Abstract
Purpose of review
Hypoxia represents one of the strongest transcriptional stimuli known to us. In most cases, hypoxia-induced changes in gene expression are directed towards adapting tissues to conditions of limited oxygen availability.
Recent findings
As a well-known example, physical exercise at high-altitude results in the transcriptional induction of erythropoietin that functions to increase oxygen carrying capacity and red cell volume. Studies of the transcriptional pathway responsible for the induction of erythropoietin during conditions of hypoxia led to the discovery of the transcription factor hypoxia-inducible factor (HIF) that is known today as the key transcription factor for hypoxia adaptation. Surgical patients are frequently at risk for experiencing detrimental effects of hypoxia or ischemia, for example in the context of acute kidney injury, myocardial, intestinal or hepatic ischemia, acute lung injury or during organ transplantation.
Summary
In the present review we discuss mechanisms of transcriptional adaptation to hypoxia and provide evidence supporting the hypothesis that targeting hypoxia-induced inflammation can represent novel pharmacologic strategies to improve perioperative outcomes. Currently, such strategies are being explored at an experimental level, but we hope that some of these targets can be translated into perioperative patient care within the next decade.
Keywords: Erythropoietin, hypoxia-inducible factor, HIF, adenosine receptor, A2BAR, Prolyl hydroxylase, PHD
Introduction
Despite significant advances in patient monitoring, airway management approaches and the safety of anesthetics that are in use today, perioperative organ failure remains one of the biggest threats for patients undergoing major surgeries1, 2. For example, a patient who is undergoing an aortic valve replacement develops acute kidney injury in his postoperative course, and subsequently becomes dialysis dependent. Similarly, a patient who is having vascular surgery develops postoperative myocardial infarction and an ischemic stroke. Patients who are undergoing coronary surgery with cardiopulmonary bypass may go on to develop acute lung injury in the postoperative course. We believe that in many instances, perioperative organ failure involves a hypoxic or an ischemic tissue injury3. Here is another example: during solid organ transplantation the ischemic injury to the organ correlates with early graft failure and inflammatory responses4–7. While hypoxia causes organ injury and inflammation, hypoxia can also elicit anti-inflammatory responses that help to dampen hypoxia-induced inflammation1. In the present review, we discuss the hypothesis that hypoxia-elicited anti-inflammatory responses can be targeted to treat or prevent acute organ injury. We will provide evidence that pharmacological strategies to target hypoxia-induced changes in gene expression can be therapeutic in acute myocardial ischemia8, acute kidney9–14 or lung injury15–18. While these pharmacological strategies are currently investigated in experimental models, we hope that at least some of these approaches may become available for the treatment of perioperative patients in the near future.
The relationship between hypoxia and inflammation
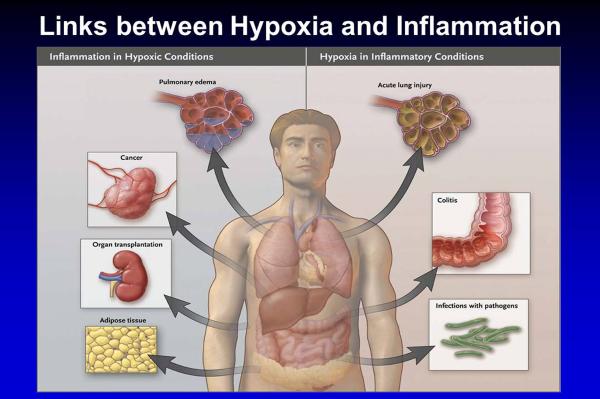
Hypoxia and inflammation share an interdependent relationship. On the one hand, hypoxia can elicit tissue inflammation. For instance, exposure to high altitude is associated with elevated inflammatory mediators in humans19. Similarly, mice that are exposed to acute hypoxia (e.g. to 8% oxygen over 8h) develop elevated plasma levels of cytokines, in conjunction with pulmonary edema and inflammatory cell accumulation in the lungs and other organs16, 20–23. There are many examples in the perioperative period that are characterized by hypoxia-induced inflammation. During organ transplantation, the ischemic graft becomes inflamed and targeting graft inflammation could represent a therapeutic approach to improve early organ function and to decrease the chances of acute rejection24. Similarly, during acute organ ischemia – such as intestinal or hepatic ischemia – the ischemic organ becomes severely inflamed and experimental strategies to dampen hypoxia-induced inflammation are currently under investigation in mouse models25–32. Other examples for inflammation occurring in hypoxic conditions are displayed in Figure 1 (left panel).
Figure 1.
Overview of clinical conditions characterized primarily by tissue hypoxia resulting in inflammatory changes (left), or inflammatory diseases that lead to tissue hypoxia (right; from the New England Journal of Medicine with permission1).
While hypoxia can induce inflammation; inflamed tissues frequently become hypoxic. For example, patients with inflammatory bowel disease experience elevated levels of hypoxia-inducible factors (HIF)33. HIF is a transcription factor that is stabilized during conditions of hypoxia. Studies of tissue hypoxia during experimentally induced colitis in mice also indicate that the inflamed intestine becomes severely hypoxic. Inflammation-induced tissue hypoxia is most likely caused by an imbalance in metabolic supply and demand ratios for metabolites and oxygen34–36. In the inflamed microenvironment, vascular stasis, occlusion or thrombosis limits the supply of oxygen and metabolites. At the same time, the oxygen consumption of invading inflammatory cells and resident tissue cells is dramatically increased. Particularly neutrophils and neutrophil derived nucleotides can further enhance hypoxia induced inflammation37–40. Together, these studies indicate that inflammatory conditions can result in robust tissue hypoxia. Clinical examples for tissue hypoxia caused by inflammatory conditions include acute lung injury, cancer or infections with pathogens (see Figure 1, right panel)1.
