Abstract
Purpose of review
Trauma, surgery and burns are three common clinical scenarios that are associated with significant acute pain. This review describes the pathophysiology of acute pain utilizing three preclinical models: surgery, burn, and fracture.
Recent findings
In general, there is greater interest directed toward peripheral mediators of acute pain. Studies indicate that treatment against nerve growth factor, interleukins, and ischemic-like mediators may provide valuable avenues for treatment of acute pain. By targeting the periphery, analgesic therapies may be reduced side effects.
Summary
Peripheral mediators of acute pain will vary depending upon the type of injury. Treatment aimed toward those mediators specific to the injury could improve acute pain management in the future. It will be important to translate these findings into clinical trials in the future.
Keywords: Incision, fracture, burn, nociception, hyperalgesia
Introduction
Surgery, burn injury and fracture can lead to severe acute pain. Adequate pain control is a key priority for patients to start early rehabilitation, which is crucial for restoring function and reducing morbidity and mortality after these acute events [1-3]. The effectiveness of these currently utilized analgesic therapies is often limited [4] and these treatments can, in some cases, have deleterious side effects. Thus, novel analgesics without side effects are needed for pain after these injuries; furthermore, a better understanding of pain caused by these acute events is needed to guide development new therapeutic approaches.
Animal models of fracture pain
Two preclinical animal models of fracture pain have recently been developed in rodents, a closed femur fracture pain model in mouse and rat [5, 6] and a closed tibia fracture pain model in mouse [7]. These two models are adapted from common fracture models, which have been used for studies of bone reconstruction and bone healing [8, 9].
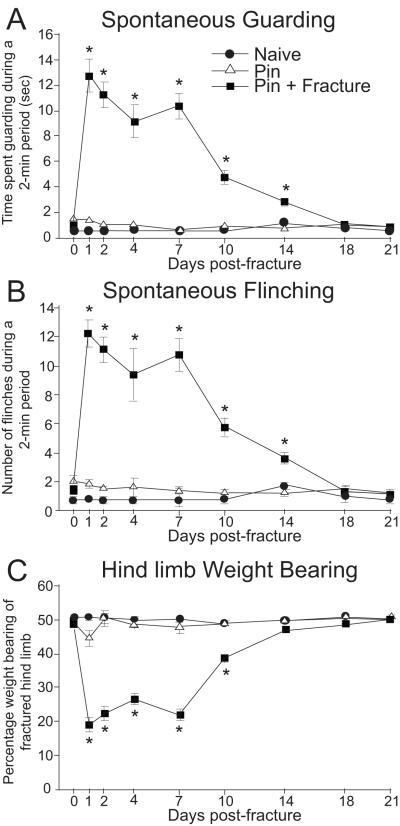
In the femur fracture model, fracture induced spontaneous pain-related behaviors including flinching and guarding [5, 10]. These behaviors were sustained through approximately 18 days after fracture. Fracture-induced pain was also assessed by measuring movement-evoked flinching and guarding [10]. Similar to the time-course of spontaneous behaviors, movement-evoked flinching and guarding peaked at day 2 post-fracture, decreased gradually and continued through day 18 post-fracture (Fig. 1). Compared to spontaneous behaviors, these behaviors evoked by movement were significantly greater in magnitude and appear to mirror exacerbated pain experienced by patients when they utilize their fractured limb [11]. In addition, reduced ability to bear weight on the fractured limb was also observed in this model [5, 12].
Fig. 1.
Guarding, flinching and weight bearing in female rats after closed fracture of the femur. Three groups of rats were studied, naïve, those with a pin in the femur only, and those with a pin plus a fracture. P <0.05 vs. pin. There is no difference between naïve and pin rats. Reprinted with permission from Freeman K, Koewler N, Jimenez-Andrade J, et al. A Fracture Pain Model in the Rat: Adaptation of a Closed Femur Fracture Model to Study Skeletal Pain. Anesthesiology 108(3), 477.
In the tibia fracture model, guarding behavior was observed as well [7]. This spontaneous pain-related behavior peaked immediately after tibial fracture (2 hr post-fracture) and remained evident until 7 days afterwards. In contrast to the femur fracture pain model, spontaneous flinching was not observed following tibial fracture. Tibial fracture-induced pain was also assessed by measuring responses of animals to mechanical and heat stimuli. Both mechanical and heat nociceptive behaviors were the greatest immediately after fracture and sustained for 7 days.
