Abstract
The interaction between the protozoan parasite Trypanosoma cruzi and the human host dates back 9000 years, as demonstrated by molecular analysis of material obtained from Andean mummies indicating the presence of the parasite’s kinetoplast DNA in populations from Chile and Peru. This long-established interaction, which persists today, demonstrates that T. cruzi has established a very well adapted relationship with the human host. From a host-parasite relationship point-of-view this is desirable, however, such a high degree of adaptation is perhaps the foundation for many of the unknowns that surround this disease. Unveiling of the immunological mechanisms that underlie the establishment of pathology, identification of parasite-associated factors that determine strain-differential tissue tropism, discovery of host genetic elements that influence the development of different clinical forms of the disease, and understanding environmental factors that may influence the host-parasite interactions, are some of the key questions remaining to be answered. The response to these questions will aid in addressing some of the current challenges in Chagas disease: fulfilling the need for efficient diagnosis, developing effective prophylactic measures, discovering effective therapeutics, and finding methods to control disease progression.
Keywords: Chagas disease, pathology, cardiomyopathy, treatment, Trypanosoma cruzi
Acute Chagas disease: the impact of efficient diagnosis on early treatment and prevention of pathology
The contact with contaminated feces/urine of the invertebrate vector during the blood meal is still considered to be the most prevalent form of transmission of Trypanosoma cruzi to the human host. However, in recent years, oral transmission due to ingestion of contaminated fruit juices has been responsible for several outbreaks of acute Chagas disease mostly in Brazil, but also in other countries in South America e.g., Venezuela [Alarcon de Noya et al. 2010; Benchimol-Barbosa 2010; Steindel et al. 2008]. Special concern has been raised with regards to human infection via blood transfusion and organ transplantation since these routes break the boundaries of the endemic countries, potentially spreading the disease worldwide [Bern et al. 2008].
After the initial infection by the trypomastigote form of T. cruzi, patients enter an acute phase, with intense parasite replication, in which T. cruzi can infect a wide range of cell populations. Diagnosis can be made through direct evidence of parasite on fresh blood samples or by indirect methods such as xenodiagnosis or hemoculture [Chiari 1999; Dubner et al. 2008]. Classic serological tests may be negative during this phase, although anti-parasite IgM and IgG detection is possible [Antas et al. 1999]. While the above-mentioned tests are currently used, they present several pitfalls: they require trained personnel to be performed and interpreted, cannot be performed in the field and, due to cross-reactivity with other trypanosomatids (in the case of serological tests), additional confirmatory tests are necessary. These facts delay the detection of infection, which, in turn, may delay the administration of therapeutic measures. The detection of the acute phase of Chagas disease is particularly important since the great majority of patients that receive treatment in this phase are cured. Thus, the search for inexpensive, efficient and specific tests for early diagnosis of T. cruzi infection is still an urgent need.
It is not always possible to determine the exact route of T. cruzi infection in most patients in endemic countries. While infections due to blood transfusion and transplantation are easier to be identified, vectorial and oral infections are not easily distinguishable due to overlapping possibilities. Determination of the route of infection is not only important from the epidemiological point of view, but also may be helpful in clinical management. Recent studies have shown that individuals infected orally have a highly symptomatic acute phase [Bastos et al. 2010], suggesting that the symptoms in patients infected orally are much more severe than the ones described in earlier studies of patients infected via contact of contaminated vector feces/urine. It is not clear whether the inoculum or parasite strains are related to these apparent differences in severity of acute phase. However, as early events may influence disease progression, determining the factors that lead to a severe acute phase is another important problem that needs to be resolved. Interestingly, despite presenting with more severe manifestations, individuals infected orally by T. cruzi respond satisfactory to the treatment, although it has been reported that 3 out of 11 patients infected orally who were treated and cured, displayed eletrocardiographic alterations four years after treatment, possibly as sequela of the severe acute phase [Pinto et al. 2009]. Other studies have also shown that approximately 10–20% of acute patients, especially children, can develop acute myocarditis of variable degrees, and/or meningoencephalitis. Occasionally, acute cardiomyopathy caused by severe parasite-induced inflammation, may lead to death (observed in about 5%) [Morris et al. 1990]. Acute myocarditis is also observed in patients who present recurrence of acute phase, especially in those immunocompromised either due to immunodeficiency such as HIV infection or induced immunosuppression [Marin-Neto et al. 1998]. Thus, detection of the acute phase, either primary or recurrent, is critical for eliminating the parasite and also for preventing the maintenance of the acute cardiomyopathy, which may last even after effective treatment and may progress to more severe cardiac disease.
Conventional Chagas disease treatment: balancing parasite elimination versus side effects
Conventional treatment of Trypanosoma cruzi infection consists of pathogen elimination to decrease the chances of developing the illness and to interrupt the chain of parasite transmission [Sosa-Estani et al. 2009]. Treatment for Chagas disease is recommended for all people diagnosed with an acute infection, cases of congenital infection, immunosupressed patients, and to all children with chronic infection [WHO report]. Adults with chronic infection may also benefit from treatment, although there is some controversy regarding administration of treatment to symptomatic patients [Coura 2009]. While some studies have shown the benefit of treating chronic patients [Viotti and Vigliano 2007; Viotti et al. 2006], the potential benefits of medication in preventing or delaying the development of Chagas disease should be weighed against the long duration of treatment (up to 2 months) and the possible adverse reactions that occur in up to 40% of treated patients. The contraindications for specific treatment are: pregnancy, liver failure, kidney failure, neurological diseases unrelated to Chagas disease, advanced Chagas disease cardiomyopathy and other diseases that might be worsened by this treatment [Marin-Neto et al. 2009]. While these aspects have to be considered to determine treatment administration, some studies have shown that treatment during the chronic phase decreases (or halts) pathology, suggesting that it should be administered to all patients, regardless of disease stage. Controversy still persists and it is important to determine what is more detrimental to the patient: the severe side effects associated with the anti-Trypanosoma drugs or the persistence of the parasite. While the establishment of standard procedures is critical for general clinical management, in some instances it is important to evaluate the pros and cons on a case-by-case basis. Treatment of T. cruzi infection seems to be one of these situations. Two anti-trypanosomacide drugs are commonly indicated for Chagas disease treatment: benznidazole and nifurtimox.
