Abstract
This study demonstrates the application of metal-assisted and microwave-accelerated evaporative crystallization (MA-MAEC) technique to rapid crystallization of L-alanine on surface engineered silver nanostructures. In this regard, silver island films (SIFs) were modified with hexamethylenediamine (HMA), 1-undecanethiol (UDET), and 11-mercaptoundecanoic acid (MUDA), which introduced -NH2, -CH3 and -COOH functional groups to SIFs, respectively. L-Alanine was crystallized on these engineered surfaces and blank SIFs at room temperature and using MA-MAEC technique. Significant improvements in crystal size, shape, and quality were observed on HMA-, MUDA- and UDET-modified SIFs at room temperature (crystallization time = 144, 40 and 147 min, respectively) as compared to those crystals grown on blank SIFs. Using the MA-MAEC technique, the crystallization time of L-alanine on engineered surfaces were reduced to 17 sec for microwave power level 10 (i.e., duty cycle 100%) and 7 min for microwave power level 1 (duty cycle 10%). Raman spectroscopy and powder x-ray diffraction (XRD) measurements showed that L-Alanine crystals grown on engineered surfaces using MA-MAEC technique had identical characteristic peaks of L-alanine crystals grown using traditional evaporative crystallization.
Keywords: L-alanine, crystallization, engineered surfaces, silver island films, self-assembled monolayers
Introduction
The importance of the amino acid L-Alanine in the quest to understand molecular structure and function has been previously described in literature.1 While L-alanine is one of the most structurally simple amino acids, it constitutes a large component of proteins. It also provides energy for organs such as the brain and central nervous system, and is also highly active in the immune and metabolic systems of the human body.2 In addition, due to recent studies predicting its health benefits, demand has risen sharply for a solid form in which the amino acid can be consumed.3 Subsequently, development of new or improvement of the existing crystallization methods for L-alanine has become of large interest to researchers. In crystal form, L-Alanine not only acts as an essential nutrient for the body but its organic properties make it a potential candidate for a nonlinear optical crystal. In this regard, new crystal growth methods are also being developed in order to obtain suitable crystals in terms of their size and desired morphology.4
One can find a number of crystallization methods for L-alanine and other amino acids, including polarized laser light irradiation in solution,5 and engineered surfaces such as nanoscale cylindrical pores,6 platforms for micro-droplet solvent evaporation and self-assembled monolayers (SAMs) of alkane thiols on patterned surfaces through solvent evaporation.7 Recently, Trout et. al. described a detailed investigation of the role of surface chemistry and morphology of porous polymer surfaces on heterogeneous nucleation of aspirin.8 In this study, the authors employed carboxyl, hydroxyl, tertiary amide and amine and phenyl ring as functional groups and found out that these functional groups have a profound effect on the nucleation and growth of aspirin crystals. Engineered surfaces clearly demonstrated the important role of surface chemistry in crystal nucleation and growth.
However, in all these reports, crystallization of amino acids and small molecules at room temperature by traditional evaporation techniques takes several hours or longer to complete. Recently, our research laboratory described a new method, called the metal-assisted and microwave-accelerated evaporative crystallization (MA-MAEC),9 for “on-demand” crystallization of amino acids in a matter of seconds. MA-MAEC technique is based on the combined use of microwave heating and plasmonic nanostructures, which affords for speeding up the crystallization process and functions as selective nucleation sites for amino acid crystal growth, respectively. To date, we have demonstrated the application of MA-MAEC technique to rapid crystallization of glycine9 and L-alanine1 on blank SIFs, where the crystallization process was accelerated from hours to several seconds.1 However, using blank (unmodified) SIFs in MA-MAEC-based crystallization scheme did not always yield all crystals with desired size distribution or high enough quality for optimum harvest.1
In an attempt to further improve the L-Alanine crystal size, shape and quality, this study presents the results of the combined use of engineered surfaces with MA-MAEC, where SIFs were modified with HMA, UDET and MUDA to introduce –NH2, -CH3 and -COOH functional groups to the surface of SIFs. Significant improvements were observed in the size and shape of crystals produced on these engineered surfaces using both room temperature crystallization and MA-MAEC. The total time complete evaporation varied between 40-147 min and 17 sec to 7 min for room temperature crystallization and MA-MAEC, respectively. In addition, the growth of crystal was monitored, at time intervals starting with the initial deposition of L-alanine solution on engineered surfaces and until the complete evaporation of solvent occurs, using optical microscopy. L-alanine crystals were characterized by Raman spectroscopy and powder XRD measurements, which showed that L-Alanine crystals grown on engineered surfaces using MA-MAEC technique had identical characteristic peaks of L-alanine crystals grown using traditional evaporative crystallization. As observed by other research groups, L-alanine crystals on engineered surfaces using MA-MAEC technique had the same polymorph. These results showed that one can collect crystals with desired size and shape before all the molecules in the initial solution are crystallized.