These animal models of fracture pain have been further validated with existing analgesics effective in treating pain in patients experiencing fracture. If analgesics used in patients also reduce pain-related behaviors in these models, it suggests similar pathophysiology between patients with fracture and the models. Administration of morphine dose-dependently reduced spontaneous flinching and guarding and also improved weight bearing on the fractured limb [5]. On day 1 or day 2 following tibial fracture, both morphine and ketoprofen, a non-selective cyclooxygenase −1 and −2 inhibitor, attenuated spontaneous pain-related behaviors as well as mechanical and heat nociception [7].
Animal models of burn pain
Several preclinical animal models have been developed to study underlying mechanisms of burn injury-induced pain. To prepare these models, varied thermal injury parameters (temperature range and burn duration), injury sites and thermal devices were utilized and, consequently, generated differential pain-related behaviors. All burns were performed while animals underwent general anesthesia. A mild burn injury (52.5 ± 1.0 °C for 45 sec) at the rat plantar hindpaw with a hot plate induced consistent but short-lasting primary heat hyperalgesia which was present only at 30 min post-injury [13, 14]. No evident primary mechanical hyperalgesia was observed. Outside the mild burn area, secondary mechanical hyperalgesia peaked at 30 to 60 min post-injury and gradually resolved in 3 hours. Secondary heat hyperalgesia did not develop. Finally, Wang et al. established another model of burn pain by immersing the dorsal part of rat hindpaw into a hot water bath (85 °C for 12 sec) and measured behavioral responses at the uninjured plantar side [15]. Such a burn injury induced profound secondary mechanical and heat hyperalgesia which were apparent by day 1 post-injury and sustained throughout the next 7 days.
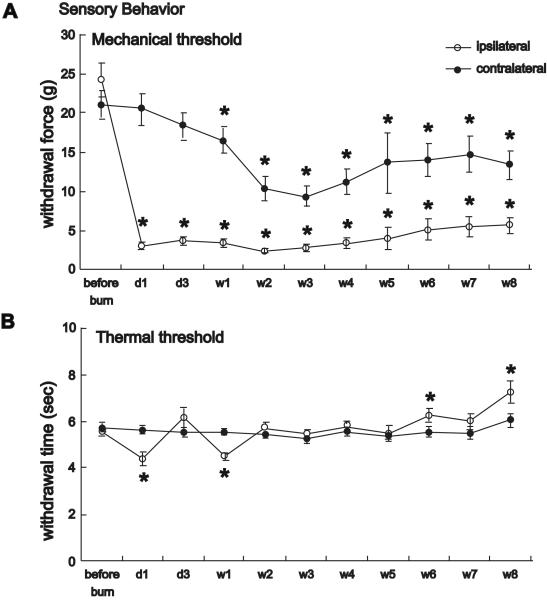
Primary mechanical hyperalgesia at the burn site (procedural pain) is the most intense type of pain in burn patients [16, 17]. In an effort to develop an animal model exhibiting burn-induced primary mechanical hyperalgesia, Summer et al. [18] used a thermal probe with fine temperature control (52.5 ± 0.1 °C for 45 sec). In this model, a 60% reduction in mechanical withdrawal threshold at the injured site was observed at 30 min post-injury. This primary hypersensitivity peaked at day 3 and persisted throughout the 7-day study. Profound primary mechanical hyperalgesia was also present in a model developed more recently by Chang et al. [19]. In this model, the rat plantar hindpaw was placed on a hot plate at 85.0 ± 0.5 °C for 15 sec. Primary mechanical hyperalgesia was observed 1 day after injury and persisted for the entire duration of the 8-week study (Fig. 2).
Fig. 2.
Behavioral studies after surface burn. A. Time course of mechanical withdrawal threshold in a burn injury model. Mechanical hyperalgesia persisted through 8 weeks. Hyperalgesia was also evident in the non-injured contralateral paw. B. Heat hyperalgesia was transient, only apparent one day and one week after surface burn. Data are graphed as mean ± standard error of the mean. * P < 0.05 after injury, compared to before injury. D=day; W=week. Reprinted from The Journal of Pain, 11(2), Chang Y-W, Tan A, Saab C, Waxman S, Unilateral Focal Burn Injury Is Followed by Long-Lasting Bilateral Allodynia and Neuronal Hyperexcitability in Spinal Cord Dorsal Horn, 121, Copyright 2010, with permission from Elsevier.
Mechanisms of burn pain
Peripheral and spinal mechanisms underlying burn pain have been investigated using these preclinical animal models. The cannabinoid receptor (CB) was recently reported as a therapeutic target that could be utilized in the future for the treatment of burn pain [20]. Local injection of cannabinoid receptor agonists into the burn injury site at the rat hindpaw dose-dependently reversed both primary mechanical and heat hyperalgesia. Such antinociceptive action was largely blunted when the CB agonist was co-injected with a CB1 antagonist, but not when the CB agonist was co-injected with CB2 antagonist.