Benznidazole (N-benzyl-2-nitroimidazole-1-acetamide) acts against T. cruzi through nitro-reduction, leading to protein synthesis inhibition and degradation of macromolecule biosynthesis [Apt 2010; Munoz et al. 2011]. This drug was released in 1966 by Hoffman-La Roche and is still largely utilized. The efficacy and tolerance of benznidazole are inversely related to the age of the patient, while its side effects are more frequent in elderly patients [Viotti et al. 2009]. The recommended benznidazole dose for children and adults is 5–10 mg/kg and 5 mg/kg daily, respectively. Treatment duration is 60 days (Brazilian Health Ministry).
Nifurtimox is a 5-nitrofuran derivative, yjre mechanism of action of which consists of enhancing the production of toxic oxygen metabolites by the parasite: increasing oxygen consumption and H2O2/superoxide radical production [Maya et al. 2007]. Although nifurtimox changes parasite metabolism, some strains are resistant to it, due to lower drug intake and elimination abilities. This resistance causes the differential efficacy of nifurtimox observed among patients infected with different T. cruzi strains [Apt 2010]. The most common nirfutimox treatment regimen is a 60–120 day course of 10 mg/kg/day [Coura 2009]. Bayer first released this drug in 1970, but its production was discontinued, due to low efficacy and toxicity [Boiani et al. 2010].
Cure is variable amongst different groups of patients: while approximately 80% can be achieved in acute patients, 8% of cure was observed in chronic adults, after an 18-year follow up [Cancado 2002]. However, treatment of children at the chronic indeterminate phase of disease led to 62% cure, as determined by serology [Sosa Estani et al. 1998]. Thus, treatment, while efficient in some cases, is still far from being ideal. Moreover, new and more efficient strategies for monitoring treatment are necessary, to allow for accurate determination of drug efficacy. Important challenges are associated with the treatment using the currently available drugs: they produce serious collateral effects that can lead to treatment discontinuation, some strains of T. cruzi have developed resistance to the drugs, specific formulations for children are still a need. Another point of concern is the fact that neither nifurtimox nor benznidazole are appropriate for using during pregnancy. Sosa-Estani et al., [2009} suggest the treatment of young infected women in reproductive age (requiring contraceptive practices during the treatment) as an alternative and highly effective strategy for preventing congenital transmission of Trypanosoma cruzi [Sosa-Estani et al. 2009]. Thus, while there is current medication available for the treatment of Chagas disease, many important aspects still need to be addressed.
New drugs for treating Chagas disease: new hopes in need of action
Due to the above-mentioned problems with the currently available drugs, other anti-Trypanosoma compounds have been tested in order to find better options to treat the illness. Although in a clearly underfunded area of research, many promising candidates have arisen. Some alternative drugs, their associated results and mechanisms of action are summarized in Table 1 and discussed below.
Table 1.
Tested alternative drugs to Chagas disease treatment
| Alternative treatment |
Class/Subclass | Observed results | Mechanism of action | Other comments | References |
|---|---|---|---|---|---|
| Ketaconazole Itraconazole |
Inhibitors of ergostherol synthesis/C14 lanosterol demethylase (CYP51) | Presents suppressive but not curative effects against T. cruzi infections in humans or experimental animals and are unable stop the progression of the disease. | Inhibition of cytochrome P450-dependent lanosterol C14 demethylase, thus reducing ergosterol synthesis. | Itraconazole has been administered to indeterminate and cardiac Chagas disease patients based on the results in experimental investigations. |
Urbina, 2002 Urbina & Docampo 2003 McCabe et al.,1986 Apt, 2010 |
|
Posaconazole |
Inhibitors of ergostherol synthesis/C14 lanosterol demethylase (CYP51) | Induces parasitological cure in murine models of acute and chronic T. cruzi infection. | Inhibition of cytochrome P450 dependent lanosterol C14 demethylase, thus reducing ergosterol synthesis. | It was shown to eliminate intracellular amastigotes from infected cardiac cells. | Urbina et al. 1996 Urbina 2002 Urbina & Docampo 2003 Silva et al. 2006 |
| Tak-187 UR-9825 Ravuconazole |
Inhibitors of ergostherol synthesis/C14 Lanosterol demethylase (CYP51) |
Exhibit trypanocidal activity both in vivo and in vitro. Present activity against T. cruzi strains partially resistant to conventional drugs and in which ketaconazole does not work. |
Inhibition of sterol biosynthesis, which is essential for parasite viability and proliferation. |
Recently, success was demonstrated with posaconazole in a patient with chronic Chagas disease and systemic lupus erythematosus. Ravuconazole is a prime candidate for clinical trials with Chagas disease patients |
Urbina, 2003 Pinazo et al., 2010 |
|
E5700 ER-119884 |
Inhibitors of ergostherol synthesis/Squalene synthase |
Are under development for their cholesterol and triglyceride lowering activity and have also shown in vitro activity against T. cruzi. |
Inhibition of sterol biosynthesis, which is essential for parasite viability and proliferation. |
E5700 was able to provide full protection against death and completely arrested development of parasitemia in a murine model of acute disease when orally administrated. |
Urbina, 2009 Urbina et al., 2004 |
|
Amiodarone |
Inhibitors of ergostherol synthesis/lanosterol synthase |
Amiodarone has direct activity against T. cruzi, both in vitro and in vivo. It is an antiarrhythmic, frequently prescribed for the symptomatic treatment of Chagas' disease patients. |
In addition to disrupting the parasites' Ca(2+) homeostasis, it also blocks ergosterol biosynthesis. | The combined use of amiodarone and posaconazole has synergistic effects that lead to an improvement in the parasitological burden in patients treated with both drugs. |
