Abstract
Neovascularisation is a major cause of visual loss in a number of ophthalmic diseases. This review aims to outline the basic regulators of vessel growth in corneal neovascularisation. An understanding of the underlying principles of physiological and pathophysiological vascular development helps to appreciate current approaches to prevent or treat corneal neovascularisation. Options for future interventions will be discussed in the light of recent evidence provided by animal models of corneal neovascularisation.
Keywords: cornea, vascular development, gene therapy, translational research
Introduction
Our ability to see is a highly specialised function, which relies on sophisticated architecture of the human eye. Each ocular structure or tissue has distinct properties and tasks — this pertains also to the vasculature. The perfectly organised vascular tree of the retinal circulation and the avascularity of the cornea serve as examples. Where the delicate homeostasis of vessel growth and inhibition is disturbed, neoformation of blood vessels in areas that were previously avascular can disrupt visual function and cause disease. Indeed, abnormal vascularisation underlies or accompanies some important ocular pathologies, including the neovascular form of age-related macular degeneration, proliferative diabetic retinopathy, retinopathy of prematurity, retinal vein occlusion, and corneal neovascularisation. The public health impact of ocular neovascularisation therefore is significant.1
Michaelson2, 3 was the first to suggest that a diffusible factor liberated by retinal or corneal tissue would stimulate vascular growth and development.2, 3 Subsequently, a number of factors to activate and guide healthy or pathological vascularisation were identified. This review intends to provide an overview of important factors and potential therapeutic targets in the context of corneal neovascularisation.
Vascular growth during development entails vasculogenesis and angiogenesis
During ontogenesis, de novo formation of a capillary lattice occurs within each organ by a process referred to as vasculogenesis.4, 5 This involves blood vessel precursor cells called angioblasts, sharing with haematopoietic cells a common progenitor of mesodermal origin, the haemangioblast.6 Aggregates of angioblasts differentiate into endothelial cells (ECs) that line a lumen containing blood precursor cells. Fusion of these ‘blood islands' forms the so-called primary capillary plexus.
Subsequently, additional vessels are formed and the primitive network is remodelled through a process termed angiogenesis. It entails sprouting and intussusception (splitting), functional maturation of ECs, and recruitment of smooth muscle cells or pericytes. This also lends primitive vessels the distinct properties of arteries and veins.5
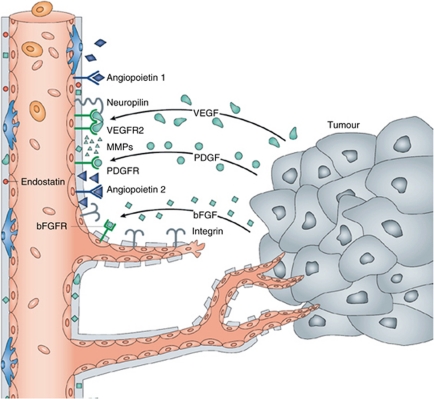
To enable sprouting from pre-established vessels, cell–cell contacts between ECs are loosened, and the extracellular matrix (ECM) is degraded.7 ECs can then extend filopodia, migrate, and lead vascular growth in response to gradients of environmental mitogens.8 Promotion and inhibition of vascularisation is orchestrated with the help of such pro- and antiangiogenic mediators, both during and after development.9, 10 Vasculogenesis is seen predominantly during embryogenesis, whereas angiogenesis occurs also in adults in the context of wound healing, pregnancy, and uterine cycling.11 However, angiogenesis has also been found to have a major role in pathological processes such as tumour growth and metastasis, as well as ocular neovascularisation (Figure 1).10, 12 Mechanisms and mediators of pathologic angiogenesis are thought to differ somewhat from physiological angiogenesis, exemplified by the fact that the latter does not usually carry an inflammatory component.13 In a rat model, angiogenesis has been identified as the underlying mechanism of corneal neovascularisation. Here, initial events are vasodilation of the limbal vessels and recruitment of leucocytes (which release additional pro-angiogenic mediators), followed by vascular sprouts, which emerge from pericorneal venules and capillaries.14
Figure 1.
Soluble angiogenic factors are released from tumour cells to induce and regulate key steps in angiogenesis. Many of these factors have also been found to have a role in ocular and, more specifically, corneal neovascularisation. Angiopoietin-1 binds to endothelial Tie-2 receptors to stabilise the established vasculature. Angiopoietin-2, however, which is secreted by tumour cells, and which competes with angiopoietin-1 for the Tie-2 receptor, increases vascular basal membrane degradation and EC migration. Vascular endothelial growth factor (VEGF), platelet-derived growth factor (PDGF), and basic fibroblast growth factor (bFGF) may also be secreted by tumour cells, and exert pro-angiogenic effects via their respective EC receptors (with VEGF-receptors requiring assistance from neuropilins). Tumours or ECs may also release matrix metalloproteinases (MMPs). These have some pro-angiogenic effects, but also cleave antiangiogenic endostatin from collagen XVIII of the extracellular matrix, and angiostatin from circulating plasminogen (not depicted; adapted from Folkman,100 with permission from Macmillan Publishers Ltd).
Corneal avascularity is the result of an active regulatory process
Although vascularisation is vital for the survival of most tissues, some structures require avascularity to ensure proper functioning. These include cartilage, heart valves, and in the eye cornea, vitreous and lens.15, 16, 17, 18 In these tissues, mechanisms are in place to inhibit ingrowth of blood vessels. To maintain what has been termed the ‘angiogenic privilege' in the cornea, a delicate balance exists between pro- and antiangiogenic factors (Figure 2). Pro-angiogenic factors include fibroblast growth factor (FGF), vascular endothelial growth factor (VEGF), platelet-derived growth factor (PDGF), and angiopoietin, among others. Factors with antiangiogenic properties include endostatin, angiostatin, thrombospondin, pigment epithelium-derived factor, and others.19 Their balance is actively maintained, as exemplified by evidence, showing that after corneal injury, antiangiogenic factors are upregulated to maintain corneal avascularity.20 However, these mechanisms are not fail-proof, and numerous clinical conditions are known to involve ingrowth of vessels into the corneal tissue. Most pathological processes of the cornea that lead to vascularisation can be assigned to one of the three main categories: hypoxic (mainly contact lens wear), inflammatory (eg, infectious keratitis or corneal graft rejection), and loss of limbal barrier function (limbal stem cell deficiency, for instance, due to aniridia).21, 22, 23
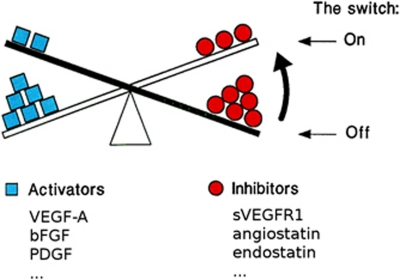
Figure 2.
The ‘angiogenic switch' hypothesis. In health or mild disease, pro-angiogenic factors are counteracted by the inhibitors of angiogenesis. Quiescent vasculature is stimulated to cause neovascularisation, if increasing levels of activators of angiogenesis tilt the balance towards vessel growth. Likewise, increased presence of inhibiting factors or removal of activators can tilt it back towards maintaining avascularity. VEGF, vascular endothelial growth factor; bFGF, basic fibroblast growth factor; PDGF, platelet-derived growth factor; sVEGFR1, soluble VEGF receptor 1. (Adapted from Hanahan and Folkman,12 with permission from Elsevier).
Presence of aberrant vessels in turn increases corneal oedema and leads to lipid deposition, haemorrhage, and scarring, further compromising corneal transparency and visual acuity.24 Neovascularisation also increases the rate of failure and rejection of corneal grafts.25 This has been attributed, at least in part, to clinically invisible lymphatic vessels, which abrogate the immunological privilege of the cornea.26, 27
Although aetiologies of corneal neovascularisation vary, the common endpoint is a breakdown of the angiogenic privilege.28 The following sections briefly characterise a selection of prominent pro- and antiangiogenic mediators, which may threaten or maintain this privilege.
