Abstract
The blockbuster drug paradigm is under increasing scrutiny across the biopharmaceutical industry. Intraocular inflammation poses particular challenges to this, given the heterogeneity of conditions in the uveitis spectrum, and the increasing acknowledgement of individual patient and disease variance in underlying immune responses. This need has triggered a drive towards personalised and stratified medicine, supported and enabled as a result of continued development of both experimental models and molecular biological techniques and improved clinical classification. As such we have the ability now to systematically appraise at a genomic, transcriptomic, and proteomic level individual immunophenotype, and the promise that in the eye this can be augmented by in vivo immune imaging to identify individual immunopathology. With such advances all running in parallel, we are entering an era of experimental medicine that will facilitate early diagnosis, generate biomarkers for accurate prognostication, and enable the development of individualised and targeted therapies, which can progress rapidly into clinical practice.
Keywords: uveitis, immunosuppression, inflammation, personalised medicine, biomarkers
Introduction
The aetiology of intraocular inflammatory disease, which represents the spectrum of disorders we manage as uveitis has, over the past century, swung from infective to autoimmune. The advent of corticosteroids in the 1950s defined a new era in treatment, and since then advances in the management of principally posterior uveitides have been achieved through improved diagnostics, the ability to more closely assess severity as well as monitor response to treatment, improved understanding of underlying immunology, better identification of patients at risk of long-term visual loss, and finally the development of more targeted therapy.
Intraocular inflammation accounts for 10–15% of bilateral and 22% of unilateral blindness in the United States, and 10% of visual impaired registration in the UK. Although it is second only to diabetic retinopathy as a major cause of treatable blindness in 20–65 year olds, it comprises a heterogeneous group of ocular inflammatory disorders and consequently there are formidable barriers to organising, financing, and recruiting patients for randomised controlled trials (RCTs) resulting in a dearth of evidence to inform patient management. As a result, care and recognition of the sight-threatening potential of immune-mediated intraocular inflammatory disease is frequently subobtimal.1
Intraocular inflammation is estimated to affect up to 115 people per 100 000 in Western populations,2 just under a quarter of whom will require systemic immunosuppression. Despite which around 35% remain visually disabled,3 often after a protracted chronic relapsing and debilitating disease course. However, published evidence and investigations of use and outcomes of immunosuppressive medications continues to accrue, emphasising the need and requirement to treat appropriately,4, 5, 6, 7 especially as ours and others data demonstrate that poor vision secondary to uveitis impacts the general quality-of-life.8, 9, 10, 11 Until recently its prevalence within the ageing population has also been grossly underestimated. So what have we achieved and how are we to improve further?
Understanding immunopathology
Uveitis is traditionally considered an autoimmune disease initiated by loss of immune tolerance to retinal proteins (eg, S-antigen and retinoid-binding protein, RBP-3) and tyrosinase-related products,12 and orchestrated by two subsets of CD4+ T cells that secrete their signature cytokines interferon (IFN)-γ for Th1 and interleukin (IL-17) for Th17 cells. To prevent autoimmunity, active peripheral (outside the thymus) tolerance mechanisms, including suppression and anergy (functional inactivation of T cells), are thought to control antigen-specific T-cell responses that escape thymic deletion during development. Naturally occurring phenotypically categorised CD4+CD25+FoxP3+ T regulatory cells (nTregs) are able to silence autoreactive T cells that have escaped thymic tolerance. The importance of this is evidenced in humans by the syndrome associated with a forkhead gene—FoxP3 mutations, called immunodysregulation polyendocrinopathy enteropathy X-linked syndrome, characterized by the development of overwhelming systemic autoimmunity. In man, nTreg percentages in Behçet's disease patients with ocular complications are decreased before an ocular attack.13, 14 In Vogt Koyanagi Harada (VKH) syndrome, nTreg cells are similarly depleted and also less functional in their suppressive ability, and therefore overall loss of nTregs in active uveitis may contribute to patients' disease susceptibility and severity.14, 15 Immune regulation may not require antigen specificity, as in experimental models of uveitis (EAU), non-retinal antigen-specific nTregs can functionally suppress disease. Peripheral tolerance to retinal antigens is thought to be weak and likely to explain why peripheral antigen-specific T cells can be activated by ocular antigens or via autoantigen molecular mimicry during unrelated infection or inflammation and lead to induction of ocular inflammation. That is the bridge between innate (principal lines of immune defence against infection) and adaptive immunity (highly specialised immune defence with memory).
There is now increasingly compelling data that supports activation of innate immune responses, which drive the inflammation we observe clinically via ‘hidden' concomitant infection (eg, latent TB) or other non-antigen specific mechanisms; the innate and adaptive immune bridge.16, 17, 18 In the absence of defined autoantigens, the definition of an autoimmune vs an autoinflammatory (non-infectious, non-autoimmune) response becomes potentially blurred. Rapid detection and elimination of microbial infection is essential for protection against pathogens and the innate immune system is pivotal to this, as well as orchestrating adaptive immunity. Such speedy responses, in part, are likely to be due to conserved molecules expressed by microorganisms (pathogen-associated molecular patterns)19 that are recognised by host receptors (pattern recognition receptors; PRRs). This mechanism is corroborated by rare yet informative genetic polymorphisms or mutations in these detection receptors, such as polymorphisms and/or mutations in one type of such receptors, human NOD-like receptors (NLRs), which display a wide range of disease phenotypes, but characteristically all have recurrent inflammatory episodes and pyrexias, and are termed hereditary periodic fevers.20, 21
The immune system, requiring both innate and adaptive immune mechanisms is not only concerned with discriminating self from non-self (a responsibility of adaptive immunity) and consequently developing memory, but also fundamentally detecting and protecting against danger signals (including extracellular pathogens and damaged tissue).22 To this end the innate immune system in part relies on activation of inflammasomes. Inflammasomes are cytoplasmic multiprotein complexes that activate proinflammatory cytokines, principally IL-1β via activation of caspases. Assembly of inflammasomes depends on NLR family members such as NALPs and NAIP (see Appendix). Various microbial and endogenous stimuli activate different types of inflammasomes. This article focuses on the Pyrin domain-containing NLRs, known as NALP proteins. Recent findings provide exciting insights into how these proteins might be activated and also provide evidence of the critical role of the NALP inflammasomes in innate immunity and inflammatory diseases.
For example, cold induced autoinflammatory syndrome 1-associated periodic syndrome, is the collective name for Muckle–Wells syndrome, familial cold urticaria, and chronic infantile neurological cutaneous articular syndrome. This group of autosomal dominant inherited disorders all have skin, joint, and eye involvement as a result of a mutation in the NACHT domain of NALP3, leading to spontaneous caspase-1 activation and increased pro-inflammatory cytokine IL-1β levels.20 Not surprisingly therefore, IL-1R antagonist and anti-IL-1 monoclonal antibodies (mAbs) have transformed the previously poor historical prognostic outcome of these diseases.23, 24 Although NALP3 is functionally similar to other PRRs, including the NOD-like receptor, NOD2, in humans, a rare point mutation in NOD2 results in granulomatous inflammation in the eye, joints, and skin called Blau's syndrome.25 Additionally, polymorphic variations in NOD2 increase susceptibility to Crohn's disease, which is not infrequently associated with uveitis. However, peripheral blood mononuclear cells from Blau patients show no increased IL-1β production when stimulated with NOD2's cognate ligand muramyl dipeptide,25 and despite early reports ascribing efficacy to the anti-IL-1mAb, Anakinra, in Blau's syndrome, this has since been refuted, but anecdotally patients respond to anti-TNF therapy.