Urbina, 2009 Benaim et al., 2009 |
|
K777 |
Inhibitor of cruzipain (a major cysteine protease of T. cruzi) | It has been shown to lower parasitemia levels and also improves cardiac damage in dogs. | Inhibition of vinyl sulfone (K777) cysteine protease. | The drug is now a candidate for clinical trial in partnership with DNDi. |
Barr et al., 2005 McKerrow et al., 2009 |
|
Aryl-imidamine DB766 (AIA) |
Aromatic diamidines (AD) and analogues |
Effectively reduces the parasite load in the blood and cardiac tissue. |
Targets the minor groove of DNA. Localizes in DNA-enriched compartments and induces considerable damage to the parasite mitochondria. | Advantage of being active at a low temperature (4°C), a condition that makes it suitable for the treatment of donated blood suspected of being infected with T. cruzi. |
Batista et al., 2010 |
|
Allopurinol (HPP) |
Analog of hypoxanthine |
HPP inhibits the epimastigote forms in culture. In mice infected with T. cruzi and treated with allopurinol, an important reduction of the parasitemia is obtained, although some parasite strains are resistant to the drug. |
Decreases uric acid and the conversion of hypoxanthine to xanthine. |
In a multinational study, chronic chagasic patients treated with HPP presented no parasitological cure. In some patients, improvement of the electrocardiographic alterations was demonstrated. |
Avila and Avila, 1981 Apt et al., 2005 |
Recently, ergosterol synthesis inhibitors (ESI) have emerged as good candidates against T. cruzi. Ergosterol is a component of fungal and some protists, such as trypanosomes, cell membranes where it plays an essential structural role [Urbina and Docampo 2003]. The ESI can be divided into subclasses, depending on which of the following enzymes they inhibit: C14 lanosterol demethylase (CYP51), lanosterol synthase and squalene synthase. Those enzymes are involved with sterol biosynthesis, which is essential for parasite survival. Ketaconazole and itraconazole are C14 lanosterol demethylase inhibitors, which have suppressive effects against the parasite but their efficacy has not been demonstrated in humans [Urbina 2002; Urbina and Docampo 2003]. Posaconazole, another ESI, induces parasitological cure in murine models of acute and chronic Chagas disease, and is effective against intracellular amastigotes [Silva et al. 2007]. Other ESI such as Tak-187, UR-9825 and ravuconazole exhibited trypanocidal activity in vivo and in vitro [Pinazo et al. 2010]. Interestingly, amiodarone, an antiarrhythmic drug used in chronic Chagas patients with cardiac symptoms, is a lanosterol synthesis inhibitor, which impairs de novo ergosterol biosynthesis. The combined use of amiodarone and posaconazole lead to an improvement in the parasitological burden in patients treated with both drugs [Benaim et al. 2006]. E5700 and ER-119884, squalene synthase inhibitors, developed for their cholesterol and triglyceride lowering activity, have also shown activity against T. cruzi in vitro [Herrera et al. 2009; Urbina et al. 2004]. Based on the obtained results with ESI, their high potency in both acute and chronic infections, better tolerability and safety profiles, these compounds are seen as potential alternatives to Chagas disease treatment. In fact, posaconazole (Merck- Schering) is already part of a Phase II clinical trial that is recruiting participants, and ravuconazole (Eisai) is poised for clinical trials in Chagas disease patients in the near term.
The cruzipain enzymes are parasite cysteine proteases that have been identified as critical to T. cruzi’s ability to invade cells and to evade the host immune response [Cazzulo et al. 1990]. For these reasons, cysteine protease inhibitors have been proposed as potential therapeutics for T. cruzi infection. Studies with K777, a vinyl sulfone protease inhibitor of cruzipain, have shown that this drug is effective in curing or alleviating T. cruzi infection in acute and non-acute models of infection and also ameliorates cardiac damage in dogs [Barr et al. 2005]. K777 has recently entered formal preclinical drug development investigations [McKerrow et al. 2009].
Allopurinol, 4-hydroxypirazole(3,4-d)pyrimidine (HPP), is an analog of hypoxanthine, which decreases uric acid and the conversion of hypoxanthine to xanthine. As T. cruzi is unable to synthesize purines de novo, HPP has been presented as a good candidate to parasite suppression [Apt 2010]. Mice infected with T. cruzi and treated with allopurinol, presented reduction of parasitemia, although some parasite strains are resistant to the drug [Avila and Avila 1981]. Patients presenting acute Chagas disease treated with allopurinol (20–30 mg/day) for 60 days, showed no reduction of parasite burden. However, in patients with chronic Chagas disease, improvement of the electrocardiographic alterations was demonstrated [Apt et al. 1998]. Thus, the efficacy of allopurinol is controversial, depending on the phase of the disease.
Another class of compounds, aromatic diamidines (AD) and their analogues, target the minor groove of DNA and has been tested against pathogenic microorganisms [Hu et al. 2008]. The effect of several AD analogues was demonstrated against T. cruzi, and among them, significant in vitro activity was found for arylimidamides (AIAs), including DB889, DB786, DB702 and DB766 [Silva et al. 2007]. DB766, has marked trypanocidal activity, especially against trypomastigotes and intracellular amastigotes. DB766 also exerts striking effects upon different parasite stocks, including those naturally resistant to benznidazole, displaying higher activity in vitro than the reference drug. Furthermore, DB766 ameliorates electrocardiographic alterations, reduces hepatic and heart lesions induced by the infection, and provides 90–100% protection against mortality, similar to that provided by benznidazole [Batista Dda et al. 2010].