While hypoxia and inflammation share an interdependent relationship, hypoxia-induced changes in gene transcription are predominantly directed towards helping to adapt tissues to hypoxia, and to dampen hypoxia induced inflammation1. In fact, understanding and targeting hypoxia induced tissue adaptation could represent a powerful therapeutic approach for perioperative medicine. This can be achieved by two different strategies. On the one hand, pharmacological strategies can be utilized to directly enhance hypoxia-dependent changes in gene transcription, for example by utilizing pharmacologic agents that enhance stabilization of HIFs8, 41, 42. Alternatively, hypoxia-induced genes that were shown to dampen hypoxia-induced inflammation can be targeted directly10, 15–18, 20, 40, 43–50.
Molecular mechanisms of hypoxia sensing and signaling
Hypoxia is among the strongest transcriptional stimuli known. A famous example of hypoxia-induced changes in gene transcription comes from the transcriptional regulation of erythropoietin during conditions of limited oxygen availability. When the oxygen supply becomes diminished e.g. in the context of ambient hypoxia, or due to tissue limited oxygen availability to the tissues during anemia, erythropoietin production is increased to counterbalance tissue hypoxia via increasing oxygen carrying capacity. This is well known to perioperative physicians, as we can hold off from transfusing red blood cells despite low hemoglobin levels if the bleeding is controlled, trusting that a healthy patient will be able to restore their red blood cell volume subsequently on their own. This response is mediated by a transcriptional induction of erythropoietin. Ground breaking studies from the laboratory of Dr. Greg Semenza identified the transcriptional mechanism responsible for erythropoietin during hypoxia. In fact, Dr. Semenza and his team identified a transcription factor that binds to the promoter of erythropoietin during conditions of hypoxia and is responsible for a hypoxia-elicited increase in promoter activity51–53. This transcription factor that is stabilized during hypoxia and drives hypoxia-induced induction or repression of gene expression was called “HIF”.
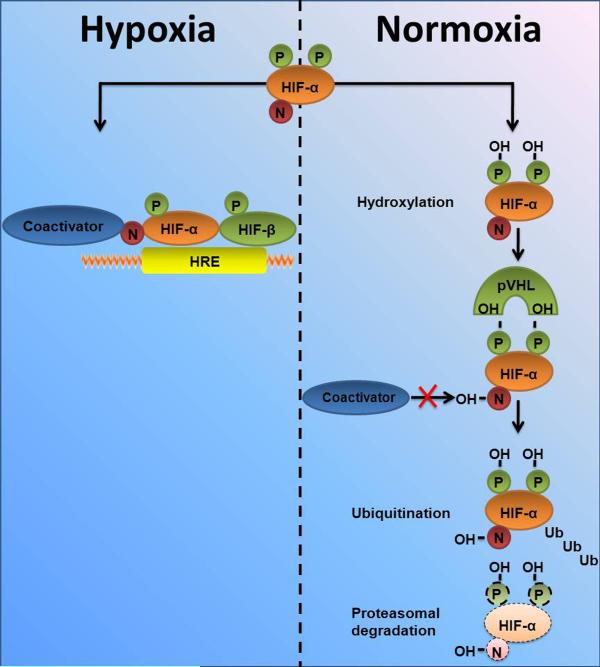
Subsequent studies determined that HIF is an α/β heterodimeric transcription factor with a constitutively expressed β-subunit and a post-translationally regulated α subunit. In fact, the α-subunit is rapidly destroyed via the proteasomal degradation pathway during normal oxygen availability. In contrast, hypoxia is associated with stabilization of the α-subunit. When the α-subunit is stabilized, it can form a heterodimer with the HIF-β subunit allowing for transcriptional activity. The heterodimer binds to the promoter region of genes that contain a consensus sequence for HIF binding – a so called hypoxia responsive element (HRE) within their promoters. Binding of HIF can either induce enhanced transcription of a target gene20, 45, 46, 53–57, or it can result in repression of gene expression21, 47–49. It is currently not well understood, why HIF can induce gene transcription in some instances, while it can repress promoter activity of genes in other circumstances. However, HIF-dependent alterations in gene-expression frequently result in coordinated adaptive responses that help to prepare tissues to limited oxygen availability1. More recently, it was discovered that α subunits of HIF exists in two iso-forms – HIF-1α and HIF-2α. While they are closely related, and both activate HRE-dependent gene transcription58, knockout studies in mice demonstrate that HIF-1α and HIF-2α play non-redundant roles, and inactivation of each one results in a distinctly different phenotype. This may result, in part, from differences in tissue-specific and temporal patterns of induction of each isoform59, 60, but, not uncommonly, both isoforms are expressed within a given cell type, and the results of several studies suggest that HIF-1α and HIF-2α may have distinct transcriptional targets61, 62. For example, the transcription of genes encoding enzymes that operate in a coordinated way in the glycolytic pathway appears to be driven by HIF-1α and not HIF-2α61–65 (Figure 2).
Figure 2. Regulation of hypoxia-inducible factor (HIF) protein levels under normoxic or hypoxic conditions.
In normoxia, hydroxylation at 2 proline residues promotes HIF-α association with pVHL and HIF-α destruction via the ubiquitin/proteasome pathway, while hydroxylation of an asparagine residue blocks association with coactivators. In hypoxia, these processes are suppressed, allowing HIF-α subunits (both HIF-1α and HIF-2α) to escape proteolysis, dimerize with HIF-1β, recruit coactivators, and activate transcription via HREs. N, asparagine; P, proline; OH, hydroxyl group; Ub, ubiquitin [modified, from the Journal of Clinical Investigation with permission 62].
