Abstract
The natural isoquinoline alkaloid berberine possesses potential to treat Alzheimer's disease (AD) by targeting multiple pathogenic factors. In the present study, docking simulations were performed to gain deeper insights into the molecular basis of berberine's inhibitory effects against the important pathogenic enzymes of AD, that is, acetylcholinesterase, butyrylcholinesterase, and two isoforms of monoamine oxidase. It was found that the theoretical binding affinities of berberine to the four enzymes are very close to the experimental values, which verify the methodology. Further inspection to the binding modes found that hydrophobic interactions between the hydrophobic surface of berberine and neighboring hydrophobic residues are the principal forces contributing to the ligand-receptor interactions. Although berberine cation also has potential to form electrostatic interaction with neighboring residues, it is interesting to find that electrostatic force is excluded in the four cases unexpectedly. These results have important implications for the berberine-based anti-AD drug design.
1. Introduction
As a natural isoquinoline alkaloid isolated from the Chinese herb Rhizoma coptidis, berberine (Figure 1) has gained considerable attention because of its wide spectrum of biochemical and pharmacological potentials, including antioxidant, antiinflammatory, anticancer activities, and so forth, [1–6]. Alzheimer's disease (AD) is the most common form of degenerative dementia with an estimated prevalence of 30 million people worldwide, and with the accelerated aging of human society, its prevalence is expected to rise steadily [7–10]. In recent years, multiple lines of evidence support that berberine also possesses potential to act as a multipotent agent to treat AD [11–14]. For instance, many experimental studies reported that berberine exhibits inhibitory effects against several key enzymes implicated in the pathogenesis of AD, including acetylcholinesterase (AChE), butyrylcholinesterase (BChE), and monoamine oxidase (MAO) [14–22]. With the aim to elucidate the molecular basis of berberine's inhibitory effects against the pathogenic enzymes in AD, in the present study, the binding modes of berberine with four enzymes, that is, AChE, BChE, MAO-A, and MAO-B, were investigated by means of docking simulations. The results indicate that hydrophobic interactions are the principal forces contributing to the binding of berberine to the four enzymes. Despite the cation ion in berberine structure (Figure 1) can interact readily with the negatively charged acidic residues, no electrostatic force is observed unexpectedly in the four cases. The findings have important implications for the berberine-based anti-AD drug design.
Figure 1.
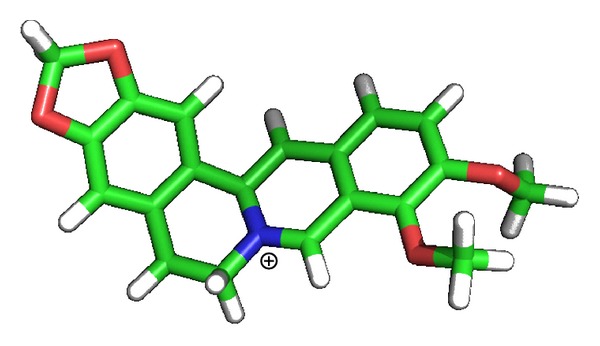
Chemical structure of berberine.
2. Methods
2.1. Structural Models
Structure coordinates for AChE, BChE, MAO-A, and MAO-B were taken from the Protein Data Bank (PDB codes: 1EA5 [23], 1P0I [24], 1O5 W [25], and 1GOS [26], resp.). The 3D structure of berberine was firstly constructed using standard geometric parameters of SYBYL software and then was optimized using Powell method with the Tripos force field (distance-dependent dielectric) to reach a final energy convergence gradient value of 0.001 kcal/mol.
2.2. Docking Methods
The Surflex-Dock program interfaced with SYBYL software is employed to perform docking experiments in this study, which uses an empirically derived scoring function based on the binding affinities of protein-ligand complexes and on their X-ray structures [27]. As a flexible docking method, Surflex-Dock has been proven to be efficient in treating numerous protein receptors [27, 28]. The active sites for four targets were selected on the basis of experimentally reported key residues, which play key roles in their catalytic activities [23–26]. During the simulations, the Kollman-all atom charges were assigned to protein atoms using SYBYL software. For berberine molecule, 30 conformations were selected to dock with target in each run. Standard parameters were used to estimate the binding affinity characterized by Surflex-Dock scores. Surflex-Dock scores (total scores) are expressed in −log10(K d) units to represent binding affinities [29, 30].
3. Results and Discussion
The theoretical binding constants of berberine to AChE, BChE, MAO-A, and MAO-B are estimated and listed in Table 1. It can be seen that berberine possesses inhibitory activity against the four enzymes and the respective binding affinities vary largely. The theoretical K d of berberine to AChE (0.66 μM), BChE (3.31 μM), MAO-A (105.2 μM), and MAO-B (66.0 μM) are very close to the experimental values (Table 1), which verify the accuracy of the present methodology. According to the theoretical K d, the inhibitory activity of berberine against AChE is the highest among the four enzymes, which is in agreement with the experimental results.