The small sample of drugs tested against T. cruzi and discussed here, represents the efforts of researchers in finding a new compound for Chagas’ disease treatment, which must be effective against the parasite and less toxic to the patients. Interestingly, the majority of the compounds under investigation are compounds previously tested and re-visited now, under a different perspective. While a few candidates have emerged in the past years, much research still needs to be done in a timely fashion, allowing for a faster-moving process of clinical trials and production. These processes require qualified research capacities, close collaboration between endemic countries and high technology centers for drug discovery and, importantly, consistent and appropriate funding.
Chronic phase of Chagas disease: the quest for prognostic markers and means to prevent pathology
If not treated or if treatment fails, the acute phase progresses into the chronic phase of the disease, characterized by a marked decrease of parasite levels in the blood and tissues. Despite this control, it is in this phase that approximately 30% of the individuals develop severe pathology. Most patients (approximately 60–70%), however, do not develop clinical symptoms, and are classified as indeterminate. The indeterminate form of Chagas disease is a long lasting phase, which may persist for 10–30 years or even throughout life. Amongst the 30–40% of individuals who develop the symptomatic forms of the disease, cardiac involvement (cardiac form), digestive involvement (digestive form), or both (cardio-digestive form), can be observed. One clinical hallmark of chronic Chagas disease is a great clinical pleomorphism and individual variability, even in patients within the same clinical form [Rocha et al. 2007].
Patients classified as indeterminate are characterized by a virtual absence of clinical symptoms, as well as normal electrocardiogram (ECG) and chest radiological tests [Rocha et al. 2007]. However, approximately 25% of patients classified as indeterminate by the above-mentioned tests show significant alterations on more sensitive exams such as ergometry, Holter and echocardiogram [Molina et al. 2006; Rocha et al. 2007]. A longitudinal study has shown that approximately 30% of indeterminate patients with normal ECG, but showing early abnormalities of the left ventricular segment on echocardiogram exam, evolved to the cardiac form after a 5 year follow-up [Espinosa et al. 1985]. Given this observation, Rocha et al. [2003] proposed a detailed clinical classification, allowing for stratification into more homogeneous sub-groups of patients. This new classification has impacted disease management, as well as allowed better interpretation of scientific data that seek an understanding of disease progression mechanisms [Dutra et al. 2005]. In this new classification, only patients with no signs of disease, even upon refined exams, are classified as indeterminate. Patients without symptoms, normal ECG and x-ray but with minor alterations in ergometric test, Holter or echocardiogram, are classified as CCC1 (chronic chagasic cardiomyopathy grade 1), indicating that they may be in clinical evolution. One of the most important goals of studying the mechanisms of pathology establishment in Chagas disease is the identification of prognostic markers of disease progression, especially markers that appear early on, at the initial progression phases. Clinical and immunological studies are currently being developed to fulfill this important need.
Cardiomyopathy is the most important symptomatic manifestation of Chagas disease, with great social and economic impact in Brazil and other endemic countries, given its high morbidity and mortality [Prata 2001; Rocha et al. 2003]. The presence of a diffuse inflammatory infiltrate has been correlated with tissue damage, which leads to chronic progressive myocardial fibrosis [Rocha et al. 2003]. Tissue fibrosis triggers structural changes of the cardiac syncitium, with functional impairment [Morris et al. 1990]. Together, these events lead to the three basic syndromes observed in cardiac Chagas disease: heart failure, arrhythmias, and thromboembolism. Chagasic cardiomyopathy can occur with or without heart enlargement (dilated and non-dilated cardiomyopathy, respectively). Non-dilated cardiomyopathy (NDC) comprises damages on the heart intrinsic conductive nervous system, leading to defective generation and conduction of nervous impulse. Prognostic studies have shown that slight alterations on ECG exams can point to further significant cardiac damage which could identify patients with potential to evolve to a more severe cardiomyopathy [Molina et al. 2006; Rassi et al. 2006; Rocha et al. 2003; Rocha et al. 2007]. Complex arrhythmias tend to be correlated with cardiac insufficiency, although it is not uncommon to find patients with advanced arrhythmias but preserved left ventricular ejection fraction (LVEF), which is the main clinical parameter for heart failure [Rassi et al. 2006]. NDC patients may also be stratified into two different groups: patients who develop advanced conduction system disturbances, such as right bundle branch block (RBBB), and those with more severe abnormalities such as RBBB associated with complete atrioventricular (AV) block (CCC3 and CCC4, respectively, as proposed by Rocha and colleagues, 2003). Dilated cardiomyopathy (DC) patients are those with worse prognosis, who have gone through cardiac remodeling process, which involves cardiomyocyte hypertrophy (CCC5, according to Rocha et al., 1993). Therefore, the main clinical aspect of DC is a radiological-based evidence of cardiomegaly, which may be associated or not with left (LVD) and right (RVD) ventricular dysfunction [Morris et al. 1990; Rocha et al. 2007].
In an attempt to identify the most relevant predictors of morbidity in Chagas disease, several clinical parameters were evaluated in a large cohort of Chagas patients [Rassi et al. 2006]. A multivariate statistical analysis pointed to some relevant parameters, and the authors formulated a risk score for each parameter, which could be used for identifying risk groups. Left ventricular dysfunction (LVD) was the main independent predictor of death in Chagas disease. This corroborated an earlier study that showed a 52% of mortality in patients with LVD after 5 years [Mady et al. 1994]. LVD can be accessed by echocardiogram exam, by measurement of left ventricular ejection fraction (LVEF), but also high levels of serum brain natriuretic peptide (BNP) has been correlated with LVD and, therefore, could be useful as an alternative, easy test [Ribeiro et al. 2003]. Evidence of cardiomegaly, based on x-ray results, was another important predictor of death.
