Abstract
Mesenchymal chondrosarcomas are rare malignant tumors of the bone and soft tissue. Spinal mesenchymal chondrosarcomas are even rarer and, to the best of our knowledge those that are concomitantly located in the intradural and extradural regions, have never been reported. We report a case of a 25-year-old man with back pain and bilateral progressive weakness of the lower extremities. Magnetic resonance imaging revealed a markedly enhanced dumbbell-shaped mass at the T7 level. The lesion was intradurally located at the left side of the spinal cord, and extended extradurally to the extraforminal space through the T7-8 intervertebral foramen. The tumor was completely excised through a posterior approach. Microscopic examination and immunohistochemical studies confirmed mesenchymal chondrosarcoma. Postoperative radiation therapy and chemotherapy were also performed to prevent local recurrence and metastasis. The patient has been symptom-free for two years after surgery. Herein, we reviewed and discussed the clinical characteristics, treatments, and outcomes of primary intraspinal mesenchymal chondrosarcomas in the literature.
Keywords: Mesenchymal chondrosarcoma, Spinal tumor, Intradural, Extradural
INTRODUCTION
Chondrosarcomas are extremely rare cartilaginous tumors that typically are associated with bone. Chondrosarcomas of the central nervous system are very rare and spinal intradural meningeal chondrosarcomas are even rarer. Mesenchymal chondrosarcoma is a variant of meningeal chondrosarcomas6). We report a case of spinal mesenchymal chondrosarcoma concomitantly located in the intradural and extradural regions at the T7-8 level.
CASE REPORT
A 44-year-old male presented with back pain and paraparesis. He experienced the back pain intermittently for a year. He had suffered from persistent severe back pain and felt his legs weakened progressively two weeks prior to hospitalization.
Examination
Physical and neurological examinations on admission showed hypoesthesia below the dermatome of T7. The muscle strength of left hip flexion was 3/5, and the others in lower extremities were 4/5, except the right ankle and toes (5/5). Hyperactivity of deep tendon reflex was observed in both knees and ankles; ankle clonus and Barbinsky's sign were positive bilaterally. Laboratory tests, including determination of complete blood count, clotting profiles, electrolytes, and blood chemistry values, were all within the normal range.
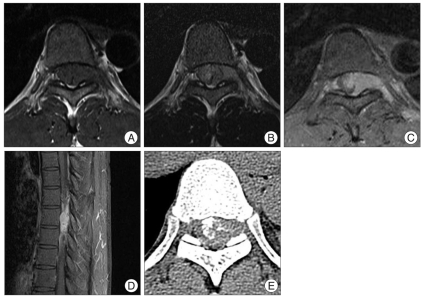
Thoracic spinal magnetic resonance imaging (MRI) revealed an intraspinal mass measuring 3×1.5×3 cm at the level of T7. The mass was intradurally located at the left side of the spinal cord, pushing the cord to the right side, and extended extradurally to the extraforminal space through the T7 neural foramen (Fig. 1). The mass appeared isointense on T1-weighted images (Fig. 1A) and mildly hyperintense on T2-weighted images (Fig. 1B). After intravenous gadolinium administration, a very strong enhancement of the tumor was observed (Fig. 1C, D). Computed tomography (CT) of the lesion showed calcification within the tumor (Fig. 1E).
Fig. 1.
Thoracic spinal MRI revealed an intraspinal mass at the level of T7. The mass is intradurally located at the left side of the spinal cord, pushing the cord to the right side, and extended extradurally to the extraforminal space through the T7 neural foramen. The mass appears isointense on T1-weighted images (A) and mildly hyperintense on T2-weighted images (B). Very strong enhancement of the tumor is observed after intravenous gadolinium administration (C and D). Non-enhanced CT of the lesion shows calcification within the tumor (E). CT : computed tomography.
