Abstract
Mechanical cues affect tendon healing, homeostasis, and development in a variety of settings. Alterations in the mechanical environment are known to result in changes in the expression of extracellular matrix proteins, growth factors, transcription factors, and cytokines that can alter tendon structure and cell viability. Loss of muscle force in utero or in the immediate postnatal period delays tendon and enthesis development. The response of healing tendons to mechanical load varies depending on anatomic location. Flexor tendons require motion to prevent adhesion formation, yet excessive force results in gap formation and subsequent weakening of the repair. Excessive motion in the setting of anterior cruciate ligament reconstruction causes accumulation of macrophages, which are detrimental to tendon graft healing. Complete removal of load is detrimental to rotator cuff healing, yet large forces are also harmful. Controlled loading can enhance healing in most settings; however, a fine balance must be reached between loads that are too low (leading to a catabolic state) and too high (leading to micro-damage). This review will summarize existing knowledge of the mechanobiology of tendon development, homeostasis, and healing.
Keywords: shoulder, mechanotransduction
Tendon structure-function
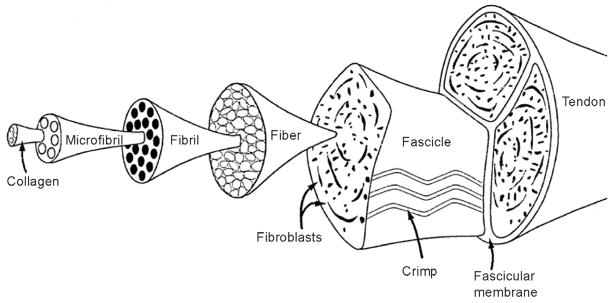
Tendons are critical for the function of joints, connecting muscles to bones and allowing for the transmission of forces between the tissues, leading to joint motion. The mechanical properties of tendons derive largely from type I collagen fibers that are arranged in dense, parallel arrays.127 This arrangement results in a resilient tissue with high tensile stiffness in the direction of fiber orientation. The organization of tendon and ligament is hierarchical in nature, from the molecular to the tissue scales (Figure 1).63 Triple-helix type I collagen molecules (300 nm in length, 1.5 nm in diameter) pack together to form microfibrils.57 Microfibrils are typically defined as five collagen molecules, stacked in a quarter-stagger array. Neighboring microfibrils interdigitate, imposing order upon a mildly twisted lattice that forms the next level structure termed a fibril (50–200nm in diameter). At the next level of structural hierarchy, fibrils close-pack into larger structures to form fibers (3–7 μm in diameter). Fibers combine to form fascicles (with diameters on the order of micrometers); at this level a characteristic “crimp” pattern can be seen histologically.62,63 Finally, fascicles are bundled together through a fascicular membrane to form tendon (diameter on the order of millimeters or centimeters).
Figure 1.
The organization of tendon is hierarchical in nature, from the molecular (i.e., collagen molecule) to the tissue scales. [Adapted from: Kastelic, J., A. Galeski, and E. Baer. 1978. The multicomposite structure of tendon. Connective Tissue Research 6 (1):11–23].
Approximately 20–30% of the dry weight of tendon is made up of proteoglycans, glycosaminoglycans, minor collagens (e.g., type III, type XII), elastin, and cellular material.71,119,127 These minor compositional constituents play important roles in the development of the tissue. For example, type V collagen and the proteoglycans biglycan and decorin regulate fibril diameter during collagen fibrillogenesis 15,95,124.
The attachment of tendon to bone is of major concern in many repair scenarios, including rotator cuff tears. Due to the large difference in mechanical properties between tendon and bone19,125, large stress concentrations will arise at the attachment point of these two materials and the connection will be at risk for failure. The natural (uninjured) tendon insertion site (the “enthesis”) overcomes this challenge via a number of strategies76: 1) gradations in its composition and structure45,76,86,106, 2) a shallow attachment angle at the insertion76,111, 3) shaping of tissue morphology of the transitional tissue76, and 4) interdigitation of transitional tissue with bone84. These four mechanisms provide a nano- through macro-mechanical description of how a robust tendon-to-bone attachment is achieved.45,76 However, the complex composition, structure, and mechanical behavior of the tendon/ligament-to-bone insertion result in a particularly difficult challenge for effective response to injury.
Tendon injury and repair
Tendinopathies limit mobility and joint function and often lead to disability and pain.93 Damage to tendons can be the result of an acute injury (e.g., laceration or sports injury) or chronic impairment (e.g., overuse injury or degeneration), and the capacity of the tendon to heal varies depending on its magnitude, duration, and location.96,103,128 Effective repair of short and intracapsular tendons (e.g., rotator cuff tendons) often relies on tendon-to-bone integration59,112 whereas effective repair of long and sheathed tendons (e.g., flexor tendons) often depends on prevention of repair-site gapping and maintenance of tendon gliding.20,44,126 Tendon healing, in general, follows a typical wound-healing course75: a short inflammatory phase (lasting on the order of days) is followed by a proliferative phase (lasting on the order of weeks), which in turn is followed by a remodeling phase (lasting on the order of months). The inflammatory phase is characterized by increased vascular permeability and an influx of local inflammatory cells including platelets, macrophages, monocytes, and neutrophils that release chemotactic agents to recruit blood vessels, fibroblasts, and intrinsic tenocytes. In the proliferative phase of healing, fibroblasts at the injury site multiply and begin producing collagen. During the remodeling phase, cellularity decreases and collagen is crosslinked and oriented parallel to the direction of muscle force.
Tendon healing depends on the contributions of mulitple cell sources, which may include fibroblasts from the endotendon and epitendon2, inflammatory cells from the vasculature21,39, cells from the synovial sheath1,3,9, and mesenchymal stem cells128, which may migrate to the area of tendon injury or proliferate from within the tendon. The contribution of each cell type to the repair depends on the anatomy and physiology of the particular tendon. For example, healing of flexor tendon injuries begins with angiogenesis and epitenon fibroblast migration to the wound site.40,43 Cells from the intrasynovial sheath infiltrate to the repair site, leading to adhesions between the sheath and the tendon surface, which impairs tendon gliding (and hence decreases digital range of motion).40,43 In the rotator cuff, on the other hand, injuries typically require repair of tendon to bone.59 In this case, abundant fibroblasts from the tendon and surrounding tissues produce a disorganized collagen scar tissue at the attachment site of the two tissues.109,112 Osteoclasts are also attracted to the repair site, and resorption of bone at the repair site can impair healing.24,28 Understanding how different tendons heal is an important consideration for post-operative treatment and rehabilitation.
Tendon mechanobiology during development and homeostasis
The importance of mechanical loading on the development and homeostasis of tendon is evident from a number of studies.14,80,107,108,122 Mechanical loading has been implicated in changes to tendon size29,50,68 and mechanical properties29,34,77, and this mechanosensitive tissue responds to loading in an adaptive manner.8 Mechanotransduction, or the ability of cells to respond to mechanical cues with biochemical signals, is an important component of musculoskeletal tissue development, homeostasis, healing, and degeneration.11,122 Tendon homeostasis is maintained with regulated levels of extracellular matrix turnover via production of both matrix degrading enzymes (e.g., matrix metalloproteinases [MMPs]) and extracellular matrix (ECM; e.g., collagen).80,81 Of particular recent interest are the roles of the transcription factor scleraxis (necessary for tenogenesis) and transforming growth factor-β (TGF-β; a master regulator of differentiation, proliferation, and ECM production), both of which have been shown to act as mediators of tendon development.88,94,97,100 Altered mechanical loading can promote changes in the expression of scleraxis as well as TGF-β, leading to changes in tendon structure, cell viability, and ECM production.18,73,107,109 Murcheson et al. demonstrated that the regulation of tendon differentiation by scleraxis distinguished force-transmitting tendons from muscle anchoring tendons, implying a mechanosensitive role for the transcription factor.88 A number of MMPs81, cytokines (e.g., interleukin-15,6,13,61,72,85,114–116,130, and cyclo-oxygenase-25,13,72,123,130), and growth factors (e.g., platelet-derived growth factor) 14,87 can also be affected by mechanical loading. This can result in either a catabolic environment leading to decreased tendon mechanical properties or an anabolic environment leading to increases in tendon mechanical properties.