Burn-induced nociception is also mediated by nerve growth factor (NGF) and its cognate receptor, the tyrosine kinase A (TrkA) pathway. Following a large burn injury (25% of total body surface), NGF was upregulated in healing skin and the occurrence of systemic hyperalgesia by the injury was prevented with anti-NGF treatment [21]. Burn-induced hyperalgesia was also attenuated by down-regulation of TrkA in primary sensory neurons with antisense oligodeoxynucleotides (ODN) [18].
The pathogenesis of burn pain also involves an inflammatory component. In burn-injured tissue, a sustained local increase of interleukin-6 (IL-6) was observed for 6 days [22]. Disruption of IL-6 signaling by knocking down glycoprotein (gp130), the signal transducing subunit of IL-6 receptor, in primary sensory neurons with antisense ODN or by functional blocking the cytokine with anti-IL-6 antibodies attenuated burn-induced hyperalgesia.
In the spinal cord, burn injury induced microglial activation as evidenced by increased cell density of microglia in the spinal dorsal horn and enhanced phosphorylation of p38 MAPK (P-p38 MAPK), which was predominantly localized to microglia [19, 23]. Suppression of glial p38 MAPK activation with a specific inhibitor (SB203580 and SD-282) or minocycline suppressed the development of hyperalgesia after burn injury [23, 24], suggesting a contribution of spinal microglia to the development of burn pain.
Animal model of postoperative pain
Several rodent models have been developed for studying the pathophysiology of postoperative pain. Among them, the plantar incision model has been most widely used [25]. This model is produced by a simple incision that includes skin, fascia and deep muscle tissue at the plantar side of rat hindpaw under general anesthesia. Following the plantar incision, animals exhibited non-evoked guarding behavior, a posture noted after hindpaw incision. It peaked immediately after incision (4hr post-incision) and resolved within 3-4 days. Non-evoked guarding likely correlates to pain at rest in patients after surgery. An incision in rat plantar hindpaw also induced hyperalgesia to mechanical and heat stimulation [26]. The greatest level of primary mechanical and heat hyperalgesia was present early after incision (2 hr post-incision). Primary mechanical hyperalgesia usually resolved within 5-6 days after incision, whereas primary heat hyperalgesia tended to last longer and returned to pre-incision level over 7-10 days. In addition, secondary mechanical hyperalgesia, but not secondary heat hyperalgesia, was observed in the plantar incision model [26].
Pain-related behaviors in the plantar incision model could be differentially inhibited by systemic morphine at doses similar to those applied in human postoperative pain studies [27]. Non-evoked guarding was most responsive to morphine and could be attenuated by a dose of 0.03 mg/kg. Greater doses were needed to reduce primary mechanical (1 mg/kg) and heat (0.3 mg/kg) hyperalgesia.
Other types of surgical procedures have been employed to model postoperative pain in animals. In comparison to the plantar incision model involving an incision in glabrous skin, a hairy-skin incision model was developed by making an incision in skin on the back of rats [28]. Mechanical hypersensitivity was induced at sites both close and distant to the incision in this model. To focus on secondary hyperalgesia, a rat gastrocnemius incision model involved an incision in the rat posterior hindlimb and behavioral responses were measured from the ipsilateral hindpaw [29, 30]. Abdominal surgery models including ovariohysterectomy and subcostal incision model closely mimicked surgical procedures to patients [31, 32]. These models possessed apparent clinical relevance and also included visceral injury component. Prolonged tissue retraction is a common practice in clinical surgeries. It was involved two animal models, postthoracotomy pain model and skin/muscle incision and retraction (SMIR) model [33, 34]. Incision in tissue followed by prolonged retraction induced long-lasting pain-related behaviors (approximately 20 to 40 days) in these two models.
Mechanisms of postoperative pain
Based on the plantar incision model, a series of studies using behavioral and neurophysiological approaches have enriched our understanding of the etiology of postoperative pain. Incision in rat plantar hindpaw induced hyperexcitability of both primary afferent nociceptors and nociceptive neurons in the spinal cord [35-41]. These processes of peripheral and central sensitization are believed to be the neural basis of postoperative pain. Spontaneous activity in pain transmission pathways was significantly increased following incision [35-40]. This elevation in neuronal spontaneous activity correlated the occurrence of non-evoked guarding behavior. Further, increased spontaneous activity in spinal dorsal horn neurons could be reversed by infiltration of local anesthetic into the incised tissue, indicating that peripheral inputs drove the neuronal hyperexcitability at the spinal level [38-40].