Pro-angiogenic factors in the cornea
VEGF has been shown to be a key mediator of vasculogenesis and angiogenesis.29, 30 Its important contribution to vascular development is reflected in the fact that the deletion of a single VEGF allele is lethal in the mouse embryo.31 Members of the VEGF family promote a number of steps in the process of angiogenesis, including proteolytic activities, vascular EC proliferation and migration, inhibition of EC apoptosis, and recruitment of EC precursors.28, 32
Particularly VEGF-A is a potent survival factor and mitogen for ECs.33 It binds the tyrosine kinase receptors VEGFR-1 and VEGFR-2, with the pro-angiogenic signal being conveyed predominantly via VEGFR-2 in many tissues.34 Involvement of VEGF-A in corneal vessel growth was demonstrated in animal models of corneal neovascularisation, using VEGF-A blocking monoclonal antibodies; when implanted into the corneal stroma, these inhibit neovascularisation.35, 36
VEGF-C and VEGF-D, acting via VEGFR-3, make important contributions to growth and development of lymphatic vessels. Indeed, antibodies against VEGFR-3 specifically block lymphangiogenesis in the cornea.37
Expression of VEGF is increased by hypoxia and inflammation.38, 39 Hence, it is upregulated in the cornea following hypoxic injury, ocular wounding, and during acute inflammation in different animal models.36, 40, 41
FGF is a heparin-binding peptide, which stimulates migration and proliferation of ECs.42, 43 FGF binds to ECs of corneal blood and lymphatic vessels.44 Ellenberg et al19 demonstrated a role of FGF in promoting corneal angiogenesis, and suggested this to occur via upregulation of VEGF. Indeed, interplay between VEGF and FGF was proposed to occur at the receptoral and postreceptoral level.45, 46 One factor involved in linking the two pathways may be membrane-type 1 matrix metalloproteinase (MMP). Membrane-type 1 MMP was shown to increase FGF-induced VEGF upregulation and corneal neovascularisation in a mouse model and in an in vitro model.47
In fact, MMPs appear to be involved in angiogenesis in the most varied ways. For instance, apart from their general abilities to remodel the ECM and pave the way for growing vessels, MMP-9 releases VEGF from the ECM.48 However, antiangiogenic properties have also been detected for MMPs, as will be discussed below.
PDGF acts to stabilise vessels by attracting pericyte progenitor cells.49, 50 It has been suggested that VEGF antagonists are more effective in vessels which lack pericytes.51 Hence, a combination of VEGF- and PDGF-antagonists could be envisaged to inhibit neovascularisation. Indeed, blocking both VEGF and PDGF pathways was more effective in inhibiting corneal neovascularisation in rabbits than VEGF pathway blockade alone.52
Angiopoietins are protein growth factors whose action (via the tyrosine kinase receptor Tie-2) is required for formation of mature blood vessels.9 Angiopoietin-1 and angiopoietin-2 were found to modulate sensitivity to VEGF (Figure 1).53 In the cornea, inhibition of angiopoietin-2 suppressed angiogenesis; however, additional inhibition of angiopoietin-1 yielded no further suppression of angiogenesis.54
Antiangiogenic factors in the cornea
To counteract vasoproliferative effects of VEGF-A, soluble forms of VEGFR-1 are expressed by corneal epithelium.55 Ambati et al55 showed that in the absence of soluble VEGFR-1, mice develop corneal neovascularisation. Furthermore, VEGFR-1 is expressed in healthy human corneal epithelium, whereas in neovascularised human corneas, VEGFR-1 expression is significantly reduced.56
In addition, VEGFR-3 is ectopically expressed in corneal epithelium.57 This acts as a decoy mechanism to neutralise VEGF-C and VEGF-D, classically regarded as lymphangiogenic factors.
A protein showing structural relationship to hypoxia-inducible transcription factors (HIF) has been reported to impair HIF1α-mediated expression of VEGF.58 Mouse corneal epithelial cells strongly expressed this protein in vitro, particularly under hypoxic conditions. In these cells, expression of VEGF was low even under hypoxic conditions, but increased when the HIF-related protein was antagonised. In vivo, antagonisation induced neovascularisation already at normal levels of oxygen, suggesting an important role in maintaining corneal avascularity.
Pigment epithelium-derived factor has been identified as an important factor opposing ocular angiogenesis.59 It is expressed in the corneal tissue,60 and was detected in human tear fluid.61
The antiangiogenic properties of angiostatin, a fragment of plasminogen, were first shown in the context of tumour growth and metastasis.62 Presence of angiostatin was demonstrated in human corneal epithelium,63 and in mouse corneas during wound healing.20 It was found to inhibit corneal neovascularisation in different rodent models.64
Endostatin is a fragment of collagen XVIII, which induces EC apoptosis,65 and which is present in human cornea.66 MMPs were reported to be involved in generating endostatin via cleavage of collagen;67 together with pro-angiogenic properties of these proteolytic enzymes (vide supra), this exemplifies their ambiguous role in angiogenesis.
Thrombospondins are matricellular proteins able to inhibit migration and survival of vascular ECs.68 They are expressed in the normal cornea, and both thrombospondin-1 and thrombospondin-2 were found to suppress the inflammation-induced corneal neovascularisation in rodents.69, 70
Current therapeutic approaches towards corneal neovascularisation
The method of choice to treat corneal neovascularisation depends on the state of maturation of these vessels. Mature vessels often no longer rely on angiogenic mediators.71, 72 Here, surgical interventions such as fine-needle cauterisation, first reported by Pillai et al,73 may constitute the most effective treatment. However, during active vessel growth, pharmacological manipulation of molecular cues for vascular ECs suggests itself as a therapeutic approach. It has been suggested from ultrastructural and immunohistochemical analysis of vascularised human corneas that vessel maturation by pericyte recruitment may occur within less than 2 weeks after clinical diagnosis of corneal neovascularisation.74 This is likely to limit the time-frame available for successful antiangiogenic therapy. However, blockade of angiogenic growth factors may still be beneficial to prevent further sprouting of vascular ECs in cases in which the angiogenic stimulus persists.
Table 1 provides an overview of current indications for antiangiogenic therapy at the cornea. Anti-inflammatory agents (eg, steroids, cyclosporine A) are a classic means to suppress corneal inflammation and corneal neovascularisation.75 On top of their anti-inflammatory properties, steroids have been shown to inhibit proliferation and migration of vascular ECs.19 Using a rodent model to compare anti-lymphangiogenic effects of different topically applied corticosteroids, the strongest effect was measured for prednisolone, which may therefore render this substance particularly suitable to prevent rejection of corneal allografts.76 However, side effects of steroids are an important cause of ocular complications, whereas efficacy in cases of non-inflammatory-mediated corneal neovascularisation is limited. Hence, it appears desirable to target molecular factors of corneal angiogenesis more selectively, as occurs already in cancer treatment outside the eye and in macular disease. This field is currently emerging; important milestones are pointed out in the following section.
