Abstract
The hormone leptin has a variety of functions. Originally known for its role in satiety and weight loss, leptin more recently has been shown to augment tumor growth in a variety of cancers. Within gliomas, there is a correlation between tumor grade and tumor expression of leptin and its receptor. This suggests that autocrine signaling within the tumor microenvironment may promote the growth of high-grade gliomas. Leptin does this through stimulation of cellular pathways that are also advantageous for tumor growth and recurrence: antiapoptosis, proliferation, angiogenesis, and migration. Conversely, a loss of leptin expression attenuates tumor growth. In animal models of colon cancer and melanoma, a decline in the expression and secretion of leptin resulted in a reduction of tumor growth. In these models, positive mental stimulation through environmental enrichment decreased leptin secretion and improved tumor outcome. This review explores the link between leptin and glioblastoma.
1. Introduction
Leptin is the product of the obese gene, located on chromosome 7 in humans. Mice with mutation in the obese gene are obese and insatiable [1]. When exogenous leptin is injected into leptin-deficient obese mice (ob/ob mice), the protein promotes satiety and weight loss [2–5]. The effects of leptin on these obese mice sparked a leptin intense focus in obesity research over the past 15 years. Unlike the ob/ob mice, obese humans are not leptin deficient. Obese humans have high circulating leptin levels which are directly correlated to the total amount of adipose tissue [6]. Leptin helps regulate bodyweight in humans by negative feedback promoting satiety when energy stores are elevated [7]. The current model suggests that obesity in humans is due to a desensitization to leptin. Obese subjects have a diminished response to leptin, and in some subjects the diminished response is due to a mutation in the leptin receptor gene [8]. The high prevalence of obesity in the USA is strongly correlated with the risk of multiple diseases, including cancer [9]. The association between cancer and obesity may, in part, be explained by elevated circulating leptin.
2. Leptin in Cancer
Leptin has been classified as a growth factor because it stimulates three key pathways well known for their roles in cell growth: proliferation, survival, and motility and migration (Figure 1). It is well documented that the binding of leptin to the leptin receptor (ObR) activates the Janus kinase-signal transducer and activator of transcription (JAK-STAT), the mitogen-activated protein kinase (MAPK), and the phosphatidylinositol 3-kinase (PI3K) pathways in both normal [10–22] and malignant cells [20, 23–41]. Supporting a role of leptin in cancer pathogenesis are reports that DNA polymorphisms in the leptin and ObR genes are associated with increased risk and progression of breast [42], prostate [43], and oral cancer [44].
Figure 1.
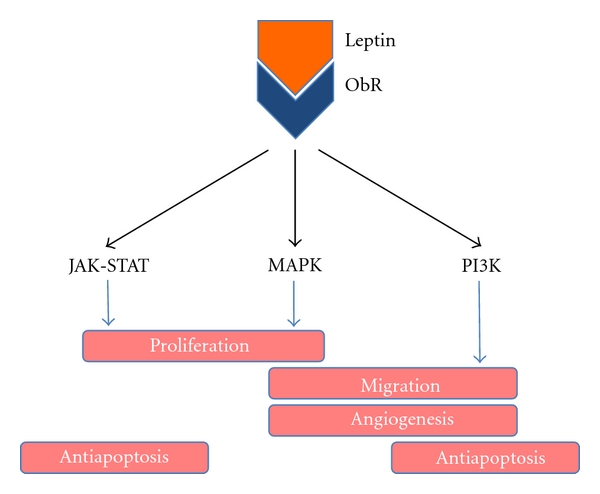
Cellular pathways activated through leptin receptor (ObR) stimulation.
Evidence generally supports leptin as a growth factor, promoting cell division and evasion of cell death [45]. Numerous reports indicate that leptin has both antiapoptotic [28, 33, 34, 36, 46–54] and proliferative effects [24, 25, 27, 29–31, 33, 34, 36, 41, 47, 49, 50, 52, 53, 55–59] (Table 1). It appears that leptin-mediated proliferation of these cancers occurs through the activation of the JAK-STAT [25, 27, 29–31, 34, 41], PI3K [24, 31, 33, 36], and MAPK [24, 31] pathways, whereas apoptosis avoidance is promoted by leptin via the JAK-STAT [28, 34] and PI3K [33, 36] pathways (Figure 1).
Table 1.
Summary of the Literature: leptin's role in cancer promotion*.
| Cancer type | Antiapoptosis | Proliferation | Migration | Angiogenesis |
|---|---|---|---|---|
| Bone | 24 | |||
| Breast | 28, 46 | 27, 56 | 23, 65 | 38, 69 |
| Cartilage | 32 | |||
| Colon | 48 | 57, 58 | 20, 62, 64 | 58 |
| Endometrial | 34 | 30, 31, 34 | ||
| Esophageal | 51 | |||
| Gallbladder | 53 | 53 | ||
| Gastric | 25, 59 | |||
| Glioma | 77 | 74 | ||
| Kidney | 29 | |||
| Large B-cell lymphoma | 33 | |||
| Leukemia | 47 | 47 | 71 | |
| Liver | 52 | 41, 52 | 26 | 70 |
| Lung | 53 | |||
| Neuroblastoma | 49 | 49 | ||
| Ovarian | 55 | |||
| Prostate | 50 | 50 | 37, 39, 40 | |
| Thyroid | 36 | 36 | 63 | 37 |
| Uterine | 68 |
*Numbers correspond to works cited.