Mostly, translation of pre-clinical immunotherapeutic studies have utilised experimental models of uveitis, such as EAU, and have led successfully to the current common clinical use of cyclosporine A (CsA), cellcept, tacrolimus, and biologics such as TNF-α blockers.26, 27 EAU continues to serve as a very useful platform to dissect immunopathogenic mechanisms, and although it, together with corroborative evidence from limited human studies, supports the assertion that uveitis is a CD4 Th1 and Th17-mediated disease, other innate immune cells including macrophages have a central role and are thought to largely govern the extent of tissue damage.28, 29 Moreover, even with clinical suppression of inflammation there remains a subgroup of patients where in the absence of clinical inflammation, an attrition of vision and persistent retinal degeneration occurs. Such clinical scenarios imply that targeting predominantly T cell infiltration does not restore retinal immune homeostasis and suggests that inhibiting other inflammatory cell types may improve therapeutic effect, akin to modifying behaviour at cellular and molecular level (vis a vis macrophage involvement) in age-related macular degeneration (AMD).30, 31 In support of this concept of ongoing chronic immune dysregulation retinal cell analysis in EAU shows persistence of a significant sustained infiltrate and an appearance, which is atypical for normal retina long after resolution of clinically evident disease.32, 33
So irrespective of the danger signal that drives inflammation (autoimmunity vs autoinflammation vs degeneration), dysregulation of immunity within the eye, innate immune activation, and macrophage infiltration remain common to many chorioretinal diseases. A current paradigm of macrophage activation recognises that their phenotype is dependent upon the signals (from cytokines and ligands expressed on other cell types) they receive.34, 35 This may be characterised experimentally by the induction of enzymes arginase I regulating, and nitric oxide synthase 2 promoting, nitric oxide production, which in turn is governed by the effects of tissue environment (eg, type of T cell responses) via newly recruited cells.36, 37, 38, 39 Macrophages express numerous cell surface receptors that interact with both natural and altered host components as well as pathogens.40 For example, the retina has extensive expression of neuronal CD200, a member of the immunoglobulin superfamily whose receptor is largely expressed on myeloid cells.41, 42 In vivo studies in EAU, particularly of CD200−/− mice, suggest that the interaction of CD200 with its receptor delivers inhibitory signals to myeloid cells via CD200R.43, 44 As a result, the retina's resident CD200R+ macrophages (microglia) remain tonically deactivated patrolling and governing retinal homeostasis.45 On the other side of the fence, complement activation will also generate further macrophage activation via C5a, promoting and promulgating the pro-inflammatory macrophage status.46
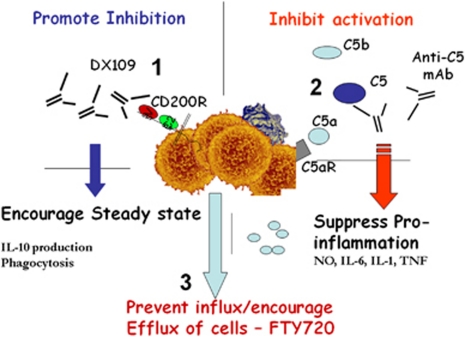
The benefits of keeping immune cell activation in check, particularly within tissues, is an effective means of inducing a rapid activation response when the constraint of the negative signal is removed, irrespective of whether this occurs in the context of autoimmunity or even in AMD. Moreover, it is increasingly clear that a broad range of activating and inhibitory receptors are available to regulate the activation status of myeloid cells and these can be utilised for therapeutic benefit. The issue remains as to how best to redress homeostasis and thereby maintain remission in either autoimmune disease or the immune activation and dysregulation observed in AMD; where currently no available therapy delivers. Preventing cell infiltrate may be a significant step in controlling chronic relapsing disease and is currently in clinical trials, particularly for multiple sclerosis, where several clinical strategies of targeted-mAb inhibition of integrins and adhesion molecules have had success (see below). However preventing efflux from secondary lymphoid tissue has also proven beneficial and is now in phase 3 trials with Fingolimod (FTY720). (See Figure 1).
Figure 1.
Schematic representation of a targeted approach to disarming macrophages. (1) The monoclonal antibody, DX109, utilises the inhibitory myeloid receptor CD200R to inhibit macrophage activity and redress homeostasis in the tissue, while enabling maintenance of anti-inflammatory IL-10 production. (2) Similarly, inhibiting macrophage activation (as shown by anti-complement therapy) will suppress EAU, principally by switching off pro-inflammatory, nitric oxide (NO), IL-6, IL-1, and TNF secretion. (3) Preventing the influx of T cells to the eye through inhibition of their efflux from lymph nodes with FTY720 (Fingolimod) also successfully induces disease remission in animal models of intraocular inflammation.
Clinical classification
Arguably many of the constraints in generating evidence for the efficacy of treatments have been due to the heterogeneity and spectrum of disorders of uveitis, their relatively low prevalence, and the lack of robust outcome measures. In order to gain understanding and uniformity, a global initiative has been established called the Standardization of Uveitis Nomenclature (SUN) working group (http://research.mssm.edu/sun). The initial workshop developed some of the common terms, definitions, and outcome criteria that are now in common use today for reporting and undertaking the increasing number of clinical studies in the field. In brief, this forum has with consensus agreed basic fundamentals of clinical descriptors. This has generated uniformity in defining an anatomical classification of inflammation, the metrics of activity (AC cells, flare, vitreous haze) and, most importantly, how to define disease onset, course, deterioration, improvement, and remission.
Nevertheless, there still remains inconsistency in ophthalmic practice, with a central issue of agreeing non-anatomical diagnostic definitions. Accordingly, SUN is currently addressing the need to generate classification criteria for ophthalmic disorders within the previously defined generic domains of anterior, posterior, intermediate, and pan uveitis. This is not re-inventing the wheel of other workshops in uveitic disorders over the years, but more assimilating data and gaining consensus in a wider user-forum to agree what constitutes the major descriptors and features for each diagnosis. Although in itself this exercise does not a priori define aetiology or indeed pathogenesis, it does facilitate a unified platform for future clinical studies, including immunophenotyping, genomic, and proteomic studies.
Treatment outcomes to date
Corticosteroids remain the most frequently used initial drug therapy for non-infectious posterior segment intraocular inflammation, despite their inherent morbidity.47 However, over the past 30 years there has been increasing use of alternative non-steroid immunosuppressants, and more recently the use of biologic therapy has accelerated5, 6 to achieve two key goals: first an attempt to minimise long-term steroid side effects and reduce steroid use by aiming to taper this to <10 mg of prednisolone a day; and second, as will be discussed further in the section below, to introduce a more targeted approach to immunomodulation earlier in the disease process.48
The non-steroidal immunosuppressive drugs can be broadly classified into anti-metabolites (methotrexate, azathiorpine, and mycophenolate mofetil (cellcept)), T cell inhibitors (tacrolimus, cyslosporin, and voclosporin), and alkylating agents (cyclosphosphamide, chlorambucil). Over the last few years, assisted by major expert opinion overview articles that provided treatment recommendations,49 there have been further seminal trials and patient cohort studies highlighting the evidence for treatment with non-biologic conventional immunosuppressants.4, 7, 50, 51, 52, 53, 54, 55, 56, 57, 58 The most compelling studies of recent years include evidence from patient cohort analytical studies for the use of cellcept displaying its effectiveness as a corticosteroid-sparing agent with an acceptable adverse event profile.4, 7, 59 In particular, there are two studies that presented their results in accordance with SUN guidelines showing concordance in effect, with around an 80% chance of achieving disease control at a prednisolone dose of <10 mg/day. Most recently a large US retrospective study (the Systemic Immunosuppressive Therapy for Eye Diseases (SITE) Cohort) showed disease remission occurred in 1 year of 73% and 55% of 236 patients who were also able to reduce their steroid dose to below 10 mg/day. Methotrexate displayed less positive data, with a 38 to 76% remission rate in 384 patients within 1 year, depending on anatomical location of uveitis, and steroid sparing success (≤10 mg/day of prednisolone) of between 20 and 51%.
Since its translation from the animal model in 1983, CsA remains the dominant T-cell inhibitor used in uveitis practice globally. Again, the US SITE cohort recently showed that 51.9% of cases achieved remission by 1 year, but only 36.1% of patients were able to reduce steroids to below 10 mg/day.60 Contemporaneously, there have been developments in other T cell inhibitors acting via inhibition of calcineurin-dependent IL-2 transcription, including the use of tacrolimus. Such developments were timely because the adverse effects of CsA were increasingly apparent and highlighted again in the SITE study, which demonstrated a threefold increase in drug cessation due to intolerance in patients over 55 years of age. A small RCT of tacrolimus vs CsA showed that the treatment effect over a 6-month period was similar, as was quality-of-life, but CsA therapy was associated with a higher incidence of elevated blood pressure and cholesterol.55 A cohort analysis of 62 patients has since revealed that patients on tacrolimus had an 85% probability of achieving remission and <10 mg/day of prednisolone with an excellent cardiovascular risk profile.58 The efficacy of voclosporin has also since been assessed in the international Lux Uveitis Multicenter Investigation of a New Approach to Treatment (LUMINATE) studies.61, 62 These included, for active non-infectious uveitis, a RCT of 218 patients, which showed that 0.4 mg/kg achieved its primary endpoint of superiority to placebo, using suppression of vitreous haze as a readout of intraocular inflammation. Additionally, there was a suggestion that it reduced the relapse rate in patients with quiescent uveitis, and on steroid dose reduction the relapse rate was 50% less than placebo. Finally, with regard to alkylating agents, the SITE project has shown that in 215 patients with various forms of ocular inflammatory disease, cyclophosphamide induced remission in 76% within 1 year, and 61.2% of patients were able to reduce steroids to <10 mg/day.