Table 1. Current indications for antiangiogenic therapya at the cornea (adapted from Cursiefen et al23).
| Infectious keratitis | Herpetic |
| Bacterial | |
| Fungal | |
| Parasitic | |
| Inflammatory conditions | Mucous membrane pemphigoid |
| Atopic conjunctivitis | |
| Rosacea | |
| Lyell's syndrome | |
| Stevens–Johnson syndrome | |
| Corneal graft | Preoperative conditioning |
| Postoperative prevention of graft rejection/failure | |
| Loss of limbal barrier function | Limbal stem cell deficiency |
| Corneal burns or other injury |
Note that antiangiogenic therapy does not exclude or replace treating the cause of the underlying condition, where applicable.87
Translational aspects of corneal angiogenesis and clinical experience with novel therapies
In 1971, Folkman77 published a seminal article in which he suggested that tumour growth depended on angiogenesis, making it a suitable target for therapeutic interventions. Some of the work leading to this hypothesis, and much of the work undertaken since, used the cornea as an experimental model. Because of its angiogenic privilege, the cornea is suitable to show vessel-inducing effects of tumour cells or putative pro- or antiangiogenic factors in vivo.78
It is worth stressing that despite similarities, vascular growth may slightly differ between distinct anatomic locations.79, 80 For instance, VEGFR-1 acts as a decoy receptor in the cornea, but induces neovascularisation in the retina, as shown by reduction of retinal neovascularisation upon experimental disruption of VEGFR-1.81 Overall, the natural avascularity of the cornea makes it quite an atypical environment to study vascular development.82
Nevertheless, experimental data from corneal angiogenesis assays contributed to the development of antiangiogenic drugs such as VEGF-inhibitors, which have been formally approved for cancer treatment, and form the mainstay for treating neovascular age-related macular degeneration.75
Curiously, development of specific antiangiogenic agents for clinical use in the anterior ocular segment remains at a less advanced stage, and use of available agents occurs ‘off-label'. Actively growing vascular sprouts can be targeted using topical application of VEGF-inhibitors such as the humanised monoclonal antibody bevacizumab, initially approved for the treatment of metastatic colorectal cancer.72 The use of this and structurally related anti-VEGF antibodies have shown clinical effects in the anterior segment of the eye. A Medline search identifies a number of case series reporting the clinical use of bevacizumab in corneal neovascularisation (Table 2). At large, these studies suggest regression of neovascularisation following anti-VEGF treatment. This is despite the considerable variability in the treatment regimens used, and in the nature and severity of the conditions treated. Although most reports conclude that topical anti-VEGF therapy for corneal neovascularisation appears safe, adverse effects such as corneal thinning and reduced epithelial healing have also been acknowledged.83 These may be due to neurotrophic effects of VEGF, leading to reduction of the numerous corneal nerves when VEGF is inhibited.84 Currently, no data from randomised controlled clinical trials is available. Such studies are warranted to confirm safety and efficacy of these anti-VEGF treatment for corneal neovascularisation, with inhibition of corneal neovascularisation having been proposed as a clinically relevant endpoint.23
Table 2. Clinical reports of the use of bevacizumab in corneal neovascularisation.
| Authors (year) | Study design | Sample size | Patient characteristics | Application mode | Dosage | Duration of treatment | Adverse reactions | Effect on corneal vascularisation |
|---|---|---|---|---|---|---|---|---|
| DeStafeno and Kim (2007)105 | Prospective case series | 2 patients (2 eyes) | Ocular trauma and pemphigoid, steroid treatment failed | Eye drops | 10 mg/ml (1%), 4 drops per day | Not specified | None | Regression |
| Uy et al (2008)106 | Retrospective case series | 2 patients (3 eyes) | Stevens–Johnson syndrome | Eye drops | 25 mg/ml (2.5%), 4 drops per day | 3 months | None | Regression |
| Bock et al (2008)107 | Prospective case series | 5 patients | Following limbal stem cell transplant and/or keratoplasty | Eye drops | 5 mg/ml (0.5%), 5 drops per day | 0.5/6 months | None | Regression |
| Kim et al (2008)83 | Prospective case series | 7 patients (10 eyes) | Various aetiologies | Eye drops | 12.5 mg/ml (1.25%) 2 drops per day | 3 months | Epitheliopathy and corneal thinning | Regression |
| Saxena et al (2009)108 | Case report | 1 patient (1 eye) | Corneal graft rejection | Eye drops | 1 mg/ml (0.1%) 2 drops per day | 15 days | None | Regression |
| Jacobs et al (2009)109 | Prospective case series | 5 patients (7 eyes) | Patients wearing the Boston Ocular Surface Prosthesis | Eye drops | 10 mg/ml (1%), 2 drops per day | 3 months | None | Regression of small vessels |
| Koenig et al (2009)110 | Prospective case series | 27 patients (30 eyes) | Progressive vascularisation, various aetiologies | Eye drops | 5 mg/ml (0.5%), 5 drops per day | 0.5–12 months | None | Vessel diameter reduction |
| Dastjerdi et al (2009)111 | Prospective case series | 10 patients (10 eyes) | Stable vascularisation, various aetiologies | Eye drops | 10 mg/ml (1%), 2 vs 4 drops per day | 3 weeks | None | Regression |
| Doctor et al (2008)112 | Retrospective case series | 7 patients (8 eyes) | Various aetiologies | Subconjunctival injection | 2.5 mg/0.1 ml, monthly injections | Up to 3 months | None | Regression |
| Bahar et al (2008)113 | Retrospective case series | 10 patients (10 eyes) | Steroid treatment failed, various aetiologies | Subconjunctival injection | 2.5 mg/0.1 ml, 2.1±0.8 (SD) injections | Not specified | None | Regression |
| Edurmus and Totan (2007)114 | Retrospective case study | 2 patients (2 eyes) | Dry eye keratitis, graft failure | Subconjunctival injection | 2.5 mg/0.1 ml | Single application | None | Regression |
| Zaki and Farid (2010)115 | Prospective case series | 10 patients (10 eyes) | Chronic inflammation, healed ulcers | Subconjunctival injection | 2.5 mg/0.1 ml | Single application | None | Regression |
| Jeong et al (2011)116 | Prospective case series | 15 patients (15 eyes) | Steroid treatment failed, various aetiologies | Subconjunctival injection | 5 mg/0.2 ml | Single application | Punctate epithelial erosions | Regression |
| Vassileva and Hergeldzhieva (2009)117 | Prospective case series | 14 patients (14 eyes) | Pre- or post-keratoplasty | Subconjunctival, perilimbal, and/or intracorneal injection | 2.5 mg/0.1 ml per affected quadrant, 1 or 2 injections | Not specified | None | Regression |
| Oh et al (2009)118 | Prospective case series | 3 patients (3 eyes) | Lipid keratopathy, corneal vascularisation of unknown origin | Combined subconjunctival and intracorneal injection | 1.25 mg/0.05 ml subconjunctivally, 1.25 mg/0.05 ml intracorneally, 2–3 injections | Monthly intervals | Intrastromal haemmorhage (resolved spontaneously) | Regression |
| Yeung et al (2011)119 | Retrospective case series | 12 patients (12 eyes) | Progressive vascularisation, steroid treatment failed, various aetiologies | Combined subconjunctival and intracorneal injection | 1.25 mg/0.05 ml subconjunctivally, 1.25 mg/0.05 ml intracorneally, 1 to 3 injections | Up to 8 months | None | Regression |
The only antiangiogenic compound for corneal neovascular disease, which has reached a controlled clinical testing, is an antisense oligonucleotide, designed to inhibit the expression of insulin receptor substrate-1 (IRS-1).85 IRSs are cytosolic adaptor proteins involved in the organisation of growth hormone and cytokine receptor signalling. Pre-clinical studies had shown targeting IRS-1 to inhibit corneal neovascularisation in rats, possibly mediated via downregulation of interleukin-1β.86 Currently, this antisense oligonucleotide against IRS-1 is being investigated in a phase III clinical trial to determine its clinical value for topical inhibition of corneal neovascularisation.85, 87
This example points out that, apart from administration of neutralising antibodies, targeting gene expression has now received some attention as a potential means to control corneal angiogenic and antiangiogenic mediators. Recent evidence from this field of study will be discussed next.
