases from liver, lung, colon, uterus, head and neck, bladder, thymus, pancreas, stomach and esophagus cancer, and cancer of unknown primary site1,3,4,19,22,24,25). However, our survival analysis couldn't show statistically significant difference among the 15 tumor types. This result seems to be related with smaller numbers of each tumor type, which is the limitation of our study. To simplify further analysis, we classified tumors into the above good and poor prognostic types1,3,4,19,22,24,25). So, these two groups which were classified with different primary tumor origin showed significant difference on OS.
Postoperative ambulation
Similar to previous results, we found that preoperative ambulation was prognostic for postoperative ambulation18,21). We also assessed preoperative motor power of hip flexion because it is important for walking and is more vulnerable than extension. Indeed, we found that preoperative motor power of hip flexion greater than grade III was associated with postoperative ambulation, with or without aid. This result seems to be important finding that we can expect longer survival and better functional recovery of patients and determine the proper surgical timing before operation.
Comparison of surgical methods
En bloc resection of metastases from slow growing tumors and from patients with higher Tokuhashi score (≥12) has been recommended to improve OS. However, en bloc surgical resections are technically demanding, are associated with higher complication rates, and do not necessarily increase OS in patients with MSCC1,3,4,13,18). Our patients underwent palliative tumor removal using either posterior or 360° surgery, because all patients had Tokuhashi scores ranging from 0 to 11 were candidates for palliative surgery18). We attempted to remove metastatic vertebra by piecemeal instead of en bloc resection in all cases. We did not observe any statistically significant differences between these two surgical methods in OS, post-operative ambulation, pain, Barthel index, and symptomatic recurrence requiring reoperation. Although we can't discuss the efficacy of en bloc resection in the curative surgery because of limitation of our study which includes the patient with Tokuhashi score below 12, our results suggest that the extent of tumor removal in the palliative surgery is not associated with better outcome. Another limitation of our study is that patient who showed preoperative weakness was included. In addition, there is one report which showed better survival when they removed metastatic spinal tumor more in the patients whose Tomita's classification is more than type 411). We also believe that our different results have the limitation because that the survival of the metastatic spinal tumor should consider too much things, such as primary origin, the status of primary cancer, medical co-morbidities, the various kinds of chemotherapy, etc.
CONCLUSION
We found that postoperative ambulation, preoperative Tokuhashi score, and primary tumor origin were significantly associated with longer OS. Moreover, preoperative motor power of hip flexion greater than grade III was critical for postoperative ambulation. The extent of tumor removal in palliative surgery was not associated with post operative outcomes.
Acknowledgements
This research was supported by the Pioneer Research Center Program through the National Research Foundation of Korea funded by the Ministry of Education, Science and Technology (2011-0001693).
References
- 1.Chaichana KL, Pendleton C, Sciubba DM, Wolinsky JP, Gokaslan ZL. Outcome following decompressive surgery for different histological types of metastatic tumors causing epidural spinal cord compression. Clinical article. J Neurosurg Spine. 2009;11:56–63. doi: 10.3171/2009.1.SPINE08657. [DOI] [PubMed] [Google Scholar]