This review is not an attempt to be exhaustive in providing evidence or be a systematic review of all agents studied. Nonetheless, there remains compelling data that immunosuppression (over and above corticosteroids) is of benefit; as patients achieve clinical remission while reducing their steroid requirement. A caveat to the interpretation of the plethora of information detailed above, is that although well performed, most studies include a heterogeneic mix of uveitic disorders in their analysis and in particular, the SITE cohort included patients with cictatricial and scleritic disorders. Which immunosuppressive agent to choose is more difficult to determine, as there are no head-to-head studies, risk stratification studies, or indeed clinical or immune phenotyping to assess who may respond to any of the individual groups of drugs. Even with these concerns raised, in summary, the likelihood of any additional immunosuppressant to work is at best a 60–80% probability to induce or maintain remission, with a 60–70% chance of reducing steroids to <10 mg/day within 1 year of treatment.
Finally, it is important to state that there are only a couple of specifically designed studies to show whether additional treatment can remove the need for steroids. First, the LUMINATE trial in quiescent uveitis, and second, a small RCT, which demonstrated that tacrolimus monotherapy is as effective as tacrolimus and prednisolone for the maintenance of disease remission (Lee et al, submitted). In this study, 62.5% of patients demonstrated treatment success. There is therefore persuasive evidence to support, wherever possible, discontinuation of steroid therapy, and this should be a goal that all future treatments achieve, setting a paradigm shift in our treatment regimen for intraocular inflammatory disease.
Recent and future treatments
The balance of risk to benefit has to be considered carefully. In a cohort study looking at over 66 802 person years of treatment it was noted that for the most commonly used immunosuppressive drugs there was no increase in overall or cancer mortality.63, 64 However, whether that is true for the biologics, which are in increasing use, will require longer follow-up and improved clinical outcome surveillance. Since the early reports of CAMPATH-1H,65, 66 most evidence for the success of biologics has accumulated using anti-TNF therapies, which have followed an iterative process of evidence for their efficacy in animal models, through to early phase studies, small RCTs and are now undergoing more definitive assessment in commercially-led RCTs.27, 57
Biologics in the main are recombinant antibodies to, or antagonists of, particular cytokines or cell-surface receptors, but may also include recombinant cytokines such as IFN-α. Biologic agents are attractive as they exert relatively specific effects, and thus with more targeted suppression may induce, much more rapidly and effectively, remission and longer term suppression.67 An example of success, which followed experimental work neutralising TNF activity via a TNF receptor 1 fusion protein,68, 69 is the finding in early phase trials that 71% of patients with refractory uveitis achieved complete cessation of intraocular inflammation, and reduction in concomitant immunosuppression was possible in 65% of cases.70, 71 There have since been multicentred trials in anti-TNF therapy using the chimeric anti-TNF-α mAb infliximab for treatment of Behcet's disease (BD).72, 73 Furthermore, most recently, reports show in favour of infliximab compared with conventional therapy with CsA,74 and indeed challenges us to think of earlier use of such targeted therapy. Although criticisms can be levied at this trial or other comparators being largely cohort studies, we are now entering the arena of adequately powered commercially driven RCTs, which will provide level I evidence for or against anti-TNF therapy (using humanised anti-TNF agent, Humira), both in JIA-uveitis and in adult uveitis, and these will define future treatment.
The understanding of underlying immune responses operative in uveitis and autoimmune disease has led to a panoply of non-TNF biologics. Most have been developed in the treatment of rheumatoid arthritis and are now tested in small case series in ocular inflammatory disease, including inhibition of T-cell activation via suppression of growth factor by inhibiting IL-2 receptor signalling with dacluzimab,75 co-stimulation of T cells via a fusion protein of CTLA-4 (Abatacept),76, 77, 78 inhibiting B-cell responses via the anti-CD20mAb Rituximab,72, 79, 80, 81, 82, 83 or blocking pro-inflammatory cytokines (anti-IL-1, Anakinra; anti-IL-6, Tocilizumab).59 As for anti-TNF-α treatment, which costs ∼£10–15 000/patient/year (compared with <£3000/year for conventional therapy) all these treatments are expensive to develop and then institute into clinical practice. The evidence therefore must be compelling. Other strategies include preventing cells from entering the target organ by inhibition of either adhesion or migration through endothelium (anti-α4-integrin (Natalizumab))84 or via preventing efflux from lymph nodes by blocking sphingosine-1-phosphate receptor (FTY720 (Fingolimod)),85 both of which have demonstrated dramatic benefit in multiple sclerosis trials and indeed in experimental models of uveitis.86 For BD, and also in other forms of non-infectious uveitis, IFN-α (which are delivered as monotherapy or in conjunction with corticosteroids) have also been successfully utilised, with both a small RCT and long-term prospective and retrospective studies demonstrating that almost 60% of patients can achieve remission and discontinue treatment.87, 88, 89, 90, 91, 92
Generating potential local treatments may circumvent many of the systemic adverse events of biologic therapy and also potentially reduce cost. Local anti-TNF inhibition is a new and promising therapeutic direction where pre-clinical studies of the TNF inhibitior ESBA105 suggest good intravitreal and neuroretinal bioavailability. Further pre-clinical studies are highlighting the potential of intravitreal therapy via inhibiting activation of macrophages through complement inhibition (prevention of C5 cleavage) and via stimulating inhibitory receptors (CD200receptor). Both pathways are highly amenable to clinical translation in the future. Currently, there have been RCTs in the use of local steroid therapy, including the MUST (Multicenter Uveitis Steroid Treatment) trial, which compares an intravitreal flouricinolone acetonide implant with systemic steroid therapy.93 The combination of both local and systemic delivery of targeted therapy will undoubtedly enhance the outcome for our patients, however, local steroid use is not without problems relating to ocular morbidity (cataracts, glaucoma, and endophthalmitis) as highlighted in the Retisert trial.94 Nevertheless, there remains a consensus for the need to reduce patients' use of systemic steroids, and local administration, either singly or combined with non-steroid systemic immunosuppression, coupled with advances in drug delivery such as the recently FDA-approved intravitreal dexamethasone implant (Ozurdex),95 have the potential, to facilitate control of intraocular inflammation.
How do we harness our approaches?
Building on the success of traditional and, more recently, biologic therapy, will rely on the ability to: (i) detect disease early, (ii) prognosticate (or provide a surrogate of how active is the disease going to be), (iii) determine which agents to use for any given disease, (iv) tailor treatment to patients immunophenotype (personalising therapy), (v) detect treatment responses and determine whether true disease remission has been achieved, and (vi) predict relapses.
To date there are compelling examples of the direction we can take. First, as mentioned, SUN is moving to generate a consensus in clinical diagnosis that will enable us to identify underlying immune gene profiles of patient groups with well-categorised disease entities. This has already generated tangible benefits, and in BD a recent genome-wide association study (GWAS) has not only confirmed the association with know HLA-B*51 but identified further associations within MHC class I and also an association at IL-10 and IL23R-IL12RB2 loci.96, 97 This is concordant with other smaller candidate single nucleotide polymorphism (SNP) analysis studies that show in sympathetic ophthalmia an association with IL-10-1082 SNP and three haplotype-tagging SNPs(htSNPs) in the IL10 gene, rs6703630, rs2222202, and rs3024490, are significantly associated with susceptibility to non-infectious uveitis, whereas a LTA+252AA/TNFhtSNP2GG haplotype (rs909253 and rs361525) is protective.98, 99 Whether this will predict the outcome on an individual basis is unknown,100 but it highlights how further GWAS and, more importantly, genomics, RNA sequencing, and transcriptomics, will shape our future approach by identifying new genes, potential biomarkers, and new targets for therapy. Examples are seen in cancer, where recently gene expression-based biomarkers have facilitated targeted chemotherapy but this is only recently being translated into autoimmunity, and in systemic vasculitis, a CD8 T cell transcriptomic profile has now been shown to predict prognosis in two distinct patient subgroups.101, 102 Such approaches are readily transferrable to investigation in ocular inflammatory disease.
Diagnosis
One of the major clinical conundrums is identifying patients at risk. Although patients may present with non-infectious uveitic disorders known to pose a high likelihood of visual loss in the short to mid term (BD, pan uveitis, VKH syndrome), there are significant number of patients wherein such prediction is not possible. Nevertheless, of equal importance is the exclusion of infection or masquerade, and AC sampling is increasingly being used for this purpose, the most classic example of which is the advances achieved in the diagnosis of primary intraocular lymphoma by measurement of aqueous IL-10 and/or IL-10: IL-6 ratios, and by vitreous biopsy, utilising modern immunohistochemistry, flow cytometry, and PCR to facilitate diagnosis.103 The utility of sampling AC or vitreous is also to discriminate infectious causes from autoimmune/inflammatory disease. By PCR or by Goldmann–Witmer co-efficient of specific antibody production against microbes/viruses we are now able to discern more precisely possible infectious causes, which as a result will direct treatment appropriately. For non-infectious inflammatory disease, the utility of AC sampling remains as a research tool. However, with the ability to now undertake multiplex analysis of cytokines in the local microenvironment, we may be able to differentiate in the future distinct patterns of cytokines that will differentiate idiopathic uveitis.104, 105, 106 Combination of this with bioinformatics derived from transcriptomics there are real possibilities to support diagnosis and enter the arena of prognostication.