Operation
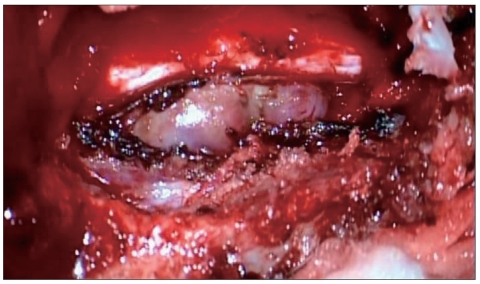
Hemilaminectomy of T7 and concomitant left T7-8 partial facetectomy were performed with the patient in the prone position. After the laminectomy, a purplish inhomogeneously firm tumor was found in the left extradural space. The mass was compressing and displacing the dural sac, extended to the left T7-8 neural foramen, and totally encroached on the foramen (Fig. 2). After resection of extradural mass, we removed the intradural mass through the dural defect. Intradural mass was found to be extramedullary tumor. It was found flowing along the T7 root to the extradural space, and not adherent to the cord or roots. We totally removed the mass without injury of cord or roots, and carried the primary suture of dura in a water-tight fashion.
Fig. 2.
After laminectomy, purplish inhomogeneously firm tumor is found in the left extradural space. The mass is compressing and displacing the dural sac, and extending to the left T7-8 neural foramen.
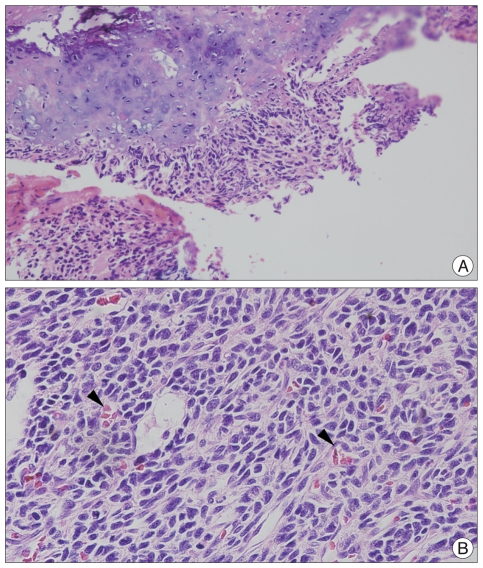
Histopathological examination revealed islands of well differentiated cartilage surrounded by a diffuse proliferation of small, primitive, undifferentiated mesenchymal cells. There were numerous dilated vascular channels resembling the histological pattern of a hemangiopericytoma. Foci of calcification were also seen among the islands of cartilage (Fig. 3). All immunochemical analyses including CD99, Myo-D1, GFAP, NSE, S-100 and CK were negative. A final diagnosis of mesenchymal chondrosarcoma was made.
Fig. 3.
A : Mesenchymal chondroarcoma in microscopic finding, characterized by bimorphic pattern in which area well differentiated cartilage (upper portion) alternate with undifferentiated stroma (lower portion) (HE ×200). B : Numerous dilated vascular channels (arrows) resembling the histological pattern of a hemangiopericytoma (HE ×400).
Postoperative course
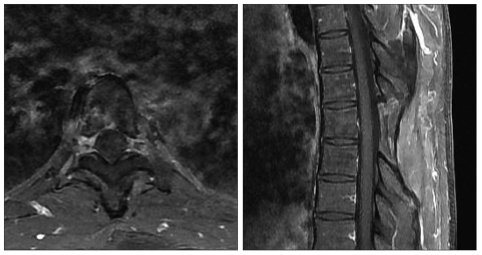
He improved gradually after surgery and his sensory and motor deficits were normalized. Six weeks after surgery, he received local irradiation (total dose : 5040 cGy) and was treated with four cycles of chemotherapy with mesna, doxorubicin, ifosfamide, and dacarbazine regimen, two months after surgery. Several MR images performed during two years after surgery have not shown any residual or recurring intraspinal mass (Fig. 4).
Fig. 4.
Postoperative MR images with gadolinium enhancement at two years after the surgery show no evidence of tumor recurrence. MR : magnetic resonance.