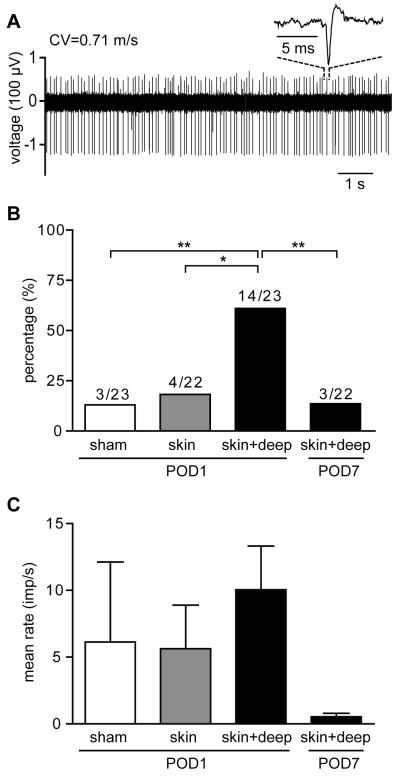
More recent studies have closely examined the specific contribution of different tissues to pain-related behaviors and neuronal hyperexcitability after incision. Incised deep tissue (fascia and muscle) rather than skin had a predominant role in the genesis of non-evoked guarding and spontaneous activity in nociceptors (Fig. 3) and nociceptive transmitting dorsal horn neurons, whereas incision in skin alone was sufficient to induce mechanical and heat hyperalgesia [39, 42]. Such differential contributions to incisional pain by cutaneous versus deep tissue have also been indicated by human studies [43-46].
Fig. 3.
Spontaneous activity of afferent fibers in 4 groups: one day after sham procedure, one day after skin, or one day after skin plus deep tissue incision, and 7 days after skin plus deep tissue incision. (A) Digitized oscilloscope trace of action potentials (inset is a representative single action potential) of a C-nociceptor 1 day after skin plus deep tissue incision. (B) Percentage of afferents with spontaneous activity in the 4 groups. (C) Comparison of average activity among the 4 groups. Imp = impulse. * P < 0.5, ** P < 0.01 versus sham. Reprinted with permission from Freeman K, Koewler N, Jimenez-Andrade J, et al. A Fracture Pain Model in the Rat: Adaptation of a Closed Femur Fracture Model to Study Skeletal Pain. Anesthesiology 108(3), 477. Reprinted with permission from Xu J, Brennan TJ. Guarding Pain and Spontaneous Activity of Nociceptors after Skin versus Skin Plus Deep Tissue Incision. Anesthesiology 108(3), 477.
Several mediators of incisional pain have been suggested based on preclinical studies. Incision in plantar hindpaw induced a decrease in tissue pH (lowest pH = 6.8) and an increase in lactate concentration (peak = 5 mM) locally for several days [47, 48]. These changes in local chemicals paralleled pain-related behaviors after incision. In addition, nociceptors in incised tissue exhibited greater sensitivity to lactic acid compared to those in intact tissue [36, 49]. Together, it suggests that an ischemic-like signal contributes to pain caused by incisions.
NGF is another component of the local chemical environment, which can sensitize nociceptive nerve endings surrounding the incision site. An increase of NGF expression in injured tissue occurred within hours after incision and returned to baseline in approximately 7 days [50-52]. Fibroblasts and Schwann cells adjacent to the incision were sources of NGF [51, 52]. Treatment of anti-NGF antibody dose-dependently attenuated non-evoked guarding [50]. Anti-NGF also induced moderate inhibition of heat hyperalgesia but had no effect on mechanical responses of incised hindpaw.
The transient receptor potential vanilloid 1 (TRPV1) receptor is considered a molecular integrator of various nociceptive stimuli. In incision-induced pathological conditions, blockade of TRPV1 with an antagonist (AMG0347) only exerted moderate inhibition of heat hyperalgesia and did not affect guarding or mechanical responses [27]. Similar findings were observed in TRPV1 knockout mice when incision was made in the mouse plantar hindpaw [53].
Although the TRPV1 receptor itself does not appear to be a key mediator of postoperative pain, TRPV1 expressing nociceptors are likely the critical afferents transmitting postoperative pain. Treatment of dilute capsaicin (0.025-0.10%), a specific activator of TRPV1, attenuated non-evoked guarding in a dose-dependent manner [54]. It also inhibited heat hyperalgesia but did not alter hypersensitivity to mechanical stimulation. Further studies suggested that capsaicin at low concentrations might selectively affect nociceptive transducing pathways since it interfered with chemo- and heat sensitivity, but not mechanosensitivity of C-nociceptors [55].