Migration is enhanced by leptin in several normal [10, 20–22, 60, 61] and cancerous tissues [20, 23, 26, 32, 35, 37, 39, 40, 62–64] (Table 1). Leptin treatment increases the growth and migration of cholangiocarcinoma cells in vitro and cholangiocarcinoma is inducible in obese fa/fa Zucker (faulty ObR) rats [53]. In metastatic colon cancer cells, leptin provokes the formation of lamellipodia and augments invasion through the MAPK and PI3K pathways [62]. It has since been confirmed that leptin increases migration through the MAPK and PI3K pathways in prostate [37, 39, 40], liver [26], cartilage [32], and breast [23, 40, 64] cancers, as well as the JAK-STAT pathway in colon [35], prostate [39], liver [26], and breast [23] cancers. Compounding the complexity of leptin's role in carcinogenesis is that leptin may have differential responses in closely related cells; leptin induces migration in papillary thyroid cancer cells but not in anaplastic and follicular thyroid cancer cells [63].
In addition to its role in cellular proliferation, apoptosis avoidance, and migration, leptin is a potent angiogenic factor. Using an in vitro angiogenesis assay, leptin enhances the formation of capillary-like tubes by human umbilical venous endothelial cells [65]. In 5- to 6-week-old C57BL/6J mice, leptin induces fenestrated blood vessel growth [66]. This response is synergistic with vascular endothelial growth factor (VEGF) and fibroblast growth factor-2 [66]. Myometrial cells and the blood-vessel walls of uterine myomas contain leptin, though the surrounding normal tissue does not. This suggests that leptin may be involved in angiogenesis and the development of uterine cancer [67]. VEGF levels are augmented by leptin in various cancers [37, 38, 58, 68]. It has been reported that the leptin-induced upregulation of VEGF may be due to activation of the IL-1 system [38]. This leptin-mediated IL-1 up-regulation appears to be accomplished by activation of the MAPK and PI3K pathways, among others [37, 38]. Leptin and ObR expression are correlated with the grade of the tumor, differentiation, and microvessel density [58, 69]. VEGF expression is also correlated to these variables [58]. It is noteworthy to mention that Per Ole Iverson and coworkers blocked the ObR which suppressed rat leukemia cell growth by inhibiting angiogenesis [70]. Interestingly, hypoxia can induce VEGF production in cells, and it has been demonstrated that leptin expression is also augmented under similar conditions [71].
3. The Leptin GBM Connection
It was once thought that adipocytes were the sole producers of leptin. However, leptin expression and secretion has since been demonstrated in several tissues of the body (cancerous and noncancerous) including the pituitary gland and hypothalamus [72]. Barbara Morash and colleagues provided the first report of leptin expression in glioma following detection of leptin expression in the rat C6 glioma cell line [72]. It was later shown that C6 cells express more leptin and ObR than normal glial tissue [73]. Leptin and ObR expression subsequently has been confirmed in human primary GBM tissue as well as established human GBM cells lines [74]. Leptin and ObR are overexpressed in human primary brain tumors when compared to normal glial tissue [74]. Furthermore, the expression of the leptin-ObR system correlates with histological grade: GBM has the greatest levels of leptin and ObR while low-grade gliomas have the least [74]. This suggests that leptin/ObR autocrine/paracrine signaling increases the malignant characteristics of gliomas.
Leptin/ObR overexpression in glioma [74], coupled with recent evidence that the release of leptin from adipose tissue promotes melanoma and colon cancer [75], provides strong evidence that leptin plays a role in cancer pathogenesis. In the rat C6 cell line, leptin knockdown using RNA interference produced a reduction of both leptin mRNA and leptin protein. This knockdown caused a twofold increase in cell death suggesting that endogenous leptin promotes cell survival [76]. Furthermore, exogenous leptin enhances migration and invasion of the rat C6 cells through increased levels of matrix metalloproteinase-13 (MMP-13) [73]. The leptin-mediated up-regulation of MMP-13 occurs through the MAPK pathway [73].
While there is increasing evidence of leptin's role in angiogenesis [37, 38, 58, 68], no studies (to our knowledge) have indicated how leptin might affect angiogenesis in GBM. However, hypoxia, which is a characteristic of solid tumors, is more pronounced with higher grades of glioma [77] and may explain the increased expression of leptin and ObR in GBM compared to lower-grade glioma [74].
