Abstract
There have been very few reports in the literature of Guillain-Barré syndrome (GBS) after spinal surgery. We present a unique case of GBS following spinal fusion for thoracic vertebral fracture. The aim of this report is to illustrate the importance of early neurological assessment and determining the exact cause of a new neurological deficit that occurs after an operation.
Keywords: Guillain-Barre syndrome, Spinal surgery, Vertebral fracture
INTRODUCTION
Guillain-Barré syndrome (GBS) typically presents following a mild respiratory and gastrointestinal illness and has an annual incidence of 0.6 to 4 cases per 100,000 people per year4,5). It is a symmetric, rapidly progressive polyneuropathy of unknown cause that has been described most frequently after non-specific viral infection4,5). GBS has rarely been reported following trauma and operation2,6,7,12). In addition, only three cases in the literature have been reported after spine surgery8-10). We report a unique case of GBS following spinal fusion for thoracic vertebral fracture.
CASE REPORT
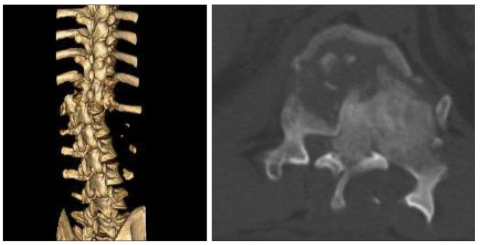
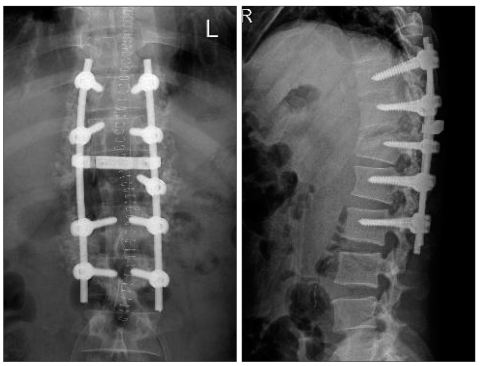
A fifty-year-old man was referred to our emergency room because of paraplegia after traffic accident. Computed tomography showed the burst fracture with dislocation on T12 and near complete obstruction of spinal canal (Fig. 1). There were also bilateral pneumothorax, hepatic and splenic laceration. Emergent operation of spinal canal decompression and spinal fusion was performed (Fig. 2) after steroid mega dose therapy. Paraplegia was not improved after operation. However, general patient's condition was improved.
Fig. 1.
Preoperative 3 dimensional computed tomography shows the burst fracture with dislocation of T12 and marked spinal canal stenosis.
Fig. 2.
Postoperative T-spine X-ray images show realignment spinal column and accurate pedicle screws instrumentation.
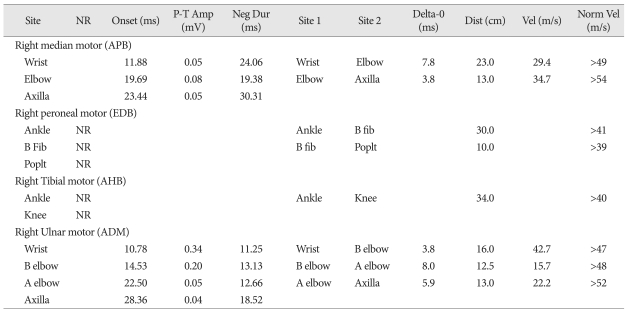
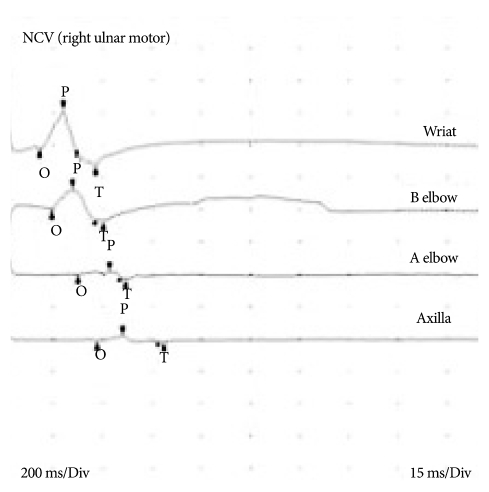
He complained of abdominal pricky pain 8 days after operation. There was no specific finding in abdominal cavity which was evaluated further with transabdominal ultrasonography by a general surgeon. Following a day, numbness in both hands was evident. Then, he noted chest discomfort and dyspnea at the evening. Chest X-ray, arterial blood gas analysis (ABGA) and cardiac ultrasonography were performed. Results of examinations were normal. He had been medicated with small amount of sedative because of anxiety and insomnia. We thought that sedatives medication was the reason of respiratory difficulty. In spite of careful observation with supportive care, he complained of the progression of dyspnea. Serial ABGA studies were performed. Level PaCO2 on serial ABGAs were within normal ranges. Grade IV motor weakness on both upper extremities and bilateral facial palsy were detected 10 days after operation. Subsequently, abrupt respiratory holding was occurred. His level of consciousness was deteriorated. We performed rapid resuscitation. After resuscitation his awareness was recovered to be alert state. But, he showed bilateral facial weakness, quadriplegia (grade 0 in bilateral upper and lower limbs) and generalized areflexia. His clinical course suggests acute inflammatory demyelinating peripheral polyneuropathy, Brain and cervical spinal imaging study revealed no abnormal lesions on CNS. Cerebrospinal fluid analysis (CSFA) was not performed because of postoperative back wound and low reliability of CSFA from injury of spinal cord and dura matter. Nerve conduction studies showed slowing conduction velocity, prolonged terminal latencies (Table 1) and conduction block in the median and ulnar nerves (Fig. 3). The results of nerve conduction velocity highly suggested of GBS. Human immunoglobulin (Liv-gamma®, SK chemicals Life Science, Korea) 2 g/kg for 5 days was infused. Motor power of both upper extremities were improved to grade "1" at 22 days after respiratory holding event. Mechanical ventilator was weaned off at 26 days. Within two months, the neurological deficits, except for minor weakness of the intrinsic muscles of the hands, had completely resolved. The original symptoms of paraplegia however persisted. The patient was satisfied with the improvement of his condition, and he was continuously monitored.