With the combination of surface engineering and MA-MAEC, perfect L-Alanine crystals can be rapidly grown on-demand. Further development of this technique may be applicable to molecules such as peptides, proteins, and inorganic molecules, and would greatly reduce the time that is required for perfect, useable crystals to be grown.
Materials and Methods
Materials
Silver nitrate was purchased from Spectrum Chemical MFG Corp. 190-proof ethanol (95%) was purchased from Pharmco Products Inc. Sodium hydroxide, ammonium hydroxide, D-glucose, hexamethylenediamine (HMA), 1-undecanethiol (UDET), 11-mercaptoundecanoic acid (MUDA), and L-Alanine were purchased from Sigma-Aldrich. All chemicals were used as received.
Methods. Preparation of SIFs
SIFs were prepared as previously described.10 Silver nanoparticles were precipitated by the addition of NaOH to AgNO3 then quickly redissolved again by the addition of NH4OH and cooled to 5°C. Blank glass slides (Corning) were submerged in the silver solution for several minutes until coated with a yellow-green tinge.
Preparation of Surface Engineering Solutions
50 mM solutions of HMA, UDET, and MUDA were prepared by the mixture of 190-proof ethanol and appropriate amounts of each surface modifying chemical. Solutions were stored in glass bottles (Corning) in a dry room temperature environment in between uses. Dry SIFs were completely submerged in HMA, UDET, and MUDA solutions for three hours, then removed and air- dried before L-Alanine solution was deposited.
Preparation of L-Alanine solution
A 2.70 M solution of L-Alanine (pH = 5.3) was prepared as previously described.1 L-Alanine was dissolved in double-distilled water (Millipore) and heated to 60°C. Solution was examined for colorlessness, transparency, and absence of undissolved particles before being deposited onto SIFs. To prevent alterations to the solubility curve of the L-Alanine solution, a new solution was prepared and used for crystallization each day.
Crystallization of L-Alanine
L-Alanine was crystallized as described in previous literature.1 Solution was applied to SIFs in 20 FL drops and was crystallized at room temperature or through microwave heating. The MA-MAEC technique was performed in a Frigidaire microwave oven at power levels 1, 5, and 10. At power level 10, 700 watts of microwave heating were applied to the sample for 100% of the designated time. At power level 5, 700 watts were applied for 50% of the time; at power level 1, 700 watts were applied for 10% of the time.
Characterization of L-Alanine Crystals
Crystal growth images were recorded with a Swift Digital M10L Monocular Microscope (Swift). Crystals were characterized by raman spectroscopy through a Raman spectrometer system (i-Raman).
Powder X-ray diffraction (XRD) data for L-alanine crystals placed in a capillary tube with thin walls (0.02 mm) were collected using an in-house X-ray generator (MicroMax 7, Rigaku/MSC, The Woodlands, TX) and a Raxis4++ image plate detector (Rigaku/MSC). The distance between the detector and samples were kept constant at 75 mm. The radiation source was CuKα (wavelength: 0.154 nm). The 2-D XRD data was collected at 0° ≤δ≤ 120° at values of 0°≤2θ≤ 40°.
The ADXV software (http://www.scripps.edu/~arvai/adxv.html) was used for interconversion of images between different formats. The 2D images were integrated to generate the 1-D Intensity vs. 2θ plots using FIT2D software11 (http://www.esrf.eu/computing/scientific/FIT2D/).