4. Environmental Enrichment Modulates Leptin Levels
It is increasingly evident that the enhanced mental stimulation from environmental enrichment (EE) delays the advancement of neurodegenerative disorders such as Huntington's, Parkinson's, and Alzheimer's [78], slows the progression of cancer [75, 79–81], and increases the activity of natural killer cells [82]. Environmental enrichment refers to the living conditions of the subject. In the context of the rodent, EE is achieved through conditions that allow the rodent to roam more freely, engage with the surroundings, be housed with other rodents, and have better access to exercise equipment. For humans, increased social and physical activity leads to EE. Interestingly, EE can reduce peripheral leptin expression and release [75].
The response to EE is related to the type of stress the subject experiences: EE increases eustress and decreases distress. Eustress is the result of positive stressors like exercise and social interaction whereas distress is the result of negative stressors like mental stress and social isolation. The augmentation of eustress and the reduction of distress are associated with longer survival and slower tumor growth [75, 79, 81]. Probably the most significant human data to date are those reported by Barbara Andersen and her colleagues who showed that distress reduction through psychological intervention resulted in a 45% decrease in the risk of breast cancer recurrence [79] and a 59% reduction in the risk of dying following breast cancer recurrence [81]. The physiological basis for this finding is an active area of investigation. Using mouse models for melanoma and colon cancer, Cao et al. demonstrated that EE enhances brain-derived neurotrophic factor (BDNF) expression [75]. BDNF in turn activates sympathetic nerve fibers innervating white adipose tissue. This beta-adrenergic stimulation suppresses leptin secretion resulting in cancer inhibition and remission [75].
5. Environmental Enrichment and GBM
A study has yet to be designed that blocks ObR or alters leptin levels in GBM subjects or animal models. One viable option for GBM treatment may be through EE. Recall that EE-induced activation of the brain-adipocyte BDNF/leptin axis causes cancer remission and inhibition in mice [75], and distress reduction lowers the rate of recurrence in breast cancer patients [79]. Environmental enrichment and psychological treatment increase BDNF and thereby reduce systemic leptin via sympathetic activation of beta-adrenergic receptors in adipose tissue. This hypothalamic-sympathoneuronal-adipocyte axis does not address the potential leptin-ObR autocrine signaling loop of GBM. Factors that influence the transcriptional regulation of the leptin gene in the rat C6 cells are different than those in adipose tissue [83, 84], and therefore successful treatments may need to be more specific to GBM. Therapies that are successful at crossing the blood-brain barrier and reducing the leptin-ObR signaling loop in GBM are needed and should be a focus of future research.
6. Summary
Leptin, which may be controlled by specific stimulation of the brain via EE or psychological intervention, has significant influence on tumor growth. In GBM and other cancer cells, leptin promotes cancer by stimulating cellular pathways that are advantageous for proliferation, angiogenesis, and evasion of death. Unfortunately, most of what is known about leptin and glioma stems from the rat C6 cell line. Future studies should focus on established human GBM cell lines and primary GBM neurosphere cultures both in vitro and in vivo.
References
- 1.Zhang Y, Proenca R, Maffei M, Barone M, Leopold L, Friedman JM. Positional cloning of the mouse obese gene and its human homologue. Nature. 1994;372(6505):425–432. doi: 10.1038/372425a0. [DOI] [PubMed] [Google Scholar]
- 2.Campfield LA, Smith FJ, Guisez Y, Devos R, Burn P. Recombinant mouse OB protein: evidence for a peripheral signal linking adiposity and central neural networks. Science. 1995;269(5223):546–549. doi: 10.1126/science.7624778. [DOI] [PubMed] [Google Scholar]
- 3.Halaas JL, Gajiwala KS, Maffei M, et al. Weight-reducing effects of the plasma protein encoded by the obese gene. Science. 1995;269(5223):543–546. doi: 10.1126/science.7624777. [DOI] [PubMed] [Google Scholar]