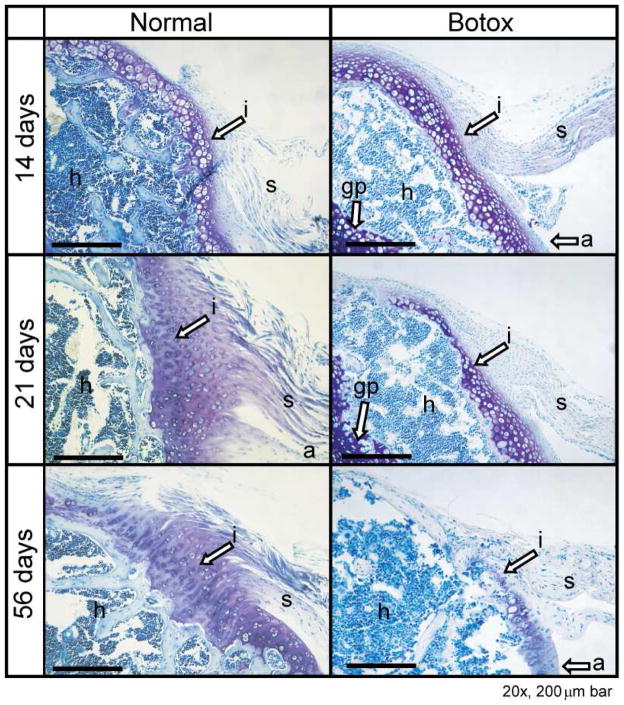
Mechanical cues are necessary for the development of tendon microstructure and strength, especially during prenatal and postnatal growth.18,32,66,83,108,110 Embryonic immobilization leads to a decrease in tenascin expression and protein levels in avian synovial joints.83 Compressive loading is critical for the production of proteoglycans in developing flexor tendons.32 Other skeletal structures are dependent on tendon mobility in utero as well. For example, the development of sesamoid bones has been shown to be dependent on embryonic mobilization, specifically localized mechanical stress and musculotendinous loading.82,99 In neonates, muscle paralysis has been shown to result in delayed tendon and fibrocartilage maturation as well as impaired mineralization at the enthesis.66,110 Muscle paralysis of supraspinatus muscles induced at birth via botulinum toxin A110 or microsurgical transection of the superior trunk of the brachial plexus66 led to musculoskeletal deformities66, delayed maturation of tendon-to-bone insertion110 (Figure 2), and increased intramuscular fat accumulation26. Understanding the role of mechanical loading during development may aid in designing rehabilitation strategies and other therapies for adults with tendon injuries.
Figure 2.
Development of the tendon-to-bone insertion was dramatically delayed in the Botox group compared to the Normal group. i, tendon-to-bone insertion; s, supraspinatus tendon; a, articular surface of the humeral head; h, humeral head; gp, growth plate. Scale bar: 200 μm. [Adapted, with permission, from: Thomopoulos S, Kim HM, Rothermich SY, Biederstadt C, Das R, Galatz LM. Decreased muscle loading delays maturation of the tendon enthesis during postnatal development. J Orthop Res 2007;25:1154–63.]
The sensitivity of tendon fibroblasts to their mechanical loading environment has been well characterized in vitro and in vivo.11,12,47,52,53,79,97,117,118,120–122,129 Nuclei of tendon fibroblasts have been shown to deform when subjected to tensile strain in situ.80 Arnoczky et al. demonstrated that tendon fibroblasts, when subjected to tensile loads in vitro, respond biochemically via the stress-activated protein kinases (SAPK) in both strain- and frequency-dependent manners12. While over-expression of biochemical pathways such as the c-Jun N-terminal kinase (JNK) have been linked to deleterious effects such as apoptosis and increased cytokine activity60, such loading may also have a therapeutic role in tendon biology. For example, Scott et al. identified upregulation of insulin-like growth factor 1 (IGF-1) following increased loading101, which may encourage cellular proliferation and tendon remodeling55. Stress deprivation induces a catabolic state in tendon cells11,35,52. Removal of load for one day led to a marked increase in mRNA and protein expression of MMP-1 in an ex vivo model11. Similarly, while cyclic loading increased the ratio of TIMP-1 to MMP-13 (resulting in an anabolic state), stress deprivation reversed this ratio through an increase in MMP-13 expression (resulting in a catabolic state)35. In the section that follows, we will discuss both beneficial and detrimental effects of mechanical loading on tendon healing.
Tendon mechanobiology during healing
Joint immobilization and temporary muscle paralysis
Proper post-surgical rehabilitation strategies for tendon repair are persistently debated in the field of orthopaedics. Recent research has suggested a beneficial effect of sling or cast immobilization to prevent post-repair rupture and aid in healing of repaired rotator cuff tendons.89,112 In some animal models of tendon injury and healing, cast immobilization has been shown to enhance healing of tendon to bone when compared to other post-repair loading regimes like exercise or complete tendon unloading.25,34,46,112 For example, in the repaired rotator cuff, immobilization has been shown to play a beneficial role in tendon-to-bone healing.34,46,112 Using a rat model of rotator cuff injury and repair, Thomopoulos et al. found that cast immobilization led to reduced expression of collagen type III (indicative of scar) and increased expression of aggrecan, collagen type II, and collagen type XII when compared to injured rats with exercise.112 Immobilization promoted the improvement of viscoelastic parameters compared to exercise post-repair.112 Similarly, Gimbel et al. found that immobilization led to increased elastic properties and improved collagen organization at the repaired insertion in the rat rotator cuff repair model compared to cage activity or post-repair exercise.46
Understanding the role of immobilization on other connective tissues, such as ligaments, may provide insight into tendon repair as well. Rodeo et al. has described the role of macrophage infiltration on the healing tendon-to-bone following anterior cruciate ligament (ACL) reconstruction.17,23,25,54,64,98 Macrophage activity has been shown to be deleterious to the healing tendon graft, and macrophage-depleted rats (treated with the bisphosphonate, liposomal clodronate) have demonstrated accelerated ACL graft healing compared to untreated rats,64 Immobilization has also led to reduced phagocytic macrophage accumulation in the healing graft tunnel following ACL repair, with concurrent improvements in tendon-to-bone integration.25
Short-term muscle paralysis has been used as a rehabilitation strategy for tendon injuries. The implementation of botulinum toxin A, a pharmacological agent that temporarily inhibits the pre-synaptic release of acetylcholine, has been used in tendon healing experiments in an effort to protect the repair from excessive muscle loads. Such treatment has been shown to lead to favorable clinical results in patients with spasticity.22,70 Additionally, botulinum toxin A has been implemented as a tool for unloading the tendon during the healing process post-repair.34,56,78 However, as described in the next section, caution must be taken when completely unloading the repair site.
While immobilization has been shown to be beneficial for post-repair treatment in rotator cuff tendons46,89,112 and ACL reconstruction25, such post-operative repair methods may be detrimental for other types of injured tendons20,44. For example, immobilization after flexor tendon repair leads to fibrous adhesions between the tendon and its synovial sheath42, which can severely limit range of motion. Complete immobilization of flexor tendon repairs can also lead to a reduction in repair strength.40,44,126 Long-term (9 week) immobilization of the knee leads to a reduction in tissue stiffness, particularly of the patellar tendon and medial collateral ligament, which has been attributed not to atrophy but rather to increased collagen turnover.4
Complete repair-site unloading
Stress deprivation by immobilization or short-term paralysis can benefit recovery and healing of tendons in certain scenarios.27,78 However, complete unloading of the repair site is detrimental to healing. Complete removal of load has been shown to limit the organization and structure of the healing tendon and insertion as well as to decrease the mechanical properties of the unloaded tendon.34,113,116 Galatz et al. showed that complete removal of load from the healing site using immobilization and botulinum toxin A application to the muscle resulted in decreased structural properties of the repair tissue (Figure 3).34 This was largely due to a decreased production of extracellular matrix. Hettrich et al. found similar results using chemical removal of load in the same rat model without immobilization.56 Repairs treated with botulinum toxin A had significantly lower cross sectional area at later time points with subsequent reduction in load to failure. Experiments in vitro have demonstrated a quick and sustained release of MMPs as well as a decrease in tissue inhibitors of MMPs when tendon cells are deprived of mechanical stress.35 Rotator cuff tendon unloading following tenotomy and muscle denervation leads to an increase in fatty degeneration and atrophy of muscle34,56,65, increased adipogenesis65,110, and increased fibrosis16,110. Similarly, removal of load after flexor tendon-to-bone repairs via a proximal tenotomy led to unfavorable results in mechanical strength and collagen organization.113
Figure 3.