It is important to point out that oxygen sensing does not occur through HIFs, but through a group of oxygen-dependent enzymes that regulate the stability of the α subunit of HIF – the so called prolyl-hydroxylases (PHDs). Under well-oxygenated conditions, HIFα becomes hydroxylated at one (or both) of two highly conserved prolyl residues by members of the prolyl hydroxylase domain (PHD) family (also called EgIN family)60, 66. Hydroxylation of either of these prolyl residues generates a binding site for the von Hippel-Lindau (pVHL) tumor suppressor protein, which is a component of a ubiquitin ligase complex. As a result, HIFα is polyubiquitylated and subjected to proteasomal degradation when oxygen is available. The PHD proteins belong to the Fe(II) and 2-oxoglutarate-dependent oxygenase superfamily, whose activity is absolutely dependent on oxygen. Accordingly, the rate of HIF hydroxylation is suppressed by hypoxia. Under low oxygen conditions, or in cells lacking functional pVHL, HIFα accumulates, dimerizes with an HIFβ family member, translocates to the nucleus, and transcriptionally activates different genes, including genes involved in erythropoiesis, angiogenesis, autophagy, and energy metabolism66–71. Factor Inhibiting HIF (FIH)1, like the PHD family members, is an Fe(II)- and 2-oxoglutarate-dependent dioxygenase. When oxygen is available, FIH1 hydroxylates a conserved asparaginyl residue within the HIF1α and HIF2α, leading to a steric clash that prevents the recruitment of the coactivators p300 and CBP66 (see Figure 2). FIH1 remains active at lower oxygen concentrations than the PHDs and so might suppress the activity of HIFα proteins that escape destruction in moderate hypoxia72. HIFα stability is also regulated by other signaling pathways. For example, HSP90 inhibitors and histone acetylase inhibitors promote HIFα degradation in a pVHL-independent manner73.
The PHD-HIF pathway as a pharmacologic target
Several studies have explored pharmacologic approaches to directly target the PHD-HIF pathways. In fact, several pharmacologic compounds have been identified that efficiently inhibit the activity of PHDs. Inhibition of PHDs prevents HIF hydroxylation and proteasomal degradation. As such, PHD inhibitors can be used to stabilize HIF in the presence of normal oxygen concentrations, or to provide higher HIF levels under hypoxic or inflammatory conditions. Some of these compounds were initially evaluated for the treatment of renal anemia – as an alternative to treatment with erythropoietin. However, recent studies indicate that such compounds can be efficiently used to treat inflammatory conditions. For example, the laboratory of Dr. Sean Colgan has performed very elegant studies on the role of the PHD-HIF pathway in intestinal inflammation34. They initially set out to study mice with tissue specific deletion of HIF during experimentally induced colitis. These studies revealed that deletion of HIF in intestinal epithelia is associated with a more severe course of the disease, including more profound weight loss of animals and increased intestinal inflammation74. Based on these studies, Dr. Colgan and his team hypothesized that treatment with a pharmacologic inhibitor of PHDs could be associated with an improved outcome of intestinal inflammation35. To address this hypothesis, they used a novel PHD inhibitor FG-4497 that readily stabilizes HIF-1α and subsequently drives the expression downstream of HIF target genes (eg, erythropoietin). They found that that the FG-4497-mediated induction of HIF-1α provides an overall beneficial influence on clinical symptoms [weight loss, colon length, tissue tumor necrosis factor-alpha (TNFalpha)] in experimentally induced colitis, most likely because of their barrier protective function and wound healing during severe tissue hypoxia at the site of inflammation41. Similar studies in a different experimental model of colitis and with a different PHD inhibitor from the laboratory of Dr. Cormac Taylor revealed strikingly similar results42. Together both studies provide a strong basis for a therapeutic use of PHD inhibitors in inflammatory mucosal diseases34. Other examples for experimental studies indicating a therapeutic use of PHD inhibitors include acute myocardial infarction. In fact, pre-treatment with a PHD inhibitor was associated with a similar reduction in myocardial infarct size as ischemic preconditioning8, 75.
Pharmacologic approaches to target specific hypoxia-induced genes
Using PHD inhibitors for the treatment of hypoxia-induced inflammation represents a relatively non-specific approach and pharmacologic HIF inhibitors may have many off-target effects. To limit the off-target effects of PHD inhibitors, an alternative approach for the treatment of hypoxia-induced inflammation is given by directly targeting specific HIF-target genes. For example, extracellular adenosine is a signaling molecule that has been shown to dampen hypoxia-induced inflammation in many animal models of disease2, 9, 12, 13, 15–18, 43, 44, 76–78. Several studies have revealed that HIF plays a direct role in the biology of extracellular adenosine. For example, HIF has been shown to transcriptionally induce enzymes that produce adenosine from precursor nucleotides on the extracellular surface55. Extracellular adenosine signaling can occur through four distinct adenosine receptors (ARs), the A1, A2A, A2B and A3AR. Hypoxia and HIF-dependent alterations in gene expression have provided strong evidence that the A2BAR represents a HIF target gene46. Consistent with the notion that A2BAR signaling plays an important role in dampening hypoxia-induced inflammation12, 15, 16, 22, 23, 27, 29, 31, 43, 76, 79–83, the A2BAR has emerged as a direct target for treating inflammation in the context of tissue hypoxia.