- 4.Pelleymounter MA, Cullen MJ, Baker MB, et al. Effects of the obese gene product on body weight regulation in ob/ob mice. Science. 1995;269(5223):540–543. doi: 10.1126/science.7624776. [DOI] [PubMed] [Google Scholar]
- 5.Rentsch J, Levens N, Chiesi M. Recombinant ob-gene product reduces food intake in fasted mice. Biochemical and Biophysical Research Communications. 1995;214(1):131–136. doi: 10.1006/bbrc.1995.2266. [DOI] [PubMed] [Google Scholar]
- 6.Maffei M, Halaas J, Ravussin E, et al. Leptin levels in human and rodent: measurement of plasma leptin and ob RNA in obese and weight-reduced subjects. Nature Medicine. 1995;1(11):1155–1161. doi: 10.1038/nm1195-1155. [DOI] [PubMed] [Google Scholar]
- 7.Friedman JM. Leptin, leptin receptors, and the control of body weight. Nutrition Reviews. 1998;56(2):S38–S46. doi: 10.1111/j.1753-4887.1998.tb01685.x. [DOI] [PubMed] [Google Scholar]
- 8.Clément K, Vaisse C, Lahlou N, et al. A mutation in the human leptin receptor gene causes obesity and pituitary dysfunction. Nature. 1998;392(6674):398–401. doi: 10.1038/32911. [DOI] [PubMed] [Google Scholar]
- 9.Pi-Sunyer X. The medical risks of obesity. Postgraduate Medicine. 2009;121(6):21–33. doi: 10.3810/pgm.2009.11.2074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nath AK, Brown RM, Michaud M, Sierra-Honigmann MR, Snyder M, Madri JA. Leptin affects endocardial cushion formation by modulating EMT and migration via Akt signaling cascades. Journal of Cell Biology. 2008;181(2):367–380. doi: 10.1083/jcb.200708197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Martín-Romero C, Sánchez-Margalet V. Human leptin activates PI3K and MAPK pathways in human peripheral blood mononuclear cells: possible role of Sam68. Cellular Immunology. 2001;212(2):83–91. doi: 10.1006/cimm.2001.1851. [DOI] [PubMed] [Google Scholar]
- 12.Sanchez-Margalet V, Martin-Romero C. Human leptin signaling in human peripheral blood mononuclear cells: activation of the JAK-STAT pathway. Cellular Immunology. 2001;211(1):30–36. doi: 10.1006/cimm.2001.1815. [DOI] [PubMed] [Google Scholar]
- 13.Machinal-Quélin F, Dieudonné MN, Leneveu MC, Pecquery R, Giudicelli Y. Proadipogenic effect of leptin on rat preadipocytes in vitro: activation of MAPK and STAT3 signaling pathways. American Journal of Physiology. 2002;282(4):C853–C863. doi: 10.1152/ajpcell.00331.2001. [DOI] [PubMed] [Google Scholar]
- 14.Najib S, Sánchez-Margalet V. Human leptin promotes survival of human circulating blood monocytes prone to apoptosis by activation of p42/44 MAPK pathway. Cellular Immunology. 2002;220(2):143–149. doi: 10.1016/s0008-8749(03)00027-3. [DOI] [PubMed] [Google Scholar]
- 15.Pérez-Pérez A, Maymó J, Dueñas JL, et al. Leptin prevents apoptosis of trophoblastic cells by activation of MAPK pathway. Archives of Biochemistry and Biophysics. 2008;477(2):390–395. doi: 10.1016/j.abb.2008.06.015. [DOI] [PubMed] [Google Scholar]
- 16.Mattioli B, Giordani L, Quaranta MG, Viora M. Leptin exerts an anti-apoptotic effect on human dendritic cells via the PI3K-Akt signaling pathway. FEBS Letters. 2009;583(7):1102–1106. doi: 10.1016/j.febslet.2009.02.029. [DOI] [PubMed] [Google Scholar]
- 17.Arias-Álvarez M, García-García RM, Torres-Rovira L, González-Bulnes A, Rebollar PG, Lorenzo PL. Influence of leptin on in vitro maturation and steroidogenic secretion of cumulus-oocyte complexes through JAK2/STAT3 and MEK 1/2 pathways in the rabbit model. Reproduction. 2010;139(3):523–532. doi: 10.1530/REP-09-0309. [DOI] [PubMed] [Google Scholar]
- 18.Chen YC, Chen CH, Hsu YH, et al. Leptin reduces gentamicin-induced apoptosis in rat renal tubular cells via the PI3K-Akt signaling pathway. European Journal of Pharmacology. 2011;658(2-3):213–218. doi: 10.1016/j.ejphar.2011.02.025. [DOI] [PubMed] [Google Scholar]
- 19.Guo Z, Jiang H, Xu X, Duan W, Mattson MP. Leptin-mediated cell survival signaling in hippocampal neurons mediated by JAK STAT3 and mitochondrial stabilization. Journal of Biological Chemistry. 2008;283(3):1754–1763. doi: 10.1074/jbc.M703753200. [DOI] [PubMed] [Google Scholar]
- 20.Attoub S, Noe V, Pirola L, et al. Leptin promotes invasiveness of kidney and colonic epithelial cells via phosphoinositide 3-kinase-, Rho-, and Rac-dependent signaling pathways. The FASEB Journal. 2000;14(14):2329–2338. doi: 10.1096/fj.00-0162. [DOI] [PubMed] [Google Scholar]