Table 1.
Theoretically estimated binding constants (K d) of berberine with AChE, BChE, MAO-A, and MAO-B, and experimental IC50.
To elucidate the forces contributing to the binding affinity, the binding modes of berberine in AChE, BChE, MAO-A, and MAO-B are shown in Figure 2. From the molecular structure point of view, berberine has a large hydrophobic surface and a cation ion, which is ideal for interacting with the hydrophobic residues and the negatively charged acidic residues (Figure 1). As shown in Figure 2, the neighboring residues to berberine in the four enzymes are almost all aromatic and/or hydrophobic amino acids. Therefore, these residues can readily form hydrophobic interactions with the hydrophobic surface of berberine. According to Figure 2, there are eight hydrophobic residues (four phenylalanine, three tyrosine, and one tryptophan) interacting with berberine in AChE, while only six hydrophobic residues (one phenylalanine, two tyrosine, two tryptophan, and one isoleucine) with respect to the binding pocket in BChE. Also, a hydrogen bond is formed between berberine and Tyr121 in AChE (Figure 2), which will strengthen the binding affinity and enhance the inhibitory activity of berberine against AChE. These two aspects may account for the relatively stronger binding of berberine to AChE than BChE. In addition, there are less hydrophobic residues involved in the binding of berberine to MAO-A and MAO-B (Figure 2), which results in their much lower binding affinity.
Figure 2.
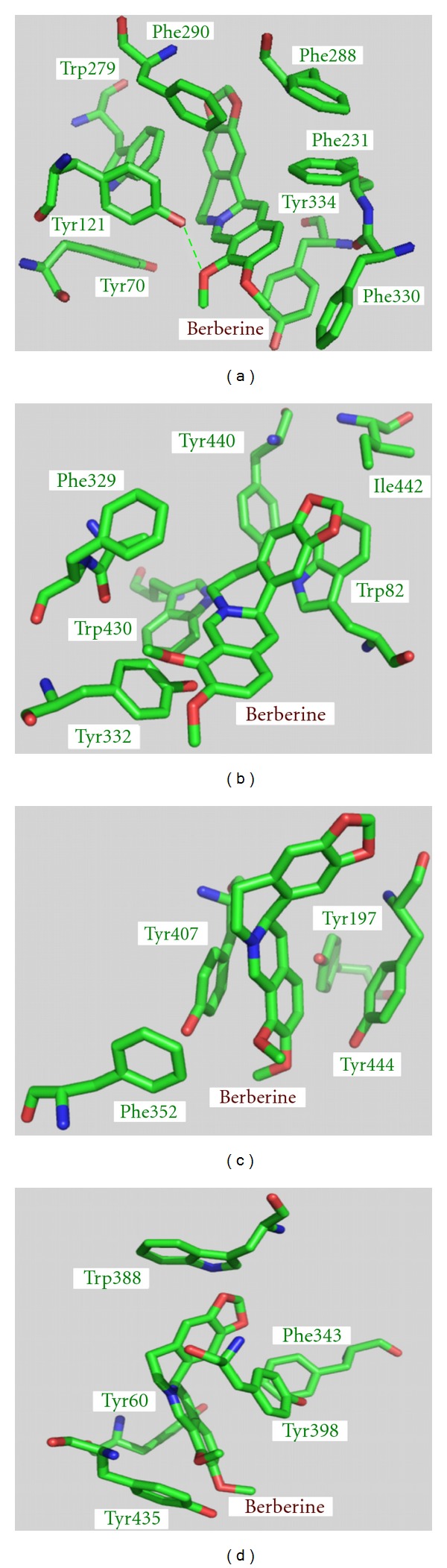
Close-up views of binding modes of berberine in AChE (a), BChE (b), MAO-A (c), and MAO-B (d). The hydrogen bond is marked in green dotted lines.
Although berberine cation also has the potential to form electrostatic interaction with neighboring residues in four enzymes, it is interesting to find that as no corresponding negatively charged acidic residues exist at proper positions, no electrostatic interaction is observed. Therefore, according to the present results, the inhibitory activities of berberine against four targets mainly arise from hydrophobic interactions.