Results and Discussion
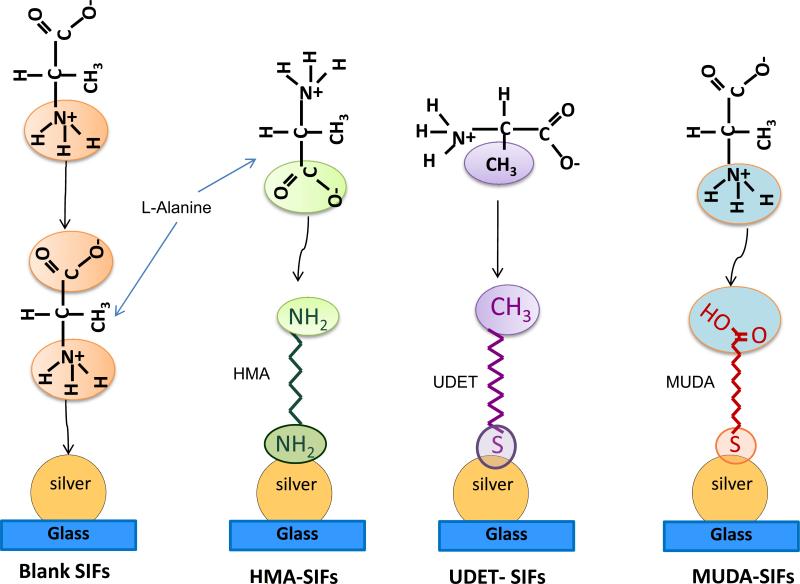
Since this study employs SIFs and surface engineered (i.e., surface modified) SIFs, it is important to comment on both the nature of SIFs and the interaction of L-alanine with functional groups present on surface engineered SIFs. The use of surface modification procedure with HMA, MUDA and UDET is only effective when plasmonic structures such as silver nanostructures are present on the surface of glass slides and thus blank glass slides were not modified with HMA, MUDA and UDET. SEM image (Supporting Information, Figure S1) of SIFs show that SIFs were deposited onto glass slides in a homogeneous manner and the size of SIFs was 50-90 nm. In this regard, the distance between the individual nanoparticles varied between > 10 – 150 nm (150 nm is observed in < 5% of the imaged area), which is thought to have an important role in crystal growth. Since the size of the L-alanine crystals (>100 m) is significantly larger than the size and the distance between the SIFs, it is thought that a large number of nanoparticles serve as multiple nucleation sites for single crystals. In this regard, Scheme 1 depicts the predicted assembly of L-alanine molecules on blank SIFs and on surface engineered SIFs. The assembly of a monolayer of L-alanine on blank SIFs takes place through the interaction of primary amine (-NH +3) group of L-alanine with the silver surface (i.e., chemisorption). Subsequent L-alanine molecules assemble onto the L-alanine monolayer through - NH3+ / -COO- group interactions (i.e., electrostatic). The orientation of L-alanine assembly onto the engineered surfaces is dependent on the type of functional group present on SIFs as depicted in Scheme 1. The assembly of L- alanine on to HMA-modified SIFs, which present -NH2 functional groups, takes place through the electrostatic interaction of –COO- group of L-alanine with the -NH2 group of HMA. In the case of MUDA-modified surfaces, the orientation of assembly of L-alanine is reversed as compared to HMA surfaces. L-alanine is expected to assemble onto UDET-modified SIFs through hydrophobic interactions between –CH3 groups.
Scheme 1.
Schematic representation of proposed attachment mechanisms of L-Alanine functional groups to SIFs and surface engineered SIFs. Please note that L-alanine is a zwitterion molecule in water at pH =5.3, that is, -COOH and NH2 groups are present in COO- and NH3+ form.
The effect of using blank SIFs, surface engineered SIFs and evaporative crystallization conditions (room temperature and microwave-accelerated, minimum = 5 samples per condition) on the time of crystallization was studied and summarized in Table 1. For a fixed volume of 20 μL drop of 2.70 M L-Alanine solution at pH=5.3, the total crystallization time (i.e., complete evaporation of solvent) on blank SIFs at room temperature was 41 min, which is similar to that on MUDA-modified surfaces. On the other hand, the total crystallization time was increased to 144 and 147 min for HMA- and UDET-modified surfaces at room temperature. Average crystallization time for L-alanine was decreased as the microwave power level was increased when using the MA-MAEC technique. For example, crystallization of L-alanine on blank SIFs and MUDA-modified SIFs was completed in 17-22 sec when using the MA-MAEC technique at microwave power level 10 and in 7-9 min at microwave power level 1. Similar to the observation made for UDET- and HMA-modified surfaces at room temperature evaporation, the total crystallization time on these engineered surfaces using MA-MAEC technique were longer than blank SIFs and MUDA-modified SIFs.
Table 1.
Summary of results for the crystallization of L-alanine from 2.70 M solution on glass slides and silver island films (SIFs) at room temperature and using MA-MAEC technique. Each experiment was repeated at least 5 times.