DISCUSSION
Chondrosarcoma is a primary malignant neoplasm arising from bone and soft tissue with both cartilaginous and undifferentiated round-cell components. Chondrosarcomas are classified into three main categories : classic, mesenchymal and myxoid types6,13). In mesenchymal chondrosarcomas, groups and sheets of small, undifferentiated cells alternate with islands of hyaline cartilage. Electron microscopy confirms that the neoplastic cells represent primitive mesenchyme, displaying focal cartilaginous differentiation6). Although chondrocytes are thought to be the cells of origin, chondrosarcomas are occasionally found in extraskeletal tissues. Extraskeletal mesenchymal chondrosarcoma accounts for 33-50% of total mesenchymal chondrosarcomas10), and the most common site of extraskeletal mesenchymal chondrosarcoma is the meninges13). Most of these tumors were reported to be intracranial in location3). Harsh and Wilson7) reviewed 16 mesenchymal chondrosarcomas of the primary central nervous system; only five occurred in intraspinal regions. These tumors have been found more frequently in an extradural location, with the majority having a dural attachment. Forbes and Eljamel5) reviewed 31 meningeal chondrosarcomas from the literature, and reported 11 spinal meningeal chondrosarcomas. Intradural involvements of spinal meningeal chondrosarcoma are very rare, with an incidence of 6% of all these spine tumors2). We searched the literature and found six cases of spinal intradural mesenchymal chondrosarcomas1,8,9,11,12,14). Our case is thus unusual in that it is not only the seventh case of spinal intradural mesenchymal chondrosarcoma, but, to the best of our knowledge, is also the first reported case involving intradural and extradural space concomitantly.
The exact histogenesis of intradural chondrosarcomas is obscure because these lesions are generally associated with cartilage. As mentioned above, chondrosarcomas are occasionally found in extraskeletal tissues; therefore, some hypotheses have been suggested. One is the tumors may arise from metaplastic changes in fibroblasts4). It seems possible that chondrosarcomas could arise from the dura because it has this periosteal component over the spinal extradural vault. These chondrosarcomas may arise from embriyonic rest cells of cartilage within the dura. In our case, we think that the tumor might have arisen from the dura of the spine and extended intradurally and extradurally. Another hypothesis states that chondrosarcomas originate from primitive multipotential mesenchymal cells which might arise from the perforating cerebrospinal blood vessels within the pia-arachnoid extensions that form the Vircow-Robins spaces14).
In immunohistochemical staining of chondrosarcomas, cytokeratin and EMA were negative and S-100, the most commonly observed marker in chondrosarcoma and a frequently referred marker in differentiation diagnosis8), however, displayed negative results in this case. Because S-100 protein represents differentiation of the cartilage, the cartilaginous portion of the tumor typically shows strong positivity. In the undifferentiated areas, however only the isolated cells can show a weak positive result or there can be negative results6).
MRI is the imaging modality of choice for intraspinal tumors, but there are no pathognomonic signs in the intradural chondrosarcomas. Because the image characteristics are similar to those of chordomas, differential diagnosis is difficult. On T1-weighted images, the signals are usually isointense with respect to the normal spinal cord while T2-weighted images show a high intensity or isointensity. The overall signal intensity is homogeneous with both short and long repetition times, but if there is calcification, which is shown frequently in chondrosarcoma, it appears as various signals in MRI, depending on the degree of differentiation. In our case, despite not finding evidence of calcification in the MRI, CT revealed intratumoral calcification. However, it is believed that there is not a significant correlation between the histologic findings and prognosis3). Images obtained after administration of gadolinium show a homogenous strongly enhanced tumor.
Despite mesenchymal chondrosarcomas' slow-growing nature, the prognosis is poor because of their tendency of local recurrence and metastasis, and their resistance to chemotherapy or radiation therapy. For these reasons, radical surgery with complete removal of the tumor is considered the best choice of therapy4,7,9,10). Although there are only few cases to draw conclusions about the effect of irradiation or chemotherapy on the prognosis, most authors agree that radiotherapy is recommended in cases in which the tumor is resectable, and chemotherapy is the treatment of choice in the selected cases, especially in recurrent cases4,5,9). We treated the patient with postoperative radiotherapy and chemotherapy, regardless of the postoperative course of disease. In our case, because the tumor was concomitantly located in the intradural and extradural spaces, we couldn't resect radically around it. We also thought that there could have been remnant malignant cells in cerebrospinal fluid.
Because the local or distant metastases may appear 20 or more years after the appearance of the primary tumor, we must follow up the patient for long term. However, we are convinced that total removal is the treatment of choice for the primary tumor and following adjuvant radiotherapy and chemotherapy must be considered.