Regardless of the clinical manifestation, additional therapeutic intervention needs to be applied to patients with cardiomyopathy, in order to treat other syndromes that may accompany the cardiac abnormal function [Rocha et al. 2007]. One of the most common health alterations associated with Chagas disease cardiomyopathy is hypertension [Gurgel and Almeida 2007] and introduction of anti-hypertensive medication is performed. Usually, the drugs utilized to treat hypertensive cardiac Chagas patients are the same that are used to treat other hypertensive patients. One of these drugs commonly used is captopril. Captopril is an inhibitor of angiotensin-converting enzyme that also has anti-inflammatory properties [Leon et al. 2003]. However, a recent study demonstrated that captopril increases infectivity of human monocytes in vitro, and induces the expression of IL-17 by T cells [Coelho dos Santos et al. 2010]. Thus, clinical management of associated morbidities need to take under consideration the co-existence of Chagas disease-induced cardiomyopathy.
Given that cardiomyopathy is the main cause of death among Chagas disease patients, measures to avoid its establishment and the introduction of adequate clinical management is critical. Thus, early therapeutic intervention in the acute phase, avoiding the establishment of events that may lead to severe heart disease during the chronic phase is a critical point. While the exact mechanisms that lead to the establishment of heart inflammation and subsequent tissue destruction are not completely clear, it is currently accepted that the persistence of the parasite and its antigens, is of unquestionable importance for pathology development [Dutra and Gollob 2008]. Whether solely the anti-parasite response is involved in tissue destruction is another point of discussion in the literature, given that anti-host responses are also associated with the development and maintenance of cardiomyopathy [Dutra and Gollob 2008]. Even under these circumstances, given that the infection with the parasite is the trigger to a possibly deleterious response, which can be amplified over time, the premature elimination of the trigger may be of importance to prevent pathology. Determining what is the earliest point in which this intervention needs to be applied to be fully successful is another challenge related to diagnosis and treatment of Chagas disease. Studies concerning the host’s immune response and clinical aspects during the acute phase of infection are critical for deciphering this enigma.
Concluding remarks
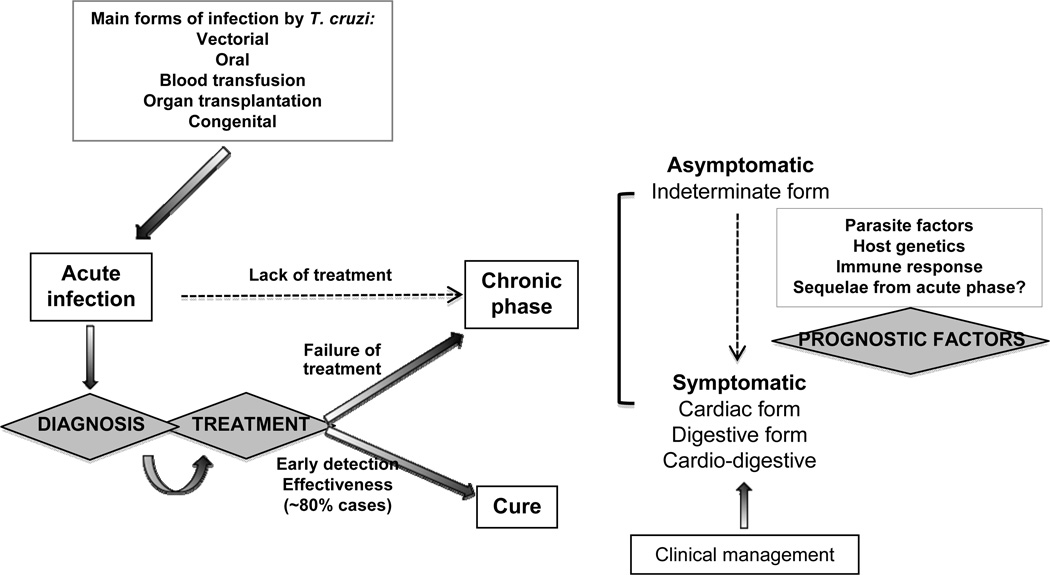
Chagas disease-associated pathology is multifactorial. Parasite-related aspects, such as differential tissue tropism [Vago et al. 2000], as well as host factors, such as genetic susceptibility [Dutra et al. 2009] and characteristics of the immune response [Dutra and Gollob 2008], need to be taken under consideration when designing studies to further our understanding in pathology development. Ideally the elimination of the parasite early in infection would protect the infected individuals from pathology development. However, early detection of infection, via fast, specific and convenient diagnostics, as well as truly efficient therapeutics, with high parasiticidal properties and low collateral effects, are major challenges in combating Chagas disease. Figure 1 shows a chart of these challenges, discussed in our text. Although significant progress has been achieved in many different areas of research related to Chagas disease, much still needs to be learned in order to solidify our intervention strategies, minimizing risk for the patients. Importantly, translational research amongst different areas of expertise needs to be vigorously implemented, to bring benefits from the bench top to the field.
Figure 1.
Regardless of the route of infection, it is critical to detect Trypanosoma cruzi infection early on in order to provide immediate treatment to the patients. It is estimated that treatment efficacy is observed in at least of 80% of the treated acute patients. Lack of detection of the acute phase, or treatment failure lead to disease chronification. During the chronic phase of Chagas disease, most individuals remain in an asymptomatic clinical form, named indeterminate. However, approximately 30% of the patients will develop severe clinical forms of Chagas disease, which often lead to death. The reasons why patients progress from the indeterminate to the symptomatic forms of Chagas disease are not completely understood, although host and parasite factors are involved. The search of prognostic markers of disease progression is a critical aspect for preventing pathology and introducing better clinical measures. The figure highlights three challenges in Chagas disease: better diagnosis, better treatment and discovery of prognostic factors.
Acknowledgements
The authors would like to acknowledge WHO/TDR Program, CNPq, FAPEMIG and NIH/NIAID for continued support of their work. We also present our apologies to the authors of many important papers not cited here due to format limitations and thank all the researchers whose scientific contributions have allowed for great progress towards the treatment and clinical management of Chagas disease.
Funding: WOD and KJG are CNPq Fellows; part of the work cited here, and performed by the authors was funded by CNPq, CAPES, FAPEMIG and the NIH/RO3 A1066044.