Future therapies may rely on local gene therapy to influence (anti-)angiogenic factors
With more knowledge now available regarding mediators of angiogenesis, targeting these pathways by gene therapy emerges as a promising means of fighting neovascularisation in the eye.28 This approach has been taken into clinical testing for subfoveal choroidal neovascularisation,80 with the anterior segment now striving to follow suit. Here, injection of an adenovirus vector encoding a soluble Tie-2 receptor inhibited neovascularisation in a mouse model of corneal injury.88 In a similar vein, ex vivo transduction of corneal tissue with a lentivirus containing the human endostatin gene has been proposed as a viable method to prevent corneal graft neovascularisation and subsequent rejection in high-risk corneal transplants.89 Adenovirus-mediated transduction of corneal ECs with soluble VEGFR-1 successfully inhibited corneal neovascularisation in a rodent model.90 The same group also used adeno-associated virus to reduce the development of experimental corneal neovascularisation.91 A somewhat different approach uses intracorneal gene therapy to express VEGFR-1 intracellularly, leading to disruption of autocrine feedback loops, decreased VEGF secretion, and inhibition of neovascularisation.92 This group was also able to show viability gene transfer for the same receptor using a non-viral vector.93 Another non-viral method employed experimentally for corneal antiangiogenic gene therapy was electroporation.94 Small interfering RNA (siRNA) constitutes another promising technique, which can be used locally in the eye to silence relevant genes.95 Here, difficulties may arise when it comes to determining an effective target sequence. Despite the help of online tools, the best siRNA sequence currently needs to be selected empirically.28 Nevertheless, siRNA targeting VEGF-A and/or its receptors was successfully shown to inhibit inflammation-induced corneal neovascularisation in mice.96
Interestingly, amidst all the efforts to counteract corneal neovascularisation, some authors have chosen to use gene transfer to promote corneal angiogenesis.97, 98, 99 Here, induced corneal neovascularisation serves as an assay to investigate the efficacy of gene transfer to the cornea or indeed the impact of the transfected pro-angiogenic gene on corneal angiogenic privilege. Through these efforts, data obtained in the cornea may — once again — support the endeavour to promote angiogenesis as a therapeutic approach towards ischaemic disorders elsewhere.
Conclusions
Corneal neovascularisation is one out of a multitude of angiogenesis-dependent diseases.100 Although many of the early studies on tumour angiogenesis were carried out using the angiogenic privilege of the cornea as a model system,101, 102 anticancer drugs such as bevacizumab are now proving to be of benefit for the treatment of corneal disease.103 However, clinical data on safety and efficacy of bevacizumab is currently limited to non-randomised, largely non-comparative case series, and antiangiogenic agents developed and approved specifically for corneal neovascularisation are not yet available.
The relative accessibility and segregation of the ocular compartment makes it a good candidate for local gene therapy.80 A plethora of in vivo studies to test this approach have been carried out in recent years, so far yielding at least one multicenter trial that aims to bring a specific corneal angiogenesis inhibitor into the ophthalmic clinic.85 Future challenges include the achievement of successful delivery and stable expression of therapeutic genes.104
In summary, increased understanding of molecules relevant in vascular development is on the cusp of translating into specific therapeutic agents, which will be useful in the ophthalmic clinic to specifically target angiogenesis, and treat or prevent corneal neovascularisation. In this context, randomised controlled trials to establish safe and effective treatment regimens for these agents are obligatory.
The author declares no conflict of interest.
References
- Lee P, Wang CC, Adamis AP. Ocular neovascularization: an epidemiologic review. Surv Ophthalmol. 1998;43:245–269. doi: 10.1016/s0039-6257(98)00035-6. [DOI] [PubMed] [Google Scholar]
- Michaelson IC. The mode of development of the vascular system of the retina. With some observations on its significance for certain retinal diseases. Trans Ophthalmol Soc UK. 1948;68:137. [Google Scholar]
- Campbell FW, Michaelson IC. Blood-vessel formation in the cornea. Br J Ophthalmol. 1949;33:248–255. doi: 10.1136/bjo.33.4.248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pardanaud L, Yassine F, Dieterlen-Lievre F. Relationship between vasculogenesis, angiogenesis and haemopoiesis during avian ontogeny. Development. 1989;105:473–485. doi: 10.1242/dev.105.3.473. [DOI] [PubMed] [Google Scholar]
- Risau W. Mechanisms of angiogenesis. Nature. 1997;386:671–674. doi: 10.1038/386671a0. [DOI] [PubMed] [Google Scholar]
- Choi K, Kennedy M, Kazarov A, Papadimitriou JC, Keller G. A common precursor for hematopoietic and endothelial cells. Development. 1998;125:725–732. doi: 10.1242/dev.125.4.725. [DOI] [PubMed] [Google Scholar]
- Hiraoka N, Allen E, Apel IJ, Gyetko MR, Weiss SJ. Matrix metalloproteinases regulate neovascularization by acting as pericellular fibrinolysins. Cell. 1998;95:365–377. doi: 10.1016/s0092-8674(00)81768-7. [DOI] [PubMed] [Google Scholar]
- Gerhardt H, Golding M, Fruttiger M, Ruhrberg C, Lundkvist A, Abramsson A, et al. VEGF guides angiogenic sprouting utilizing endothelial tip cell filopodia. J Cell Biol. 2003;161:1163–1177. doi: 10.1083/jcb.200302047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yancopoulos GD, Davis S, Gale NW, Rudge JS, Wiegand SJ, Holash J. Vascular-specific growth factors and blood vessel formation. Nature. 2000;407:242–248. doi: 10.1038/35025215. [DOI] [PubMed] [Google Scholar]
- Folkman J. Angiogenesis in cancer, vascular, rheumatoid and other disease. Nat Med. 1995;1:27–31. doi: 10.1038/nm0195-27. [DOI] [PubMed] [Google Scholar]
- Dorrell M, Uusitalo-Jarvinen H, Aguilar E, Friedlander M. Ocular neovascularization: basic mechanisms and therapeutic advances. Surv Ophthalmol. 2007;52:S3–S19. doi: 10.1016/j.survophthal.2006.10.017. [DOI] [PubMed] [Google Scholar]
- Hanahan D, Folkman J. Patterns and emerging mechanisms of the angiogenic switch during tumorigenesis. Cell. 1996;86:353–364. doi: 10.1016/s0092-8674(00)80108-7. [DOI] [PubMed] [Google Scholar]