Targeting hypoxia-induced inflammation in perioperative medicine
As outlined above, there are many examples where hypoxia-elicited changes in gene expression represent endogenous pathways to dampen unhealthy inflammatory responses. In the final paragraph, we will discuss an example of how one of these pathways can be targeted to treat surgical patients. An important example for hypoxia-induced inflammation in the perioperative setting is represented by acute lung injury (ALI)18. ALI is a syndrome consisting of acute hypoxemic respiratory failure with bilateral pulmonary infiltrates, not attributable to left heart failure84. Despite optimal management consisting of aggressive treatment of the initiating cause, vigilant supportive care, and the prevention of nosocomial infections, mortality ranges between 35 and 60%17, 84. In fact, approximately 200,000 patients develop ALI annually in the USA, leading to 75,000 deaths and accounting for up to 3.6 million hospital days85. The pathogenesis of ALI is characterized by the influx of a protein-rich edema fluid into the interstitial and intra-alveolar spaces as a consequence of increased permeability of the alveolar–capillary barrier.
Despite the large impact of ALI on morbidity and mortality in critically ill patients84, many episodes of acute lung injury are self-limiting, and resolve spontaneously through unknown mechanisms. For example, patients undergoing major thoracic surgery for lung cancer have an overall incidence of ALI of less than 5%86, open heart surgery with cardiopulmonary bypass less than 0.5%87 or kidney transplantation of less than 0.2%88. Based on these clinical observations, we hypothesized that there may be innate adaptive pathways to dampen acute increases in the capillary-alveolar barrier associated with pulmonary stretch by mechanical ventilation. A series of experiments led to the identification of hypoxia-dependent increases in extracellular adenosine production and signaling during acute lung injury17, 50, 83. In the course of these studies, we profiled the response to ventilator induced lung injury in mice with genetic deletions of each of the 4 ARs and found that deletion of the A2BAR gene was specifically associated with reduced survival time and increased pulmonary albumin leakage after injury. In WT mice, treatment with an A2BAR-selective antagonist resulted in enhanced pulmonary inflammation, edema, and attenuated gas exchange, while an A2BAR agonist attenuated VILI. Taken together, these studies reveal a role for A2BAR signaling in attenuating VILI and implicate this receptor as a potential therapeutic target during acute lung injury15.
Conclusions
Pharmacologic studies for the treatment of hypoxia-induced inflammation provide evidence that direct activation of the PHD-HIF pathway can be utilized to treat inflammatory conditions. Alternatively, HIF-target genes can be targeted directly for the treatment of inflammatory conditions that are characterized by tissue hypoxia. At present, these therapeutic studies are carried out in animal models and have yet to be translated from bench to bedside. As many instances of organ failure in surgical patients are characterized by hypoxia-induced inflammation, these strategies are particularly promising for improving outcomes in surgical patients. We hope that in the course of the next decade, at least some of these treatment approaches can be introduced into the clinical practice of perioperative and critical care medicine.
Key points
Tissue hypoxia can lead to inflammation. Tissue inflammation can lead to hypoxia.
The key transcription factors for hypoxia adaptation is the factor hypoxia-inducible factor (HIF)
Prolylhydroxylases (PHD) and adenosine signaling are two major pathways that interact with HIF signaling events and change the outcome of ischemia-reperfusion injury in an experimental setting
Drugs that interact with these pathways are in the transition from bench to bedside
Acknowledgement
The present review is supported by U.S. National Institutes of Health grant R01-HL0921, R01-DK083385 and R01HL098294 to HKE, K08 HL102267 to TE and German National Science Foundation (DFG) grant to MK.
Footnotes
Conflict of Interest Statement None of the authors has a conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- **1.Eltzschig HK, Carmeliet P. Hypoxia and inflammation. N Engl J Med. 2011;364:656–65. doi: 10.1056/NEJMra0910283. [DOI] [PMC free article] [PubMed] [Google Scholar]; This comprehensive review article summerizes the molelcular mechanism of the interdependence between hypoxia and inflammation. It furthermore explains in detail how HIF is regulated and how this regulation might alter the course of disease in various pathophysioloigcal settings.
- 2.Eltzschig HK. Adenosine: an old drug newly discovered. Anesthesiology. 2009;111:904–15. doi: 10.1097/ALN.0b013e3181b060f2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Eltzschig HK. Targeting Hypoxia-induced Inflammation. Anesthesiology. 2011;114:239–42. doi: 10.1097/ALN.0b013e3182070c66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Andrade CF, Kaneda H, Der S, et al. Toll-like receptor and cytokine gene expression in the early phase of human lung transplantation. J Heart Lung Transplant. 2006;25:1317–23. doi: 10.1016/j.healun.2006.09.017. [DOI] [PubMed] [Google Scholar]
- 5.De Perrot M, Sekine Y, Fischer S, et al. Interleukin-8 release during early reperfusion predicts graft function in human lung transplantation. Am J Respir Crit Care Med. 2002;165:211–5. doi: 10.1164/ajrccm.165.2.2011151. [DOI] [PubMed] [Google Scholar]
- 6.Del Rizzo DF, Menkis AH, Pflugfelder PW, et al. The role of donor age and ischemic time on survival following orthotopic heart transplantation. J Heart Lung Transplant. 1999;18:310–9. doi: 10.1016/s1053-2498(98)00059-x. [DOI] [PubMed] [Google Scholar]