- 21.Oda A, Taniguchi T, Yokoyama M. Leptin stimulates rat aortic smooth muscle cell proliferation and migration. Kobe Journal of Medical Sciences. 2001;47(3):141–150. [PubMed] [Google Scholar]
- 22.Goetze S, Bungenstock A, Czupalla C, et al. Leptin induces endothelial cell migration through Akt, which is inhibited by PPARγ-ligands. Hypertension. 2002;40(5):748–754. doi: 10.1161/01.hyp.0000035522.63647.d3. [DOI] [PubMed] [Google Scholar]
- 23.Kim S, Nagalingam A, Saxena NK, Singh SV, Sharma D. Benzyl isothiocyanate inhibits oncogenic actions of leptin in human breast cancer cells by suppressing activation of signal transducer and activator of transcription 3. Carcinogenesis. 2011;32(3):359–367. doi: 10.1093/carcin/bgq267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Burguera B, Brunetto A, Garcia-Ocana A, et al. Leptin increases proliferation of human steosarcoma cells through activation of PI(3)-K and MAPK pathways. Medical Science Monitor. 2006;12(11):BR341–BR349. [PubMed] [Google Scholar]
- 25.Judd LM, Bredin K, Kalantzis A, Jenkins BJ, Ernst M, Giraud AS. STAT3 activation regulates growth, inflammation, and vascularization in a mouse model of gastric tumorigenesis. Gastroenterology. 2006;131(4):1073–1085. doi: 10.1053/j.gastro.2006.07.018. [DOI] [PubMed] [Google Scholar]
- 26.Saxena NK, Sharma D, Ding X, et al. Concomitant activation of the JAK/STAT, PI3K/AKT, and ERK signaling is involved in leptin-mediated promotion of invasion and migration of hepatocellular carcinoma cells. Cancer Research. 2007;67(6):2497–2507. doi: 10.1158/0008-5472.CAN-06-3075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Saxena NK, Vertino PM, Anania FA, Sharma D. Leptin-induced growth stimulation of breast cancer cells involves recruitment of histone acetyltransferases and mediator complex to CYCLIN D1 promoter via activation of Stat3. Journal of Biological Chemistry. 2007;282(18):13316–13325. doi: 10.1074/jbc.M609798200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jiang H, Yu J, Guo H, Song H, Chen S. Upregulation of survivin by leptin/STAT3 signaling in MCF-7 cells. Biochemical and Biophysical Research Communications. 2008;368(1):1–5. doi: 10.1016/j.bbrc.2007.04.004. [DOI] [PubMed] [Google Scholar]
- 29.Li L, Gao Y, Zhang LL, He DL. Concomitant activation of the JAK/STAT3 and ERK1/2 signaling is involved in leptin-mediated proliferation of renal cell carcinoma Caki-2 cells. Cancer Biology and Therapy. 2008;7(11):1787–1792. doi: 10.4161/cbt.7.11.6837. [DOI] [PubMed] [Google Scholar]
- 30.Catalano S, Giordano C, Rizza P, et al. Evidence that leptin through STAT and CREB signaling enhances cyclin D1 expression and promotes human endometrial cancer proliferation. Journal of Cellular Physiology. 2009;218(3):490–500. doi: 10.1002/jcp.21622. [DOI] [PubMed] [Google Scholar]
- 31.Gao J, Tian J, Lv Y, et al. Leptin induces functional activation of cyclooxygenase-2 through JAK2/ STAT3, MAPK/ERK, and PI3K/AKT pathways in human endometrial cancer cells. Cancer Science. 2009;100(3):389–395. doi: 10.1111/j.1349-7006.2008.01053.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yang SN, Chen HT, Tsou HK, et al. Leptin enhances cell migration in human chondrosarcoma cells through OBRl leptin receptor. Carcinogenesis. 2009;30(4):566–574. doi: 10.1093/carcin/bgp023. [DOI] [PubMed] [Google Scholar]
- 33.Uddin S, Bu R, Ahmed M, et al. Leptin receptor expression and its association with PI3K/AKT signaling pathway in diffuse large B-cell lymphoma. Leukemia and Lymphoma. 2010;51(7):1305–1314. doi: 10.3109/10428191003802365. [DOI] [PubMed] [Google Scholar]
- 34.Liu Y, et al. Leptin activates STAT3 and ERK1/2 pathways and induces endometrial cancer cell proliferation. Journal of Huazhong University of Science and Technology Medical. 2011;31(3):365–370. doi: 10.1007/s11596-011-0382-7. [DOI] [PubMed] [Google Scholar]
- 35.Ratke J, Entschladen F, Niggemann B, Zänker KS, Lang K. Leptin stimulates the migration of colon carcinoma cells by multiple signaling pathways. Endocrine-Related Cancer. 2010;17(1):179–189. doi: 10.1677/ERC-09-0225. [DOI] [PubMed] [Google Scholar]
- 36.Uddin S, Bavi P, Siraj AK, et al. Leptin-R and its association with PI3K/AKT signaling pathway in papillary thyroid carcinoma. Endocrine-Related Cancer. 2010;17(1):191–202. doi: 10.1677/ERC-09-0153. [DOI] [PubMed] [Google Scholar]