Prognosis
We currently require biomarkers to assist in our clinical classification and to predict prognosis. Serological markers of immune activation are largely fruitless and do not act as a sensitive indicator of either severity or long-term prognosis, and where patient case series may suggest possible uses, the analysis has never been expanded into larger scale studies. Although HLA typing can assist in diagnosis, for example HLA-A29 in Birdshot chorioretinopathy, or HLA-B*51 in BD, they are largely confirmatory of clinical diagnostic criteria. For other disorders there remain a strong genetic association, common to many autoimmune diseases, particularly associated with MHC class II alleles such as HLA-DRB1*0405 and *0410 in VKH and HLA-DR associations in sympathetic ophthalmia, tubulointerstitial nephritis and uveitis, and intermediate uveitis.107, 108, 109 Nevertheless, in general the positive predictive value of HLA typing in affirming a diagnosis is low. As with most other serological tests to establish a specific diagnosis of uveitis, the diagnosis is not confirmed on the result of the test, and in this case HLA typing alone and moreover the negative result does not exclude the diagnosis. An exception to this is HLA-B27, which may assist in previously undiagnosed uveitis-related spondyloarthropathy.110 With regard to prognosis, however, there remains little evidence unless, for example, we can determine shared epitopes that confer susceptibility to more severe disease and hone to a specific diagnosis, as proposed most recently for VKH.111
Are there any imaging modalities that assist? We increasingly use OCT, autoflourescence, and angiography (fluorescein and indocyanine) in determining the extent of the disease and help determine the level of immunosuppression required by determining the extent of sight-threatening disease present (vaso-occlusive, macular ischaemia, profound oedema). The use of spectral domain OCT is gaining prominence and may define in the future which therapy is appropriate vis a vis immunosuppression, anti-VEGF, and RPE pump stimulators.112
Treatment response
Again we lack the specificity and sensitivity of any biomarker to aid in prediction of treatment response. Clinically we may appreciate disease remission (as defined by SUN guidelines) and can assess angiographic resolution of lesions or leakage of vasculature. Most pertinently, one of the major causes of long-term visual loss is persistent CMO, and in this regard OCT has assisted in monitoring the response to therapy. However, determination of the resolution is not as obvious as we think we recognise clinically, and indeed without the ability to image cellular detail, we are currently unable to predict remission at the tissue level.
Detecting remission
Again although we can observe the resolution of CMO, absence of inflammatory cells, resolution of chorioretinal lesions, or scarring of them, none detect immunopathological remission. This remains at best an empirical judgement based principally on the observation that we are able to withdraw treatment slowly without relapse. We have to date no satisfactory serological biomarkers or bio-informatic evidence that support remission, such as suppression of acute-phase response proteins, changes to T-cell profiles, or systemic or intracellular cytokine levels. Although there have been encouraging examples in small populations, including to name a few: changes to sIL-2R, ICAM-1, ANCA, T-cell subsets, and pDC in biologic and IFN-α treated patients, most assays remain to be validated and all may lack the specificity and sensitivity for wider use.113, 114, 115, 116, 117, 118 Nevertheless with the advent of transcriptomics we should be able to develop an ability to predict treatment response and possibly remission in the future. Increased high resolution imaging and the ability to assess labelled cells entering, or being excluded from, tissue sites will help define the level of cell infiltrate within tissues and overcome the dilemma of the unexpected relapse in a clinically quiescent patient. Similarly, measurement of electrodiagnostics and autoflourescence can assist in a composite assessment of the function and tissue damage and provide ancillary evidence of remission
Personalised and stratified medicine
Although we are on the threshold to tailor treatment to individual needs, generic treatment ladders are currently applied to all patients requiring systemic immunosuppression for sight-threatening disease. We then refine our choice of treatment, first by evaluating patients response to corticosteroids, and then by trial and error with a range of second-line drugs, ultimately resorting to more targeted biologic therapies when everything else fails. This achieves an individualised care of sorts, but only after patients have traversed a stepwise hierarchy of therapies (corticosteroids, immunosuppressants, cytotoxics, and biologics) based on the evidence and rationale we have presented here. Hence, it is not uncommon for patients to endure recurrent cycles of corticosteroid rescue (either local depot delivery or systemic) followed by the introduction of additional or alternative agents with each uveitic reactivation. Given the challenges in establishing RCTs in uncommon diseases, it has historically been difficult to generate the evidence base required to support early introduction of high cost interventions, although the argument for the proactive use of biologics, such as anti-TNF-α, in otherwise classically treatmented refractory blinding conditions such as BD, is now being made,48 supported by the establishment of large-scale RCTs (see above). However, we remain in a prime position to utilise the strength of exploiting an orphan disease. Such conditions such as Birdshot chorioretinopathy can act, with further understanding of specific immunopathology and immune mechanisms related to that condition, as proof of concept for targeted and personalised, stratified therapy, that may also inform other related conditions. This is germane to inflammatory conditions, where understanding of underlying immune mechanisms can deliver step change in outcome.119
Drug responses are likely to be better informed by an individual's underlying immunophenotype, and this may be independent of their clinical presentation. To this end, we have identified a subset of CD4+ T helper cells, which are unresponsive to corticosteroid treatment and are prevalent in patients with corticosteroid refractory uveitis. The presence of these cells had a 90% positive predictive value for steroid refractory disease in a small pilot study, and therefore has potential to be developed as a predictive biomarker of corticosteroid treatment success.120 Such an immune cell subset-driven approach has already demonstrated benefits in other inflammatory diseases where the application of genome-wide technologies to interrogate CD4+, CD8+, and monocyte transcriptomes has revealed characteristic signatures, which correlate with long-term prognosis.102 Given that the eye is also uniquely placed as a window on the immune response, we will be able to harness and translate developments in ocular imaging, alongside immunophenotyping, to inform both basic understanding of intraocular inflammation and also a personalised readout of response to therapy in terms of immune cell trafficking, and such strategies have already been successfully applied in mice.121
As the translational divide continues to be bridged there is real potential and optimism using the platforms discussed to revolutionise our understanding of immunity in general and, more specifically, deliver personalised care for patients with intraocular inflammatory diseases.
Acknowledgments
RWL is funded as a clinical lecturer by the NIHR and both ADD and RWL are faculty of the NIHR Biomedical Research Centre for Ophthalmology (Moorfields Eye Hospital and UCL Institute of Ophthalmology). The review was in part supported by grants from the National Eye Research Centre, Dunhill Medical Foundation, and Underwood Trust.
Appendix
Caspase-1
One of the cysteine proteases family is involved in cleaving IL-1β and is activated in the inflammasome and contributes to inflammasome activation and generation of pro-inflammatory state.
Pyrin
Pyrin is a protein produced by white blood cells (neutrophils, eosinophils, and macrophages) encoded by MEFV (Mediterranean Fever) and various pathogen recognition receptors (PPRs) have pyrin-binding domains.
NOD
Nucleotide oligomerization domain. There are several genes encoding for NOD and NOD2 gene (nucleotide-binding oligomerization domain containing 2) encodes for a protein, NOD2, also known as the caspase recruitment domain family, member 15 (CARD15), and an intracellular PPR. Other NLR proteins include NALPs and NAIP.
NALPs
NACHT (neuronal apoptosis inhibitory protein (NAIP), MHC class II transcription activator (CIITA), incompatibility locus protein from Podospora anserine (HET-E), and telomerase-associated protein (TP1)), leucine-rich repeat (LRR), and pyrin domain (PYD)-containing protein; a member of the nucleotide-binding oligomerization domain NLR family of intracellular proteins that sense components of pathogens and dying cells.
NAIP
Neuronal apoptosis inhibitory protein.
The authors declare no conflict of interest.