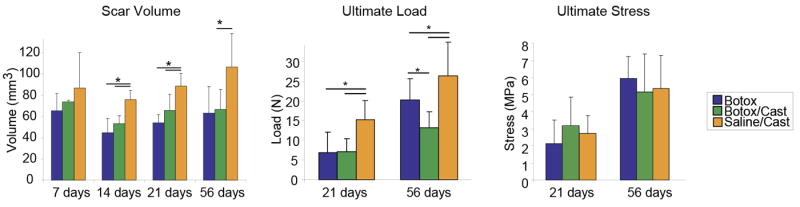
Complete removal of load (Botox/Cast group) led to significantly lower scar volume and ultimate load (a structural property), but did not affect ultimate stress (a material property). * p < 0.05. [Adapted, with permission, from: Galatz LM, Charlton N, Das R, Kim HM, Havlioglu N, Thomopoulos S. Complete removal of load is detrimental to rotator cuff healing. Journal of Shoulder and Elbow Surgery 2009;18:669–75.].
Passive motion
Tendons requiring long excursions for function (e.g., the flexor tendons of the hand) are typically encased in synovial sheaths. Successful repair of these tendons requires recovery of tissue strength and maintenance of intrasynovial gliding properties. In order to maintain gliding, adhesions between the tendon surface and its sheath must be prevented. Therefore, post-operatively rehabilitation strategies must balance the detrimental effects of immobilization (which will lead to adhesions) with the detrimental effects of active motion (which increase the risk for repair-site rupture). Passive motion, where the tendon is moved within its sheath without active muscle forces, has been established as optimal loading environment for healing.20,37,40,41,44,126 Passive mechanical rehabilitation methods have been shown to be an effective post-repair treatment for preventing fibrotic adhesions in long tendons, and the optimal timepoint of initiation of such treatment is about 5 days post-repair31. By mobilizing the tendon in the synovial sheath, adhesion formations are reduced, injured tendons become stronger than if immobilized, and cell activity and collagen deposition at the repair site are improved40.
Although passive mechanical loading of the repaired flexor tendon is clearly beneficial, the same may not be true of the repaired rotator cuff. Recent work by Peltz et al. using the rat model of rotator cuff injury demonstrated that passive mobilization post-repair can lead to decreased range of motion in joints compared to immobilization.90 However, clinical studies have demonstrated a reduction in pain scores for patients undergoing continuous passive motion following rotator cuff repair30, and some suggest continuous passive motion to be clinically more beneficial than immobilization36. In contrast, active motion rehabilitation protocols have been proposed for flexor tendon healing which involve moving the tendon within its sheath using active muscle force. This can lead to high loads on the tendon repair site, and a higher risk for rupture.74 Similarly, high loads across the repair site during tendon-to-bone healing may lead to gaps, microtears, or repair-site rupture and poor healing outcomes.20,38,67,102,132
Overuse
Tendon overuse is a common problem in sport and work settings.8,10 Repeated overloading can lead to tendinosis, tendinitis, and/or micro-tearing of collagen fibrils, weakening the tendon and eventually leading to rupture.48,58,92,104,105 This is a significant problem for individuals who perform repetitive activities and manual labor.8,10,33,105 A number animal models have been used to investigate the influence of overuse on rotator cuff and Achilles tendon degeneration.48,58,92,104,105 These studies have demonstrated that overuse activity can lead to increased inflammation, changes in tendon structure, and decreased material properties. In a rat model of rotator cuff tendinosis, Soslowsky et al. demonstrated that increased loading on the supraspinatus due to exercise resulted in decreased collagen organization and increased cellularity, implicating overuse as one likely etiology for the development of tendinopathy.105
Repetitive mechanical overloading has been shown to lead to elevated inflammatory markers such as prostaglandin E2 (PGE2)130,131, IL-1β7 and tumor necrosis factor-α (TNF-α)7. In an animal study on healthy tendon, Archambault et al. found that chronic repetitive loading did not induce inflammatory or degenerative changes to Achilles tendons in rabbits7. However, changes in cytokine expression were observed, leading to increased expression of collagen type III (indicative of scar) and MMP-3 (indicative of a catabolic state). In the injured Achilles tendon, loading with treadmill exercise has been shown to induce macrophage activation, scar formation, and loss of tendon function.49 In the rat rotator cuff model, repair of the supraspinatus tendon to its humeral head insertion followed by exercise demonstrated significant decreases in mechanical, structural and compositional properties of the tendon-to bone insertion site. Exercise also led to reduced joint range of motion compared with rats exposed to immobilization or normal cage activity.91,112 Overloading via exercise led to increased production of extracellular matrix at the repair site. However, the material properties of the repair were not improved with increased load; rather, more material of lesser quality was produced. Exposure to overloading has detrimental effects for tendon properties and joint biomechanics in tendon mid-substance healing as well. After injury and repair of canine flexor digitalis profundus, increased levels of force applied during postoperative rehabilitation did not improve tendon strength nor accelerate healing.51
Clinical application of mechanobiology for tendon healing
Tendon is a mechanosensitive tissue; this responsiveness provides the opportunity for treatments based on mechanical loading. However, care must be taken in the application of load for enhanced tendon healing. The rehabilitation protocol should be chosen based on the particular tendon injured and the pathology associated with the injury (Figure 4). Multiple factors impact tendon healing in a clinical setting including age, tear size, tear chronicity, and patient biology. However, consideration of the existing research can guide an informed approach to postoperative rehabilitation after tendon repair.
Figure 4.
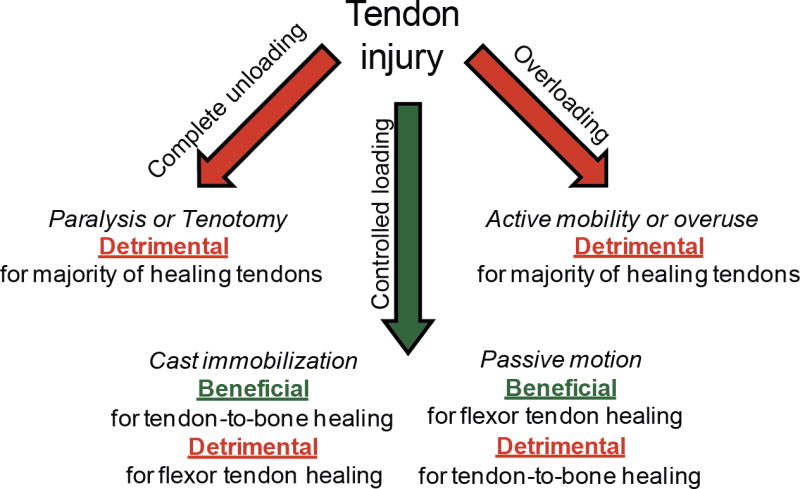
Complete removal of load is detrimental to tendon healing, yet large forces are also harmful. Controlled loading can enhance healing in most settings; however, a fine balance must be reached between loads that are too low (leading to a catabolic state) and too high (leading to micro-damage).
In general, a fine balance must be met between understimulating and overloading the healing tendon-to-bone interface (Figure 4). Studies reviewed in earlier sections demonstrate that complete removal of load from the healing site is detrimental, but excessive load is also harmful. For example, in the study by Galatz et al., complete removal of load from the repair site led to decreased matrix production and decreased structural properties.34 Similar results were reported by Hettrich et al.56 These studies indicate that the production of extracellular matrix at the repair site is largely dependent upon mechanical stimulation. However, excessive motion and load can also be detrimental to healing. Thomopoulos et al. compared immobilization, cage activity, and treadmill running in the rat model after rotator cuff repair.112 Although, higher load across the repair site stimulated the production of extracellular matrix, this additional scar-like material did not improve the material properties of the repair. These studies illustrate the sensitivity of the repair to postoperative rehabilitation for the example of rotator cuff repair.
Clinical practice and clinical research have influenced postoperative rehabilitation approaches. For example, in the past decade there has been a gradual transition from open rotator cuff repair to arthroscopic repair. The use of minimally invasive techniques for repair, and studies showing a failure of tendon-to-bone healing, have initiated a trend toward slower, more conservative rehabilitation protocols. Unfortunately, few clinical studies have addressed this directly. Parsons et al evaluated patients who underwent a 6 week period of immobilization after rotator cuff repair compared to patients who underwent early initiation of passive motion.89 In the early postoperative group, 23% of patients had relative loss of motion at the early time point of 6 weeks. However, there was no difference between the patients with early stiffness compared to the rest of the group one year after surgery. Although the numbers were small, there was a trend toward better healing in the stiffer group as assessed by MRI. Duzgun, et al. compared a slow and accelerated postoperative range of motion protocol with patients initiating active range of motion at 6 and 3 weeks respectively.31 The accelerated program was associated with less pain and improved DASH (Disabilities of the Shoulder and Hand) scores. Another small study comparing two rehabilitation protocols, one initiating early motion and muscle activation and another with early immobilization, found no differences in functional outcome at 2 years after surgery.69 However, there was no assessment of healing in either study. Overall, strong clinical evidence for a specific rehabilitation protocol over another does not exist, but early mobilization in a controlled fashion does not appear to be harmful.