Conclusion
Several preclinical animal models for surgery, fracture and burn pain have been developed and exhibit different forms of pain-related behaviors. The complex mechanisms underlying pain in these models may include inflammatory, ischemic and neuropathic components. Key mediators contributing to the pathogenesis of pain after these acute injuries like NGF, cannabinoids, IL-6, ischemic mediators and TRPV1 containing afferents are being explored at both the peripheral and spinal levels.
Key points.
* Optimizing preclinical animal pain models is critical for elucidating the pathophysiology of acute pain.
* In contrast to chronic pain models induced by chemicals or nerve ligations, clinically relevant injuries are utilized to model acute pain preclinically.
* Different types of acute tissue injury can result in specific patterns of pain-related behaviors in these rodent models.
* Non-evoked ongoing pain is an important, overlooked component of acute pain and is likely transmitted by increased spontaneous activity in nociceptive pathways.
* Peripheral sensitization appears to make a major contribution to the development of acute pain and several key targets in the periphery have been suggested.
Acknowledgements
This work was supported by the Department of Anesthesia at the University of Iowa and by National Institutes of Health, Bethesda, Maryland grant GM-55831 Nociception from Deep Muscle Incision to T.J.B.
T.J.B. currently is a consultant for Cubist Pharmaceuticals. He has served as a consultant in the past for PainReform, Xenon Pharmaceuticals, Covidian, AnaptysBio, Trevena, Inc., and Angiochem. T.J.B. has served on the Pfizer Advisory Board in the past. T.J.B. has current grants from Hydra Biosciences and Ironwood Pharmaceuticals. He has an ongoing grant with Galleon Pharmaceuticals. He has received grants in the past from Kai Pharmaceuticals and Adynxx, Inc.
J.X. is currently employed by Abbott Laboratories, Abbott Park, IL.
Footnotes
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Chudyk AM, Jutai JW, Petrella RJ, Speechley M. Systematic review of hip fracture rehabilitation practices in the elderly. Arch Phys Med Rehabil. 2009;90:246–62. doi: 10.1016/j.apmr.2008.06.036. [DOI] [PubMed] [Google Scholar]
- 2.Johnell O, Kanis JA. An estimate of the worldwide prevalence and disability associated with osteoporotic fractures. Osteoporos Int. 2006;17:1726–33. doi: 10.1007/s00198-006-0172-4. [DOI] [PubMed] [Google Scholar]
- 3.Roth T, Kammerlander C, Gosch M, et al. Outcome in geriatric fracture patients and how it can be improved. Osteoporos Int. 2010;21:S615–9. doi: 10.1007/s00198-010-1401-4. [DOI] [PubMed] [Google Scholar]
- 4.Montgomery RK. Pain management in burn injury. Crit Care Nurs Clin North Am. 2004;16:39–49. doi: 10.1016/j.ccell.2003.10.005. [DOI] [PubMed] [Google Scholar]
- 5.Freeman KT, Koewler NJ, Jimenez-Andrade JM, et al. A fracture pain model in the rat: adaptation of a closed femur fracture model to study skeletal pain. Anesthesiology. 2008;108:473–83. doi: 10.1097/ALN.0b013e3181649351. [DOI] [PubMed] [Google Scholar]
- 6.Koewler NJ, Freeman KT, Buus RJ, et al. Effects of a monoclonal antibody raised against nerve growth factor on skeletal pain and bone healing after fracture of the C57BL/6J mouse femur. J Bone Miner Res. 2007;22:1732–42. doi: 10.1359/jbmr.070711. [DOI] [PubMed] [Google Scholar]
- 7.Minville V, Laffosse JM, Fourcade O, et al. Mouse model of fracture pain. Anesthesiology. 2008;108:467–72. doi: 10.1097/ALN.0b013e3181649333. [DOI] [PubMed] [Google Scholar]
- 8.Bonnarens F, Einhorn TA. Production of a standard closed fracture in laboratory animal bone. J Orthop Res. 1984;2:97–101. doi: 10.1002/jor.1100020115. [DOI] [PubMed] [Google Scholar]
- 9.Manigrasso MB, O’Connor JP. Characterization of a closed femur fracture model in mice. J Orthop Trauma. 2004;18:687–95. doi: 10.1097/00005131-200411000-00006. [DOI] [PubMed] [Google Scholar]
- 10.Jimenez-Andrade JM, Martin CD, Koewler NJ, et al. Nerve growth factor sequestering therapy attenuates non-malignant skeletal pain following fracture. Pain. 2007;133:183–96. doi: 10.1016/j.pain.2007.06.016. [DOI] [PubMed] [Google Scholar]
- 11.MacDermid JC, Roth JH, Richards RS. Pain and disability reported in the year following a distal radius fracture: a cohort study. BMC Musculoskelet Disord. 2003;4:24. doi: 10.1186/1471-2474-4-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *12.Jimenez-Andrade JM, Mantyh WG, Bloom AP, et al. The effect of aging on the density of the sensory nerve fiber innervation of bone and acute skeletal pain. Neurobiol Aging. 2010 doi: 10.1016/j.neurobiolaging.2010.08.008. This study examined nerve fiber density, bone density, and aging in rats. Even though bone mass decreased, sensory innervation was unchanged. This study suggests pain after bone injury is likely to be maintained in the aging population.