- 2.Chaichana KL, Woodworth GF, Sciubba DM, McGirt MJ, Witham TJ, Bydon A, et al. Predictors of ambulatory function after decompressive surgery for metastatic epidural spinal cord compression. Neurosurgery. 2008;62:683–692. doi: 10.1227/01.neu.0000317317.33365.15. discussion 683-692. [DOI] [PubMed] [Google Scholar]
- 3.Choi D, Crockard A, Bunger C, Harms J, Kawahara N, Mazel C, et al. Review of metastatic spine tumour classification and indications for surgery: the consensus statement of the Global Spine Tumour Study Group. Eur Spine J. 2010;19:215–222. doi: 10.1007/s00586-009-1252-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Eleraky M, Papanastassiou I, Vrionis FD. Management of metastatic spine disease. Curr Opin Support Palliat Care. 2010;4:182–188. doi: 10.1097/SPC.0b013e32833d2fdd. [DOI] [PubMed] [Google Scholar]
- 5.Gerszten PC, Welch WC. Current surgical management of metastatic spinal disease. Oncology (Williston Park) 2000;14:1013–1024. discussion 1024, 1029-1030. [PubMed] [Google Scholar]
- 6.Hirabayashi H, Ebara S, Kinoshita T, Yuzawa Y, Nakamura I, Takahashi J, et al. Clinical outcome and survival after palliative surgery for spinal metastases: palliative surgery in spinal metastases. Cancer. 2003;97:476–484. doi: 10.1002/cncr.11039. [DOI] [PubMed] [Google Scholar]
- 7.Hur JH, Gwak HS, Chang UK, Lee CH. The analysis of primary origin in spinal metastasis occurring as the initial manifestation of malignancy. J Korean Neurosurg Soc. 2003;33:30–35. [Google Scholar]
- 8.Jang JS, Kim JK, Park WM, Heon Y, Rhee CH, Lee SH. Surgical treatment of metastatic spinal tumor. J Korean Neurosurg Soc. 1999;28:1491–1497. [Google Scholar]
- 9.Kim HJ. Neurosurgical treatment of spinal metastatic tumor. J Korean Neurosurg Soc. 1997;26:1814–1817. [Google Scholar]
- 10.Klimo P, Jr, Thompson CJ, Kestle JR, Schmidt MH. A meta-analysis of surgery versus conventional radiotherapy for the treatment of metastatic spinal epidural disease. Neuro Oncol. 2005;7:64–76. doi: 10.1215/S1152851704000262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kwon YM, Kim KS, Kuh SU, Chin DK, Jin BH, Cho YE. Survival rate and neurological outcome after operation for advanced spinal metastasis (Tomita's classification > or = type 4) Yonsei Med J. 2009;50:689–696. doi: 10.3349/ymj.2009.50.5.689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Leviov M, Dale J, Stein M, Ben-Shahar M, Ben-Arush M, Milstein D, et al. The management of metastatic spinal cord compression : a radiotherapeutic success ceiling. Int J Radiat Oncol Biol Phys. 1993;27:231–234. doi: 10.1016/0360-3016(93)90232-k. [DOI] [PubMed] [Google Scholar]
- 13.Li H, Gasbarrini A, Cappuccio M, Terzi S, Paderni S, Mirabile L, et al. Outcome of excisional surgeries for the patients with spinal metastases. Eur Spine J. 2009;18:1423–1430. doi: 10.1007/s00586-009-1111-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Maranzano E, Latini P. Effectiveness of radiation therapy without surgery in metastatic spinal cord compression: final results from a prospective trial. Int J Radiat Oncol Biol Phys. 1995;32:959–967. doi: 10.1016/0360-3016(95)00572-g. [DOI] [PubMed] [Google Scholar]
- 15.Melcher I, Disch AC, Khodadadyan-Klostermann C, Tohtz S, Smolny M, Stöckle U, et al. Primary malignant bone tumors and solitary metastases of the thoracolumbar spine: results by management with total en bloc spondylectomy. Eur Spine J. 2007;16:1193–1202. doi: 10.1007/s00586-006-0295-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.North RB, LaRocca VR, Schwartz J, North CA, Zahurak M, Davis RF, et al. Surgical management of spinal metastases: analysis of prognostic factors during a 10-year experience. J Neurosurg Spine. 2005;2:564–573. doi: 10.3171/spi.2005.2.5.0564. [DOI] [PubMed] [Google Scholar]