- Carmeliet P. Mechanisms of angiogenesis and arteriogenesis. Nat Med. 2000;6:389–395. doi: 10.1038/74651. [DOI] [PubMed] [Google Scholar]
- Burger PC, Chandler DB, Klintworth GK. Corneal neovascularization as studied by scanning electron microscopy of vascular casts. Lab Invest. 1983;48:169–180. [PubMed] [Google Scholar]
- Beebe DC. Maintaining transparency: a review of the developmental physiology and pathophysiology of two avascular tissues. Semin Cell Dev Biol. 2008;19:125–133. doi: 10.1016/j.semcdb.2007.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pufe T, Petersen WJ, Miosge N, Goldring MB, Mentlein R, Varoga DJ, et al. Endostatin/collagen XVIII--an inhibitor of angiogenesis--is expressed in cartilage and fibrocartilage. Matrix Biol. 2004;23:267–276. doi: 10.1016/j.matbio.2004.06.003. [DOI] [PubMed] [Google Scholar]
- Yoshioka M, Yuasa S, Matsumura K, Kimura K, Shiomi T, Kimura N, et al. Chondromodulin-I maintains cardiac valvular function by preventing angiogenesis. Nat Med. 2006;12:1151–1159. doi: 10.1038/nm1476. [DOI] [PubMed] [Google Scholar]
- Goldberg MF. Persistent fetal vasculature (PFV): an integrated interpretation of signs and symptoms associated with persistent hyperplastic primary vitreous (PHPV). LIV Edward Jackson Memorial Lecture. Am J Ophthalmol. 1997;124:587–626. doi: 10.1016/s0002-9394(14)70899-2. [DOI] [PubMed] [Google Scholar]
- Ellenberg D, Azar DT, Hallak JA, Tobaigy F, Han KY, Jain S, et al. Novel aspects of corneal angiogenic and lymphangiogenic privilege. Prog Retin Eye Res. 2010;29:208–248. doi: 10.1016/j.preteyeres.2010.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabison E, Chang J-H, Hernández-Quintela E, Javier J, Lu PC, Ye H, et al. Anti-angiogenic role of angiostatin during corneal wound healing. Exp Eye Res. 2004;78:579–589. doi: 10.1016/j.exer.2003.09.005. [DOI] [PubMed] [Google Scholar]
- Chang JH, Gabison EE, Kato T, Azar DT. Corneal neovascularization. Curr Opin Ophthalmol. 2001;12:242–249. doi: 10.1097/00055735-200108000-00002. [DOI] [PubMed] [Google Scholar]
- Azar DT. Corneal angiogenic privilege: angiogenic and antiangiogenic factors in corneal avascularity, vasculogenesis, and wound healing (an American Ophthalmological Society thesis) Trans Am Ophthalmol Soc. 2006;104:264–302. [PMC free article] [PubMed] [Google Scholar]
- Cursiefen C, Colin J, Dana R, Diaz-Llopis M, Faraj LA, Garcia-Delpech S, et al. Consensus statement on indications for anti-angiogenic therapy in the management of corneal diseases associated with neovascularisation: outcome of an expert roundtable Br J Ophthalmol 2011. e-pub ahead of print 28 June 2011; doi: 10.1136/bjo.2011.204701 [DOI] [PubMed]
- Qazi Y, Maddula S, Ambati BK. Mediators of ocular angiogenesis. J Genet. 2009;88:495–515. doi: 10.1007/s12041-009-0068-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachmann B, Taylor RS, Cursiefen C.Corneal neovascularization as a risk factor for graft failure and rejection after keratoplasty: an evidence-based meta-analysis Ophthalmology 20101171300–1305.e7. [DOI] [PubMed] [Google Scholar]
- Cursiefen C, Chen L, Dana MR, Streilein JW. Corneal lymphangiogenesis: evidence, mechanisms, and implications for corneal transplant immunology. Cornea. 2003;22:273–281. doi: 10.1097/00003226-200304000-00021. [DOI] [PubMed] [Google Scholar]
- Dietrich T, Bock F, Yuen D, Hos D, Bachmann BO, Zahn G, et al. Cutting edge: lymphatic vessels, not blood vessels, primarily mediate immune rejections after transplantation. J Immunol. 2010;184:535–539. doi: 10.4049/jimmunol.0903180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajappa M, Saxena P, Kaur J. Ocular angiogenesis: mechanisms and recent advances in therapy. Adv Clin Chem. 2010;50:103–121. [PubMed] [Google Scholar]
- Millauer B, Wizigmann-Voos S, Schnürch H, Martinez R, Møller NP, Risau W, et al. High affinity VEGF binding and developmental expression suggest Flk-1 as a major regulator of vasculogenesis and angiogenesis. Cell. 1993;72:835–846. doi: 10.1016/0092-8674(93)90573-9. [DOI] [PubMed] [Google Scholar]
- Leung DW, Cachianes G, Kuang WJ, Goeddel DV, Ferrara N. Vascular endothelial growth factor is a secreted angiogenic mitogen. Science. 1989;246:1306–1309. doi: 10.1126/science.2479986. [DOI] [PubMed] [Google Scholar]
- Ferrara N, Carver-Moore K, Chen H, Dowd M, Lu L, O'Shea KS, et al. Heterozygous embryonic lethality induced by targeted inactivation of the VEGF gene. Nature. 1996;380:439–442. doi: 10.1038/380439a0. [DOI] [PubMed] [Google Scholar]
- Terman BI, Dougher-Vermazen M, Carrion ME, Dimitrov D, Armellino DC, Gospodarowicz D, et al. Identification of the KDR tyrosine kinase as a receptor for vascular endothelial cell growth factor. Biochem Biophys Res Commun. 1992;187:1579–1586. doi: 10.1016/0006-291x(92)90483-2. [DOI] [PubMed] [Google Scholar]
- Penn JS, Madan A, Caldwell RB, Bartoli M, Caldwell RW, Hartnett ME. Vascular endothelial growth factor in eye disease. Prog Retin Eye Res. 2008;27:331–371. doi: 10.1016/j.preteyeres.2008.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaipainen A, Korhonen J, Pajusola K, Aprelikova O, Persico MG, Terman BI, et al. The related FLT4, FLT1, and KDR receptor tyrosine kinases show distinct expression patterns in human fetal endothelial cells. J Exp Med. 1993;178:2077–2088. doi: 10.1084/jem.178.6.2077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binétruy-Tournaire R, Demangel C, Malavaud B, Vassy R, Rouyre S, Kraemer M, et al. Identification of a peptide blocking vascular endothelial growth factor (VEGF)-mediated angiogenesis. EMBO J. 2000;19:1525–1533. doi: 10.1093/emboj/19.7.1525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amano S, Rohan R, Kuroki M, Tolentino M, Adamis AP. Requirement for vascular endothelial growth factor in wound- and inflammation-related corneal neovascularization. Invest Ophthalmol Vis Sci. 1998;39:18–22. [PubMed] [Google Scholar]
- Bock F, Onderka J, Dietrich T, Bachmann B, Pytowski B, Cursiefen C. Blockade of VEGFR3-signalling specifically inhibits lymphangiogenesis in inflammatory corneal neovascularisation. Graefes Arch Clin Exp Ophthalmol. 2008;246:115–119. doi: 10.1007/s00417-007-0683-5. [DOI] [PubMed] [Google Scholar]
- Cheng T, Cao W, Wen R, Steinberg RH, LaVail MM. Prostaglandin E2 induces vascular endothelial growth factor and basic fibroblast growth factor mRNA expression in cultured rat Müller cells. Invest Ophthalmol Vis Sci. 1998;39:581–591. [PubMed] [Google Scholar]
- Behzadian MA, Wang XL, Al-Shabrawey M, Shabrawey M, Caldwell RB. Effects of hypoxia on glial cell expression of angiogenesis-regulating factors VEGF and TGF-beta. Glia. 1998;24:216–225. [PubMed] [Google Scholar]