- 13.Jones DL, Sorkin LS. Systemic gabapentin and S(+)-3-isobutyl-gamma-aminobutyric acid block secondary hyperalgesia. Brain Res. 1998;810:93–9. doi: 10.1016/s0006-8993(98)00890-7. [DOI] [PubMed] [Google Scholar]
- 14.Nozaki-Taguchi N, Yaksh TL. A novel model of primary and secondary hyperalgesia after mild thermal injury in the rat. Neurosci Lett. 1998;254:25–8. doi: 10.1016/s0304-3940(98)00648-x. [DOI] [PubMed] [Google Scholar]
- 15.Wang S, Lim G, Yang L, et al. A rat model of unilateral hindpaw burn injury: slowly developing rightwards shift of the morphine dose-response curve. Pain. 2005;116:87–95. doi: 10.1016/j.pain.2005.03.044. [DOI] [PubMed] [Google Scholar]
- 16.Byers JF, Bridges S, Kijek J, LaBorde P. Burn patients’ pain and anxiety experiences. J Burn Care Rehabil. 2001;22:144–9. doi: 10.1097/00004630-200103000-00011. [DOI] [PubMed] [Google Scholar]
- 17.Summer GJ, Puntillo KA, Miaskowski C, et al. Burn injury pain: the continuing challenge. J Pain. 2007;8:533–48. doi: 10.1016/j.jpain.2007.02.426. [DOI] [PubMed] [Google Scholar]
- 18.Summer GJ, Puntillo KA, Miaskowski C, et al. TrkA and PKC-epsilon in thermal burn-induced mechanical hyperalgesia in the rat. J Pain. 2006;7:884–91. doi: 10.1016/j.jpain.2006.04.009. [DOI] [PubMed] [Google Scholar]
- *19.Chang YW, Tan A, Saab C, Waxman S. Unilateral focal burn injury is followed by long-lasting bilateral allodynia and neuronal hyperexcitability in spinal cord dorsal horn. J Pain. 2010;11:119–30. doi: 10.1016/j.jpain.2009.06.009. After unilateral burn injury central hyperexcitability occurs and shares features with central hyperexcitability after nerve injury. This study identifies the complexity of burn injury and the potential for developing persistent post-burn pain.
- 20.Johanek LM, Simone DA. Activation of peripheral cannabinoid receptors attenuates cutaneous hyperalgesia produced by a heat injury. Pain. 2004;109:432–42. doi: 10.1016/j.pain.2004.02.020. [DOI] [PubMed] [Google Scholar]
- 21.Ueda M, Hirose M, Takei N, et al. Nerve growth factor induces systemic hyperalgesia after thoracic burn injury in the rat. Neurosci Lett. 2002;328:97–100. doi: 10.1016/s0304-3940(02)00456-1. [DOI] [PubMed] [Google Scholar]
- 22.Summer GJ, Romero-Sandoval EA, Bogen O, et al. Proinflammatory cytokines mediating burn-injury pain. Pain. 2008;135:98–107. doi: 10.1016/j.pain.2007.05.012. [DOI] [PubMed] [Google Scholar]
- 23.Sorkin L, Svensson CI, Jones-Cordero TL, et al. Spinal p38 mitogen-activated protein kinase mediates allodynia induced by first-degree burn in the rat. J Neurosci Res. 2009;87:948–55. doi: 10.1002/jnr.21905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chang YW, Waxman SG. Minocycline attenuates mechanical allodynia and central sensitization following peripheral second-degree burn injury. J Pain. 2010;11:1146–54. doi: 10.1016/j.jpain.2010.02.010. [DOI] [PubMed] [Google Scholar]
- 25.Brennan TJ, Vandermeulen EP, Gebhart GF. Characterization of a rat model of incisional pain. Pain. 1996;64:493–501. doi: 10.1016/0304-3959(95)01441-1. [DOI] [PubMed] [Google Scholar]
- 26.Zahn PK, Brennan TJ. Primary and secondary hyperalgesia in a rat model for human postoperative pain. Anesthesiology. 1999;90:863–72. doi: 10.1097/00000542-199903000-00030. [DOI] [PubMed] [Google Scholar]
- 27.Wu C, Gavva NR, Brennan TJ. Effect of AMG0347, a transient receptor potential type V1 receptor antagonist, and morphine on pain behavior after plantar incision. Anesthesiology. 2008;108:1100–8. doi: 10.1097/ALN.0b013e31817302b3. [DOI] [PubMed] [Google Scholar]
- 28.Duarte AM, Pospisilova E, Reilly E, et al. Reduction of postincisional allodynia by subcutaneous bupivacaine: findings with a new model in the hairy skin of the rat. Anesthesiology. 2005;103:113–25. doi: 10.1097/00000542-200507000-00018. [DOI] [PubMed] [Google Scholar]