References
- Nguyen QD, Hatef E, Kayen B, Macahilig CP, Ibrahim M, Wang J, et al. A cross-sectional study of the current treatment patterns in noninfectious uveitis among specialists in the United States. Ophthalmology. 2011;118 (1:184–190. doi: 10.1016/j.ophtha.2010.03.029. [DOI] [PubMed] [Google Scholar]
- Gritz DC, Wong IG.Incidence and prevalence of uveitis in Northern California; the Northern California Epidemiology of Uveitis Study Ophthalmology 2004111(3491–500.discussion. [DOI] [PubMed] [Google Scholar]
- Suttorp-Schulten MS, Rothova A. The possible impact of uveitis in blindness: a literature survey. Br J Ophthalmol. 1996;80 (9:844–848. doi: 10.1136/bjo.80.9.844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniel E, Thorne JE, Newcomb CW, Pujari SS, Kacmaz RO, Levy-Clarke GA, et al. Mycophenolate mofetil for ocular inflammation Am J Ophthalmol 2010149(3423–432.e1–e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imrie FR, Dick AD. Nonsteroidal drugs for the treatment of noninfectious posterior and intermediate uveitis. Curr Opin Ophthalmol. 2007;18 (3:212–219. doi: 10.1097/ICU.0b013e3281107fef. [DOI] [PubMed] [Google Scholar]
- Imrie FR, Dick AD. Biologics in the treatment of uveitis. Curr Opin Ophthalmol. 2007;18 (6:481–486. doi: 10.1097/ICU.0b013e3282f03d42. [DOI] [PubMed] [Google Scholar]
- Thorne JE, Jabs DA, Qazi FA, Nguyen QD, Kempen JH, Dunn JP. Mycophenolate mofetil therapy for inflammatory eye disease. Ophthalmology. 2005;112 (8:1472–1477. doi: 10.1016/j.ophtha.2005.02.020. [DOI] [PubMed] [Google Scholar]
- Durrani OM, Meads CA, Murray PI. Uveitis: a potentially blinding disease. Ophthalmologica. 2004;218 (4:223–236. doi: 10.1159/000078612. [DOI] [PubMed] [Google Scholar]
- Gardiner AM, Armstrong RA, Dunne MC, Murray PI. Correlation between visual function and visual ability in patients with uveitis. Br J Ophthalmol. 2002;86 (9:993–996. doi: 10.1136/bjo.86.9.993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy CC, Greiner K, Plskova J, Frost NA, Forrester JV, Dick AD. Validity of using vision-related quality of life as a treatment end point in intermediate and posterior uveitis. Br J Ophthalmol. 2007;91 (2:154–156. doi: 10.1136/bjo.2006.105528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy CC, Hughes EH, Frost NA, Dick AD. Quality of life and visual function in patients with intermediate uveitis. Br J Ophthalmol. 2005;89 (9:1161–1165. doi: 10.1136/bjo.2005.067421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caspi RR. Understanding autoimmune uveitis through animal models. The Friedenwald lecture. Invest Ophthalmol Vis Sci. 2011;52 (3:1872–1879. doi: 10.1167/iovs.10-6909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nanke Y, Kotake S, Goto M, Ujihara H, Matsubara M, Kamatani N. Decreased percentages of regulatory T cells in peripheral blood of patients with Behcet's disease before ocular attack: a possible predictive marker of ocular attack. Mod Rheumatol. 2008;18 (4:354–358. doi: 10.1007/s10165-008-0064-x. [DOI] [PubMed] [Google Scholar]
- Yeh S, Li Z, Forooghian F, Hwang FS, Cunningham MA, Pantanelli S, et al. CD4+Foxp3+ T-regulatory cells in noninfectious uveitis. Arch Ophthalmol. 2009;127 (4:407–413. doi: 10.1001/archophthalmol.2009.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L, Yang P, Zhou H, He H, Ren X, Chi W, et al. Diminished frequency and function of CD4+CD25high regulatory T cells associated with active uveitis in Vogt-Koyanagi-Harada syndrome. Invest Ophthalmol Vis Sci. 2008;49 (8:3475–3482. doi: 10.1167/iovs.08-1793. [DOI] [PubMed] [Google Scholar]
- Gallucci S, Matzinger P. Danger signals: SOS to the immune system. Curr Opin Immunol. 2001;13 (1:114–119. doi: 10.1016/s0952-7915(00)00191-6. [DOI] [PubMed] [Google Scholar]
- Nathan C. Neutrophils and immunity: challenges and opportunities. Nat Rev Immunol. 2006;6 (3:173–182. doi: 10.1038/nri1785. [DOI] [PubMed] [Google Scholar]
- Nathan C, Ding A. Nonresolving inflammation. Cell. 2010;140 (6:871–882. doi: 10.1016/j.cell.2010.02.029. [DOI] [PubMed] [Google Scholar]
- Carneiro LA, Travassos LH, Girardin SE. Nod-like receptors in innate immunity and inflammatory diseases. Ann Med. 2007;39 (8:581–593. doi: 10.1080/07853890701576172. [DOI] [PubMed] [Google Scholar]
- McDermott MF. Genetic clues to understanding periodic fevers, and possible therapies. Trends Mol Med. 2002;8 (12:550–554. doi: 10.1016/s1471-4914(02)02425-5. [DOI] [PubMed] [Google Scholar]
- McDermott MF, Aksentijevich I. The autoinflammatory syndromes. Curr Opin Allergy Clin Immunol. 2002;2 (6:511–516. doi: 10.1097/00130832-200212000-00006. [DOI] [PubMed] [Google Scholar]
- Matzinger P. Tolerance, danger, and the extended family. Annu Rev Immunol. 1994;12:991–1045. doi: 10.1146/annurev.iy.12.040194.005015. [DOI] [PubMed] [Google Scholar]
- Lachmann HJ, Kone-Paut I, Kuemmerle-Deschner JB, Leslie KS, Hachulla E, Quartier P, et al. Use of canakinumab in the cryopyrin-associated periodic syndrome. N Engl J Med. 2009;360 (23:2416–2425. doi: 10.1056/NEJMoa0810787. [DOI] [PubMed] [Google Scholar]
- Lachmann HJ, Lowe P, Felix SD, Rordorf C, Leslie K, Madhoo S, et al. In vivo regulation of interleukin 1beta in patients with cryopyrin-associated periodic syndromes. J Exp Med. 2009;206 (5:1029–1036. doi: 10.1084/jem.20082481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin TM, Zhang Z, Kurz P, Rose CD, Chen H, Lu H, et al. The NOD2 defect in Blau syndrome does not result in excess interleukin-1 activity. Arthritis Rheum. 2009;60 (2:611–618. doi: 10.1002/art.24222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dick AD. Immune regulation of uveoretinal inflammation. Dev Ophthalmol. 1999;30:187–202. doi: 10.1159/000060744. [DOI] [PubMed] [Google Scholar]
- Dick AD, Forrester JV, Liversidge J, Cope AP. The role of tumour necrosis factor (TNF-alpha) in experimental autoimmune uveoretinitis (EAU) Prog Retin Eye Res. 2004;23 (6:617–637. doi: 10.1016/j.preteyeres.2004.06.005. [DOI] [PubMed] [Google Scholar]
- Dick AD, Carter D, Robertson M, Broderick C, Hughes E, Forrester JV, et al. Control of myeloid activity during retinal inflammation. J Leukoc Biol. 2003;74 (2:161–166. doi: 10.1189/jlb.1102535. [DOI] [PubMed] [Google Scholar]
- Forrester JV, Xu H, Kuffova L, Dick AD, McMenamin PG. Dendritic cell physiology and function in the eye. Immunol Rev. 2010;234 (1:282–304. doi: 10.1111/j.0105-2896.2009.00873.x. [DOI] [PubMed] [Google Scholar]
- Forrester JV. Macrophages eyed in macular degeneration. Nat Med. 2003;9 (11:1350–1351. doi: 10.1038/nm1103-1350. [DOI] [PubMed] [Google Scholar]
- Xu H, Chen M, Forrester JV. Para-inflammation in the aging retina. Prog Retin Eye Res. 2009;28 (5:348–368. doi: 10.1016/j.preteyeres.2009.06.001. [DOI] [PubMed] [Google Scholar]
- Kerr EC, Copland DA, Dick AD, Nicholson LB. The dynamics of leukocyte infiltration in experimental autoimmune uveoretinitis. Prog Retin Eye Res. 2008;27 (5:527–535. doi: 10.1016/j.preteyeres.2008.07.001. [DOI] [PubMed] [Google Scholar]
- Kerr EC, Raveney BJ, Copland DA, Dick AD, Nicholson LB. Analysis of retinal cellular infiltrate in experimental autoimmune uveoretinitis reveals multiple regulatory cell populations. J Autoimmun. 2008;31 (4:354–361. doi: 10.1016/j.jaut.2008.08.006. [DOI] [PubMed] [Google Scholar]
- Gordon S. Alternative activation of macrophages. Nat Rev Immunol. 2003;3 (1:23–35. doi: 10.1038/nri978. [DOI] [PubMed] [Google Scholar]