Table 1.
This table shows prolonged terminal latencies and slowing nerve conduction velocity
NR : no response, P-T : peak-terminal, Amp : amplitude, Dur : duration, APB : abductor pollicis brevis, EDB : extensor dgitorum brevis, AHB : abductor hallucis brevis, ADM : abductor digiti minimi
Fig. 3.
This graph shows the conduction block at the right ulnar nerve.
DISCUSSION
GBS is a symmetric, rapidly progressive demyelinating polyradiculopathy of unknown cause. The antecedent events may or may not present. Approximately, two-thirds of cases occur following a simple, trivial infection, usually viral in nature4,5). Antecedent events or assocaited illness include viral exanthems and other viral illnesses (Epstein-Barr virus, cytomegalovirus, and Human-Immuno deficiency virus), bacterial infections (Campylobacter, Mycoplasma pneumoniae, Lyme disease), exposure to thrombolytic agent, and lymphoma4,5). Trauma and surgical operations may precede GBS, but only one case of GBS following thoracic vertebral fracture has been reported9) and only three cases in the literature have occurred after spine surgery8-10). No clear explanation exists for the development of GBS after surgical procedure and traumatic spinal injury. GBS is typically caused by an autoimmune attack of peripheral nerve. It is likely that a variety of agent (viral, bacterial, certain vaccines, and peripheral nerve itself) are all capable of precipitating an immune response against components of autologous peripheral myelin in susceptible individuals4,5). Scozzafava et al.9) suggested that traumatized tissues, including peripheral nerve, may lead to the exposure of antigens such as myelin protein, with subsequent generation of autoantibodies against the peripheral nerves. The presence of antibodies to myelin basic protein has been identified in trauma patients and in surgical patients2). The connection of GBS and surgery and trauma also has been postulated as the result of alternations in T-cell function and major stress during surgery and trauma2,4,7,12). In our patients, the only clearly identified precipitating event was the thoracic vertebral fracture and spinal fusion operation, which we consider to be the antecedent event for the development of GBS.
The typical case of GBS can be readily identified. In typical case, the first symptoms of GBS are pain, numbness, paresthesia in the limbs. The major clinical manifestation is weakness that evolves symmetrically over a period of several days to a week or two, or more longer. The proximal as well as distal muscles of the limbs are involved, usually the lower extremities before the upper. The facial nerve are often affected and less often the ocular motor nerves. In 5% of cases, the weakness progresses to total motor paralysis with respiratory failure within a few days4,5,7). Autonomic dysfuntion is common and causes tachycardia and other arrhythmia, postural hypotension, hypertension, urinary retention, and vasomotor symptoms4,5). The interesting point of our case is that many of the hallmark sign and symptoms that lead to a clinical diagnosis of GBS (such as paresthesia with ascending weakness), were obscured by spinal cord injury. In our case, the patient was initially paraplegic state after spinal cord injury, therefore we could not recognize the beginning leg symptoms of paresthesia and weakness. Although our patient reported dyspnea and chest discomfort 2 days before respiratory holding events, the appropriate reason of symptoms could not explained. The important point is that we did not concern about the GBS at that time, in spite of increasing tendency of PaCO2 was detected on the arterial blood gas analysis. After the onset of bilateral facial weakness, quadriplegia and generalized areflexia, presumtive diagnosis of GBS could be made.
The greatest value of the current case is to demonstrate the diagnostic confusion that results when progressive neurological deterioration occurs during the postoperative period. Operation on the spine can lead to many complications. Neurological deficits after surgery are infrequent, but they need immediate evaluation because some of the causes can be treated successfully. Neurological deficits that develop postoperatively suggest a fairly narrow differential diagnosis. Complications that are related to an operative implant, an epidural hematoma, instability, or a displaced bone must all be considered, and they can usually be rapidly excluded by the appropriate imaging studies8). Progressive neurological deterioration unrelated to the spinal surgery itself may present a confusing clinical impression.