References
- Alarcon de Noya B, Diaz-Bello Z, Colmenares C, Ruiz-Guevara R, Mauriello L, Zavala-Jaspe R, Suarez JA, Abate T, Naranjo L, Paiva M. Large urban outbreak of orally acquired acute Chagas disease at a school in Caracas, Venezuela. J Infect Dis. 2010;201(9):1308–1315. doi: 10.1086/651608. others. [DOI] [PubMed] [Google Scholar]
- Antas PR, Medrano-Mercado N, Torrico F, Ugarte-Fernandez R, Gomez F, Correa Oliveira R, Chaves AC, Romanha AJ, Araujo-Jorge TC. Early, intermediate, and late acute stages in Chagas' disease: a study combining anti-galactose IgG, specific serodiagnosis, and polymerase chain reaction analysis. Am J Trop Med Hyg. 1999;61(2):308–314. doi: 10.4269/ajtmh.1999.61.308. [DOI] [PubMed] [Google Scholar]
- Apt W. Current and developing therapeutic agents in the treatment of Chagas disease. Drug Des Devel Ther. 2010;4:243–253. doi: 10.2147/dddt.s8338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apt W, Aguilera X, Arribada A, Perez C, Miranda C, Sanchez G, Zulantay I, Cortes P, Rodriguez J, Juri D. Treatment of chronic Chagas' disease with itraconazole and allopurinol. Am J Trop Med Hyg. 1998;59(1):133–138. doi: 10.4269/ajtmh.1998.59.133. [DOI] [PubMed] [Google Scholar]
- Avila JL, Avila A. Trypanosoma cruzi: allopurinol in the treatment of mice with experimental acute Chagas disease. Exp Parasitol. 1981;51(2):204–208. doi: 10.1016/0014-4894(81)90109-0. [DOI] [PubMed] [Google Scholar]
- Barr SC, Warner KL, Kornreic BG, Piscitelli J, Wolfe A, Benet L, McKerrow JH. A cysteine protease inhibitor protects dogs from cardiac damage during infection by Trypanosoma cruzi. Antimicrob Agents Chemother. 2005;49(12):5160–5161. doi: 10.1128/AAC.49.12.5160-5161.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bastos CJ, Aras R, Mota G, Reis F, Dias JP, de Jesus RS, Freire MS, de Araujo EG, Prazeres J, Grassi MF. Clinical outcomes of thirteen patients with acute chagas disease acquired through oral transmission from two urban outbreaks in northeastern Brazil. PLoS Negl Trop Dis. 2010;4(6):e711. doi: 10.1371/journal.pntd.0000711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batista Dda G, Batista MM, de Oliveira GM, do Amaral PB, Lannes-Vieira J, Britto CC, Junqueira A, Lima MM, Romanha AJ, Sales Junior PA. Arylimidamide DB766, a potential chemotherapeutic candidate for Chagas' disease treatment. Antimicrob Agents Chemother. 2010;54(7):2940–2952. doi: 10.1128/AAC.01617-09. others. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benaim G, Sanders JM, Garcia-Marchan Y, Colina C, Lira R, Caldera AR, Payares G, Sanoja C, Burgos JM, Leon-Rossell A. Amiodarone has intrinsic anti-Trypanosoma cruzi activity and acts synergistically with posaconazole. J Med Chem. 2006;49(3):892–899. doi: 10.1021/jm050691f. others. [DOI] [PubMed] [Google Scholar]
- Benchimol-Barbosa PR. Trends on acute Chagas' disease transmitted by oral route in Brazil: steady increase in new cases and a concealed residual fluctuation. Int J Cardiol. 2010;145(3):494–496. doi: 10.1016/j.ijcard.2009.08.030. [DOI] [PubMed] [Google Scholar]
- Bern C, Montgomery SP, Katz L, Caglioti S, Stramer SL. Chagas disease and the US blood supply. Curr Opin Infect Dis. 2008;21(5):476–482. doi: 10.1097/QCO.0b013e32830ef5b6. [DOI] [PubMed] [Google Scholar]
- Boiani M, Piacenza L, Hernandez P, Boiani L, Cerecetto H, Gonzalez M, Denicola A. Mode of action of nifurtimox and N-oxide-containing heterocycles against Trypanosoma cruzi: is oxidative stress involved? Biochem Pharmacol. 2010;79(12):1736–1745. doi: 10.1016/j.bcp.2010.02.009. [DOI] [PubMed] [Google Scholar]
- Cancado JR. Long term evaluation of etiological treatment of chagas disease with benznidazole. Rev Inst Med Trop Sao Paulo. 2002;44(1):29–37. [PubMed] [Google Scholar]
- Cazzulo JJ, Cazzulo Franke MC, Martinez J, Franke de Cazzulo BM. Some kinetic properties of a cysteine proteinase (cruzipain) from Trypanosoma cruzi. Biochim Biophys Acta. 1990;1037(2):186–191. doi: 10.1016/0167-4838(90)90166-d. [DOI] [PubMed] [Google Scholar]
- Chiari E. Chagas disease diagnosis using polymerase chain reaction, hemoculture and serologic methods. Mem Inst Oswaldo Cruz. 1999;94 Suppl 1:299–300. doi: 10.1590/s0074-02761999000700054. [DOI] [PubMed] [Google Scholar]
- Coelho dos Santos JS, Menezes CA, Villani FN, Magalhaes LM, Scharfstein J, Gollob KJ, Dutra WO. Captopril increases the intensity of monocyte infection by Trypanosoma cruzi and induces human T helper type 17 cells. Clin Exp Immunol. 2010;162(3):528–536. doi: 10.1111/j.1365-2249.2010.04270.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coura JR. Present situation and new strategies for Chagas disease chemotherapy: a proposal. Mem Inst Oswaldo Cruz. 2009;104(4):549–554. doi: 10.1590/s0074-02762009000400002. [DOI] [PubMed] [Google Scholar]