- 7.Thabut G, Mal H, Cerrina J, et al. Graft ischemic time and outcome of lung transplantation: a multicenter analysis. Am J Respir Crit Care Med. 2005;171:786–91. doi: 10.1164/rccm.200409-1248OC. [DOI] [PubMed] [Google Scholar]
- 8.Eckle T, Kohler D, Lehmann R, et al. Hypoxia-Inducible Factor-1 Is Central to Cardioprotection: A New Paradigm for Ischemic Preconditioning. Circulation. 2008;118:166–175. doi: 10.1161/CIRCULATIONAHA.107.758516. [DOI] [PubMed] [Google Scholar]
- 9.Bauerle JD, Grenz A, Kim JH, et al. Adenosine generation and signaling during acute kidney injury. J Am Soc Nephrol. 2011;22:14–20. doi: 10.1681/ASN.2009121217. [DOI] [PubMed] [Google Scholar]
- 10.Grenz A, Dalton HJ, Bauerle J, et al. Partial netrin-1 deficiency aggravates acute kidney injury. PLoS One. 2011 doi: 10.1371/journal.pone.0014812. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Grenz A, Eckle T, Zhang H, et al. Use of a hanging-weight system for isolated renal artery occlusion during ischemic preconditioning in mice. Am J Physiol Renal Physiol. 2007;292:F475–F485. doi: 10.1152/ajprenal.00275.2006. [DOI] [PubMed] [Google Scholar]
- 12.Grenz A, Osswald H, Eckle T, et al. The Reno-Vascular A2B Adenosine Receptor Protects the Kidney from Ischemia. PLoS Medicine. 2008;5:e137. doi: 10.1371/journal.pmed.0050137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Grenz A, Zhang H, Eckle T, et al. Protective role of ecto-5'-nucleotidase (CD73) in renal ischemia. J Am Soc Nephrol. 2007;18:833–45. doi: 10.1681/ASN.2006101141. [DOI] [PubMed] [Google Scholar]
- 14.Grenz A, Zhang H, Weingart J, et al. Lack of effect of extracellular adenosine generation and signalling on renal erythropoietin secretion during hypoxia. Am J Physiol Renal Physiol. 2007:00243. doi: 10.1152/ajprenal.00243.2007. 2007. [DOI] [PubMed] [Google Scholar]
- 15.Eckle T, Grenz A, Laucher S, et al. A2B adenosine receptor signaling attenuates acute lung injury by enhancing alveolar fluid clearance in mice. J Clin Invest. 2008;118:3301–3315. doi: 10.1172/JCI34203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Eckle T, Faigle M, Grenz A, et al. A2B adenosine receptor dampens hypoxia-induced vascular leak. Blood. 2008;111:2024–35. doi: 10.1182/blood-2007-10-117044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Eckle T, Fullbier L, Wehrmann M, et al. Identification of ectonucleotidases CD39 and CD73 in innate protection during acute lung injury. J Immunol. 2007;178:8127–37. doi: 10.4049/jimmunol.178.12.8127. [DOI] [PubMed] [Google Scholar]
- 18.Eckle T, Koeppen M, Eltzschig HK. Role of extracellular adenosine in acute lung injury. Physiology (Bethesda) 2009;24:298–306. doi: 10.1152/physiol.00022.2009. [DOI] [PubMed] [Google Scholar]
- 19.Hartmann G, Tschop M, Fischer R, et al. High altitude increases circulating interleukin-6, interleukin-1 receptor antagonist and C-reactive protein. Cytokine. 2000;12:246–52. doi: 10.1006/cyto.1999.0533. [DOI] [PubMed] [Google Scholar]
- 20.Rosenberger P, Schwab JM, Mirakaj V, et al. Hypoxia-inducible factor-dependent induction of netrin-1 dampens inflammation caused by hypoxia. Nat Immunol. 2009;10:195–202. doi: 10.1038/ni.1683. [DOI] [PubMed] [Google Scholar]
- 21.Eltzschig HK, Abdulla P, Hoffman E, et al. HIF-1-dependent repression of equilibrative nucleoside transporter (ENT) in hypoxia. J. Exp. Med. 2005;202:1493–1505. doi: 10.1084/jem.20050177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Eltzschig HK, Ibla JC, Furuta GT, et al. Coordinated adenine nucleotide phosphohydrolysis and nucleoside signaling in posthypoxic endothelium: role of ectonucleotidases and adenosine A2B receptors. J Exp Med. 2003;198:783–96. doi: 10.1084/jem.20030891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Eltzschig HK, Thompson LF, Karhausen J, et al. Endogenous adenosine produced during hypoxia attenuates neutrophil accumulation: coordination by extracellular nucleotide metabolism. Blood. 2004;104:3986–3992. doi: 10.1182/blood-2004-06-2066. [DOI] [PubMed] [Google Scholar]
- 24.Kruger B, Krick S, Dhillon N, et al. Donor Toll-like receptor 4 contributes to ischemia and reperfusion injury following human kidney transplantation. Proc Natl Acad Sci U S A. 2009;106:3390–5. doi: 10.1073/pnas.0810169106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kim M, Park SW, Pitson SM, et al. Isoflurane protects human kidney proximal tubule cells against necrosis via sphingosine kinase and sphingosine-1-phosphate generation. Am J Nephrol. 2010;31:353–62. doi: 10.1159/000298339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Park SW, Kim M, Brown KM, et al. Paneth cell-derived IL-17A causes multi-organ dysfunction after hepatic ischemia and reperfusion injury. Hepatology. 2011 doi: 10.1002/hep.24253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hart M, Jacobi B, Schittenhelm J, et al. A2B Adenosine Receptor Signaling Provides Potent Protection during Intestinal Ischemia/Reperfusion Injury. J Immunol. 2009 doi: 10.4049/jimmunol.0802193. in press. [DOI] [PubMed] [Google Scholar]
- 28.Hart ML, Gorzolla IC, Schittenhelm J, et al. SP1-dependent induction of CD39 facilitates hepatic ischemic preconditioning. J Immunol. 2010;184:4017–24. doi: 10.4049/jimmunol.0901851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *29.Hart ML, Grenz A, Gorzolla IC, et al. Hypoxia-Inducible Factor-1alpha-Dependent Protection from Intestinal Ischemia/Reperfusion Injury Involves Ecto-5'-Nucleotidase (CD73) and the A2B Adenosine Receptor. J Immunol. 2011 doi: 10.4049/jimmunol.0903617. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]; This study demonstrated the dramatic protective properties of HIF1alpha in the setting of ischemia repefusion injury through enhancing the adenosine signalling pathway.