- Gordon S, Taylor PR. Monocyte and macrophage heterogeneity. Nat Rev Immunol. 2005;5 (12:953–964. doi: 10.1038/nri1733. [DOI] [PubMed] [Google Scholar]
- Krausgruber T, Blazek K, Smallie T, Alzabin S, Lockstone H, Sahgal N, et al. IRF5 promotes inflammatory macrophage polarization and TH1-TH17 responses. Nat Immunol. 2011;12 (3:231–238. doi: 10.1038/ni.1990. [DOI] [PubMed] [Google Scholar]
- Munder M, Eichmann K, Modolell M. Alternative metabolic states in murine macrophages reflected by the nitric oxide synthase/arginase balance: competitive regulation by CD4+ T cells correlates with Th1/Th2 phenotype. J Immunol. 1998;160 (11:5347–5354. [PubMed] [Google Scholar]
- Robertson M, Liversidge J, Forrester JV, Dick AD. Neutralizing tumor necrosis factor-alpha activity suppresses activation of infiltrating macrophages in experimental autoimmune uveoretinitis. Invest Ophthalmol Vis Sci. 2003;44 (7:3034–3041. doi: 10.1167/iovs.02-1156. [DOI] [PubMed] [Google Scholar]
- Robertson MJ, Erwig LP, Liversidge J, Forrester JV, Rees AJ, Dick AD. Retinal microenvironment controls resident and infiltrating macrophage function during uveoretinitis. Invest Ophthalmol Vis Sci. 2002;43 (7:2250–2257. [PubMed] [Google Scholar]
- Taylor PR, Martinez-Pomares L, Stacey M, Lin HH, Brown GD, Gordon S. Macrophage receptors and immune recognition. Annu Rev Immunol. 2005;23:901–944. doi: 10.1146/annurev.immunol.23.021704.115816. [DOI] [PubMed] [Google Scholar]
- Dick AD, Broderick C, Forrester JV, Wright GJ. Distribution of OX2 antigen and OX2 receptor within retina. Invest Ophthalmol Vis Sci. 2001;42:170–176. [PubMed] [Google Scholar]
- Wright GJ, Puklavec MJ, Willis AC, Hoek RM, Sedgwick JD, Brown MH, et al. Lymphoid/neuronal cell surface OX2 glycoprotein recognises a novel receptor on macrophages implicated in their control of function. Immunity. 2000;13:233–238. doi: 10.1016/s1074-7613(00)00023-6. [DOI] [PubMed] [Google Scholar]
- Broderick C, Hoek RM, Forrester JV, Liversidge J, Sedgwick JD, Dick AD. Constitutive retinal CD200 expression regulates resident microglia and activation state of inflammatory cells during experimental autoimmune uveoretinitis. Am J Pathol. 2002;161 (5:1669–1677. doi: 10.1016/S0002-9440(10)64444-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoek RM, Ruuls SR, Murphy CA, Wright GJ, Goddard R, Zurawski SM, et al. Down-regulation of the macrophage lineage through interaction with OX2 (CD200) Science. 2000;290 (5497:1768–1771. doi: 10.1126/science.290.5497.1768. [DOI] [PubMed] [Google Scholar]
- Copland DA, Calder CJ, Raveney BJ, Nicholson LB, Phillips J, Cherwinski H, et al. Monoclonal antibody-mediated CD200 receptor signaling suppresses macrophage activation and tissue damage in experimental autoimmune uveoretinitis. Am J Pathol. 2007;171 (2:580–588. doi: 10.2353/ajpath.2007.070272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Copland DA, Hussain K, Baalasubramanian S, Hughes TR, Morgan BP, Xu H, et al. Systemic and local anti-C5 therapy reduces the disease severity in experimental autoimmune uveoretinitis. Clin Exp Immunol. 2010;159 (3:303–314. doi: 10.1111/j.1365-2249.2009.04070.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothova A. Corticosteroids in uveitis. Ophthalmol Clin North Am. 2002;15 (3:389–394. doi: 10.1016/s0896-1549(02)00023-8. [DOI] [PubMed] [Google Scholar]
- Lee RW, Dick AD. Treat early and embrace the evidence in favour of anti-TNF-alpha therapy for Behcet's uveitis. Br J Ophthalmol. 2010;94 (3:269–270. doi: 10.1136/bjo.2009.176750. [DOI] [PubMed] [Google Scholar]
- Jabs DA, Rosenbaum JT, Foster CS, Holland GN, Jaffe GJ, Louie JS, et al. Guidelines for the use of immunosuppressive drugs in patients with ocular inflammatory disorders: recommendations of an expert panel. Am J Ophthalmol. 2000;130 (4:492–513. doi: 10.1016/s0002-9394(00)00659-0. [DOI] [PubMed] [Google Scholar]
- Galor A, Jabs DA, Leder HA, Kedhar SR, Dunn JP, Peters GB, III, et al. Comparison of antimetabolite drugs as corticosteroid-sparing therapy for noninfectious ocular inflammation. Ophthalmology. 2008;115 (10:1826–1832. doi: 10.1016/j.ophtha.2008.04.026. [DOI] [PubMed] [Google Scholar]
- Hogan AC, McAvoy CE, Dick AD, Lee RW. Long-term efficacy and tolerance of tacrolimus for the treatment of uveitis. Ophthalmology. 2007;114 (5:1000–1006. doi: 10.1016/j.ophtha.2007.01.026. [DOI] [PubMed] [Google Scholar]
- Kilmartin DJ, Forrester JV, Dick AD. Cyclosporin A therapy in refractory non-infectious childhood uveitis. Br J Ophthalmol. 1998;82 (7:737–742. doi: 10.1136/bjo.82.7.737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilmartin DJ, Forrester JV, Dick AD. Rescue therapy with mycophenolate mofetil in refractory uveitis. Lancet. 1998;352 (9121:35–36. doi: 10.1016/S0140-6736(05)79515-5. [DOI] [PubMed] [Google Scholar]
- Kilmartin DJ, Forrester JV, Dick AD. Tacrolimus (FK506) in failed cyclosporin A therapy in endogenous posterior uveitis. Ocul Immunol Inflamm. 1998;6 (2:101–109. doi: 10.1076/ocii.6.2.101.4051. [DOI] [PubMed] [Google Scholar]
- Murphy CC, Greiner K, Plskova J, Duncan L, Frost NA, Forrester JV, et al. Cyclosporine vs tacrolimus therapy for posterior and intermediate uveitis. Arch Ophthalmol. 2005;123 (5:634–641. doi: 10.1001/archopht.123.5.634. [DOI] [PubMed] [Google Scholar]
- Pasadhika S, Kempen JH, Newcomb CW, Liesegang TL, Pujari SS, Rosenbaum JT, et al. Azathioprine for ocular inflammatory diseases Am J Ophthalmol 2009148(4500–509.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma SM, Nestel AR, Lee RW, Dick AD. Clinical review: anti-TNFalpha therapies in uveitis: perspective on 5 years of clinical experience. Ocul Immunol Inflamm. 2009;17 (6:403–414. doi: 10.3109/09273940903072443. [DOI] [PubMed] [Google Scholar]
- Teoh SC, Hogan AC, Dick AD, Lee RW.Mycophenolate mofetil for the treatment of uveitis Am J Ophthalmol 2008146(5752–760.60 e1–3. [DOI] [PubMed] [Google Scholar]
- Teoh SC, Sharma S, Hogan A, Lee R, Ramanan AV, Dick AD. Tailoring biological treatment: anakinra treatment of posterior uveitis associated with the CINCA syndrome. Br J Ophthalmol. 2007;91 (2:263–264. doi: 10.1136/bjo.2006.0101477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kacmaz RO, Kempen JH, Newcomb C, Daniel E, Gangaputra S, Nussenblatt RB, et al. Cyclosporine for ocular inflammatory diseases. Ophthalmology. 2010;117 (3:576–584. doi: 10.1016/j.ophtha.2009.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anglade E, Aspeslet LJ, Weiss SL. A new agent for the treatment of noninfectious uveitis: rationale and design of three LUMINATE (Lux Uveitis Multicenter Investigation of a New Approach to Treatment) trials of steroid-sparing voclosporin. Clin Ophthalmol. 2008;2 (4:693–702. doi: 10.2147/opth.s2452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deuter CM. [Systemic voclosporin for uveitis treatment] Ophthalmologe. 2010;107 (7:672–675. doi: 10.1007/s00347-010-2217-5. [DOI] [PubMed] [Google Scholar]