- 37.Frankenberry KA, Somasundar P, McFadden DW, Vona-Davis LC. Leptin induces cell migration and the expression of growth factors in human prostate cancer cells. American Journal of Surgery. 2004;188(5):560–565. doi: 10.1016/j.amjsurg.2004.07.031. [DOI] [PubMed] [Google Scholar]
- 38.Zhou W, Guo S, Gonzalez-Perez RR. Leptin pro-angiogenic signature in breast cancer is linked to IL-1 signalling. British Journal of Cancer. 2011;104(1):128–137. doi: 10.1038/sj.bjc.6606013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Deo DD, Rao AP, Bose SS, et al. Differential effects of leptin on the invasive potential of androgen-dependent and -independent prostate carcinoma cells. Journal of Biomedicine and Biotechnology. 2008;2008(1) doi: 10.1155/2008/163902. Article ID 163902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Huang CY, Yu HS, Lai TY, et al. Leptin increases motility and integrin up-regulation in human prostate cancer cells. Journal of Cellular Physiology. 2011;226(5):1274–1282. doi: 10.1002/jcp.22455. [DOI] [PubMed] [Google Scholar]
- 41.Sharma D, Wang J, Fu PP, et al. Adiponectin antagonizes the oncogenic actions of leptin in hepatocellular carcinogenesis. Hepatology. 2010;52(5):1713–1722. doi: 10.1002/hep.23892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Snoussi K, Strosberg AD, Bouaouina N, Ahmed SB, Helal AN, Chouchane L. Leptin and leptin receptor polymorphisms are associated with increased risk and poor prognosis of breast carcinoma. BMC Cancer. 2006;6, article 38 doi: 10.1186/1471-2407-6-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ribeiro R, Vasconcelos A, Costa S, et al. Overexpressing leptin genetic polymorphism (-2548 G/A) is associated with susceptibility to prostate cancer and risk of advanced disease. Prostate. 2004;59(3):268–274. doi: 10.1002/pros.20004. [DOI] [PubMed] [Google Scholar]
- 44.Yapijakis C, Kechagiadakis M, Nkenke E, et al. Association of leptin -2548G/A and leptin receptor Q223R polymorphisms with increased risk for oral cancer. Journal of Cancer Research and Clinical Oncology. 2009;135(4):603–612. doi: 10.1007/s00432-008-0494-z. [DOI] [PubMed] [Google Scholar]
- 45.Somasundar P, McFadden DW, Hileman SM, Vona-Davis L. Leptin is a growth factor in cancer. Journal of Surgical Research. 2004;116(2):337–349. doi: 10.1016/j.jss.2003.09.004. [DOI] [PubMed] [Google Scholar]
- 46.De Assis S, Khan G, Hilakivi-Clarke L. High birth weight increases mammary tumorigenesis in rats. International Journal of Cancer. 2006;119(7):1537–1546. doi: 10.1002/ijc.21936. [DOI] [PubMed] [Google Scholar]
- 47.Hino M, Nakao T, Yamane T, Ohta K, Takubo T, Tatsumi N. Leptin receptor and leukemia. Leukemia and Lymphoma. 2000;36(5-6):457–461. doi: 10.3109/10428190009148392. [DOI] [PubMed] [Google Scholar]
- 48.Rouet-Benzineb P, Aparicio T, Guilmeau S, et al. Leptin counteracts sodium butyrate-induced apoptosis in human colon cancer HT-29 cells via NF-κB signaling. Journal of Biological Chemistry. 2004;279(16):16495–16502. doi: 10.1074/jbc.M312999200. [DOI] [PubMed] [Google Scholar]
- 49.Russo VC, Metaxas S, Kobayashi K, Harris M, Werther GA. Antiapoptotic effects of leptin in human neuroblastoma cells. Endocrinology. 2004;145(9):4103–4112. doi: 10.1210/en.2003-1767. [DOI] [PubMed] [Google Scholar]
- 50.Somasundar P, Frankenberry KA, Skinner H, et al. Prostate cancer cell proliferation is influenced by leptin. Journal of Surgical Research. 2004;118(1):71–82. doi: 10.1016/j.jss.2004.01.017. [DOI] [PubMed] [Google Scholar]
- 51.Ogunwobi O, Mutungi G, Beales ILP. Leptin stimulates proliferation and inhibits apoptosis in Barrett’s esophageal adenocarcinoma cells by cyclooxygenase-2-dependent, prostaglandin-E2-mediated transactivation of the epidermal growth factor receptor and c-Jun NH2-terminal kinase activation. Endocrinology. 2006;147(9):4505–4516. doi: 10.1210/en.2006-0224. [DOI] [PubMed] [Google Scholar]
- 52.Chen C, Chang YC, Liu CL, Liu TP, Chang KJ, Guo IC. Leptin induces proliferation and anti-apoptosis in human hepatocarcinoma cells by up-regulating cyclin D1 and down-regulating Bax via a Janus kinase 2-linked pathway. Endocrine-Related Cancer. 2007;14(2):513–529. doi: 10.1677/ERC-06-0027. [DOI] [PubMed] [Google Scholar]