- 29.Pogatzki EM, Niemeier JS, Brennan TJ. Persistent secondary hyperalgesia after gastrocnemius incision in the rat. Eur J Pain. 2002;6:295–305. doi: 10.1053/eujp.2002.0339. [DOI] [PubMed] [Google Scholar]
- 30.Pogatzki EM, Niemeier JS, Sorkin LS, Brennan TJ. Spinal glutamate receptor antagonists differentiate primary and secondary mechanical hyperalgesia caused by incision. Pain. 2003;105:97–107. doi: 10.1016/s0304-3959(03)00169-6. [DOI] [PubMed] [Google Scholar]
- 31.Lascelles BD, Waterman AE, Cripps PJ, et al. Central sensitization as a result of surgical pain: investigation of the pre-emptive value of pethidine for ovariohysterectomy in the rat. Pain. 1995;62:201–12. doi: 10.1016/0304-3959(94)00266-H. [DOI] [PubMed] [Google Scholar]
- 32.Martin TJ, Buechler NL, Kahn W, et al. Effects of laparotomy on spontaneous exploratory activity and conditioned operant responding in the rat: a model for postoperative pain. Anesthesiology. 2004;101:191–203. doi: 10.1097/00000542-200407000-00030. [DOI] [PubMed] [Google Scholar]
- 33.Buvanendran A, Kroin JS, Kerns JM, et al. Characterization of a new animal model for evaluation of persistent postthoracotomy pain. Anesth Analg. 2004;99:1453–60. doi: 10.1213/01.ANE.0000134806.61887.0D. table of contents. [DOI] [PubMed] [Google Scholar]
- 34.Flatters SJ. Characterization of a model of persistent postoperative pain evoked by skin/muscle incision and retraction (SMIR) Pain. 2008;135:119–30. doi: 10.1016/j.pain.2007.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Banik RK, Brennan TJ. Spontaneous discharge and increased heat sensitivity of rat C-fiber nociceptors are present in vitro after plantar incision. Pain. 2004;112:204–13. doi: 10.1016/j.pain.2004.08.026. [DOI] [PubMed] [Google Scholar]
- 36.Kang S, Brennan TJ. Chemosensitivity and mechanosensitivity of nociceptors from incised rat hindpaw skin. Anesthesiology. 2009;111:155–64. doi: 10.1097/ALN.0b013e3181a16443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pogatzki EM, Gebhart GF, Brennan TJ. Characterization of Adelta- and C-fibers innervating the plantar rat hindpaw one day after an incision. J Neurophysiol. 2002;87:721–31. doi: 10.1152/jn.00208.2001. [DOI] [PubMed] [Google Scholar]
- 38.Pogatzki EM, Vandermeulen EP, Brennan TJ. Effect of plantar local anesthetic injection on dorsal horn neuron activity and pain behaviors caused by incision. Pain. 2002;97:151–61. doi: 10.1016/s0304-3959(02)00014-3. [DOI] [PubMed] [Google Scholar]
- 39.Xu J, Brennan TJ. Comparison of skin incision vs. skin plus deep tissue incision on ongoing pain and spontaneous activity in dorsal horn neurons. Pain. 2009;144:329–39. doi: 10.1016/j.pain.2009.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Xu J, Richebe P, Brennan TJ. Separate groups of dorsal horn neurons transmit spontaneous activity and mechanosensitivity one day after plantar incision. Eur J Pain. 2009;13:820–8. doi: 10.1016/j.ejpain.2008.10.005. [DOI] [PubMed] [Google Scholar]
- 41.Zahn PK, Pogatzki-Zahn EM, Brennan TJ. Spinal administration of MK-801 and NBQX demonstrates NMDA-independent dorsal horn sensitization in incisional pain. Pain. 2005;114:499–510. doi: 10.1016/j.pain.2005.01.018. [DOI] [PubMed] [Google Scholar]
- **42.Xu J, Brennan TJ. Guarding pain and spontaneous activity of nociceptors after skin versus skin plus deep tissue incision. Anesthesiology. 2010;112:153–64. doi: 10.1097/ALN.0b013e3181c2952e. This study demonstrated that skin injury alone produced little evidence of spontaneous pain or spontaneous activity in nociceptors. This suggests that deep tissue may be most important to study postoperative pain mechanisms.