- Kempen JH, Daniel E, Dunn JP, Foster CS, Gangaputra S, Hanish A, et al. Overall and cancer related mortality among patients with ocular inflammation treated with immunosuppressive drugs: retrospective cohort study. BMJ. 2009;339:b2480. doi: 10.1136/bmj.b2480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kempen JH, Gangaputra S, Daniel E, Levy-Clarke GA, Nussenblatt RB, Rosenbaum JT, et al. Long-term risk of malignancy among patients treated with immunosuppressive agents for ocular inflammation: a critical assessment of the evidence Am J Ophthalmol 2008146(6802–812.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dick AD, Meyer P, James T, Forrester JV, Hale G, Waldmann H, et al. Campath-1H therapy in refractory ocular inflammatory disease. Br J Ophthalmol. 2000;84 (1:107–109. doi: 10.1136/bjo.84.1.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isaacs JD, Hale G, Waldmann H, Dick AD, Haynes R, Forrester JV, et al. Monoclonal antibody therapy of chronic intraocular inflammation using Campath-1H. Br J Ophthalmol. 1995;79 (11:1054–1055. doi: 10.1136/bjo.79.11.1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dick AD, Isaacs JD. Immunomodulation of autoimmune responses with monoclonal antibodies and immunoadhesins: treatment of ocular inflammatory disease in the next millennium. Br J Ophthalmol. 1999;83 (11:1230–1234. doi: 10.1136/bjo.83.11.1230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dick AD, Duncan L, Hale G, Waldmann H, Isaacs J. Neutralizing TNF-alpha activity modulates T-cell phenotype and function in experimental autoimmune uveoretinitis. J Autoimmun. 1998;11 (3:255–264. doi: 10.1006/jaut.1998.0197. [DOI] [PubMed] [Google Scholar]
- Dick AD, McMenamin PG, Korner H, Scallon BJ, Ghrayeb J, Forrester JV, et al. Inhibition of tumor necrosis factor activity minimizes target organ damage in experimental autoimmune uveoretinitis despite quantitatively normal activated T cell traffic to the retina. Eur J Immunol. 1996;26 (5:1018–1025. doi: 10.1002/eji.1830260510. [DOI] [PubMed] [Google Scholar]
- Murphy CC, Duncan L, Forrester JV, Dick AD. Systemic CD4(+) T cell phenotype and activation status in intermediate uveitis. Br J Ophthalmol. 2004;88 (3:412–416. doi: 10.1136/bjo.2003.028506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy CC, Greiner K, Plskova J, Duncan L, Frost A, Isaacs JD, et al. Neutralizing tumor necrosis factor activity leads to remission in patients with refractory noninfectious posterior uveitis. Arch Ophthalmol. 2004;122 (6:845–851. doi: 10.1001/archopht.122.6.845. [DOI] [PubMed] [Google Scholar]
- Rodrigues EB, Farah ME, Maia M, Penha FM, Regatieri C, Melo GB, et al. Therapeutic monoclonal antibodies in ophthalmology. Prog Retin Eye Res. 2009;28 (2:117–144. doi: 10.1016/j.preteyeres.2008.11.005. [DOI] [PubMed] [Google Scholar]
- Theodossiadis PG, Markomichelakis NN, Sfikakis PP. Tumor necrosis factor antagonists: preliminary evidence for an emerging approach in the treatment of ocular inflammation. Retina. 2007;27 (4:399–413. doi: 10.1097/MAJ.0b013e3180318fbc. [DOI] [PubMed] [Google Scholar]
- Yamada Y, Sugita S, Tanaka H, Kamoi K, Kawaguchi T, Mochizuki M. Comparison of infliximab versus ciclosporin during the initial 6-month treatment period in Behcet disease. Br J Ophthalmol. 2010;94 (3:284–288. doi: 10.1136/bjo.2009.158840. [DOI] [PubMed] [Google Scholar]
- Yeh S, Wroblewski K, Buggage R, Li Z, Kurup SK, Sen HN, et al. High-dose humanized anti-IL-2 receptor alpha antibody (daclizumab) for the treatment of active, non-infectious uveitis. J Autoimmun. 2008;31 (2:91–97. doi: 10.1016/j.jaut.2008.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenawy N, Cleary G, Mewar D, Beare N, Chandna A, Pearce I. Abatacept: a potential therapy in refractory cases of juvenile idiopathic arthritis-associated uveitis. Graefes Arch Clin Exp Ophthalmol. 2011;249 (2:297–300. doi: 10.1007/s00417-010-1523-6. [DOI] [PubMed] [Google Scholar]
- Zulian F, Balzarin M, Falcini F, Martini G, Alessio M, Cimaz R, et al. Abatacept for severe anti-tumor necrosis factor alpha refractory juvenile idiopathic arthritis-related uveitis. Arthritis Care Res (Hoboken) 2010;62 (6:821–825. doi: 10.1002/acr.20115. [DOI] [PubMed] [Google Scholar]
- Angeles-Han S, Flynn T, Lehman T. Abatacept for refractory juvenile idiopathic arthritis-associated uveitis- a case report. J Rheumatol. 2008;35 (9:1897–1898. [PubMed] [Google Scholar]
- Heiligenhaus A, Miserocchi E, Heinz C, Gerloni V, Kotaniemi K. Treatment of severe uveitis associated with juvenile idiopathic arthritis with anti-CD20 monoclonal antibody (rituximab) Rheumatology (Oxford) 2011;50 (8:1390–1394. doi: 10.1093/rheumatology/ker107. [DOI] [PubMed] [Google Scholar]
- Iaccheri B, Androudi S, Bocci EB, Gerli R, Cagini C, Fiore T. Rituximab treatment for persistent scleritis associated with rheumatoid arthritis. Ocul Immunol Inflamm. 2010;18 (3:223–225. doi: 10.3109/09273941003739928. [DOI] [PubMed] [Google Scholar]
- Miserocchi E, Pontikaki I, Modorati G, Bandello F, Meroni PL, Gerloni V. Rituximab for uveitis. Ophthalmology. 2011;118 (1:223–224. doi: 10.1016/j.ophtha.2010.07.031. [DOI] [PubMed] [Google Scholar]
- Tappeiner C, Heinz C, Specker C, Heiligenhaus A. Rituximab as a treatment option for refractory endogenous anterior uveitis. Ophthalmic Res. 2007;39 (3:184–186. doi: 10.1159/000103239. [DOI] [PubMed] [Google Scholar]
- Taylor SR, Salama AD, Joshi L, Pusey CD, Lightman SL. Rituximab is effective in the treatment of refractory ophthalmic Wegener's granulomatosis. Arthritis Rheum. 2009;60 (5:1540–1547. doi: 10.1002/art.24454. [DOI] [PubMed] [Google Scholar]
- Goodin DS, Cohen BA, O'Connor P, Kappos L, Stevens JC. Assessment: the use of natalizumab (Tysabri) for the treatment of multiple sclerosis (an evidence-based review): report of the Therapeutics and Technology Assessment Subcommittee of the American Academy of Neurology. Neurology. 2008;71 (10:766–773. doi: 10.1212/01.wnl.0000320512.21919.d2. [DOI] [PubMed] [Google Scholar]
- Brinkmann V, Billich A, Baumruker T, Heining P, Schmouder R, Francis G, et al. Fingolimod (FTY720): discovery and development of an oral drug to treat multiple sclerosis. Nat Rev Drug Discov. 2010;9 (11:883–897. doi: 10.1038/nrd3248. [DOI] [PubMed] [Google Scholar]
- Raveney BJ, Copland DA, Nicholson LB, Dick AD. Fingolimod (FTY720) as an acute rescue therapy for intraocular inflammatory disease. Arch Ophthalmol. 2008;126 (10:1390–1395. doi: 10.1001/archopht.126.10.1390. [DOI] [PubMed] [Google Scholar]
- Deuter CM, Zierhut M, Mohle A, Vonthein R, Stobiger N, Kotter I. Long-term remission after cessation of interferon-alpha treatment in patients with severe uveitis due to Behcet's disease. Arthritis Rheum. 2010;62 (9:2796–2805. doi: 10.1002/art.27581. [DOI] [PubMed] [Google Scholar]
- Plskova J, Greiner K, Forrester JV. Interferon-alpha as an effective treatment for noninfectious posterior uveitis and panuveitis. Am J Ophthalmol. 2007;144 (1:55–61. doi: 10.1016/j.ajo.2007.03.050. [DOI] [PubMed] [Google Scholar]
- Bodaghi B, Gendron G, Wechsler B, Terrada C, Cassoux N, Huong du LT, et al. Efficacy of interferon alpha in the treatment of refractory and sight threatening uveitis: a retrospective monocentric study of 45 patients. Br J Ophthalmol. 2007;91 (3:335–339. doi: 10.1136/bjo.2006.101550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotter I, Gunaydin I, Zierhut M, Stubiger N. The use of interferon alpha in Behcet disease: review of the literature. Semin Arthritis Rheum. 2004;33 (5:320–335. doi: 10.1016/j.semarthrit.2003.09.010. [DOI] [PubMed] [Google Scholar]