4. Conclusions
In conclusion, the theoretically estimated binding affinities of berberine to the four enzymes, AChE, BChE, MAO-A, and MAO-B, are very close to the experimental values. According to the binding modes, the hydrophobic interactions between berberine and surrounding hydrophobic residues in the enzymes play predominant roles, while electrostatic force is excluded in the binding of berberine to the four targets. These findings shed lights on the molecular basis of the inhibitory effects of berberine against the enzymes implicated in the pathogenesis of AD and will be helpful for the berberine-based anti-AD drug design.
Acknowledgment
This work was supported by the National Natural Science Foundation of China (Grant no. 30800184).
References
- 1.Imanshahidi M, Hosseinzadeh H. Pharmacological and therapeutic effects of Berberis vulgaris and its active constituent, berberine. Phytotherapy Research. 2008;22(8):999–1012. doi: 10.1002/ptr.2399. [DOI] [PubMed] [Google Scholar]
- 2.Vuddanda PR, Chakraborty S, Singh S. Berberine: a potential phytochemical with multispectrum therapeutic activities. Expert Opinion on Investigational Drugs. 2010;19(10):1297–1307. doi: 10.1517/13543784.2010.517745. [DOI] [PubMed] [Google Scholar]
- 3.Kuo CL, Chi CW, Liu TY. The anti-inflammatory potential of berberine in vitro and in vivo . Cancer Letters. 2004;203(2):127–137. doi: 10.1016/j.canlet.2003.09.002. [DOI] [PubMed] [Google Scholar]
- 4.Sun Y, Xun K, Wang Y, Chen X. A systematic review of the anticancer properties of berberine, a natural product from Chinese herbs. Anti-Cancer Drugs. 2009;20(9):757–769. doi: 10.1097/CAD.0b013e328330d95b. [DOI] [PubMed] [Google Scholar]
- 5.Račková L, Májeková M, Košt'álová D, Štefek M. Antiradical and antioxidant activities of alkaloids isolated from Mahonia aquifolium. Structural aspects. Bioorganic & Medicinal Chemistry. 2004;12(17):4709–4715. doi: 10.1016/j.bmc.2004.06.035. [DOI] [PubMed] [Google Scholar]
- 6.Stermitz FR, Lorenz P, Tawara JN, Zenewicz LA, Lewis K. Synergy in a medicinal plant: antimicrobial action of berberine potentiated by 5-methoxyhydnocarpin, a multidrug pump inhibitor. Proceedings of the National Academy of Sciences of the United States of America. 2000;97(4):1433–1437. doi: 10.1073/pnas.030540597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ballard C, Gauthier S, Corbett A, Brayne C, Aarsland D, Jones E. Alzheimer's disease. The Lancet. 2011;377(9770):1019–1031. doi: 10.1016/S0140-6736(10)61349-9. [DOI] [PubMed] [Google Scholar]
- 8.Holtzman DM, Morris JC, Goate AM. Alzheimer's disease: the challenge of the second century. Science Translational Medicine. 2011;3(77):p. 77sr1. doi: 10.1126/scitranslmed.3002369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cummings JL. Alzheimer's disease. The New England Journal of Medicine. 2004;351(1):56–57. doi: 10.1056/NEJMra040223. [DOI] [PubMed] [Google Scholar]
- 10.Plassman BL, Langa KM, Fisher GG, et al. Prevalence of dementia in the United States: the aging, demographics, and memory study. Neuroepidemiology. 2007;29(1-2):125–132. doi: 10.1159/000109998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhu F, Qian C. Berberine chloride can ameliorate the spatial memory impairment and increase the expression of interleukin-1 beta and inducible nitric oxide synthase in the rat model of Alzheimer’s disease. BMC Neuroscience. 2006;7, article 78 doi: 10.1186/1471-2202-7-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ji H-F, Shen L. Berberine: a potential multipotent natural product to combat Alzheimer's disease. Molecules. 2011;16(8):6732–6740. doi: 10.3390/molecules16086732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ye M, Fu S, Pi R, He F. Neuropharmacological and pharmacokinetic properties of berberine: a review of recent research. Journal of Pharmacy and Pharmacology. 2009;61(7):831–837. doi: 10.1211/jpp/61.07.0001. [DOI] [PubMed] [Google Scholar]
- 14.Jung HA, Min BS, Yokozawa T, Lee JH, Kim YS, Choi JS. Anti-Alzheimer and antioxidant activities of coptidis rhizoma alkaloids. Biological & Pharmaceutical Bulletin. 2009;32(8):1433–1438. doi: 10.1248/bpb.32.1433. [DOI] [PubMed] [Google Scholar]