| Blank SIF | MUDA -SIF | HMA -SIF | UDET- SIF | |
|---|---|---|---|---|
| RT | 41 ± 13 min | 40 ± 3 min | 144 ± 15 min | 147 ± 14 min |
| MW PL 1 | 7.0 ± 1 min | 9.0 ± 1 min | 16 ± 8 min | 17 ± 4 min |
| MW PL 5 | 45 ± 6 sec | 27 ± 7 sec | 32 ± 7sec | 22 ± 1 sec |
| MW PL 10 | 22 ± 3 sec | 17 ± 2 sec | 23 ± 4 sec | 21 ± 5 sec |
The above mentioned observations can be explained by the nature of the – COOH, –CH3 and -NH2 functional groups present on SIFs. It is well known that self assembled monolayers containing -COOH groups introduces hydrophilicity to the surfaces, while –CH3 and -NH2 groups make the surfaces more hydrophobic. In this regard, a solution of L-alanine on these engineered surfaces spreads differently: more spreading of solution was observed on MUDA-modified SIFs than UDET- and HMA surfaces at room temperature and using microwave heating. As shown in Figure S2 (see Supporting Information), the differences in spreading of initial L-alanine solution on blank SIFs and MUDA-modified SIFs resulted in the growth of crystals at room temperature (identical observation was made for surfaces exposed to microwave heating) over a large surface area. L-alanine crystals were grown on a significantly smaller surface area on UDET-modified SIFs due to the hydrophobic nature of the surfaces. We also note that variation in the pH value of the initial L-alanine solution was not attempted in this study due to extent of data presented here and will be reported in due time.
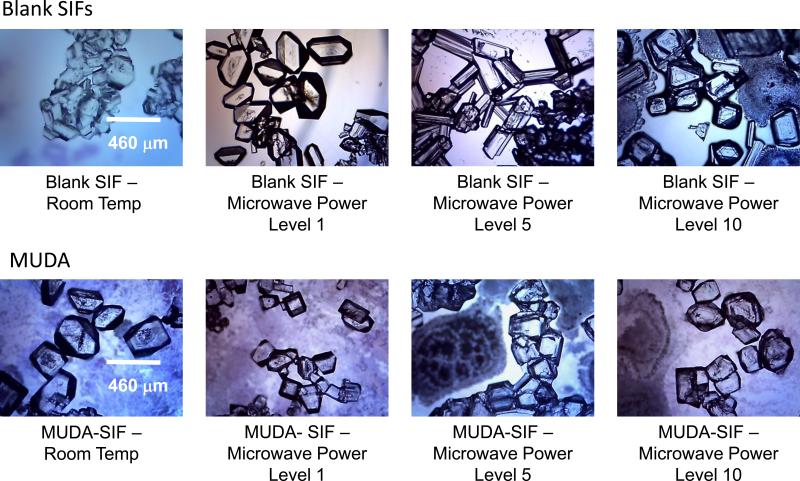
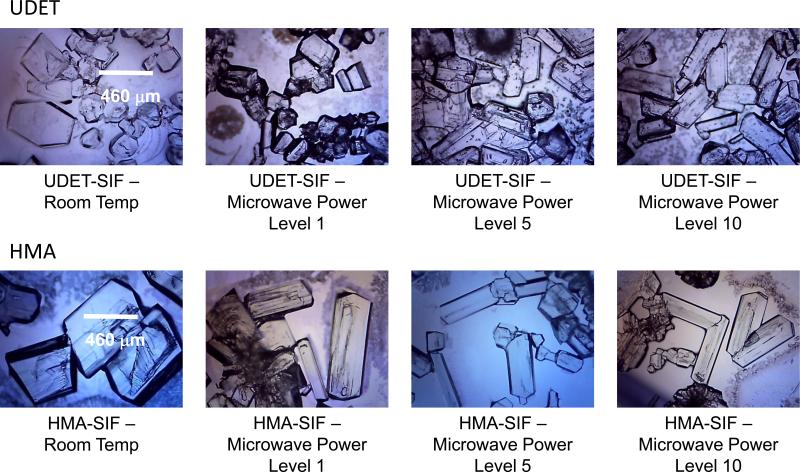
Figure 1 and 2 show the optical images of L-alanine crystals grown (after complete evaporation of the solvent) on blank SIFs and MUDA-modified SIFs (Figure 1) and UDET- and HMA-modified SIFs (Figure 2) at room temperature and using MA-MAEC technique. The optical images in Figure 1 and 2 show that larger L-alanine crystals was grown on HMA- and UDET-modified SIFs than those grown on blank SIFs and MUDA-modified SIFs. These observations can be partially explained by the spreading of the initial L-alanine solution as described in the previous paragraph. That is, as L-alanine solution was spread across a wider surface area on blank SIFs and MUDA-modified SIFs, crystal nucleation and growth occurred at a larger number of sites on the surface. Subsequently, small amount of L-alanine molecules were available for growth of crystals on all the nucleation sites. In contrast, since the spread of L-alanine solution occurred across a significantly smaller surface area on HMA- and UDET- modified SIFs, large amount of L-alanine molecules were available for growth of crystals on all the nucleation sites, and hence larger crystals were grown.
Figure 1.
Optical images of L-alanine crystals formed from 2.70 M solution on blank SIFs and MUDA-modified SIFs at room temperature and using MA-MAEC technique. Each experiment was repeated at least 5 times.