CONCLUSION
Mesenchymal chondrosarcoma is a very rare tumor, especially in the spine. In this case, the tumor is thought to have originated from the dura, and extended to the intradural and extradural spaces concomitantly. Because spinal mesenchymal chondrosarcomas easily recur and metastasize and have a poor response to chemotherapy or radiotherapy, total resection of tumor is the treatment of choice. Although debatable, postoperative adjuvant radiotherapy or chemotherapy should be considered.
References
- 1.Belhachmi A, Akhaddar A, Gazzaz M, Elasri C, Elmostarchid B, Boucetta M, et al. Primary spinal intradural mesenchymal chondrosarcoma. A pediatric case report. J Neuroradiol. 2008;35:189–191. doi: 10.1016/j.neurad.2007.11.005. [DOI] [PubMed] [Google Scholar]
- 2.Camins MB, Duncan AW, Smith J, Marcove RC. Chondrosarcoma of the spine. Spine (Phila Pa 1976) 1978;3:202–209. doi: 10.1097/00007632-197809000-00003. [DOI] [PubMed] [Google Scholar]
- 3.Cho YJ, Jin BH, Chin DK, Yoon DH, Kim YS, Jung HJ. Three cases of spinal mesenchymal chondrosarcoma. J Korean Neurosurg Soc. 1997;26:1446–1450. [Google Scholar]
- 4.Di Lorenzo N, Palatinsky E, Artico M, Palma L. Dural mesenchymal chondrosarcoma of the lumbar spine. Case report. Surg Neurol. 1989;31:470–472. doi: 10.1016/0090-3019(89)90095-5. [DOI] [PubMed] [Google Scholar]
- 5.Forbes RB, Eljamel MS. Meningeal chondrosarcomas, a review of 31 patients. Br J Neurosurg. 1998;12:461–464. doi: 10.1080/02688699844727. [DOI] [PubMed] [Google Scholar]
- 6.Greenfield JG, Love S, Louis DN, Ellison D. Greenfield's Neuropathology. ed 8. London: Edward Arnold Ltd; 2008. [Google Scholar]
- 7.Harsh GR, 4th, Wilson CB. Central nervous system mesenchymal chondrosarcoma. Case report. J Neurosurg. 1984;61:375–381. doi: 10.3171/jns.1984.61.2.0375. [DOI] [PubMed] [Google Scholar]
- 8.Kotil K, Bilge T, Olagac V. Primary intradural myxoid chondrosarcoma : a case report and review in the literature. J Neurooncol. 2005;75:169–172. doi: 10.1007/s11060-005-1739-1. [DOI] [PubMed] [Google Scholar]
- 9.Li YH, Yao XH. Primary intradural mesenchymal chondrosarcoma of the spine in a child. Pediatr Radiol. 2007;37:1155–1158. doi: 10.1007/s00247-007-0576-0. [DOI] [PubMed] [Google Scholar]
- 10.Louvet C, de Gramont A, Krulik M, Jagueux M, Hubert D, Brissaud P, et al. Extraskeletal mesenchymal chondrosarcoma : case report and review of literature. J Clin Oncol. 1985;3:858–863. doi: 10.1200/JCO.1985.3.6.858. [DOI] [PubMed] [Google Scholar]
- 11.Ranjan A, Chacko G, Joseph T, Chandi SM. Intraspinal mesenchymal chondrosarcoma. Case report. J Neurosurg. 1994;80:928–930. doi: 10.3171/jns.1994.80.5.0928. [DOI] [PubMed] [Google Scholar]
- 12.Rushing EJ, Mena H, Smirniotopoulos JG. Mesenchymal chondrosarcoma of the cauda equina. Clin Neuropathol. 1995;14:150–153. [PubMed] [Google Scholar]
- 13.Russell DS, Rubinstein LJ. Pathology of Tumors of the Nervous System. Baltimore: Williams & Wilkins; 1989. [Google Scholar]
- 14.Vanderhooft JE, Conrad EU, Anderson PA, Richardson ML, Bruckner J. Intradural recurrence with chondrosarcoma of the spine. A case report and review of the literature. Clin Orthop Relat Res. 1993:90–95. [PubMed] [Google Scholar]