- Dubner S, Schapachnik E, Riera AR, Valero E. Chagas disease: state-of-the-art of diagnosis and management. Cardiol J. 2008;15(6):493–504. [PubMed] [Google Scholar]
- Dutra WO, Gollob KJ. Current concepts in immunoregulation and pathology of human Chagas disease. Curr Opin Infect Dis. 2008;21(3):287–292. doi: 10.1097/QCO.0b013e3282f88b80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutra WO, Menezes CA, Villani FN, da Costa GC, da Silveira AB, Reis D, Gollob KJ. Cellular and genetic mechanisms involved in the generation of protective and pathogenic immune responses in human Chagas disease. Mem Inst Oswaldo Cruz. 2009;104 Suppl 1:208–218. doi: 10.1590/s0074-02762009000900027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutra WO, Rocha MO, Teixeira MM. The clinical immunology of human Chagas disease. Trends Parasitol. 2005;21(12):581–587. doi: 10.1016/j.pt.2005.09.007. [DOI] [PubMed] [Google Scholar]
- Espinosa R, Carrasco HA, Belandria F, Fuenmayor AM, Molina C, Gonzalez R, Martinez O. Life expectancy analysis in patients with Chagas' disease: prognosis after one decade (1973–1983) Int J Cardiol. 1985;8(1):45–56. doi: 10.1016/0167-5273(85)90262-1. [DOI] [PubMed] [Google Scholar]
- Gurgel CB, Almeida EA. Frequency of hypertension in patients with chronic Chagas disease and its consequences on the heart: a clinical and pathological study. Arq Bras Cardiol. 2007;89(3):174–182. 191–200. doi: 10.1590/s0066-782x2007001500008. [DOI] [PubMed] [Google Scholar]
- Herrera C, Vallejos GA, Loaiza R, Zeledon R, Urbina A, Sepulveda-Boza S. In vitro activity of thienyl-2-nitropropene compounds against Trypanosoma cruzi. Mem Inst Oswaldo Cruz. 2009;104(7):980–985. doi: 10.1590/s0074-02762009000700007. [DOI] [PubMed] [Google Scholar]
- Hu L, Arafa RK, Ismail MA, Wenzler T, Brun R, Munde M, Wilson WD, Nzimiro S, Samyesudhas S, Werbovetz KA. Azaterphenyl diamidines as antileishmanial agents. Bioorg Med Chem Lett. 2008;18(1):247–251. doi: 10.1016/j.bmcl.2007.10.091. others. [DOI] [PubMed] [Google Scholar]
- Leon JS, Wang K, Engman DM. Captopril ameliorates myocarditis in acute experimental Chagas disease. Circulation. 2003;107(17):2264–2269. doi: 10.1161/01.CIR.0000062690.79456.D0. [DOI] [PubMed] [Google Scholar]
- Mady C, Cardoso RH, Barretto AC, da Luz PL, Bellotti G, Pileggi F. Survival and predictors of survival in patients with congestive heart failure due to Chagas' cardiomyopathy. Circulation. 1994;90(6):3098–3102. doi: 10.1161/01.cir.90.6.3098. [DOI] [PubMed] [Google Scholar]
- Marin-Neto JA, Bromberg-Marin G, Pazin-Filho A, Simoes MV, Maciel BC. Cardiac autonomic impairment and early myocardial damage involving the right ventricle are independent phenomena in Chagas' disease. Int J Cardiol. 1998;65(3):261–269. doi: 10.1016/s0167-5273(98)00132-6. [DOI] [PubMed] [Google Scholar]
- Marin-Neto JA, Rassi A, Jr, Avezum A, Jr, Mattos AC, Rassi A, Morillo CA, Sosa-Estani S, Yusuf S. The BENEFIT trial: testing the hypothesis that trypanocidal therapy is beneficial for patients with chronic Chagas heart disease. Mem Inst Oswaldo Cruz. 2009;104 Suppl 1:319–324. doi: 10.1590/s0074-02762009000900042. [DOI] [PubMed] [Google Scholar]
- Maya JD, Cassels BK, Iturriaga-Vasquez P, Ferreira J, Faundez M, Galanti N, Ferreira A, Morello A. Mode of action of natural and synthetic drugs against Trypanosoma cruzi and their interaction with the mammalian host. Comp Biochem Physiol A Mol Integr Physiol. 2007;146(4):601–620. doi: 10.1016/j.cbpa.2006.03.004. [DOI] [PubMed] [Google Scholar]
- McKerrow JH, Doyle PS, Engel JC, Podust LM, Robertson SA, Ferreira R, Saxton T, Arkin M, Kerr ID, Brinen LS. Two approaches to discovering and developing new drugs for Chagas disease. Mem Inst Oswaldo Cruz. 2009;104 Suppl 1:263–269. doi: 10.1590/s0074-02762009000900034. others. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molina RB, Matsubara BB, Hueb JC, Zanati SG, Meira DA, Cassolato JL, Paiva SA, Zornoff LA. Dysautonomia and ventricular dysfunction in the indeterminate form of Chagas disease. Int J Cardiol. 2006;113(2):188–193. doi: 10.1016/j.ijcard.2005.11.010. [DOI] [PubMed] [Google Scholar]
- Morris SA, Tanowitz HB, Wittner M, Bilezikian JP. Pathophysiological insights into the cardiomyopathy of Chagas' disease. Circulation. 1990;82(6):1900–1909. doi: 10.1161/01.cir.82.6.1900. [DOI] [PubMed] [Google Scholar]
- Munoz MJ, Murcia L, Segovia M. The urgent need to develop new drugs and tools for the treatment of Chagas disease. Expert Rev Anti Infect Ther. 2011;9(1):5–7. doi: 10.1586/eri.10.144. [DOI] [PubMed] [Google Scholar]