In conclusion, tendon responds to mechanical cues in a variety of settings, including the post-injury and post-operative scenarios. In general, some controlled loading is necessary for development, homeostasis, and healing. However, excessive force will produce a negative effect on tendon resulting in injury and impaired healing post-repair. Although clinical evidence supporting specific rehabilitation protocols is limited, basic science research can contribute to the formation of logical approaches to postoperative management.
Acknowledgments
Grant support/Acknowledgements: Support was received from the National Institutes of Health (AR055580 and AR057836).
Footnotes
Financial statement: Neither the authors nor or any member of their family have received any financial remuneration related to the subject of the article.
Institutional review board (IRB) approval related to the study: Not applicable (this is a review article).
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Abrahamsson SO, Gelberman RH, Amiel D, Winterton P, Harwood F. Autogenous flexor tendon grafts: fibroblast activity and matrix remodeling in dogs. J Orthop Res. 1995;13:58–66. doi: 10.1002/jor.1100130110. [DOI] [PubMed] [Google Scholar]
- 2.Abrahamsson SO, Lundborg G, Lohmander LS. Tendon healing in vivo. An experimental model. Scandinavian Journal of Plastic & Reconstructive Surgery & Hand Surgery. 1989;23:199–205. doi: 10.3109/02844318909075118. [DOI] [PubMed] [Google Scholar]
- 3.Amiel D, Harwood FL, Gelberman RH, Chu CR, Seiler JG, 3rd, Abrahamsson S. Autogenous intrasynovial and extrasynovial tendon grafts: an experimental study of pro alpha 1(I) collagen mRNA expression in dogs. J Orthop Res. 1995;13:459–63. doi: 10.1002/jor.1100130321. [DOI] [PubMed] [Google Scholar]
- 4.Amiel D, Woo SL, Harwood FL, Akeson WH. The effect of immobilization on collagen turnover in connective tissue: a biochemical-biomechanical correlation. Acta Orthopaedica Scandinavica. 1982;53:325–32. doi: 10.3109/17453678208992224. [DOI] [PubMed] [Google Scholar]
- 5.Archambault J, Tsuzaki M, Herzog W, Banes AJ. Stretch and interleukin-1beta induce matrix metalloproteinases in rabbit tendon cells in vitro. J Orthop Res. 2002;20:36–9. doi: 10.1016/S0736-0266(01)00075-4. [DOI] [PubMed] [Google Scholar]
- 6.Archambault JM, Elfervig-Wall MK, Tsuzaki M, Herzog W, Banes AJ. Rabbit tendon cells produce MMP-3 in response to fluid flow without significant calcium transients. Journal of Biomechanics. 2002;35:303–9. doi: 10.1016/S0021-9290(01)00217-2. [DOI] [PubMed] [Google Scholar]
- 7.Archambault JM, Hart DA, Herzog W. Response of rabbit Achilles tendon to chronic repetitive loading. Connective Tissue Research. 2001;42:13–23. doi: 10.3109/03008200109014245. [DOI] [PubMed] [Google Scholar]
- 8.Archambault JM, Wiley JP, Bray RC. Exercise loading of tendons and the development of overuse injuries. A review of current literature. Sports Medicine. 1995;20:77–89. doi: 10.2165/00007256-199520020-00003. [DOI] [PubMed] [Google Scholar]
- 9.Ark JW, Gelberman RH, Abrahamsson SO, Seiler JG, 3rd, Amiel D. Cellular survival and proliferation in autogenous flexor tendon grafts. J Hand Surg Am. 1994;19:249–58. doi: 10.1016/0363-5023(94)90015-9. [DOI] [PubMed] [Google Scholar]
- 10.Arnoczky SP, Lavagnino M, Egerbacher M. The mechanobiological aetiopathogenesis of tendinopathy: is it the over-stimulation or the under-stimulation of tendon cells? Int J Exp Pathol. 2007;88:217–26. doi: 10.1111/j.1365-2613.2007.00548.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Arnoczky SP, Tian T, Lavagnino M, Gardner K. Ex vivo static tensile loading inhibits MMP-1 expression in rat tail tendon cells through a cytoskeletally based mechanotransduction mechanism. J Orthop Res. 2004;22:328–33. doi: 10.1016/S0736-0266(03)00185-2. [DOI] [PubMed] [Google Scholar]
- 12.Arnoczky SP, Tian T, Lavagnino M, Gardner K, Schuler P, Morse P. Activation of stress-activated protein kinases (SAPK) in tendon cells following cyclic strain: the effects of strain frequency, strain magnitude, and cytosolic calcium. Journal of Orthopaedic Research. 2002;20:947–52. doi: 10.1016/S0736-0266(02)00038-4. [DOI] [PubMed] [Google Scholar]
- 13.Asundi K, Rempel D. MMP-1, IL-1beta, and COX-2 mRNA expression is modulated by static load in rabbit flexor tendons. Annals of Biomedical Engineering. 2008;36:237–43. doi: 10.1007/s10439-007-9427-2. No doi. [DOI] [PubMed] [Google Scholar]
- 14.Banes AJ, Horesovsky G, Larson C, Tsuzaki M, Judex S, Archambault J, et al. Mechanical load stimulates expression of novel genes in vivo and in vitro in avian flexor tendon cells. Osteoarthritis & Cartilage. 1999;7:141–53. doi: 10.1053/joca.1998.0169. [DOI] [PubMed] [Google Scholar]
- 15.Banos CC, Thomas AH, Kuo CK. Collagen fibrillogenesis in tendon development: current models and regulation of fibril assembly. Birth Defects Res C Embryo Today. 2008;84:228–44. doi: 10.1002/bdrc.20130. [DOI] [PubMed] [Google Scholar]
- 16.Barton ER, Gimbel JA, Williams GR, Soslowsky LJ. Rat supraspinatus muscle atrophy after tendon detachment. J Orthop Res. 2005;23:259–65. doi: 10.1016/j.orthres.2004.08.018. [DOI] [PubMed] [Google Scholar]
- 17.Bedi A, Kovacevic D, Fox AJ, Imhauser CW, Stasiak M, Packer J, et al. Effect of early and delayed mechanical loading on tendon-to-bone healing after anterior cruciate ligament reconstruction. J Bone Joint Surg Am. 2010;92:2387–401. doi: 10.2106/JBJS.I.01270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Blitz E, Viukov S, Sharir A, Shwartz Y, Galloway JL, Pryce BA, et al. Bone ridge patterning during musculoskeletal assembly is mediated through SCX regulation of Bmp4 at the tendon-skeleton junction. Dev Cell. 2009;17:861–73. doi: 10.1016/j.devcel.2009.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bostrom MPG, Boskey A, Kauffman JK, Einhorn TA. Form and function of bone. In: Buckwalter JA, Einhorn TA, Simon SR, editors. Orthopaedic Basic Science: AAOS. 2000. pp. 319–70. [Google Scholar]
- 20.Boyer MI, Goldfarb CA, Gelberman RH. Recent progress in flexor tendon healing. The modulation of tendon healing with rehabilitation variables. J Hand Ther. 2005;18:80–5. doi: 10.1197/j.jht.2005.01.009. quiz 86. [DOI] [PubMed] [Google Scholar]
- 21.Boyer MI, Watson JT, Lou J, Manske PR, Gelberman RH, Cai SR. Quantitative variation in vascular endothelial growth factor mRNA expression during early flexor tendon healing: an investigation in a canine model. J Orthop Res. 2001;19:869–72. doi: 10.1016/S0736-0266(01)00017-1. [DOI] [PubMed] [Google Scholar]
- 22.Brashear A, Gordon M, Elovic E, Kassicieh V, Marciniak C, Do M, et al. Intramuscular Injection of Botulinum Toxin for the Treatment of Wrist and Finger Spasticity after a Stroke. The New England Journal of Medicine. 2002;347:395–400. doi: 10.1056/NEJMoa011892. [DOI] [PubMed] [Google Scholar]