Figure 2.
Optical images of L-alanine crystals formed from 2.70 M solution on UDET- and HMA-modified SIFs at room temperature and using MA-MAEC technique. Each experiment was repeated at least 5 times.
In order to better understand the crystallization process during room temperature and microwave heating evaporation, optical images of the initial L-alanine solution and the growing crystals on blank SIFs and MUDA-, UDET-, and HMA-modified SIFS were taken at time intervals as indicated in the Figures 3-6 and Supplementary Figures S3-S10. It is important to note that in all MA-MAEC experiments, microwave heating was stopped for a brief period of time (~10 sec) to collect optical images, while the growth of crystals at room temperature were observed on an optical microscope stage without interruption. Figure 3 (HMA_RT) shows the time progression of the growth of L-Alanine crystals on HMA-modified SIFs at room temperature. As expected, no crystals were observed in the initial L-alanine solution at the time of the first image (t=0 min) was taken. After only 15 min, a single crystal of ~60 m appeared on the HMA-modified surface, which grew to its full size ~500 m in 150 min. The change in the defect on the crystal face on the plane out of page (marked with an arrow) is clearly observed. After its appearance in the second image taken at 15 min, the defect grew across the crystal face and later shrank as the crystal face fully developed. L-alanine crystals grown on HMA-modified SIFs at room temperature ranged in size from 400 to 1108 Fm, indicating up to an 8-fold increase in size compared to blank SIFs.1 However, the crystals grown on HMA-modified SIFs required up to 100 min longer as compared to those crystals grown on blank SIFs at room temperature.
Figure 3.
Time progression of the growth of L-alanine crystals on HMA-modified SIFs at room temperature. Each experiment was repeated at least 5 times.
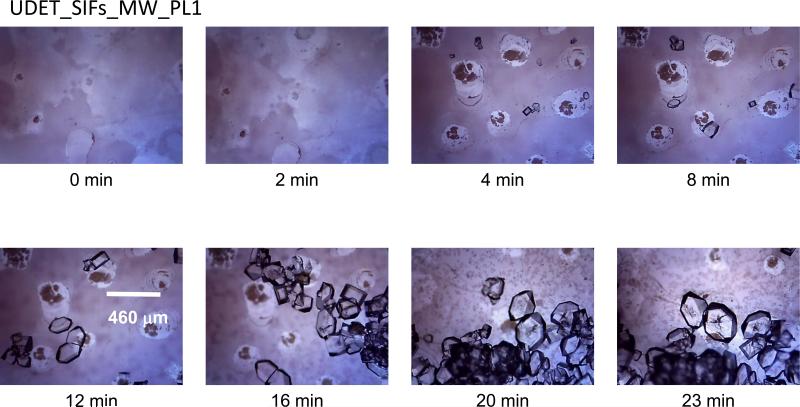
Figure 6.
Time progression of the growth of L-alanine crystals on UDET-modified SIFs using MA-MAEC technique at microwave power level 1. Each experiment was repeated at least 5 times.
Subsequently, the growth of crystals on HMA-modified SIFs using the MA-MAEC technique was also monitored and is shown in Figure 4 and Figures S3-S4 (Supporting Information). Figure 4 shows the time progression of the growth of two different types of L-alanine crystals on HMA-modified SIFs under microwave heating (power level 1). Under these conditions, within 12 min large sized crystals already appeared. It is important to note that the solvent was not completely evaporated until 26 min. Subsequently, some of the crystals were observed to move around the surface < 20 min, disappearing from the imaged area. When the power level of microwave heating was increased to 5 and 10, large L-alanine crystals appeared on after 15 sec and complete evaporation took place in 40 sec, where L-alanine crystals were fully formed (Figure S3-S4). The size of the crystals grown on HMA-modified SIFs ranged from 632 to 1452 Fm. In contrast to the short and tall trapezoidal shape that the majority of crystals grown on HMA-modified SIFs at room temperature took on, crystals grown using MA-MAEC technique were orthorhombic. In the case of the crystals grown at room temperature, the evaporation of solvent was significantly slower, affording for the growth of taller crystals. In addition, despite the improvement of crystal quality on HMA- modified SIFs using MA-MAEC technique at microwave power levels 1 and 5, the quality of crystals grown at microwave power level 10 was significantly altered. Although the crystals grown using microwave power level 10 were still large, the number of crystals (minimum of 100 crystal count on 5 different samples) were significantly less as compared to crystals grown using microwave power levels 1 and 5 and there were yet more imperfections in crystals. This can be explained by the duration of the microwave heating that the samples were exposed to: as these results show, continuous microwave heating at power level 10 (duty cycle: 100%) prevented the crystallization process of growing large and perfect L-alanine crystals, and thus resulted in the growth of smaller imperfect crystals. In this regard, the use of microwave heating at power levels 1 and 5 (heat is on for 50% or 10% of the time, respectively) is recommended for the use of MA-MAEC technique with HMA-modified SIFs.