- 43.Dorr LD, Maheshwari AV, Long WT, et al. Early pain relief and function after posterior minimally invasive and conventional total hip arthroplasty. A prospective, randomized, blinded study. J Bone Joint Surg Am. 2007;89:1153–60. doi: 10.2106/JBJS.F.00940. [DOI] [PubMed] [Google Scholar]
- 44.Kawamata M, Takahashi T, Kozuka Y, et al. Experimental incision-induced pain in human skin: effects of systemic lidocaine on flare formation and hyperalgesia. Pain. 2002;100:77–89. doi: 10.1016/s0304-3959(02)00233-6. [DOI] [PubMed] [Google Scholar]
- 45.Kawamata M, Watanabe H, Nishikawa K, et al. Different mechanisms of development and maintenance of experimental incision-induced hyperalgesia in human skin. Anesthesiology. 2002;97:550–9. doi: 10.1097/00000542-200209000-00006. [DOI] [PubMed] [Google Scholar]
- 46.Ogonda L, Wilson R, Archbold P, et al. A minimal-incision technique in total hip arthroplasty does not improve early postoperative outcomes. A prospective, randomized, controlled trial. J Bone Joint Surg Am. 2005;87:701–10. doi: 10.2106/JBJS.D.02645. [DOI] [PubMed] [Google Scholar]
- 47.Kim TJ, Freml L, Park SS, Brennan TJ. Lactate concentrations in incisions indicate ischemic-like conditions may contribute to postoperative pain. J Pain. 2007;8:59–66. doi: 10.1016/j.jpain.2006.06.003. [DOI] [PubMed] [Google Scholar]
- 48.Woo YC, Park SS, Subieta AR, Brennan TJ. Changes in tissue pH and temperature after incision indicate acidosis may contribute to postoperative pain. Anesthesiology. 2004;101:468–75. doi: 10.1097/00000542-200408000-00029. [DOI] [PubMed] [Google Scholar]
- **49.Xu J, Gu H, Brennan TJ. Increased sensitivity of group III and group IV afferents from incised muscle in vitro. Pain. 2010;151:744–55. doi: 10.1016/j.pain.2010.09.003. This study demonstrated peripheral sensitization of muscle afferents after incision persists in vitro. Sensitization in injured muscle may detect changes in mechanical, heat, and chemosensitivity.
- 50.Banik RK, Subieta AR, Wu C, Brennan TJ. Increased nerve growth factor after rat plantar incision contributes to guarding behavior and heat hyperalgesia. Pain. 2005;117:68–76. doi: 10.1016/j.pain.2005.05.017. [DOI] [PubMed] [Google Scholar]
- 51.Wu C, Boustany L, Liang H, Brennan TJ. Nerve growth factor expression after plantar incision in the rat. Anesthesiology. 2007;107:128–35. doi: 10.1097/01.anes.0000267512.08619.bd. [DOI] [PubMed] [Google Scholar]
- 52.Wu C, Erickson MA, Xu J, et al. Expression profile of nerve growth factor after muscle incision in the rat. Anesthesiology. 2009;110:140–9. doi: 10.1097/ALN.0b013e318190bc84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Banik RK, Brennan TJ. Trpv1 mediates spontaneous firing and heat sensitization of cutaneous primary afferents after plantar incision. Pain. 2009;141:41–51. doi: 10.1016/j.pain.2008.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hamalainen MM, Subieta A, Arpey C, Brennan TJ. Differential effect of capsaicin treatment on pain-related behaviors after plantar incision. J Pain. 2009;10:637–45. doi: 10.1016/j.jpain.2009.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *55.Kang S, Wu C, Banik RK, Brennan TJ. Effect of capsaicin treatment on nociceptors in rat glabrous skin one day after plantar incision. Pain. 2010;148:128–40. doi: 10.1016/j.pain.2009.10.031. This study demonstrated that capsaicin produces analgesia but does not kill sensory C-fibers in a model of postoperative pain. Capsaicin’s analgesic effect may be related to inhibition of chemosensitization produced by incision.