- 23.Brophy RH, Kovacevic D, Imhauser CW, Stasiak M, Bedi A, Fox AJ, et al. Effect of short-duration low-magnitude cyclic loading versus immobilization on tendon-bone healing after ACL reconstruction in a rat model. J Bone Joint Surg Am. 2011;93:381–93. doi: 10.2106/JBJS.I.00933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cadet ER, Vorys GC, Rahman R, Park SH, Gardner TR, Lee FY, et al. Improving bone density at the rotator cuff footprint increases supraspinatus tendon failure stress in a rat model. J Orthop Res. 2010;28:308–14. doi: 10.1002/jor.20972. [DOI] [PubMed] [Google Scholar]
- 25.Dagher E, Hays PL, Kawamura S, Godin J, Deng XH, Rodeo SA. Immobilization modulates macrophage accumulation in tendon-bone healing. Clin Orthop Relat Res. 2009;467:281–7. doi: 10.1007/s11999-008-0512-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Das R, Rich J, Kim HM, McAlinden A, Thomopoulos S. Effects of botulinum toxin-induced paralysis on postnatal development of the supraspinatus muscle. J Orthop Res. 2011;29:281–8. doi: 10.1002/jor.21234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.De Aguiar G, Chait L, Schultz D, Blelock S, Theron A, Snijman C, et al. Chemoprotection of flexor tendon repairs using botulinum toxin. Plastic & Reconstructive Surgery. 2009;124:201–09. doi: 10.1097/PRS.0b013e3181ab118c. [DOI] [PubMed] [Google Scholar]
- 28.Ditsios K, Boyer MI, Kusano N, Gelberman RH, Silva MJ. Bone loss following tendon laceration, repair and passive mobilization. J Orthop Res. 2003;21:990–6. doi: 10.1016/S0736-0266(03)00112-8. [DOI] [PubMed] [Google Scholar]
- 29.Dressler MR, Butler DL, Wenstrup R, Awad HA, Smith F, Boivin GP. A potential mechanism for age-related declines in patellar tendon biomechanics. Journal of Orthopaedic Research. 2002;20:1315–22. doi: 10.1016/S0736-0266(02)00052-9. [DOI] [PubMed] [Google Scholar]
- 30.Du Plessis M, Eksteen E, Jenneker A, Kriel E, Mentoor C, Stucky T, et al. The effectiveness of continuous passive motion on range of motion, pain and muscle strength following rotator cuff repair: a systematic review. Clin Rehabil. 2011;25:291–302. doi: 10.1177/0269215510380835. [DOI] [PubMed] [Google Scholar]
- 31.Duzgun I, Baltaci G, Atay OA. Comparison of slow and accelerated rehabilitation protocol after arthroscopic rotator cuff repair: pain and functional activity. Acta Orthop Traumatol Turc. 2011;45:23–33. doi: 10.3944/AOTT.2011.2386. [DOI] [PubMed] [Google Scholar]
- 32.Evanko SP, Vogel KG. Proteoglycan synthesis in fetal tendon is differentially regulated by cyclic compression in vitro. Arch Biochem Biophys. 1993;307:153–64. doi: 10.1006/abbi.1993.1574. [DOI] [PubMed] [Google Scholar]
- 33.Fu FH, Harner CD, Klein AH. Clinical Orthopaedics & Related Research. 1991. Shoulder impingement syndrome. A critical review; pp. 162–73. [PubMed] [Google Scholar]
- 34.Galatz LM, Charlton N, Das R, Kim HM, Havlioglu N, Thomopoulos S. Complete removal of load is detrimental to rotator cuff healing. Journal of Shoulder and Elbow Surgery. 2009;18:669–75. doi: 10.1016/j.jse.2009.02.016. [DOI] [PubMed] [Google Scholar]
- 35.Gardner K, Arnoczky S, Caballero O, Lavagnino M. The effect of stress-deprivation and cyclic loading on the TIMP/MMP ratio in tendon cells: an in vitro experimental study. Disability and Rehabilitation. 2008;30:1523–29. doi: 10.1080/09638280701785395. [DOI] [PubMed] [Google Scholar]
- 36.Garofalo R, Conti M, Notarnicola A, Maradei L, Giardella A, Castagna A. Effects of one-month continuous passive motion after arthroscopic rotator cuff repair: results at 1-year follow-up of a prospective randomized study. Musculoskelet Surg. 2010;94 (Suppl 1):S79–83. doi: 10.1007/s12306-010-0058-7. [DOI] [PubMed] [Google Scholar]
- 37.Gelberman RH, Amifl D, Gonsalves M, Woo S, Akeson WH. The influence of protected passive mobilization on the healing of flexor tendons: a biochemical and microangiographic study. Hand. 1981;13:120–8. doi: 10.1016/s0072-968x(81)80051-4. [DOI] [PubMed] [Google Scholar]
- 38.Gelberman RH, Boyer MI, Brodt MD, Winters SC, Silva MJ. The effect of gap formation at the repair site on the strength and excursion of intrasynovial flexor tendons. An experimental study on the early stages of tendon-healing in dogs. J Bone Joint Surg Am. 1999;81:975–82. doi: 10.2106/00004623-199907000-00010. [DOI] [PubMed] [Google Scholar]
- 39.Gelberman RH, Chu CR, Williams CS, Seiler JG, 3rd, Amiel D. Angiogenesis in healing autogenous flexor-tendon grafts. J Bone Joint Surg Am. 1992;74:1207–16. [PubMed] [Google Scholar]
- 40.Gelberman RH, Manske PR, Akeson WH, Woo SL, Lundborg G, Amiel D. Flexor tendon repair. J Orthop Res. 1986;4:119–28. doi: 10.1002/jor.1100040116. [DOI] [PubMed] [Google Scholar]
- 41.Gelberman RH, Menon J, Gonsalves M, Akeson WH. The effects of mobilization on the vascularization of healing flexor tendons in dogs. Clin Orthop Relat Res. 1980:283–9. [PubMed] [Google Scholar]
- 42.Gelberman RH, Vande Berg JS, Lundborg GN, Akeson WH. Flexor tendon healing and restoration of the gliding surface. An ultrastructural study in dogs. J Bone Joint Surg Am. 1983;65:70–80. [PubMed] [Google Scholar]
- 43.Gelberman RH, Vandeberg JS, Manske PR, Akeson WH. The early stages of flexor tendon healing: a morphologic study of the first fourteen days. J Hand Surg Am. 1985;10:776–84. doi: 10.1016/s0363-5023(85)80151-9. [DOI] [PubMed] [Google Scholar]
- 44.Gelberman RH, Woo SL, Lothringer K, Akeson WH, Amiel D. Effects of early intermittent passive mobilization on healing canine flexor tendons. Journal of Hand Surgery - American Volume. 1982;7:170–5. doi: 10.1016/s0363-5023(82)80083-x. [DOI] [PubMed] [Google Scholar]
- 45.Genin GM, Kent A, Birman V, Wopenka B, Pasteris JD, Marquez PJ, et al. Functional grading of mineral and collagen in the attachment of tendon to bone. Biophys J. 2009;97:976–85. doi: 10.1016/j.bpj.2009.05.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gimbel JA, Van Kleunen JP, Williams GR, Thomopoulos S, Soslowsky LJ. Long durations of immobilization in the rat result in enhanced mechanical properties of the healing supraspinatus tendon insertion site. J Biomech Eng. 2007;129:400–4. doi: 10.1115/1.2721075. [DOI] [PubMed] [Google Scholar]
- 47.Giori NJ, Beaupre GS, Carter DR. Cellular shape and pressure may mediate mechanical control of tissue composition in tendons. Journal of Orthopaedic Research. 1993;11:581–91. doi: 10.1002/jor.1100110413. [DOI] [PubMed] [Google Scholar]
- 48.Glazebrook MA, Wright JR, Jr, Langman M, Stanish WD, Lee JM. Histological analysis of achilles tendons in an overuse rat model. J Orthop Res. 2008 doi: 10.1002/jor.20546. [DOI] [PubMed] [Google Scholar]
- 49.Godbout C, Ang O, Frenette J. Early voluntary exercise does not promote healing in a rat model of Achilles tendon injury. Journal of Applied Physiology. 2006;101:1720–26. doi: 10.1152/japplphysiol.00301.2006. [DOI] [PubMed] [Google Scholar]
- 50.Goh KL, Holmes DF, Lu HY, Richardson S, Kadler KE, Purslow PP, et al. Ageing changes in the tensile properties of tendons: influence of collagen fibril volume fraction. J Biomech Eng. 2008;130:021011. doi: 10.1115/1.2898732. [DOI] [PubMed] [Google Scholar]