- Pinazo MJ, Munoz J, Posada E, Lopez-Chejade P, Gallego M, Ayala E, del Cacho E, Soy D, Gascon J. Tolerance of benznidazole in treatment of Chagas' disease in adults. Antimicrob Agents Chemother. 2010;54(11):4896–4899. doi: 10.1128/AAC.00537-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinto AY, Ferreira AG, Jr, Valente Vda C, Harada GS, Valente SA. Urban outbreak of acute Chagas disease in Amazon region of Brazil: four-year follow-up after treatment with benznidazole. Rev Panam Salud Publica. 2009;25(1):77–83. doi: 10.1590/s1020-49892009000100012. [DOI] [PubMed] [Google Scholar]
- Prata A. Clinical and epidemiological aspects of Chagas disease. Lancet Infect Dis. 2001;1(2):92–100. doi: 10.1016/S1473-3099(01)00065-2. [DOI] [PubMed] [Google Scholar]
- Rassi A, Jr, Rassi A, Little WC, Xavier SS, Rassi SG, Rassi AG, Rassi GG, Hasslocher-Moreno A, Sousa AS, Scanavacca MI. Development and validation of a risk score for predicting death in Chagas' heart disease. N Engl J Med. 2006;355(8):799–808. doi: 10.1056/NEJMoa053241. [DOI] [PubMed] [Google Scholar]
- Ribeiro AL, Reis AM, Teixeira MM, Rocha MO. Brain natriuretic peptide in Chagas' disease: further insights. Lancet. 2003;362(9380):333. doi: 10.1016/S0140-6736(03)13990-6. [DOI] [PubMed] [Google Scholar]
- Rocha MO, Ribeiro AL, Teixeira MM. Clinical management of chronic Chagas cardiomyopathy. Front Biosci. 2003;8:e44–e54. doi: 10.2741/926. [DOI] [PubMed] [Google Scholar]
- Rocha MO, Teixeira MM, Ribeiro AL. An update on the management of Chagas cardiomyopathy. Expert Rev Anti Infect Ther. 2007;5(4):727–743. doi: 10.1586/14787210.5.4.727. [DOI] [PubMed] [Google Scholar]
- Silva CF, Batista MM, Mota RA, de Souza EM, Stephens CE, Som P, Boykin DW, Soeiro Mde N. Activity of "reversed" diamidines against Trypanosoma cruzi "in vitro". Biochem Pharmacol. 2007;73(12):1939–1946. doi: 10.1016/j.bcp.2007.03.020. [DOI] [PubMed] [Google Scholar]
- Sosa Estani S, Segura EL, Ruiz AM, Velazquez E, Porcel BM, Yampotis C. Efficacy of chemotherapy with benznidazole in children in the indeterminate phase of Chagas' disease. Am J Trop Med Hyg. 1998;59(4):526–529. doi: 10.4269/ajtmh.1998.59.526. [DOI] [PubMed] [Google Scholar]
- Sosa-Estani S, Viotti R, Segura EL. Therapy, diagnosis and prognosis of chronic Chagas disease: insight gained in Argentina. Mem Inst Oswaldo Cruz. 2009;104 Suppl 1:167–180. doi: 10.1590/s0074-02762009000900023. [DOI] [PubMed] [Google Scholar]
- Steindel M, Kramer Pacheco L, Scholl D, Soares M, de Moraes MH, Eger I, Kosmann C, Sincero TC, Stoco PH, Murta SM. Characterization of Trypanosoma cruzi isolated from humans, vectors, and animal reservoirs following an outbreak of acute human Chagas disease in Santa Catarina State, Brazil. Diagn Microbiol Infect Dis. 2008;60(1):25–32. doi: 10.1016/j.diagmicrobio.2007.07.016. others. [DOI] [PubMed] [Google Scholar]
- Urbina JA. Chemotherapy of Chagas disease. Curr Pharm Des. 2002;8(4):287–295. doi: 10.2174/1381612023396177. [DOI] [PubMed] [Google Scholar]
- Urbina JA, Concepcion JL, Caldera A, Payares G, Sanoja C, Otomo T, Hiyoshi H. In vitro and in vivo activities of E5700 and ER-119884, two novel orally active squalene synthase inhibitors, against Trypanosoma cruzi. Antimicrob Agents Chemother. 2004;48(7):2379–2387. doi: 10.1128/AAC.48.7.2379-2387.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urbina JA, Docampo R. Specific chemotherapy of Chagas disease: controversies and advances. Trends Parasitol. 2003;19(11):495–501. doi: 10.1016/j.pt.2003.09.001. [DOI] [PubMed] [Google Scholar]
- Vago AR, Andrade LO, Leite AA, d'Avila Reis D, Macedo AM, Adad SJ, Tostes S, Jr, Moreira MC, Filho GB, Pena SD. Genetic characterization of Trypanosoma cruzi directly from tissues of patients with chronic Chagas disease: differential distribution of genetic types into diverse organs. Am J Pathol. 2000;156(5):1805–1809. doi: 10.1016/s0002-9440(10)65052-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viotti R, Vigliano C. Etiological treatment of chronic Chagas disease: neglected 'evidence' by evidence-based medicine. Expert Rev Anti Infect Ther. 2007;5(4):717–726. doi: 10.1586/14787210.5.4.717. [DOI] [PubMed] [Google Scholar]
- Viotti R, Vigliano C, Lococo B, Alvarez MG, Petti M, Bertocchi G, Armenti A. Side effects of benznidazole as treatment in chronic Chagas disease: fears and realities. Expert Rev Anti Infect Ther. 2009;7(2):157–163. doi: 10.1586/14787210.7.2.157. [DOI] [PubMed] [Google Scholar]
- Viotti R, Vigliano C, Lococo B, Bertocchi G, Petti M, Alvarez MG, Postan M, Armenti A. Long-term cardiac outcomes of treating chronic Chagas disease with benznidazole versus no treatment: a nonrandomized trial. Ann Intern Med. 2006;144(10):724–734. doi: 10.7326/0003-4819-144-10-200605160-00006. [DOI] [PubMed] [Google Scholar]