- 15.Ingkaninan K, Phengpa P, Yuenyongsawad S, Khorana N. Acetylcholinesterase inhibitors from Stephania venosa tuber. Journal of Pharmacy and Pharmacology. 2006;58(5):695–700. doi: 10.1211/jpp.58.5.0015. [DOI] [PubMed] [Google Scholar]
- 16.Huang L, Luo Z, He F, Shi A, Qin F, Li X. Berberine derivatives, with substituted amino groups linked at the 9-position, as inhibitors of acetylcholinesterase/butyrylcholinesterase. Bioorganic & Medicinal Chemistry Letters. 2010;20(22):6649–6652. doi: 10.1016/j.bmcl.2010.09.013. [DOI] [PubMed] [Google Scholar]
- 17.Huang L, Shi A, He F, Li X. Synthesis, biological evaluation, and molecular modeling of berberine derivatives as potent acetylcholinesterase inhibitors. Bioorganic & Medicinal Chemistry. 2010;18(3):1244–1251. doi: 10.1016/j.bmc.2009.12.035. [DOI] [PubMed] [Google Scholar]
- 18.Kim DK, Lee KT, Baek NI, et al. Acetylcholinesterase inhibitors from the aerial parts of Corydalis speciosa . Archives of Pharmacal Research. 2004;27(11):1127–1131. doi: 10.1007/BF02975117. [DOI] [PubMed] [Google Scholar]
- 19.Kong LD, Cheng CHK, Tan RX. Monoamine oxidase inhibitors from rhizoma of Coptis chinensis. Planta Medica. 2001;67(1):74–76. doi: 10.1055/s-2001-10874. [DOI] [PubMed] [Google Scholar]
- 20.Lee SS, Kai M, Lee MK. Effects of natural isoquinoline alkaloids on monoamine oxidase activity in mouse brain: inhibition by berberine and palmatine. Medical Science Research. 1999;27(11):749–751. [Google Scholar]
- 21.Castillo J, Hung J, Rodriguez M, Bastidas E, Laboren I, Jaimes A. LED fluorescence spectroscopy for direct determination of monoamine oxidase B inactivation. Analytical Biochemistry. 2005;343(2):293–298. doi: 10.1016/j.ab.2005.05.027. [DOI] [PubMed] [Google Scholar]
- 22.Kulkarni SK, Dhir A. On the mechanism of antidepressant-like action of berberine chloride. European Journal of Pharmacology. 2008;589(1–3):163–172. doi: 10.1016/j.ejphar.2008.05.043. [DOI] [PubMed] [Google Scholar]
- 23.Dvir H, Jiang HL, Wong DM, et al. X-ray structures of Torpedo californica acetylcholinesterase complexed with (+)-huperzine A and (-)-huperzine B: structural evidence for an active site rearrangement. Biochemistry. 2002;41(35):10810–10818. doi: 10.1021/bi020151+. [DOI] [PubMed] [Google Scholar]
- 24.Nicolet Y, Lockridge O, Masson P, Fontecilla-Camps JC, Nachon F. Crystal structure of human butyrylcholinesterase and of its complexes with substrate and products. The Journal of Biological Chemistry. 2003;278(42):41141–41147. doi: 10.1074/jbc.M210241200. [DOI] [PubMed] [Google Scholar]
- 25.Ma J, Yoshimura M, Yamashita E, Nakagawa A, Ito A, Tsukihara T. Structure of rat monoamine oxidase A and its specific recognitions for substrates and inhibitors. Journal of Molecular Biology. 2004;338(1):103–114. doi: 10.1016/j.jmb.2004.02.032. [DOI] [PubMed] [Google Scholar]
- 26.Binda C, Newton-Vinson P, Hubálek F, Edmondson DE, Mattevi A. Structure of human monoamine oxidase B, a drug target for the treatment of neurological disorders. Nature Structural Biology. 2001;9(1):22–26. doi: 10.1038/nsb732. [DOI] [PubMed] [Google Scholar]
- 27.Jain AN. Surflex: fully automatic flexible molecular docking using a molecular similarity-based search engine. Journal of Medicinal Chemistry. 2003;46(4):499–511. doi: 10.1021/jm020406h. [DOI] [PubMed] [Google Scholar]
- 28.Kellenberger E, Rodrigo J, Muller P, Rognan D. Comparative evaluation of eight docking tools for docking and virtual screening accuracy. Proteins. 2004;57(2):225–242. doi: 10.1002/prot.20149. [DOI] [PubMed] [Google Scholar]
- 29.Holt PA, Chaires JB, Trent JO. Molecular docking of intercalators and groove-binders to nucleic adds using autodock and surflex. Journal of Chemical Information and Modeling. 2008;48(8):1602–1615. doi: 10.1021/ci800063v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yang M, Zhou L, Zuo Z, Tang X, Liu J, Ma X. Structure-based virtual screening for glycosyltransferase51. Molecular Simulation. 2008;34(9):849–856. [Google Scholar]