- 53.Fava G, Alpini G, Rychlicki C, et al. Leptin enhances cholangiocarcinoma cell growth. Cancer Research. 2008;68(16):6752–6761. doi: 10.1158/0008-5472.CAN-07-6682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Shen Y, Wang Q, Zhao Q, Zhou J. Leptin promotes the immune escape of lung cancer by inducing proinflammatory cytokines and resistance to apoptosis. Molecular Medicine Reports. 2009;2(2):295–299. doi: 10.3892/mmr_00000099. [DOI] [PubMed] [Google Scholar]
- 55.Choi JH, Park SH, Leung PCK, Choi KC. Expression of leptin receptors and potential effects of leptin on the cell growth and activation of mitogen-activated protein kinases in ovarian cancer cells. Journal of Clinical Endocrinology and Metabolism. 2005;90(1):207–210. doi: 10.1210/jc.2004-0297. [DOI] [PubMed] [Google Scholar]
- 56.Dieudonne MN, Machinal-Quelin F, Serazin-Leroy V, Leneveu MC, Pecquery R, Giudicelli Y. Leptin mediates a proliferative response in human MCF7 breast cancer cells. Biochemical and Biophysical Research Communications. 2002;293(1):622–628. doi: 10.1016/S0006-291X(02)00205-X. [DOI] [PubMed] [Google Scholar]
- 57.Hardwick JCH, Van Den Brink GR, Offerhaus GJ, Van Deventer SJH, Peppelenbosch MP. Leptin is a growth factor for colonic epithelial cells. Gastroenterology. 2001;121(1):79–90. doi: 10.1053/gast.2001.25490. [DOI] [PubMed] [Google Scholar]
- 58.Hui L, Desen W, Zhizhong P, et al. Expression and biological significance of leptin, leptin receptor, VEGF, and CD34 in colorectal carcinoma. Cell Biochemistry and Biophysics. 2011;60(3):241–244. doi: 10.1007/s12013-010-9145-5. [DOI] [PubMed] [Google Scholar]
- 59.Pai R, Lin C, Tran T, Tarnawski A. Leptin activates STAT and ERK2 pathways and induces gastric cancer cell proliferation. Biochemical and Biophysical Research Communications. 2005;331(4):984–992. doi: 10.1016/j.bbrc.2005.03.236. [DOI] [PubMed] [Google Scholar]
- 60.Suzukawa M, Nagase H, Ogahara I, et al. Leptin enhances survival and induces migration, degranulation, and cytokine synthesis of human basophils. Journal of Immunology. 2011;186(9):5254–5260. doi: 10.4049/jimmunol.1004054. [DOI] [PubMed] [Google Scholar]
- 61.Schram K, Ganguly R, No EK, Fang X, Thong FS, Sweeney G. Regulation of MT1-MMP and MMP-2 by leptin in cardiac fibroblasts involves Rho/ROCK-dependent actin cytoskeletal reorganization and leads to enhanced cell migration. Endocrinology. 2011;152(5):2037–2047. doi: 10.1210/en.2010-1166. [DOI] [PubMed] [Google Scholar]
- 62.Jaffe T, Schwartz B. Leptin promotes motility and invasiveness in human colon cancer cells by activating multiple signal-transduction pathways. International Journal of Cancer. 2008;123(11):2543–2556. doi: 10.1002/ijc.23821. [DOI] [PubMed] [Google Scholar]
- 63.Cheng SP, Yin PH, Chang YC, Lee CH, Huang SY, Chi CW. Differential roles of leptin in regulating cell migration in thyroid cancer cells. Oncology Reports. 2010;23(6):1721–1727. doi: 10.3892/or_00000817. [DOI] [PubMed] [Google Scholar]
- 64.Saxena NK, Taliaferro-Smith L, Knight BB, et al. Bidirectional crosstalk between leptin and insulin-like growth factor-I signaling promotes invasion and migration of breast cancer cells via transactivation of epidermal growth factor receptor. Cancer Research. 2008;68(23):9712–9722. doi: 10.1158/0008-5472.CAN-08-1952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Bouloumié A, Drexler HCA, Lafontan M, Busse R. Leptin, the product of Ob gene, promotes angiogenesis. Circulation Research. 1998;83(10):1059–1066. doi: 10.1161/01.res.83.10.1059. [DOI] [PubMed] [Google Scholar]
- 66.Cao R, Brakenhielm E, Wahlestedt C, Thyberg J, Cao Y. Leptin induces vascular permeability and synergistically stimulates angiogenesis with FGF-2 and VEGF. Proceedings of the National Academy of Sciences of the United States of America. 2001;98(11):6390–6395. doi: 10.1073/pnas.101564798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Markowska A, Belloni AS, Rucinski M, et al. Leptin and leptin receptor expression in the myometrium and uterine myomas: is leptin involved in tumor development? International Journal of Oncology. 2005;27(6):1505–1509. [PubMed] [Google Scholar]