- Kotter I, Eckstein AK, Stubiger N, Zierhut M. Treatment of ocular symptoms of Behcet's disease with interferon alpha 2a: a pilot study. Br J Ophthalmol. 1998;82 (5:488–494. doi: 10.1136/bjo.82.5.488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pivetti-Pezzi P, Accorinti M, Pirraglia MP, Priori R, Valesini G. Interferon alpha for ocular Behcet's disease. Acta Ophthalmol Scand. 1997;75 (6:720–722. doi: 10.1111/j.1600-0420.1997.tb00638.x. [DOI] [PubMed] [Google Scholar]
- Kempen JH, Altaweel MM, Holbrook JT, Jabs DA, Sugar EA.The multicenter uveitis steroid treatment trial: rationale, design, and baseline characteristics Am J Ophthalmol 2010149(4550–561.e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim LL, Smith JR, Rosenbaum JT. Retisert (Bausch & Lomb/control delivery systems) Curr Opin Investig Drugs. 2005;6 (11:1159–1167. [PubMed] [Google Scholar]
- Lowder C, Belfort R, Jr, Lightman S, Foster CS, Robinson MR, Schiffman RM, et al. Dexamethasone intravitreal implant for noninfectious intermediate or posterior uveitis. Arch Ophthalmol. 2011;129 (5:545–553. doi: 10.1001/archophthalmol.2010.339. [DOI] [PubMed] [Google Scholar]
- Mizuki N, Meguro A, Ota M, Ohno S, Shiota T, Kawagoe T, et al. Genome-wide association studies identify IL23R-IL12RB2 and IL10 as Behcet's disease susceptibility loci. Nat Genet. 2010;42 (8:703–706. doi: 10.1038/ng.624. [DOI] [PubMed] [Google Scholar]
- Remmers EF, Cosan F, Kirino Y, Ombrello MJ, Abaci N, Satorius C, et al. Genome-wide association study identifies variants in the MHC class I, IL10, and IL23R-IL12RB2 regions associated with Behcet's disease. Nat Genet. 2010;42 (8:698–702. doi: 10.1038/ng.625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atan D, Fraser-Bell S, Plskova J, Kuffova L, Hogan A, Tufail A, et al. Cytokine polymorphism in noninfectious uveitis. Invest Ophthalmol Vis Sci. 2010;51 (8:4133–4142. doi: 10.1167/iovs.09-4583. [DOI] [PubMed] [Google Scholar]
- Atan D, Turner SJ, Kilmartin DJ, Forrester JV, Bidwell J, Dick AD, et al. Cytokine gene polymorphism in sympathetic ophthalmia. Invest Ophthalmol Vis Sci. 2005;46 (11:4245–4250. doi: 10.1167/iovs.05-0126. [DOI] [PubMed] [Google Scholar]
- Atan D, Fraser-Bell S, Plskova J, Kuffova L, Hogan A, Tufail A, et al. Punctate inner choroidopathy and multifocal choroiditis with panuveitis share haplotypic associations with IL10 and TNF loci. Invest Ophthalmol Vis Sci. 2011;52 (6:3573–3581. doi: 10.1167/iovs.10-6743. [DOI] [PubMed] [Google Scholar]
- McKinney EF, Lyons PA, Carr EJ, Hollis JL, Jayne DR, Willcocks LC, et al. A CD8+ T cell transcription signature predicts prognosis in autoimmune disease Nat Med 201016(5586–591.1p following 91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyons PA, McKinney EF, Rayner TF, Hatton A, Woffendin HB, Koukoulaki M, et al. Novel expression signatures identified by transcriptional analysis of separated leucocyte subsets in systemic lupus erythematosus and vasculitis. Ann Rheum Dis. 2010;69 (6:1208–1213. doi: 10.1136/ard.2009.108043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Intzedy L, Teoh SC, Hogan A, Mangwana S, Mayer EJ, Dick AD, et al. Cytopathological analysis of vitreous in intraocular lymphoma. Eye (Lond) 2008;22 (2:289–293. doi: 10.1038/sj.eye.6702965. [DOI] [PubMed] [Google Scholar]
- Sugita S, Shimizu N, Watanabe K, Mizukami M, Morio T, Sugamoto Y, et al. Use of multiplex PCR and real-time PCR to detect human herpes virus genome in ocular fluids of patients with uveitis. Br J Ophthalmol. 2008;92 (7:928–932. doi: 10.1136/bjo.2007.133967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ooi KG, Galatowicz G, Towler HM, Lightman SL, Calder VL. Multiplex cytokine detection versus ELISA for aqueous humor: IL-5, IL-10, and IFNgamma profiles in uveitis. Invest Ophthalmol Vis Sci. 2006;47 (1:272–277. doi: 10.1167/iovs.05-0790. [DOI] [PubMed] [Google Scholar]
- Curnow SJ, Falciani F, Durrani OM, Cheung CM, Ross EJ, Wloka K, et al. Multiplex bead immunoassay analysis of aqueous humor reveals distinct cytokine profiles in uveitis. Invest Ophthalmol Vis Sci. 2005;46 (11:4251–4259. doi: 10.1167/iovs.05-0444. [DOI] [PubMed] [Google Scholar]
- Martin TM, Rosenbaum JT. Genetics in uveitis. Int Ophthalmol Clin. 2005;45 (2:15–30. doi: 10.1097/01.iio.0000155939.83083.dd. [DOI] [PubMed] [Google Scholar]
- Martin TM, Kurz DE, Rosenbaum JT. Genetics of uveitis. Ophthalmol Clin North Am. 2003;16 (4:555–565. doi: 10.1016/s0896-1549(03)00071-3. [DOI] [PubMed] [Google Scholar]
- Mackensen F, David F, Schwenger V, Smith LK, Rajalingam R, Levinson RD, et al. HLA-DRB1*0102 is associated with TINU syndrome and bilateral, sudden-onset anterior uveitis but not with interstitial nephritis alone. Br J Ophthalmol. 2010;95 (7:971–975. doi: 10.1136/bjo.2010.187955. [DOI] [PubMed] [Google Scholar]
- Zamecki KJ, Jabs DA.HLA typing in uveitis: use and misuse Am J Ophthalmol 2010149(2189–193.e2. [DOI] [PubMed] [Google Scholar]
- Tiercy JM, Rathinam SR, Gex-Fabry M, Baglivo E. A shared HLA-DRB1 epitope in the DR beta first domain is associated with Vogt-Koyanagi-Harada syndrome in Indian patients. Mol Vis. 2010;16:353–358. [PMC free article] [PubMed] [Google Scholar]
- Gulati N, Forooghian F, Lieberman R, Jabs DA. Vascular endothelial growth factor inhibition in uveitis: a systematic review. Br J Ophthalmol. 2011;95 (2:162–165. doi: 10.1136/bjo.2009.177279. [DOI] [PubMed] [Google Scholar]
- Martin CM, Lacomba MS, Molina CI, Chamond RR, Galera JM, Estevez EC. Levels of soluble ICAM-1 and soluble IL-2R in the serum and aqueous humor of uveitis patients. Curr Eye Res. 2000;20 (4:287–292. [PubMed] [Google Scholar]
- Arocker-Mettinger E, Asenbauer T, Ulbrich S, Grabner G. Serum interleukin 2-receptor levels in uveitis. Curr Eye Res. 1990;9 (Suppl:25–29. doi: 10.3109/02713689008999415. [DOI] [PubMed] [Google Scholar]
- Plskova J, Greiner K, Muckersie E, Duncan L, Forrester JV. Interferon-alpha: a key factor in autoimmune disease. Invest Ophthalmol Vis Sci. 2006;47 (9:3946–3950. doi: 10.1167/iovs.06-0058. [DOI] [PubMed] [Google Scholar]
- Kilmartin DJ, Fletcher ZJ, Almeida JA, Liversidge J, Forrester JV, Dick AD. CD69 expression on peripheral CD4+ T cells parallels disease activity and is reduced by mycophenolate mofetil therapy in uveitis. Invest Ophthalmol Vis Sci. 2001;42 (6:1285–1292. [PubMed] [Google Scholar]
- Dick AD. Serological tests for monitoring and predicting disease severity, course, and outcome of autoimmune intraocular inflammation. Br J Ophthalmol. 1998;82 (8:856–857. doi: 10.1136/bjo.82.8.856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dick AD, Cheng YF, Purdie AT, Liversidge J, Forrester JV. Immunocytochemical analysis of blood lymphocytes in uveitis. Eye (Lond) 1992;6 (Pt 6:643–647. doi: 10.1038/eye.1992.138. [DOI] [PubMed] [Google Scholar]
- Pascual V, Allantaz F, Arce E, Punaro M, Banchereau J. Role of interleukin-1 (IL-1) in the pathogenesis of systemic onset juvenile idiopathic arthritis and clinical response to IL-1 blockade. J Exp Med. 2005;201 (9:1479–1486. doi: 10.1084/jem.20050473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee RW, Schewitz LP, Nicholson LB, Dayan CM, Dick AD. Steroid refractory CD4+ T cells in patients with sight-threatening uveitis. Invest Ophthalmol Vis Sci. 2009;50 (9:4273–4278. doi: 10.1167/iovs.08-3152. [DOI] [PubMed] [Google Scholar]
- Xu H, Manivannan A, Goatman KA, Liversidge J, Sharp PF, Forrester JV, et al. Improved leukocyte tracking in mouse retinal and choroidal circulation. Exp Eye Res. 2002;74 (3:403–410. doi: 10.1006/exer.2001.1134. [DOI] [PubMed] [Google Scholar]