- 30.Hart ML, Henn M, Kohler D, et al. Role of extracellular nucleotide phosphohydrolysis in intestinal ischemia-reperfusion injury. FASEB J. 2008;22:2784–97. doi: 10.1096/fj.07-103911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hart ML, Jacobi B, Schittenhelm J, et al. Cutting Edge: A2B Adenosine receptor signaling provides potent protection during intestinal ischemia/reperfusion injury. J Immunol. 2009;182:3965–8. doi: 10.4049/jimmunol.0802193. [DOI] [PubMed] [Google Scholar]
- 32.Hart ML, Much C, Gorzolla IC, et al. Extracellular adenosine production by ecto-5'-nucleotidase protects during murine hepatic ischemic preconditioning. Gastroenterology. 2008;135:1739–1750. e3. doi: 10.1053/j.gastro.2008.07.064. [DOI] [PubMed] [Google Scholar]
- 33.Giatromanolaki A, Sivridis E, Maltezos E, et al. Hypoxia inducible factor 1alpha and 2alpha overexpression in inflammatory bowel disease. J Clin Pathol. 2003;56:209–13. doi: 10.1136/jcp.56.3.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **34.Colgan SP, Taylor CT. Hypoxia: an alarm signal during intestinal inflammation. Nat Rev Gastroenterol Hepatol. 2010;7:281–7. doi: 10.1038/nrgastro.2010.39. [DOI] [PMC free article] [PubMed] [Google Scholar]; In this review the author discuss how inflammatory conditions in the intestinal tract can lead to tissue hypoxia and how this might evolve as a therapeutic target in the future.
- 35.Taylor CT, Colgan SP. Hypoxia and gastrointestinal disease. J Mol Med. 2007;85:1295–300. doi: 10.1007/s00109-007-0277-z. [DOI] [PubMed] [Google Scholar]
- 36.Taylor CT. Interdependent roles for hypoxia inducible factor and nuclear factor-kappaB in hypoxic inflammation. J Physiol. 2008;586:4055–9. doi: 10.1113/jphysiol.2008.157669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Eltzschig HK, Eckle T, Mager A, et al. ATP release from activated neutrophils occurs via connexin 43 and modulates adenosine-dependent endothelial cell function. Circ Res. 2006;99:1100–8. doi: 10.1161/01.RES.0000250174.31269.70. [DOI] [PubMed] [Google Scholar]
- 38.Eltzschig HK, Macmanus CF, Colgan SP. Neutrophils as Sources of Extracellular Nucleotides: Functional Consequences at the Vascular Interface. Trends Cardiovasc Med. 2008;18:103–107. doi: 10.1016/j.tcm.2008.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Faigle M, Seessle J, Zug S, et al. ATP release from vascular endothelia occurs across Cx43 hemichannels and is attenuated during hypoxia. PLoS ONE. 2008;3:e2801. doi: 10.1371/journal.pone.0002801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kohler D, Eckle T, Faigle M, et al. CD39/ectonucleoside triphosphate diphosphohydrolase 1 provides myocardial protection during cardiac ischemia/reperfusion injury. Circulation. 2007;116:1784–94. doi: 10.1161/CIRCULATIONAHA.107.690180. [DOI] [PubMed] [Google Scholar]
- 41.Robinson A, Keely S, Karhausen J, et al. Mucosal protection by hypoxia-inducible factor prolyl hydroxylase inhibition. Gastroenterology. 2008;134:145–55. doi: 10.1053/j.gastro.2007.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cummins EP, Seeballuck F, Keely SJ, et al. The hydroxylase inhibitor dimethyloxalylglycine is protective in a murine model of colitis. Gastroenterology. 2008;134:156–65. doi: 10.1053/j.gastro.2007.10.012. [DOI] [PubMed] [Google Scholar]
- *43.Aherne CM, Kewley EM, Eltzschig HK. The resurgence of A2B adenosine receptor signaling. Biochim Biophys Acta. 2010 doi: 10.1016/j.bbamem.2010.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]; Here the pathophysiological role of the A2B adenosine receptor in different setting is reviewed in detail
- 44.Eckle T, Fullbier L, Grenz A, et al. Usefulness of pressure-controlled ventilation at high inspiratory pressures to induce acute lung injury in mice. Am J Physiol Lung Cell Mol Physiol. 2008;295:L718–24. doi: 10.1152/ajplung.90298.2008. [DOI] [PubMed] [Google Scholar]
- 45.Eltzschig HK, Faigle M, Knapp S, et al. Endothelial catabolism of extracellular adenosine during hypoxia: the role of surface adenosine deaminase and CD26. Blood. 2006;108:1602–10. doi: 10.1182/blood-2006-02-001016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kong T, Westerman KA, Faigle M, et al. HIF-dependent induction of adenosine A2B receptor in hypoxia. Faseb J. 2006;20:2242–50. doi: 10.1096/fj.06-6419com. [DOI] [PubMed] [Google Scholar]
- 47.Loffler M, Morote-Garcia JC, Eltzschig SA, et al. Physiological roles of vascular nucleoside transporters. Arterioscler Thromb Vasc Biol. 2007;27:1004–13. doi: 10.1161/ATVBAHA.106.126714. [DOI] [PubMed] [Google Scholar]
- 48.Morote-Garcia JC, Rosenberger P, Kuhlicke J, et al. HIF-1-dependent repression of adenosine kinase attenuates hypoxia-induced vascular leak. Blood. 2008;111:5571–80. doi: 10.1182/blood-2007-11-126763. [DOI] [PubMed] [Google Scholar]