- Kvanta A, Sarman S, Fagerholm P, Seregard S, Steen B. Expression of matrix metalloproteinase-2 (MMP-2) and vascular endothelial growth factor (VEGF) in inflammation-associated corneal neovascularization. Exp Eye Res. 2000;70:419–428. doi: 10.1006/exer.1999.0790. [DOI] [PubMed] [Google Scholar]
- Mastyugin V, Mosaed S, Bonazzi A, Dunn MW, Schwartzman ML. Corneal epithelial VEGF and cytochrome P450 4B1 expression in a rabbit model of closed eye contact lens wear. Curr Eye Res. 2001;23:1–10. doi: 10.1076/ceyr.23.1.1.5422. [DOI] [PubMed] [Google Scholar]
- Terranova VP, DiFlorio R, Lyall RM, Hic S, Friesel R, Maciag T. Human endothelial cells are chemotactic to endothelial cell growth factor and heparin. J Cell Biol. 1985;101:2330–2334. doi: 10.1083/jcb.101.6.2330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gospodarowicz D, Abraham JA, Schilling J. Isolation and characterization of a vascular endothelial cell mitogen produced by pituitary-derived folliculo stellate cells. Proc Natl Acad Sci USA. 1989;86:7311–7315. doi: 10.1073/pnas.86.19.7311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soubrane G, Jerdan J, Karpouzas I, Fayein NA, Glaser B, Coscas G, et al. Binding of basic fibroblast growth factor to normal and neovascularized rabbit cornea. Invest Ophthalmol Vis Sci. 1990;31:323–333. [PubMed] [Google Scholar]
- Presta M, Dell'Era P, Mitola S, Moroni E, Ronca R, Rusnati M. Fibroblast growth factor/fibroblast growth factor receptor system in angiogenesis. Cytokine Growth Factor Rev. 2005;16:159–178. doi: 10.1016/j.cytogfr.2005.01.004. [DOI] [PubMed] [Google Scholar]
- Murakami M, Simons M. Fibroblast growth factor regulation of neovascularization. Curr Opin Hematol. 2008;15:215–220. doi: 10.1097/MOH.0b013e3282f97d98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onguchi T, Han KY, Chang J-H, Azar DT. Membrane type-1 matrix metalloproteinase potentiates basic fibroblast growth factor-induced corneal neovascularization. Am J Pathol. 2009;174:1564–1571. doi: 10.2353/ajpath.2009.080452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergers G, Brekken R, McMahon G, Vu TH, Itoh T, Tamaki K, et al. Matrix metalloproteinase-9 triggers the angiogenic switch during carcinogenesis. Nat Cell Biol. 2000;2:737–744. doi: 10.1038/35036374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindahl P, Johansson BR, Levéen P, Betsholtz C. Pericyte loss and microaneurysm formation in PDGF-B-deficient mice. Science. 1997;277:242–245. doi: 10.1126/science.277.5323.242. [DOI] [PubMed] [Google Scholar]
- Abramsson A, Lindblom P, Betsholtz C. Endothelial and nonendothelial sources of PDGF-B regulate pericyte recruitment and influence vascular pattern formation in tumors. J Clin Invest. 2003;112:1142–1151. doi: 10.1172/JCI18549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glade Bender J, Cooney EM, Kandel JJ, Yamashiro DJ. Vascular remodeling and clinical resistance to antiangiogenic cancer therapy. Drug Resist Updat. 2004;7:289–300. doi: 10.1016/j.drup.2004.09.001. [DOI] [PubMed] [Google Scholar]
- Pérez-Santonja JJ, Campos-Mollo E, Lledó-Riquelme M, Javaloy J, Alió JL.Inhibition of corneal neovascularization by topical bevacizumab (anti-VEGF) and Sunitinib (Anti-VEGF and Anti-PDGF) in an animal model Am J Ophthalmol 2010150519–528.e1. [DOI] [PubMed] [Google Scholar]
- Visconti RP, Richardson CD, Sato TN. Orchestration of angiogenesis and arteriovenous contribution by angiopoietins and vascular endothelial growth factor (VEGF) Proc Natl Acad Sci USA. 2002;99:8219–8224. doi: 10.1073/pnas.122109599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coxon A, Bready J, Min H, Kaufman S, Leal J, Yu D, et al. Context-dependent role of angiopoietin-1 inhibition in the suppression of angiogenesis and tumor growth: implications for AMG 386, an angiopoietin-1/2-neutralizing peptibody. Mol Cancer Ther. 2010;9:2641–2651. doi: 10.1158/1535-7163.MCT-10-0213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ambati BK, Nozaki M, Singh N, Takeda A, Jani PD, Suthar T, et al. Corneal avascularity is due to soluble VEGF receptor-1. Nature. 2006;443:993–997. doi: 10.1038/nature05249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ambati BK, Patterson E, Jani P, Jenkins C, Higgins E, Singh N, et al. Soluble vascular endothelial growth factor receptor-1 contributes to the corneal antiangiogenic barrier. Br J Ophthalmol. 2007;91:505–508. doi: 10.1136/bjo.2006.107417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cursiefen C, Chen L, Saint-Geniez M, Hamrah P, Jin Y, Rashid S, et al. Nonvascular VEGF receptor 3 expression by corneal epithelium maintains avascularity and vision. Proc Natl Acad Sci USA. 2006;103:11405–11410. doi: 10.1073/pnas.0506112103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makino Y, Cao R, Svensson K, Bertilsson G, Asman M, Tanaka H, et al. Inhibitory PAS domain protein is a negative regulator of hypoxia-inducible gene expression. Nature. 2001;414:550–554. doi: 10.1038/35107085. [DOI] [PubMed] [Google Scholar]
- Dawson DW, Volpert OV, Gillis P, Crawford SE, Xu H, Benedict W, et al. Pigment epithelium-derived factor: a potent inhibitor of angiogenesis. Science. 1999;285:245–248. doi: 10.1126/science.285.5425.245. [DOI] [PubMed] [Google Scholar]
- Karakousis PC, John SK, Behling KC, Surace EM, Smith JE, Hendrickson A, et al. Localization of pigment epithelium derived factor (PEDF) in developing and adult human ocular tissues. Mol Vis. 2001;7:154–163. [PubMed] [Google Scholar]
- Abdiu O, Van Setten G. Antiangiogenic activity in tears: presence of pigment-epithelium-derived factor. New insights and preliminary results. Ophthalmic Res. 2008;40:16–18. doi: 10.1159/000111153. [DOI] [PubMed] [Google Scholar]
- O'Reilly MS, Holmgren L, Shing Y, Chen C, Rosenthal RA, Moses M, et al. Angiostatin: a novel angiogenesis inhibitor that mediates the suppression of metastases by a Lewis lung carcinoma. Cell. 1994;79:315–328. doi: 10.1016/0092-8674(94)90200-3. [DOI] [PubMed] [Google Scholar]
- Twining SS, Wilson PM, Ngamkitidechakul C. Extrahepatic synthesis of plasminogen in the human cornea is up-regulated by interleukins-1alpha and -1beta. Biochem J. 1999;339 ( Pt 3:705–712. [PMC free article] [PubMed] [Google Scholar]
- Ambati BK, Joussen AM, Ambati J, Moromizato Y, Guha C, Javaherian K, et al. Angiostatin inhibits and regresses corneal neovascularization. Arch Ophthalmol. 2002;120:1063–1068. doi: 10.1001/archopht.120.8.1063. [DOI] [PubMed] [Google Scholar]