Figure 4.
Time progression of the growth of L-alanine crystals on HMA-modified SIFs using MA-MAEC technique at microwave power level 1. Each experiment was repeated at least 5 times.
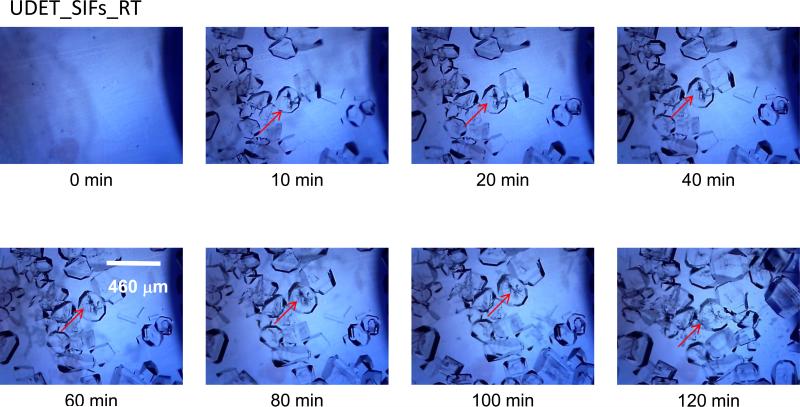
The growth of crystals on UDET-modified SIFs at room temperature and using the MA-MAEC technique was also monitored and is shown in Figures 5-6 and Figures S5-S6 (see Supporting Information). Figure 5 shows the time progression of the growth of L-Alanine crystals on UDET-modified SIFs at room temperature. As expected, no crystals were observed in the initial L-alanine solution at the time of the first image (t=0 min) was taken. Although the complete evaporation of solvent took 120 min large number of L-alanine crystals appeared on UDET-modified SIFs after only 10 min. The size of L-alanine crystals grown at room temperature ranged from 512 to 968 Fm. Between 10 and 120 min, the number and the size L-alanine of crystals were slightly increased, which implies that one can use UDET-modified SIFs in a room temperature based evaporative crystallization and harvest crystals before complete evaporation of the solvent. This implies that the assembly of L-alanine through hydrophobic interactions is more effective in growing L-alanine crystals at room temperature. In addition, as explained in the beginning of Results and Discussion section, surfaces modified with UDET are very hydrophobic and results in decrease in the spreading of the solution over the surface that may have an additional effect on the short time of crystallization on these surfaces.
Figure 5.
Time progression of the growth of L-alanine crystals on UDET-modified SIFs at room temperature. Each experiment was repeated at least 5 times.
In the combined use of UDET-modified SIFs with the MA-MAEC technique (Figure 6 and Figures S5 and S6 (Supp. Info.), L-alanine crystals appeared after 4 min of microwave heating and continued to grow until complete evaporation of solvent took place at 23 min. The size of L-alanine crystals grown under these conditions ranged from 248 to 888 Fm. L-Alanine crystal edges grown on UDET-modified SIFS using MA-MAEC appeared darker in the optical microscope images, indicating that these crystals were thicker than crystals grown on at room temperature. As observed with crystals grown on HMA-modified SIFs, crystals grown on UDET-modified SIFs using MA-MAEC (for microwave power levels 5 and 10) appeared to be more elongated and thinner than their counterparts grown at room temperature. This can be also explained by the faster evaporation of solvent caused by continuous microwave heating at power level 10. This particular power level also revealed the highest organization of growth and the most abundance of perfect crystals, suggesting that MA-MAEC is best used in combination with UDET-modified SIFs at microwave power level 10.
Lastly, MUDA-modified SIFs were employed in crystallization of L-alanine at room temperature and using the MA-MAEC technique and shown in Figures S7-S10 (Supp. Info.). While total crystallization time was shorter for the majority of MUDA- modified SIFs compared to blank SIFs, there was no significant improvement in the crystal size. Crystals grown on MUDA-modified SIFs at room temperature measured from 312 to 600 Fm and from 340 to 572 Fm in size at microwave heating, indicating up to a 2.5-fold increase from the size of crystals grown on blank SIFs without surface modification. Since the combined use of the MA-MAEC technique with MUDA-modified SIFs reduces crystallization time to 17 sec, this would be an appropriate method for the production of L-alanine crystals if a size requirement for the desired crystals is not specified. Although the use of microwave power levels 1 and 10 with MUDA-modified SIFs afforded for the growth a fairly large amount of crystals, the use of microwave power level 5 delivered the most homogenously grown crystals (minimum count of 100 crystals). At all power levels, L-alanine crystals appeared to be smaller than the crystals observed on HMA- and UDET-modified SIFs, which implies that the spreading of the initial solution on MUDA-modified SIFs were similar.