- 49.Morote-Garcia JC, Rosenberger P, Nivillac NM, et al. Hypoxia-inducible factor-dependent repression of equilibrative nucleoside transporter 2 attenuates mucosal inflammation during intestinal hypoxia. Gastroenterology. 2009;136:607–18. doi: 10.1053/j.gastro.2008.10.037. [DOI] [PubMed] [Google Scholar]
- 50.Reutershan J, Vollmer I, Stark S, et al. Adenosine and inflammation: CD39 and CD73 are critical mediators in LPS-induced PMN trafficking into the lungs. FASEB J. 2009;23:473–82. doi: 10.1096/fj.08-119701. [DOI] [PubMed] [Google Scholar]
- 51.Semenza GL, Roth PH, Fang HM, et al. Transcriptional regulation of genes encoding glycolytic enzymes by hypoxia-inducible factor 1. J Biol Chem. 1994;269:23757–63. [PubMed] [Google Scholar]
- 52.Wang G, Jiang B, Rue E, et al. Hypoxia-Inducible Factor 1 is a Basic-Helix-Loop-Helix-PAS Heterodimer Regulated by Cellular O2 Tension. PNAS. 1995;92:5510–5514. doi: 10.1073/pnas.92.12.5510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Semenza GL. Life with oxygen. Science. 2007;318:62–4. doi: 10.1126/science.1147949. [DOI] [PubMed] [Google Scholar]
- 54.Kong T, Eltzschig HK, Karhausen J, et al. Leukocyte adhesion during hypoxia is mediated by HIF-1-dependent induction of {beta}2 integrin gene expression. PNAS. 2004;101:10440–10445. doi: 10.1073/pnas.0401339101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Synnestvedt K, Furuta GT, Comerford KM, et al. Ecto-5'-nucleotidase (CD73) regulation by hypoxia-inducible factor-1 mediates permeability changes in intestinal epithelia. J Clin Invest. 2002;110:993–1002. doi: 10.1172/JCI15337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kuhlicke J, Frick JS, Morote-Garcia JC, et al. Hypoxia Inducible Factor (HIF)-1 Coordinates Induction of Toll-Like Receptors TLR2 and TLR6 during Hypoxia. PLoS ONE. 2007;2:e1364. doi: 10.1371/journal.pone.0001364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zheng W, Kuhlicke J, Jackel K, et al. Hypoxia inducible factor-1 (HIF-1)-mediated repression of cystic fibrosis transmembrane conductance regulator (CFTR) in the intestinal epithelium. FASEB J. 2009;23:204–13. doi: 10.1096/fj.08-110221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wenger RH. Cellular adaptation to hypoxia: O2-sensing protein hydroxylases, hypoxia-inducible transcription factors, and O2-regulated gene expression. FASEB J. 2002;16:1151–62. doi: 10.1096/fj.01-0944rev. [DOI] [PubMed] [Google Scholar]
- 59.Holmquist-Mengelbier L, Fredlund E, Lofstedt T, et al. Recruitment of HIF-1alpha and HIF-2alpha to common target genes is differentially regulated in neuroblastoma: HIF-2alpha promotes an aggressive phenotype. Cancer Cell. 2006;10:413–23. doi: 10.1016/j.ccr.2006.08.026. [DOI] [PubMed] [Google Scholar]
- 60.Schofield CJ, Ratcliffe PJ. Oxygen sensing by HIF hydroxylases. Nat Rev Mol Cell Biol. 2004;5:343–54. doi: 10.1038/nrm1366. [DOI] [PubMed] [Google Scholar]
- 61.Rankin EB, Biju MP, Liu Q, et al. Hypoxia-inducible factor-2 (HIF-2) regulates hepatic erythropoietin in vivo. J Clin Invest. 2007;117:1068–77. doi: 10.1172/JCI30117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ratcliffe PJ. HIF-1 and HIF-2: working alone or together in hypoxia? J Clin Invest. 2007;117:862–5. doi: 10.1172/JCI31750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hu CJ, Wang LY, Chodosh LA, et al. Differential roles of hypoxia-inducible factor 1alpha (HIF-1alpha) and HIF-2alpha in hypoxic gene regulation. Mol Cell Biol. 2003;23:9361–74. doi: 10.1128/MCB.23.24.9361-9374.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Brusselmans K, Compernolle V, Tjwa M, et al. Heterozygous deficiency of hypoxia-inducible factor-2{alpha} protects mice against pulmonary hypertension and right ventricular dysfunction during prolonged hypoxia. J. Clin. Invest. 2003;111:1519–1527. doi: 10.1172/JCI15496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Compernolle V, Brusselmans K, Acker T, et al. Loss of HIF-2alpha and inhibition of VEGF impair fetal lung maturation, whereas treatment with VEGF prevents fatal respiratory distress in premature mice. Nat Med. 2002;8:702–10. doi: 10.1038/nm721. [DOI] [PubMed] [Google Scholar]
- 66.Kaelin WG. Proline hydroxylation and gene expression. Annu Rev Biochem. 2005;74:115–28. doi: 10.1146/annurev.biochem.74.082803.133142. [DOI] [PubMed] [Google Scholar]
- 67.Kaelin WG, Jr., Ratcliffe PJ. Oxygen sensing by metazoans: the central role of the HIF hydroxylase pathway. Mol Cell. 2008;30:393–402. doi: 10.1016/j.molcel.2008.04.009. [DOI] [PubMed] [Google Scholar]