- Tonini T, Rossi F, Claudio PP. Molecular basis of angiogenesis and cancer. Oncogene. 2003;22:6549–6556. doi: 10.1038/sj.onc.1206816. [DOI] [PubMed] [Google Scholar]
- Lin H-C, Chang J-H, Jain S, Gabison EE, Kure T, Kato T, et al. Matrilysin cleavage of corneal collagen type XVIII NC1 domain and generation of a 28-kDa fragment. Invest Ophthalmol Vis Sci. 2001;42:2517–2524. [PubMed] [Google Scholar]
- O'Reilly MS, Wiederschain D, Stetler-Stevenson WG, Folkman J, Moses MA. Regulation of angiostatin production by matrix metalloproteinase-2 in a model of concomitant resistance. J Biol Chem. 1999;274:29568–29571. doi: 10.1074/jbc.274.41.29568. [DOI] [PubMed] [Google Scholar]
- Armstrong LC, Bornstein P. Thrombospondins 1 and 2 function as inhibitors of angiogenesis. Matrix Biol. 2003;22:63–71. doi: 10.1016/s0945-053x(03)00005-2. [DOI] [PubMed] [Google Scholar]
- Cursiefen C, Masli S, Ng TF, Dana MR, Bornstein P, Lawler J, et al. Roles of thrombospondin-1 and -2 in regulating corneal and iris angiogenesis. Invest Ophthalmol Vis Sci. 2004;45:1117–1124. doi: 10.1167/iovs.03-0940. [DOI] [PubMed] [Google Scholar]
- Volpert OV, Tolsma SS, Pellerin S, Feige JJ, Chen H, Mosher DF, et al. Inhibition of angiogenesis by thrombospondin-2. Biochem Biophys Res Commun. 1995;217:326–332. doi: 10.1006/bbrc.1995.2780. [DOI] [PubMed] [Google Scholar]
- Oshima Y, Oshima S, Nambu H, Kachi S, Takahashi K, Umeda N, et al. Different effects of angiopoietin-2 in different vascular beds: new vessels are most sensitive. FASEB J. 2005;19:963–965. doi: 10.1096/fj.04-2209fje. [DOI] [PubMed] [Google Scholar]
- Lin C-T, Hu F-R, Kuo K-T, Chen YM, Chu HS, Lin YH, et al. The different effects of early and late bevacizumab (Avastin) injection on inhibiting corneal neovascularization and conjunctivalization in rabbit limbal insufficiency. Invest Ophthalmol Vis Sci. 2010;51:6277–6285. doi: 10.1167/iovs.09-4571. [DOI] [PubMed] [Google Scholar]
- Pillai CT, Dua HS, Hossain P. Fine needle diathermy occlusion of corneal vessels. Invest Ophthalmol Vis Sci. 2000;41:2148–2153. [PubMed] [Google Scholar]
- Cursiefen C, Hofmann-Rummelt C, Küchle M, Schlötzer-Schrehardt U. Pericyte recruitment in human corneal angiogenesis: an ultrastructural study with clinicopathological correlation. Br J Ophthalmol. 2003;87:101–106. doi: 10.1136/bjo.87.1.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regenfuss B, Bock F, Parthasarathy A, Cursiefen C. Corneal (lymph)angiogenesis--from bedside to bench and back: a tribute to Judah Folkman. Lymphat Res Biol. 2008;6:191–201. doi: 10.1089/lrb.2008.6348. [DOI] [PubMed] [Google Scholar]
- Hos D, Saban DR, Bock F, Regenfuss B, Onderka J, Masli S, et al. Suppression of inflammatory corneal lymphangiogenesis by application of topical corticosteroids. Arch Ophthalmol. 2011;129 (4:445–452. doi: 10.1001/archophthalmol.2011.42. [DOI] [PubMed] [Google Scholar]
- Folkman J. Tumor angiogenesis: therapeutic implications. N Engl J Med. 1971;285:1182–1186. doi: 10.1056/NEJM197111182852108. [DOI] [PubMed] [Google Scholar]
- Kenyon BM, Voest EE, Chen CC, Flynn E, Folkman J, D'Amato RJ, et al. A model of angiogenesis in the mouse cornea. Invest Ophthalmol Vis Sci. 1996;37:1625–1632. [PubMed] [Google Scholar]
- Campochiaro PA. Ocular versus extraocular neovascularization: mirror images or vague resemblances. Invest Ophthalmol Vis Sci. 2006;47:462–474. doi: 10.1167/iovs.05-1494. [DOI] [PubMed] [Google Scholar]
- Campochiaro PA. Potential applications for RNAi to probe pathogenesis and develop new treatments for ocular disorders. Gene Ther. 2006;13:559–562. doi: 10.1038/sj.gt.3302653. [DOI] [PubMed] [Google Scholar]
- Luttun A, Tjwa M, Moons L, Wu Y, Angelillo-Scherrer A, Liao F, et al. Revascularization of ischemic tissues by PlGF treatment, and inhibition of tumor angiogenesis, arthritis and atherosclerosis by anti-Flt1. Nat Med. 2002;8:831–840. doi: 10.1038/nm731. [DOI] [PubMed] [Google Scholar]
- Auerbach R, Akhtar N, Lewis RL, Shinners BL. Angiogenesis assays: problems and pitfalls. Cancer Metastasis Rev. 2000;19:167–172. doi: 10.1023/a:1026574416001. [DOI] [PubMed] [Google Scholar]
- Kim SW, Ha BJ, Kim EK, Tchah H, Kim TI. The effect of topical bevacizumab on corneal neovascularization. Ophthalmology. 2008;115:e33–e38. doi: 10.1016/j.ophtha.2008.02.013. [DOI] [PubMed] [Google Scholar]
- Yu CQ, Zhang M, Matis KI, Kim C, Rosenblatt MI. Vascular endothelial growth factor mediates corneal nerve repair. Invest Ophthalmol Vis Sci. 2008;49:3870–3878. doi: 10.1167/iovs.07-1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cursiefen C, Bock F, Horn FK, Kruse FE, Seitz B, Borderie V, et al. GS-101 antisense oligonucleotide eye drops inhibit corneal neovascularization: interim results of a randomized phase II trial. Ophthalmology. 2009;116:1630–1637. doi: 10.1016/j.ophtha.2009.04.016. [DOI] [PubMed] [Google Scholar]
- Andrieu-Soler C, Berdugo M, Doat M, Courtois Y, BenEzra D, Behar-Cohen F. Downregulation of IRS-1 expression causes inhibition of corneal angiogenesis. Invest Ophthalmol Vis Sci. 2005;46:4072–4078. doi: 10.1167/iovs.05-0105. [DOI] [PubMed] [Google Scholar]
- Bock F, Regenfuß B, Cursiefen C. [Antiangiogenic therapy at the ocular surface: When, what and why?] Ophthalmologe. 2011;108:230–236. doi: 10.1007/s00347-010-2262-0. [DOI] [PubMed] [Google Scholar]
- Singh N, Macnamara E, Rashid S, Ambati J, Kontos CD, Higgins E, et al. Systemic soluble Tie2 expression inhibits and regresses corneal neovascularization. Biochem Biophys Res Commun. 2005;332:194–199. doi: 10.1016/j.bbrc.2005.04.108. [DOI] [PubMed] [Google Scholar]
- Murthy RC, McFarland TJ, Yoken J, Chen S, Barone C, Burke D, et al. Corneal transduction to inhibit angiogenesis and graft failure. Invest Ophthalmol Vis Sci. 2003;44:1837–1842. doi: 10.1167/iovs.02-0853. [DOI] [PubMed] [Google Scholar]
- Lai CM, Brankov M, Zaknich T, Lai YK, Shen WY, Constable IJ, et al. Inhibition of angiogenesis by adenovirus-mediated sFlt-1 expression in a rat model of corneal neovascularization. Hum Gene Ther. 2001;12:1299–1310. doi: 10.1089/104303401750270959. [DOI] [PubMed] [Google Scholar]
- Lai YK, Shen WY, Brankov M, Lai CM, Constable IJ, Rakoczy PE. Potential long-term inhibition of ocular neovascularisation by recombinant adeno-associated virus-mediated secretion gene therapy. Gene Ther. 2002;9:804–813. doi: 10.1038/sj.gt.3301695. [DOI] [PubMed] [Google Scholar]