- 68.Knight BB, Oprea-Ilies GM, Nagalingam A, et al. Survivin upregulation, dependent on leptin-EGFR-Notch1 axis, is essential for leptin-induced migration of breast carcinoma cells. Endocrine-Related Cancer. 2011;18(4):413–428. doi: 10.1530/ERC-11-0075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ribatti D, Belloni AS, Nico B, Di Comite M, Crivellato E, Vacca A. Leptin-leptin receptor are involved in angiogenesis in human hepatocellular carcinoma. Peptides. 2008;29(9):1596–1602. doi: 10.1016/j.peptides.2008.05.011. [DOI] [PubMed] [Google Scholar]
- 70.Iversen PO, Drevon CA, Reseland JE. Prevention of leptin binding to its receptor suppresses rat leukemic cell growth by inhibiting angiogenesis. Blood. 2002;100(12):4123–4128. doi: 10.1182/blood-2001-11-0134. [DOI] [PubMed] [Google Scholar]
- 71.Ambrosini G, Nath AK, Rocío Sierra-Honigmann M, Flores-Riveros J. Transcriptional activation of the human leptin gene in response to hypoxia. Involvement of hypoxia-inducible factor 1. Journal of Biological Chemistry. 2002;277(37):34601–34609. doi: 10.1074/jbc.M205172200. [DOI] [PubMed] [Google Scholar]
- 72.Morash B, Li A, Murphy PR, Wilkinson M, Ur E. Leptin gene expression in the brain and pituitary gland. Endocrinology. 1999;140(12):5995–5998. doi: 10.1210/endo.140.12.7288. [DOI] [PubMed] [Google Scholar]
- 73.Yeh WL, Lu DY, Lee MJ, Fu WM. Leptin induces migration and invasion of glioma cells through MMP-13 production. GLIA. 2009;57(4):454–464. doi: 10.1002/glia.20773. [DOI] [PubMed] [Google Scholar]
- 74.Riolfi M, Ferla R, Valle LD, et al. Leptin and its receptor are overexpressed in brain tumors and correlate with the degree of malignancy. Brain Pathology. 2010;20(2):481–489. doi: 10.1111/j.1750-3639.2009.00323.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Cao L, Liu X, Lin EJD, et al. Environmental and genetic activation of a brain-adipocyte BDNF/leptin axis causes cancer remission and inhibition. Cell. 2010;142(1):52–64. doi: 10.1016/j.cell.2010.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Brown R, Morash B, Ur E, Wilkinson M. RNAi-mediated silencing of leptin gene expression increases cell death in C6 glioblastoma cells. Molecular Brain Research. 2005;139(2):357–360. doi: 10.1016/j.molbrainres.2005.05.009. [DOI] [PubMed] [Google Scholar]
- 77.Evans SM, Judy KD, Dunphy I, et al. Hypoxia is important in the biology and aggression of human glial brain tumors. Clinical Cancer Research. 2004;10(24):8177–8184. doi: 10.1158/1078-0432.CCR-04-1081. [DOI] [PubMed] [Google Scholar]
- 78.Laviola G, Hannan AJ, Macrì S, Solinas M, Jaber M. Effects of enriched environment on animal models of neurodegenerative diseases and psychiatric disorders. Neurobiology of Disease. 2008;31(2):159–168. doi: 10.1016/j.nbd.2008.05.001. [DOI] [PubMed] [Google Scholar]
- 79.Andersen BL, Yang HC, Farrar WB, et al. Psychologic intervention improves survival for breast cancer patients: a randomized clinical trial. Cancer. 2008;113(12):3450–3458. doi: 10.1002/cncr.23969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Esser KA, Harpole CE, Prins GS, Diamond AM. Physical activity reduces prostate carcinogenesis in a transgenic model. Prostate. 2009;69(13):1372–1377. doi: 10.1002/pros.20987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Andersen BL, Thornton LM, Shapiro CL, et al. Biobehavioral, immune, and health benefits following recurrence for psychological intervention participants. Clinical Cancer Research. 2010;16(12):3270–3278. doi: 10.1158/1078-0432.CCR-10-0278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Benaroya-Milshtein N, Hollander N, Apter A, et al. Environmental enrichment in mice decreases anxiety, attenuates stress responses and enhances natural killer cell activity. European Journal of Neuroscience. 2004;20(5):1341–1347. doi: 10.1111/j.1460-9568.2004.03587.x. [DOI] [PubMed] [Google Scholar]
- 83.Morash B, Johnstone J, Leopold C, et al. The regulation of leptin gene expression in the C6 glioblastoma cell line. Molecular and Cellular Endocrinology. 2000;165(1-2):97–105. doi: 10.1016/s0303-7207(00)00259-8. [DOI] [PubMed] [Google Scholar]
- 84.Li AW, Morash B, Hollenberg AN, Ur E, Wilkinson M, Murphy PR. Transcriptional regulation of the leptin gene promoter in rat GH3 pituitary and C6 glioma cells. Molecular and Cellular Endocrinology. 2001;176(1-2):57–65. doi: 10.1016/s0303-7207(01)00476-2. [DOI] [PubMed] [Google Scholar]