A precise diagnosis is important because specific treatment is available for many causes of weakness. On the basis of the history, the findings of the physical examination and the analysis of cerebrospinal fluid, and the demonstration of peripheral demyelination by the nerve-conduction studies, the diagnosis of GBS can be established1,3). Management of GBS focuses on supportive care and strict monitoring of pulmonary function with vital capacity measurements as respiratory failure may be imminent. Plasmapharesis and intravenous immunoglobulin therapies have been shown to decrease the hospital time, accelerate functional recovery3,4). In the present case, cerebrospinal fluid analysis could not apply because of postoperative back wound and low reliability of cerebrospinal fluid analysis resulting from injury of spinal cord and dura matter, but electrophysiologic study comparable to GBS supported the diagnosis of GBS. The patient was treated with intravenous immuneglobulin 2 g/kg/day for 5 days.
Approximately 3 to 5 percent of patients do not survive from the illness, even in the best-equipped hospitals4). The majority of patients recover nearly completely4,5). In about 10 percent of patients, residual disability is pronounced4,11). It is rare for GBS to recur; it has been estimated that 3% of people may have a further attack4,5,11). The clinical outcome of our patient was uneventful and he had a complete recovery.
CONCLUSION
We report a unique case of GBS following spinal fusion for thoracic vertebral fracture. This case reinforces the need for neurosurgeons to maintain awareness of this potentially reversible condition, GBS, that may arise after spinal operation.
Acknowledgements
This work was supported for two years by Pusan National University Research Grant.
References
- 1.Asbury AK, Cornblath DR. Assessment of current diagnostic criteria for Guillain-Barré syndrome. Ann Neurol. 1990;27(Suppl):S21–S24. doi: 10.1002/ana.410270707. [DOI] [PubMed] [Google Scholar]
- 2.Duncan R, Kennedy PG. Guillain-Barré syndrome following acute head trauma. Postgrad Med J. 1987;63:479–480. doi: 10.1136/pgmj.63.740.479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hadden RD, Cornblath DR, Hughes RA, Zielasek J, Hartung HP, Toyka KV, et al. Electrophysiological classification of Guillain-Barré syndrome : clinical associations and outcome Plasma Exchange/Sandoglobulin Guillain-Barr Syndrome Trial Group. Ann Neurol. 1998;44:780–788. doi: 10.1002/ana.410440512. [DOI] [PubMed] [Google Scholar]
- 4.Hughes RA, Cornblath DR. Guillain-Barr� syndrome. Lancet. 2005;366:1653–1666. doi: 10.1016/S0140-6736(05)67665-9. [DOI] [PubMed] [Google Scholar]
- 5.Hughes RA, Rees JH. Clinical and epidemiologic features of Guillain-Barré syndrome. J Infect Dis. 1997;176(Suppl 2):S92–S98. doi: 10.1086/513793. [DOI] [PubMed] [Google Scholar]
- 6.Lee MC, Campbell R, Born C. Guillain-Barré syndrome after failed pelvic fracture fixation. J Trauma. 2009;67:E132–E135. doi: 10.1097/TA.0b013e31804a7fc0. [DOI] [PubMed] [Google Scholar]
- 7.Lin TM, Lee SS, Lin RT, Lai CS, Lin SD. Guillain-Barré syndrome following facial bone fracture. J Plast Reconstr Aesthet Surg. 2006;59:543–546. doi: 10.1016/j.bjps.2005.10.017. [DOI] [PubMed] [Google Scholar]
- 8.Riebel GD, Heller JG, Hopkins LC. Guillain-Barré syndrome after an operation on the spine. A case report. J Bone Joint Surg Am. 1995;77:1565–1567. doi: 10.2106/00004623-199510000-00012. [DOI] [PubMed] [Google Scholar]
- 9.Scozzafava J, Jickling G, Jhamandas JH, Jacka MJ. Guillain-Barré syndrome following thoracic spinal cord trauma. Can J Anaesth. 2008;55:441–446. doi: 10.1007/BF03016311. [DOI] [PubMed] [Google Scholar]
- 10.Stambough JL, Quinlan JG, Swanson JD. Guillain-Barré syndrome following spinal fusion for adult scoliosis. Spine (Phila Pa 1976) 1990;15:45–46. doi: 10.1097/00007632-199001000-00012. [DOI] [PubMed] [Google Scholar]
- 11.Visser LH, Schmitz PI, Meulstee J, van Doorn PA, van der Meché FG. Prognostic factors of Guillain-Barr syndrome after intravenous immunoglobulin or plasma exchange. Dutch Guillain-Barré Study Group. Neurology. 1999;53:598–604. doi: 10.1212/wnl.53.3.598. [DOI] [PubMed] [Google Scholar]
- 12.Yardimci N, Gulsen S, Avci AY, Altinors N, Zileli T, Can U. Can subdural hematoma be a trigger for Guillain-Barré syndrome? Int J Neurosci. 2009;119:366–372. doi: 10.1080/00207450802480135. [DOI] [PubMed] [Google Scholar]