- Singh N, Amin S, Richter E, Rashid S, Scoglietti V, Jani PD, et al. Flt-1 intraceptors inhibit hypoxia-induced VEGF expression in vitro and corneal neovascularization in vivo. Invest Ophthalmol Vis Sci. 2005;46:1647–1652. doi: 10.1167/iovs.04-1172. [DOI] [PubMed] [Google Scholar]
- Jani PD, Singh N, Jenkins C, Raghava S, Mo Y, Amin S, et al. Nanoparticles sustain expression of Flt intraceptors in the cornea and inhibit injury-induced corneal angiogenesis. Invest Ophthalmol Vis Sci. 2007;48:2030–2036. doi: 10.1167/iovs.06-0853. [DOI] [PubMed] [Google Scholar]
- Yu W-Z, Li X-X, She H-C, He PY, Dong JQ, Rui M, et al. Gene transfer of kringle 5 of plasminogen by electroporation inhibits corneal neovascularization. Ophthalmic Res. 2003;35:239–246. doi: 10.1159/000072143. [DOI] [PubMed] [Google Scholar]
- Reich SJ, Fosnot J, Kuroki A, Tang W, Yang X, Maguire AM, et al. Small interfering RNA (siRNA) targeting VEGF effectively inhibits ocular neovascularization in a mouse model. Mol Vis. 2003;9:210–216. [PubMed] [Google Scholar]
- Kim B, Tang Q, Biswas PS, Xu J, Schiffelers RM, Xie FY, et al. Inhibition of ocular angiogenesis by siRNA targeting vascular endothelial growth factor pathway genes: therapeutic strategy for herpetic stromal keratitis. Am J Pathol. 2004;165:2177–2185. doi: 10.1016/S0002-9440(10)63267-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stechschulte SU, Joussen AM, von Recum HA, Poulaki V, Moromizato Y, Yuan J, et al. Rapid ocular angiogenic control via naked DNA delivery to cornea. Invest Ophthalmol Vis Sci. 2001;42:1975–1979. [PubMed] [Google Scholar]
- Kuo C-N, Yang L-C, Wu P-C, Kuo HK, Kuo CJ, Tai MH. Dehydrated form of plasmid expressing basic fibroblast growth factor-polyethylenimine complex is a novel and accurate method for gene transfer to the cornea. Curr Eye Res. 2005;30:1015–1024. doi: 10.1080/02713680500330512. [DOI] [PubMed] [Google Scholar]
- Mezentsev A, Mastyugin V, Seta F, Ashkar S, Kemp R, Reddy DS, et al. Transfection of cytochrome P4504B1 into the cornea increases angiogenic activity of the limbal vessels. J Pharmacol Exp Ther. 2005;315:42–50. doi: 10.1124/jpet.105.088211. [DOI] [PubMed] [Google Scholar]
- Folkman J. Angiogenesis: an organizing principle for drug discovery. Nat Rev Drug Discov. 2007;6:273–286. doi: 10.1038/nrd2115. [DOI] [PubMed] [Google Scholar]
- Auerbach R, Arensman R, Kubai L, Folkman J. Tumor-induced angiogenesis: lack of inhibition by irradiation. Int J Cancer. 1975;15:241–245. doi: 10.1002/ijc.2910150209. [DOI] [PubMed] [Google Scholar]
- Gimbrone MA, Cotran RS, Leapman SB, Folkman J. Tumor growth and neovascularization: an experimental model using the rabbit cornea. J Natl Cancer Inst. 1974;52:413–427. doi: 10.1093/jnci/52.2.413. [DOI] [PubMed] [Google Scholar]
- Bock F, Onderka J, Dietrich T, Bachmann B, Kruse FE, Paschke M, et al. Bevacizumab as a potent inhibitor of inflammatory corneal angiogenesis and lymphangiogenesis. Invest Ophthalmol Vis Sci. 2007;48:2545–2552. doi: 10.1167/iovs.06-0570. [DOI] [PubMed] [Google Scholar]
- Klausner EA, Peer D, Chapman RL, Multack RF, Andurkar SV. Corneal gene therapy. J Controlled Release. 2007;124:107–133. doi: 10.1016/j.jconrel.2007.05.041. [DOI] [PubMed] [Google Scholar]
- DeStafeno JJ, Kim T. Topical bevacizumab therapy for corneal neovascularization. Arch Ophthalmol. 2007;125:834–836. doi: 10.1001/archopht.125.6.834. [DOI] [PubMed] [Google Scholar]
- Uy HS, Chan PS, Ang RE. Topical bevacizumab and ocular surface neovascularization in patients with stevens-johnson syndrome. Cornea. 2008;27:70–73. doi: 10.1097/ICO.0b013e318158f6ad. [DOI] [PubMed] [Google Scholar]
- Bock F, König Y, Kruse F, Baier M, Cursiefen C. Bevacizumab (Avastin) eye drops inhibit corneal neovascularization. Graefes Arch Clin Exp Ophthalmol. 2008;246:281–284. doi: 10.1007/s00417-007-0684-4. [DOI] [PubMed] [Google Scholar]
- Saxena S, Kishore P, Pandey S, Khattri M, Kumar D. Topical bevacizumab for corneal neovascularization after penetrating keratoplasty. Eur J Ophthalmol. 2009;19:870–872. doi: 10.1177/112067210901900530. [DOI] [PubMed] [Google Scholar]
- Lim M, Jacobs DS, Rosenthal P, Carrasquillo KG. The Boston Ocular Surface Prosthesis as a novel drug delivery system for bevacizumab. Semin Ophthalmol. 2009;24:149–155. doi: 10.1080/08820530902802013. [DOI] [PubMed] [Google Scholar]
- Koenig Y, Bock F, Horn F, Kruse F, Straub K, Cursiefen C. Short- and long-term safety profile and efficacy of topical bevacizumab (Avastin) eye drops against corneal neovascularization. Graefes Arch Clin Exp Ophthalmol. 2009;247 (10:1375–1382. doi: 10.1007/s00417-009-1099-1. [DOI] [PubMed] [Google Scholar]
- Dastjerdi MH, Al-Arfaj KM, Nallasamy N, Hamrah P, Jurkunas UV, Pineda R, 2nd, et al. Topical bevacizumab in the treatment of corneal neovascularization: results of a prospective, open-label, noncomparative study. Arch Ophthalmol. 2009;127:381–389. doi: 10.1001/archophthalmol.2009.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doctor PP, Bhat PV, Foster CS. Subconjunctival bevacizumab for corneal neovascularization. Cornea. 2008;27:992–995. doi: 10.1097/ICO.0b013e31817786ad. [DOI] [PubMed] [Google Scholar]
- Bahar I, Kaiserman I, McAllum P, Rootman D, Slomovic A. Subconjunctival bevacizumab injection for corneal neovascularization. Cornea. 2008;27:142–147. doi: 10.1097/ICO.0b013e318159019f. [DOI] [PubMed] [Google Scholar]
- Erdurmus M, Totan Y. Subconjunctival bevacizumab for corneal neovascularization. Graefes Arch Clin Exp Ophthalmol. 2007;245:1577–1579. doi: 10.1007/s00417-007-0587-4. [DOI] [PubMed] [Google Scholar]
- Zaki AA, Farid SF. Subconjunctival bevacizumab for corneal neovascularization. Acta Ophthalmol. 2010;88:868–871. doi: 10.1111/j.1755-3768.2009.01585.x. [DOI] [PubMed] [Google Scholar]
- Jeong JH, Chun YS, Kim ES, Kim JC. Compensatory growth factor and cytokine response in tears after subconjunctival bevacizumab injection. Cornea. 2011;30:1071–1077. doi: 10.1097/ICO.0b013e31820cd3f4. [DOI] [PubMed] [Google Scholar]
- Vassileva PI, Hergeldzhieva TG. Avastin use in high risk corneal transplantation. Graefes Arch Clin Exp Ophthalmol. 2009;247:1701–1706. doi: 10.1007/s00417-009-1170-y. [DOI] [PubMed] [Google Scholar]
- Oh JY, Kim MK, Wee WR. Subconjunctival and intracorneal bevacizumab injection for corneal neovascularization in lipid keratopathy. Cornea. 2009;28:1070–1073. doi: 10.1097/ICO.0b013e31819839f9. [DOI] [PubMed] [Google Scholar]
- Yeung SN, Lichtinger A, Kim P, Amiran MD, Slomovic AR. Combined use of subconjunctival and intracorneal bevacizumab injection for corneal neovascularization. Cornea. 2011;30:1110–1114. doi: 10.1097/ICO.0b013e31821379aa. [DOI] [PubMed] [Google Scholar]