- 68.Aragones J, Fraisl P, Baes M, et al. Oxygen Sensors at the Crossroad of Metabolism. Cell Metab. 2009;9:11–22. doi: 10.1016/j.cmet.2008.10.001. [DOI] [PubMed] [Google Scholar]
- 69.Fraisl P, Aragones J, Carmeliet P. Inhibition of oxygen sensors as a therapeutic strategy for ischaemic and inflammatory disease. Nat Rev Drug Discov. 2009;8:139–52. doi: 10.1038/nrd2761. [DOI] [PubMed] [Google Scholar]
- 70.Kaelin WG. Von Hippel-Lindau disease. Annu Rev Pathol. 2007;2:145–73. doi: 10.1146/annurev.pathol.2.010506.092049. [DOI] [PubMed] [Google Scholar]
- 71.Kaelin WG., Jr. The von Hippel-Lindau tumour suppressor protein: O2 sensing and cancer. Nat Rev Cancer. 2008;8:865–73. doi: 10.1038/nrc2502. [DOI] [PubMed] [Google Scholar]
- 72.Dayan F, Roux D, Brahimi-Horn MC, et al. The oxygen sensor factor-inhibiting hypoxia-inducible factor-1 controls expression of distinct genes through the bifunctional transcriptional character of hypoxia-inducible factor-1alpha. Cancer Res. 2006;66:3688–98. doi: 10.1158/0008-5472.CAN-05-4564. [DOI] [PubMed] [Google Scholar]
- 73.Kong X, Lin Z, Liang D, et al. Histone deacetylase inhibitors induce VHL and ubiquitin-independent proteasomal degradation of hypoxia-inducible factor 1alpha. Mol Cell Biol. 2006;26:2019–28. doi: 10.1128/MCB.26.6.2019-2028.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Karhausen J, Furuta GT, Tomaszewski JE, et al. Epithelial hypoxia-inducible factor-1 is protective in murine experimental colitis. J. Clin. Invest. 2004;114:1098–1106. doi: 10.1172/JCI21086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Eckle T, Grenz A, Kohler D, et al. Systematic evaluation of a novel model for cardiac ischemic preconditioning in mice. Am J Physiol Heart Circ Physiol. 2006;291:H2533–40. doi: 10.1152/ajpheart.00472.2006. [DOI] [PubMed] [Google Scholar]
- 76.Eckle T, Krahn T, Grenz A, et al. Cardioprotection by ecto-5'-nucleotidase (CD73) and A2B adenosine receptors. Circulation. 2007;115:1581–90. doi: 10.1161/CIRCULATIONAHA.106.669697. [DOI] [PubMed] [Google Scholar]
- 77.Grenz A, Homann D, Eltzschig HK. Extracellular Adenosine - a “Safety Signal” that Dampens Hypoxia-Induced Inflammation during Ischemia. Antioxid Redox Signal. 2011 doi: 10.1089/ars.2010.3665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Grenz A, Zhang H, Hermes M, et al. Contribution of E-NTPDase1 (CD39) to renal protection from ischemia-reperfusion injury. FASEB J. 2007;21:2863–73. doi: 10.1096/fj.06-7947com. [DOI] [PubMed] [Google Scholar]
- 79.Chen H, Yang D, Carroll SH, et al. Activation of the macrophage A2b adenosine receptor regulates tumor necrosis factor-alpha levels following vascular injury. Exp Hematol. 2009;37:533–8. doi: 10.1016/j.exphem.2009.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Csoka B, Nemeth ZH, Rosenberger P, et al. A2B adenosine receptors protect against sepsis-induced mortality by dampening excessive inflammation. J Immunol. 2010;185:542–50. doi: 10.4049/jimmunol.0901295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Eltzschig HK, Rivera-Nieves J, Colgan SP. Targeting the A2B adenosine receptor during gastrointestinal ischemia and inflammation. Expert Opin Ther Targets. 2009;13:1267–77. doi: 10.1517/14728220903241666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Frick JS, MacManus CF, Scully M, et al. Contribution of adenosine A2B receptors to inflammatory parameters of experimental colitis. J Immunol. 2009;182:4957–64. doi: 10.4049/jimmunol.0801324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Schingnitz U, Hartmann K, Macmanus CF, et al. Signaling through the A2B adenosine receptor dampens endotoxin-induced acute lung injury. J Immunol. 2010;184:5271–9. doi: 10.4049/jimmunol.0903035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ware LB, Matthay MA. The Acute Respiratory Distress Syndrome. N Engl J Med. 2000;342:1334–1349. doi: 10.1056/NEJM200005043421806. [DOI] [PubMed] [Google Scholar]
- 85.Rubenfeld GD, Caldwell E, Peabody E, et al. Incidence and Outcomes of Acute Lung Injury. N Engl J Med. 2005;353:1685–1693. doi: 10.1056/NEJMoa050333. [DOI] [PubMed] [Google Scholar]
- 86.Licker M, de Perrot M, Spiliopoulos A, et al. Risk factors for acute lung injury after thoracic surgery for lung cancer. Anesth Analg. 2003;97:1558–65. doi: 10.1213/01.ANE.0000087799.85495.8A. [DOI] [PubMed] [Google Scholar]
- 87.Milot J, Perron J, Lacasse Y, et al. Incidence and predictors of ARDS after cardiac surgery. Chest. 2001;119:884–8. doi: 10.1378/chest.119.3.884. [DOI] [PubMed] [Google Scholar]
- 88.Shorr AF, Abbott KC, Agadoa LY. Acute respiratory distress syndrome after kidney transplantation: epidemiology, risk factors, and outcomes. Crit Care Med. 2003;31:1325–30. doi: 10.1097/01.CCM.0000053645.38356.A6. [DOI] [PubMed] [Google Scholar]