- 51.Goldfarb CA, Harwood F, Silva MJ, Gelberman RH, Amiel D, Boyer MI. The effect of variations in applied rehabilitation force on collagen concentration and maturation at the intrasynovial flexor tendon repair site. J Hand Surg Am. 2001;26:841–6. doi: 10.1053/jhsu.2001.26190. [DOI] [PubMed] [Google Scholar]
- 52.Hannafin JA, Arnoczky SP, Hoonjan A, Torzilli PA. Effect of stress deprivation and cyclic tensile loading on the material and morphologic properties of canine flexor digitorum profundus tendon: an in vitro study. Journal of Orthopaedic Research. 1995;13:907–14. doi: 10.1002/jor.1100130615. [DOI] [PubMed] [Google Scholar]
- 53.Hannafin JA, Attia EA, Henshaw R, Warren RF, Bhargava MM. Effect of cyclic strain and plating matrix on cell proliferation and integrin expression by ligament fibroblasts. J Orthop Res. 2006;24:149–58. doi: 10.1002/jor.20018. [DOI] [PubMed] [Google Scholar]
- 54.Hays PL, Kawamura S, Deng XH, Dagher E, Mithoefer K, Ying L, et al. The role of macrophages in early healing of a tendon graft in a bone tunnel. J Bone Joint Surg Am. 2008;90:565–79. doi: 10.2106/JBJS.F.00531. [DOI] [PubMed] [Google Scholar]
- 55.Heinemeier KM, Olesen JL, Schjerling P, Haddad F, Langberg H, Baldwin KM, et al. Short-term strength training and the expression of myostatin and IGF-I isoforms in rat muscle and tendon: differential effects of specific contraction types. J Appl Physiol. 2007;102:573–81. doi: 10.1152/japplphysiol.00866.2006. [DOI] [PubMed] [Google Scholar]
- 56.Hettrich CM, Rodeo SA, Hannafin JA, Ehteshami J, Shubin Stein BE. The effect of muscle paralysis using Botox on the healing of tendon to bone in a rat model. J Shoulder Elbow Surg. 2010 doi: 10.1016/j.jse.2010.09.016. [DOI] [PubMed] [Google Scholar]
- 57.Hodge AJ, Petruska JA. In: Aspects of protein structure. Ramachandran GN, editor. New York: Academic Press; 1963. pp. 289–300. [Google Scholar]
- 58.Huang TF, Perry SM, Soslowsky LJ. The effect of overuse activity on Achilles tendon in an animal model: a biomechanical study. Ann Biomed Eng. 2004;32:336–41. doi: 10.1023/B:ABME.0000017537.26426.76. [DOI] [PubMed] [Google Scholar]
- 59.Iannotti JP, Naranja RJ, Gartsman GM. Surgical treatment of the intact cuff and repairable cuff defect: Arthroscopic and open techniques. In: Norris TR, editor. Orthopaedic Knowledge Update: Shoulder and Elbow. Rosemont IL: American Academy of Orthopaedic Surgeons; 1994. pp. 151–55. [Google Scholar]
- 60.Ip Y, Davis R. Signal transduction by the c-Jun N-terminal kinase (JNK)- from inflammation to development. Current Opinion in Cell Biology. 1998;10:205–19. doi: 10.1016/s0955-0674(98)80143-9. [DOI] [PubMed] [Google Scholar]
- 61.John T, Lodka D, Kohl B, Ertel W, Jammrath J, Conrad C, et al. Effect of pro-inflammatory and immunoregulatory cytokines on human tenocytes. J Orthop Res. 2010;28:1071–7. doi: 10.1002/jor.21079. [DOI] [PubMed] [Google Scholar]
- 62.Kastelic J. A structural mechanical model for tendon crimping. Journal of Biomechanics. 1980;13:887–93. doi: 10.1016/0021-9290(80)90177-3. [DOI] [PubMed] [Google Scholar]
- 63.Kastelic J, Galeski A, Baer E. The multicomposite structure of tendon. Connective Tissue Research. 1978;6:11–23. doi: 10.3109/03008207809152283. [DOI] [PubMed] [Google Scholar]
- 64.Kawamura S, Ying L, Kim HJ, Dynybil C, Rodeo SA. Macrophages accumulate in the early phase of tendon-bone healing. J Orthop Res. 2005;23:1425–32. doi: 10.1016/j.orthres.2005.01.014. [DOI] [PubMed] [Google Scholar]
- 65.Kim H, Galatz L, Lim C, Havlioglu N, Thomopoulos S. The effect of tear size and nerve injury on rotator cuff muscle fatty degeneration in a rodent animal model. J Shoulder Elbow Surg. 2011 doi: 10.1016/j.jse.2011.05.004. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kim HM, Galatz LM, Das R, Patel N, Thomopoulos S. Musculoskeletal deformities secondary to neurotomy of the superior trunk of the brachial plexus in neonatal mice. J Orthop Res. 2010;28:1391–8. doi: 10.1002/jor.21128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kim HM, Nelson G, Thomopoulos S, Silva MJ, Das R, Gelberman RH. Technical and biological modifications for enhanced flexor tendon repair. J Hand Surg Am. 2010;35:1031–7. doi: 10.1016/j.jhsa.2009.12.044. quiz 38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kjoller K, Langberg H, Heinemeier KM, Bayer M, Hansen M, Holm L, et al. From mechanical loading to collagen synthesis, structural changes and function in human tendon. Scandinavian Journal of Medicine and Science in Sports. 2009;19:500–10. doi: 10.1111/j.1600-0838.2009.00986.x. No doi. [DOI] [PubMed] [Google Scholar]
- 69.Klintberg I, Gunnarsson A-C, Svantesson U, Styf J, Karlsson J. Early loading in physiotherapy treatment after full-thickness rotator cuff repair: a prospective randomized pilot-study with a two-year follow-up. Clinical Rehabilitation. 2009;23:622–38. doi: 10.1177/0269215509102952. [DOI] [PubMed] [Google Scholar]
- 70.Koman L, Mooney, Smith B, Goodman A, Mulvaney T. Management of cerebral palsy with botulinum-A toxin: preliminary investigation. Journal of Pediatric Orthopedics. 1993;13:489–95. doi: 10.1097/01241398-199307000-00013. [DOI] [PubMed] [Google Scholar]
- 71.Koob TJ, Vogel KG. Site-related variations in glycosaminoglycan content and swelling properties of bovine flexor tendon. Journal of Orthopaedic Research. 1987;5:414–24. doi: 10.1002/jor.1100050314. [DOI] [PubMed] [Google Scholar]
- 72.Koshima H, Kondo S, Mishima S, Choi HR, Shimpo H, Sakai T, et al. Expression of interleukin-1beta, cyclooxygenase-2, and prostaglandin E2 in a rotator cuff tear in rabbits. J Orthop Res. 2007;25:92–7. doi: 10.1002/jor.20241. No doi. [DOI] [PubMed] [Google Scholar]
- 73.Kuo CK, Tuan RS. Mechanoactive tenogenic differentiation of human mesenchymal stem cells. Tissue Eng Part A. 2008;14:1615–27. doi: 10.1089/ten.tea.2006.0415. [DOI] [PubMed] [Google Scholar]
- 74.Lieber RL, Amiel D, Kaufman KR, Whitney J, Gelberman RH. Relationship between joint motion and flexor tendon force in the canine forelimb. J Hand Surg [Am] 1996;21:957–62. doi: 10.1016/S0363-5023(96)80299-1. [DOI] [PubMed] [Google Scholar]
- 75.Lin TW, Cardenas L, Soslowsky LJ. Biomechanics of tendon injury and repair. J Biomech. 2004;37:865–77. doi: 10.1016/j.jbiomech.2003.11.005. [DOI] [PubMed] [Google Scholar]
- 76.Liu Y, Birman V, Chen C, Thomopoulos S, Genin GM. Mechanisms of bimaterial attachment at the interface of tendon to bone. Journal of Engineering Materials and Technology. 2011;133 doi: 10.1115/1.4002641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Loitz B, Zernicke R, Vailas A, Kody M, Meals R. Effects of short-term immobilization versus continuous passive motion on the biomechanical and biochemical properties of the rabbit tendon. Clinical Orthopaedics & Related Research. 1989;244:265–71. [PubMed] [Google Scholar]
- 78.Ma J, Shen J, Smith B, Ritting A, Smith T, Koman L. Bioprotection of Tendon Repair: Adjunctive Use of Botulinum Toxin A in Achilles Tendon Repair in the Rat. J Bone Joint Surg Am. 2007;89:2241–49. doi: 10.2106/JBJS.D.03054. [DOI] [PubMed] [Google Scholar]