- 17.Patchell RA, Tibbs PA, Regine WF, Payne R, Saris S, Kryscio RJ, et al. Direct decompressive surgical resection in the treatment of spinal cord compression caused by metastatic cancer : a randomised trial. Lancet. 2005;366:643–648. doi: 10.1016/S0140-6736(05)66954-1. [DOI] [PubMed] [Google Scholar]
- 18.Putz C, Wiedenhöfer B, Gerner HJ, Fürstenberg CH. Tokuhashi prognosis score : An important tool in prediction of the neurological outcome in metastatic spinal cord compression: a retrospective clinical study. Spine (Phila Pa 1976) 2008;33:2669–2674. doi: 10.1097/BRS.0b013e318188b98f. [DOI] [PubMed] [Google Scholar]
- 19.Rades D, Huttenlocher S, Dunst J, Bajrovic A, Karstens JH, Rudat V, et al. Matched pair analysis comparing surgery followed by radiotherapy and radiotherapy alone for metastatic spinal cord compression. J Clin Oncol. 2010;28:3597–3604. doi: 10.1200/JCO.2010.28.5635. [DOI] [PubMed] [Google Scholar]
- 20.Rades D, Lange M, Veninga T, Stalpers LJ, Bajrovic A, Adamietz IA, et al. Final results of a prospective study comparing the local control of short-course and long-course radiotherapy for metastatic spinal cord compression. Int J Radiat Oncol Biol Phys. 2011;79:524–530. doi: 10.1016/j.ijrobp.2009.10.073. [DOI] [PubMed] [Google Scholar]
- 21.Sundaresan N, Galicich JH, Lane JM, Bains MS, McCormack P. Treatment of neoplastic epidural cord compression by vertebral body resection and stabilization. J Neurosurg. 1985;63:676–684. doi: 10.3171/jns.1985.63.5.0676. [DOI] [PubMed] [Google Scholar]
- 22.Tanaka M, Nakahara S, Ito Y, Kunisada T, Misawa H, Koshimune K, et al. Surgical treatment of metastatic vertebral tumors. Acta Med Okayama. 2009;63:145–150. doi: 10.18926/AMO/31849. [DOI] [PubMed] [Google Scholar]
- 23.Tokuhashi Y, Ajiro Y, Umezawa N. Outcome of treatment for spinal metastases using scoring system for preoperative evaluation of prognosis. Spine (Phila Pa 1976) 2009;34:69–73. doi: 10.1097/BRS.0b013e3181913f19. [DOI] [PubMed] [Google Scholar]
- 24.Tokuhashi Y, Matsuzaki H, Oda H, Oshima M, Ryu J. A revised scoring system for preoperative evaluation of metastatic spine tumor prognosis. Spine (Phila Pa 1976) 2005;30:2186–2191. doi: 10.1097/01.brs.0000180401.06919.a5. [DOI] [PubMed] [Google Scholar]
- 25.Tomita K, Kawahara N, Kobayashi T, Yoshida A, Murakami H, Akamaru T. Surgical strategy for spinal metastases. Spine (Phila Pa 1976) 2001;26:298–306. doi: 10.1097/00007632-200102010-00016. [DOI] [PubMed] [Google Scholar]
- 26.Weigel B, Maghsudi M, Neumann C, Kretschmer R, Müller FJ, Nerlich M. Surgical management of symptomatic spinal metastases. Postoperative outcome and quality of life. Spine (Phila Pa 1976) 1999;24:2240–2246. doi: 10.1097/00007632-199911010-00012. [DOI] [PubMed] [Google Scholar]
- 27.Yamashita T, Aota Y, Kushida K, Murayama H, Hiruma T, Takeyama M, et al. Changes in physical function after palliative surgery for metastatic spinal tumor: association of the revised Tokuhashi score with neurologic recovery. Spine (Phila Pa 1976) 2008;33:2341–2346. doi: 10.1097/BRS.0b013e3181878733. [DOI] [PubMed] [Google Scholar]
- 28.Zelefsky MJ, Scher HI, Krol G, Portenoy RK, Leibel SA, Fuks ZY. Spinal epidural tumor in patients with prostate cancer. Clinical and radiographic predictors of response to radiation therapy. Cancer. 1992;70:2319–2325. doi: 10.1002/1097-0142(19921101)70:9<2319::aid-cncr2820700918>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]