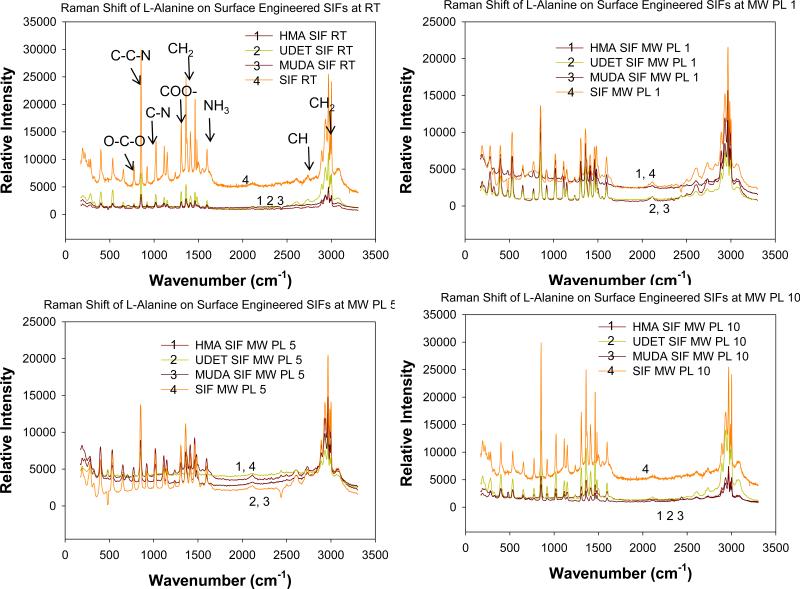
In order to characterize and compare L-alanine crystals grown on blank SIFs and engineered surfaces at room temperature and using the MA-MAEC technique, Raman spectroscopy and powder X-ray diffraction methods were employed. Figure 7 shows the Raman spectrum of L-alanine crystallized on blank SIFs and surface engineered SIFs at room temperature and using MA-MAEC technique. Since all peaks on the Raman spectra for all samples studied here are at identical locations of peaks for L-Alanine crystals grown at room temperature on glass surfaces, we conclude that the use of engineered surfaces and microwave heating did not alter the molecular structure of L-alanine during crystallization process.
Figure 7.
Raman spectrum of L-alanine crystallized on blank SIF and surface engineered SIFs at room temperature and using MA-MAEC technique.
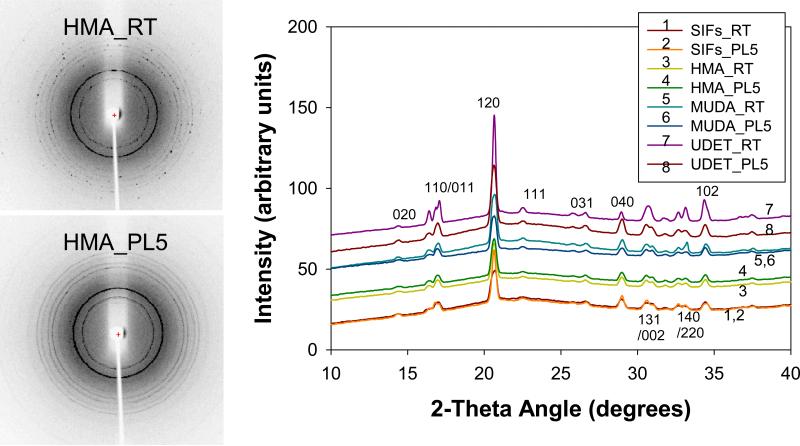
For all engineered surfaces, the rings seen in XRD grown through microwave heating (Figure 8-Left and Figure S11) appeared to be more homogenous than those grown at room temperature, which implies the presence of larger number and higher quality L-alanine crystals grown on these engineered surfaces. As shown In Figure 8-Right, on all surfaces the same polymorph was obtained. Work is currently underway for the demonstration of the combined use of engineered surfaces and MA-MAEC technique to other amino acids, small molecules and proteins. The results of these studies will be published in due course.
Figure 8.
Experimental 2-D (Left) and 1-D (Right) Powder X-Ray Diffraction patterns of L-alanine crystals grown from L-alanine solutions 2.7 M pH= 5.3 on glass at room temperature (RT) and using microwave heating (PL). The Miller indices corresponding to the peaks are also shown. The bell-shape in the 1-D plot is due to the background signal as also observed in the previous publications by others.