- 79.Magnusson SP, Langberg H, Kjaer M. The pathogenesis of tendinopathy: balancing the response to loading. Nat Rev Rheumatol. 2010;6:262–8. doi: 10.1038/nrrheum.2010.43. [DOI] [PubMed] [Google Scholar]
- 80.Magnusson SP, Narici MV, Maganaris CN, Kjaer M. Human tendon behaviour and adaptation, in vivo. The Journal of physiology. 2008;586:71–81. doi: 10.1113/jphysiol.2007.139105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Magra M, Maffulli N. Molecular events in tendinopathy: a role for metalloproteases. Foot Ankle Clin. 2005;10:267–77. doi: 10.1016/j.fcl.2005.01.012. [DOI] [PubMed] [Google Scholar]
- 82.Mikic B, Johnson T, Chhabra A, Schalet B, Wong M, Hunziker EB. Differential effects of embryonic immobilization on the development of fibrocartilaginous skeletal elements. Journal of Rehabilitation Research and Development. 2000;37:127–33. [PubMed] [Google Scholar]
- 83.Mikic B, Wong M, Chiquet M, Hunziker EB. Mechanical modulation of tenascin-C and collagen-XII expression during avian synovial joint formation. J Orthop Res. 2000;18:406–15. doi: 10.1002/jor.1100180312. [DOI] [PubMed] [Google Scholar]
- 84.Milz S, Rufai A, Buettner A, Putz R, Ralphs JR, Benjamin M. Three-dimensional reconstructions of the Achilles tendon insertion in man. J Anat. 2002;200:145–52. doi: 10.1046/j.0021-8782.2001.00016.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Miyatake S, Tohyama H, Kondo E, Katsura T, Onodera S, Yasuda K. Local administration of interleukin-1 receptor antagonist inhibits deterioration of mechanical properties of the stress-shielded patellar tendon. J Biomech. 2008;41:884–9. doi: 10.1016/j.jbiomech.2007.10.018. [DOI] [PubMed] [Google Scholar]
- 86.Moffat KL, Sun WH, Pena PE, Chahine NO, Doty SB, Ateshian GA, et al. Characterization of the structure-function relationship at the ligament-to-bone interface. Proc Natl Acad Sci U S A. 2008;105:7947–52. doi: 10.1073/pnas.0712150105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Molloy T, Wang Y, Murrell G. The roles of growth factors in tendon and ligament healing. Sports Medicine. 2003;33:381–94. doi: 10.2165/00007256-200333050-00004. [DOI] [PubMed] [Google Scholar]
- 88.Murchison ND, Price BA, Conner DA, Keene DR, Olson EN, Tabin CJ, et al. Regulation of tendon differentiation by scleraxis distinguishes force-transmitting tendons from muscle-anchoring tendons. Development. 2007;134:2697–708. doi: 10.1242/dev.001933. [DOI] [PubMed] [Google Scholar]
- 89.Parsons BO, Gruson KI, Chen DD, Harrison AK, Gladstone J, Flatow EL. Does slower rehabilitation after arthroscopic rotator cuff repair lead to long-term stiffness? J Shoulder Elbow Surg. 2010;19:1034–39. doi: 10.1016/j.jse.2010.04.006. [DOI] [PubMed] [Google Scholar]
- 90.Peltz CD, Dourte LM, Kuntz AF, Sarver JJ, Kim SY, Williams GR, et al. The effect of postoperative passive motion on rotator cuff healing in a rat model. J Bone Joint Surg Am. 2009;91:2421–9. doi: 10.2106/JBJS.H.01121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Peltz CD, Sarver JJ, Dourte LM, Wurgler-Hauri CC, Williams GR, Soslowsky LJ. Exercise following a short immobilization period is detrimental to tendon properties and joint mechanics in a rat rotator cuff injury model. J Orthop Res. 2010;28:841–5. doi: 10.1002/jor.21059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Perry SM, McIlhenny SE, Hoffman MC, Soslowsky LJ. Inflammatory and angiogenic mRNA levels are altered in a supraspinatus tendon overuse animal model. J Shoulder Elbow Surg. 2005;14:79S–83S. doi: 10.1016/j.jse.2004.09.020. [DOI] [PubMed] [Google Scholar]
- 93.Praemer A, Furner S, Rice D. Musculoskeletal Conditions in the US. Park Ridge IL: American Academy of Orthopaedic Surgeons; 1992. [Google Scholar]
- 94.Pryce BA, Watson SS, Murchison ND, Staverosky JA, Dunker N, Schweitzer R. Recruitment and maintenance of tendon progenitors by TGFbeta signaling are essential for tendon formation. Development. 2009;136:1351–61. doi: 10.1242/dev.027342. No doi. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Reed CC, Iozzo RV. The role of decorin in collagen fibrillogenesis and skin homeostasis. Glycoconj J. 2002;19:249–55. doi: 10.1023/A:1025383913444. [DOI] [PubMed] [Google Scholar]
- 96.Riley G. The pathogenesis of tendinopathy. A molecular perspective. Rheumatology (Oxford) 2004;43:131–42. doi: 10.1093/rheumatology/keg448. [DOI] [PubMed] [Google Scholar]
- 97.Robbins JR, Evanko SP, Vogel KG. Mechanical loading and TGF-beta regulate proteoglycan synthesis in tendon. Archives of Biochemistry & Biophysics. 1997;342:203–11. doi: 10.1006/abbi.1997.0102. [DOI] [PubMed] [Google Scholar]
- 98.Rodeo SA, Arnoczky SP, Torzilli PA, Hidaka C, Warren RF. Tendon-healing in a bone tunnel. A biomechanical and histological study in the dog. Journal of Bone & Joint Surgery - American Volume. 1993;75:1795–803. doi: 10.2106/00004623-199312000-00009. [DOI] [PubMed] [Google Scholar]
- 99.Sarin V, Erickson G, Giori N, Bergman A, Carter D. Coincident Development of Sesamoid Bones and Clues to Their Evolution Anatomical Record (New Anat) 1999;257:174–80. doi: 10.1002/(SICI)1097-0185(19991015)257:5<174::AID-AR6>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 100.Schweitzer R, Chyung JH, Murtaugh LC, Brent AE, Rosen V, Olson EN, et al. Analysis of the tendon cell fate using Scleraxis, a specific marker for tendons and ligaments. Development. 2001;128:3855–66. doi: 10.1242/dev.128.19.3855. [DOI] [PubMed] [Google Scholar]
- 101.Scott A, Cook JL, Hart DA, Walker DC, Duronio V, Khan KM. Tenocyte responses to mechanical loading in vivo: a role for local insulin-like growth factor 1 signaling in early tendinosis in rats. Arthritis and rheumatism. 2007;56:871–81. doi: 10.1002/art.22426. [DOI] [PubMed] [Google Scholar]
- 102.Silva MJ, Brodt MD, Boyer MI, Morris TS, Dinopoulos H, Amiel D, et al. Effects of increased in vivo excursion on digital range of motion and tendon strength following flexor tendon repair. J Orthop Res. 1999;17:777–83. doi: 10.1002/jor.1100170524. [DOI] [PubMed] [Google Scholar]
- 103.Smith RK, Birch HL, Goodman S, Heinegard D, Goodship AE. The influence of ageing and exercise on tendon growth and degeneration--hypotheses for the initiation and prevention of strain-induced tendinopathies. Comp Biochem Physiol A Mol Integr Physiol. 2002;133:1039–50. doi: 10.1016/S1095-6433(02)00148-4. [DOI] [PubMed] [Google Scholar]
- 104.Soslowsky LJ, Thomopoulos S, Esmail A, Flanagan CL, Iannotti JP, Williamson JD, 3rd, et al. Rotator cuff tendinosis in an animal model: role of extrinsic and overuse factors. Annals of Biomedical Engineering. 2002;30:1057–63. doi: 10.1114/1.1509765. [DOI] [PubMed] [Google Scholar]
- 105.Soslowsky LJ, Thomopoulos S, Tun S, Flanagan CL, Keefer CC, Mastaw J, et al. Neer Award 1999. Overuse activity injures the supraspinatus tendon in an animal model: a histologic and biomechanical study. J Shoulder Elbow Surg. 2000;9:79–84. [PubMed] [Google Scholar]
- 106.Stouffer DC, Butler DL, Hosny D. The relationship between crimp pattern and mechanical response of human patellar tendon-bone units. Journal of Biomechanical Engineering. 1985;107:158–65. doi: 10.1115/1.3138536. [DOI] [PubMed] [Google Scholar]
- 107.Thomopoulos S, Das R, Birman V, Smith L, Ku K, Elson E, et al. Fibrocartilage tissue engineering: The role of the stress environment on cell morphology and matrix expression. Tissue Eng Part A. 2011;17:1039–53. doi: 10.1089/ten.TEA.2009.0499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Thomopoulos S, Genin GM, Galatz LM. The development and morphogenesis of the tendon-to-bone insertion - what development can teach us about healing. J Musculoskelet Neuronal Interact. 2010;10:35–45. No doi. [PMC free article] [PubMed] [Google Scholar]
- 109.Thomopoulos S, Hattersley G, Rosen V, Mertens M, Galatz L, Williams GR, et al. The localized expression of extracellular matrix components in healing tendon insertion sites: an in situ hybridization study. Journal of Orthopaedic Research. 2002;20:454–63. doi: 10.1016/S0736-0266(01)00144-9. [DOI] [PubMed] [Google Scholar]
- 110.Thomopoulos S, Kim HM, Rothermich SY, Biederstadt C, Das R, Galatz LM. Decreased muscle loading delays maturation of the tendon enthesis during postnatal development. J Orthop Res. 2007;25:1154–63. doi: 10.1002/jor.20418. [DOI] [PubMed] [Google Scholar]
- 111.Thomopoulos S, Marquez JP, Weinberger B, Birman V, Genin GM. Collagen fiber orientation at the tendon to bone insertion and its influence on stress concentrations. J Biomech. 2006;39:1842–51. doi: 10.1016/j.jbiomech.2005.05.021. [DOI] [PubMed] [Google Scholar]
- 112.Thomopoulos S, Williams GR, Soslowsky LJ. Tendon to bone healing: differences in biomechanical, structural, and compositional properties due to a range of activity levels. Journal of Biomechanical Engineering. 2003;125:106–13. doi: 10.1115/1.1536660. [DOI] [PubMed] [Google Scholar]
- 113.Thomopoulos S, Zampiakis E, Das R, Silva MJ, Gelberman RH. The effect of muscle loading on flexor tendon-to-bone healing in a canine model. J Orthop Res. 2008;26:1611–7. doi: 10.1002/jor.20689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Tsuzaki M, Bynum D, Almekinders L, Yang X, Faber J, Banes AJ. ATP modulates load-inducible IL-1beta, COX 2, and MMP-3 gene expression in human tendon cells. J Cell Biochem. 2003;89:556–62. doi: 10.1002/jcb.10534. [DOI] [PubMed] [Google Scholar]
- 115.Tsuzaki M, Guyton G, Garrett W, Archambault JM, Herzog W, Almekinders L, et al. IL-1 beta induces COX2, MMP-1, -3 and -13, ADAMTS-4, IL-1 beta and IL-6 in human tendon cells. J Orthop Res. 2003;21:256–64. doi: 10.1016/S0736-0266(02)00141-9. No doi. [DOI] [PubMed] [Google Scholar]
- 116.Uchida H, Tohyama H, Nagashima K, Ohba Y, Matsumoto H, Toyama Y, et al. Stress deprivation simultaneously induces over-expression of interleukin-1beta, tumor necrosis factor-alpha, and transforming growth factor-beta in fibroblasts and mechanical deterioration of the tissue in the patellar tendon. J Biomech. 2005;38:791–8. doi: 10.1016/j.jbiomech.2004.05.009. [DOI] [PubMed] [Google Scholar]
- 117.Vogel KG. The effect of compressive loading on proteoglycan turnover in cultured fetal tendon. Connective Tissue Research. 1996;34:227–37. doi: 10.3109/03008209609000701. [DOI] [PubMed] [Google Scholar]
- 118.Vogel KG, Ordog A, Pogany G, Olah J. Proteoglycans in the compressed region of human tibialis posterior tendon and in ligaments. Journal of Orthopaedic Research. 1993;11:68–77. doi: 10.1002/jor.1100110109. [DOI] [PubMed] [Google Scholar]
- 119.Vogel KG, Sandy JD, Pogany G, Robbins JR. Aggrecan in bovine tendon. Matrix Biology. 1994;14:171–9. doi: 10.1016/0945-053x(94)90006-x. [DOI] [PubMed] [Google Scholar]
- 120.Waggett AD, Benjamin M, Ralphs JR. Connexin 32 and 43 gap junctions differentially modulate tenocyte response to cyclic mechanical load. Eur J Cell Biol. 2006;85:1145–54. doi: 10.1016/j.ejcb.2006.06.002. [DOI] [PubMed] [Google Scholar]
- 121.Wall ME, Banes AJ. Early responses to mechanical load in tendon: role for calcium signaling, gap junctions and intercellular communication. J Musculoskelet Neuronal Interact. 2005;5:70–84. No doi. [PubMed] [Google Scholar]
- 122.Wang JH. Mechanobiology of tendon. J Biomech. 2006;39:1563–82. doi: 10.1016/j.jbiomech.2005.05.011. [DOI] [PubMed] [Google Scholar]
- 123.Wang JH, Jia F, Yang G, Yang S, Campbell BH, Stone D, et al. Cyclic mechanical stretching of human tendon fibroblasts increases the production of prostaglandin E2 and levels of cyclooxygenase expression: a novel in vitro model study. Connect Tissue Res. 2003;44:128–33. doi: 10.1080/713713684. [DOI] [PubMed] [Google Scholar]
- 124.Wenstrup RJ, Smith SM, Florer JB, Zhang G, Beason DP, Seegmiller RE, et al. Regulation of collagen fibril nucleation and initial fibril assembly involves coordinate interactions with collagens V and XI in developing tendon. The Journal of biological chemistry. 2011;286:20455–65. doi: 10.1074/jbc.M111.223693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Woo SL, An K, Frank CB, Livesay GA, Ma CB, Zeminski JA, et al. Orthopaedic Basic Science. Rosemont, IL: AAOS; 2000. Anatomy, biology, and biomechanics of tendon and ligament; pp. 581–616. [Google Scholar]
- 126.Woo SL, Gelberman RH, Cobb NG, Amiel D, Lothringer K, Akeson WH. The importance of controlled passive mobilization on flexor tendon healing. A biomechanical study. Acta Orthop Scand. 1981;52:615–22. doi: 10.3109/17453678108992156. [DOI] [PubMed] [Google Scholar]
- 127.Woo SL, Lee TQ, Abramowitch SD, Gilbert TW. Structure and function of ligaments and tendons. In: Mow VC, Huiskes R, editors. Basic Orthopaedic Biomechanics and Mechano-Biology. Philadelphia: Lippincott Williams & Wilkins; 2005. pp. 301–42. [Google Scholar]
- 128.Xu Y, Murrell GA. The basic science of tendinopathy. Clin Orthop Relat Res. 2008;466:1528–38. doi: 10.1007/s11999-008-0286-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Yamamoto E, Tokura S, Hayashi K. Effects of cyclic stress on the mechanical properties of cultured collagen fascicles from the rabbit patellar tendon. J Biomech Eng. 2003;125:893–901. doi: 10.1115/1.1634286. [DOI] [PubMed] [Google Scholar]
- 130.Yang G, Im HJ, Wang JH. Repetitive mechanical stretching modulates IL-1beta induced COX-2, MMP-1 expression, and PGE2 production in human patellar tendon fibroblasts. Gene. 2005;363:166–72. doi: 10.1016/j.gene.2005.08.006. No doi. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Zhang J, Wang JH. Production of PGE(2) increases in tendons subjected to repetitive mechanical loading and induces differentiation of tendon stem cells into non-tenocytes. Journal of orthopaedic research: official publication of the Orthopaedic Research Society. 2010;28:198–203. doi: 10.1002/jor.20962. [DOI] [PubMed] [Google Scholar]
- 132.Zhao C, Amadio PC, Tanaka T, Kutsumi K, Tsubone T, Zobitz ME, et al. Effect of gap size on gliding resistance after flexor tendon repair. The Journal of bone and joint surgery. American volume. 2004;86-A:2482–8. doi: 10.2106/00004623-200411000-00019. No doi. [DOI] [PubMed] [Google Scholar]