Conclusions
In summary, a detailed investigation for the use of surface engineered SIFs (HMA-, MUDA- and UDET-modified) for rapid crystallization of L-Alanine is presented. Significant improvements in crystal size and shape were observed on engineered SIFs at room temperature as compared to those crystals grown on blank SIFs. In addition, using the MA-MAEC technique, the crystallization time of L-alanine on engineered surfaces were reduced to 17 sec for microwave power level 10 and 7 min for microwave power level 1. Raman spectroscopy and powder x-ray diffraction (XRD) measurements showed that L-Alanine crystals grown on engineered surfaces using MA-MAEC technique had identical characteristic peaks of L-alanine crystals grown using traditional evaporative crystallization. In addition, L-alanine crystals grown on engineered surfaces using MA-MAEC technique had the same polymorph as on blank glass slides. The observations made in this study imply that MA-MAEC technique has the potential to develop into a method in which well-sized high-quality L-Alanine crystals can be produced in a much faster time than is currently advertised by crystals grown through traditional crystallization techniques.
Supplementary Material
Acknowledgement
The project described was supported by Award Number 5-K25EB007565-05 from the National Institute of Biomedical Imaging and Bioengineering. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Biomedical Imaging and Bioengineering or the National Institutes of Health. The authors also acknowledge Department of Homeland Security, Office of University Programs, Science & Technology Directorate: DHS 2008-ST-062-000011 for support. The authors would also like to thank Dr. Erdogan Ergican for his valuable discussions on the interpretation of the XRD data.
Footnotes
Supporting Information Available. Additional optical images of L-alanine crystals, experimental details and powder X-ray diffraction images are provided. This material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.Alabanza AM, Aslan K. Metal-Assisted and Microwave-Accelerated Evaporative Crystallization: Application to L-Alanine. Crystal Growth and Design. 2011;11(10):4300–4301. doi: 10.1021/cg201018j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bıyık R. EPR and optical absorption studies of VO2+ doped L-alanine (C3H7NO2) single crystals. Physica B. 2009;404:3483–3486. [Google Scholar]
- 3.Koyama MSM, Sasaki K, Kon-no K. Preparation of L-Alanine Crystals Containing Gold Nanoparticles. J Disper Sci Technol. 2008;29:1266–1271. [Google Scholar]
- 4.Kumar RM, Babu DR, Jayaraman D, Jayavel R, Kitamura K. Studies on the growth aspects of semi-organic L-alanine acetate: a promising NLO crystal. J Cryst Growth. 2005;275:e1935–e1939. [Google Scholar]
- 5.Garetz BA, Matic J, Myerson AS. Polarization switching of crystal structure in the nonphotochemical light-induced nucleation of supersaturated aqueous glycine solutions. Physical Review Letters. 2002;89(17) doi: 10.1103/PhysRevLett.89.175501. [DOI] [PubMed] [Google Scholar]
- 6.Hamilton BD, Weissbuch I, Lahav M, Hillmyer MA, Ward MD. Manipulating Crystal Orientation in Nanoscale Cylindrical Pores by Stereochemical Inhibition. Journal of the American Chemical Society. 2009;131(7):2588–2596. doi: 10.1021/ja807193s. [DOI] [PubMed] [Google Scholar]
- 7.Lee AY, Lee IS, Dettet SS, Boerner J, Myerson AS. Crystallization on confined engineered surfaces: A method to control crystal size and generate different polymorphs. Journal of the American Chemical Society. 2005;127(43):14982–14983. doi: 10.1021/ja055416x. [DOI] [PubMed] [Google Scholar]
- 8.Trout BL, Diao Y, Myerson AS, Hatton TA. Surface Design for Controlled Crystallization: The Role of Surface Chemistry and Nanoscale Pores in Heterogeneous Nucleation. Langmuir. 2011;27(9):5324–5334. doi: 10.1021/la104351k. [DOI] [PubMed] [Google Scholar]
- 9.Pinard MA, Aslan K. Metal-Assisted and Microwave-Accelerated Evaporative Crystallization. Cryst Growth Des. 2010;10(11):4706–4709. doi: 10.1021/cg101059c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Geddes CD, Aslan K. Microwave-accelerated metal-enhanced fluorescence: Platform technology for ultrafast and ultrabright assays. Anal Chem. 2005;77(24):8057–8067. doi: 10.1021/ac0516077. [DOI] [PubMed] [Google Scholar]
- 11. [septermber 2011]; http://www.esrf.eu/computing/scientific/FIT2D/
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.