Abstract
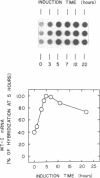
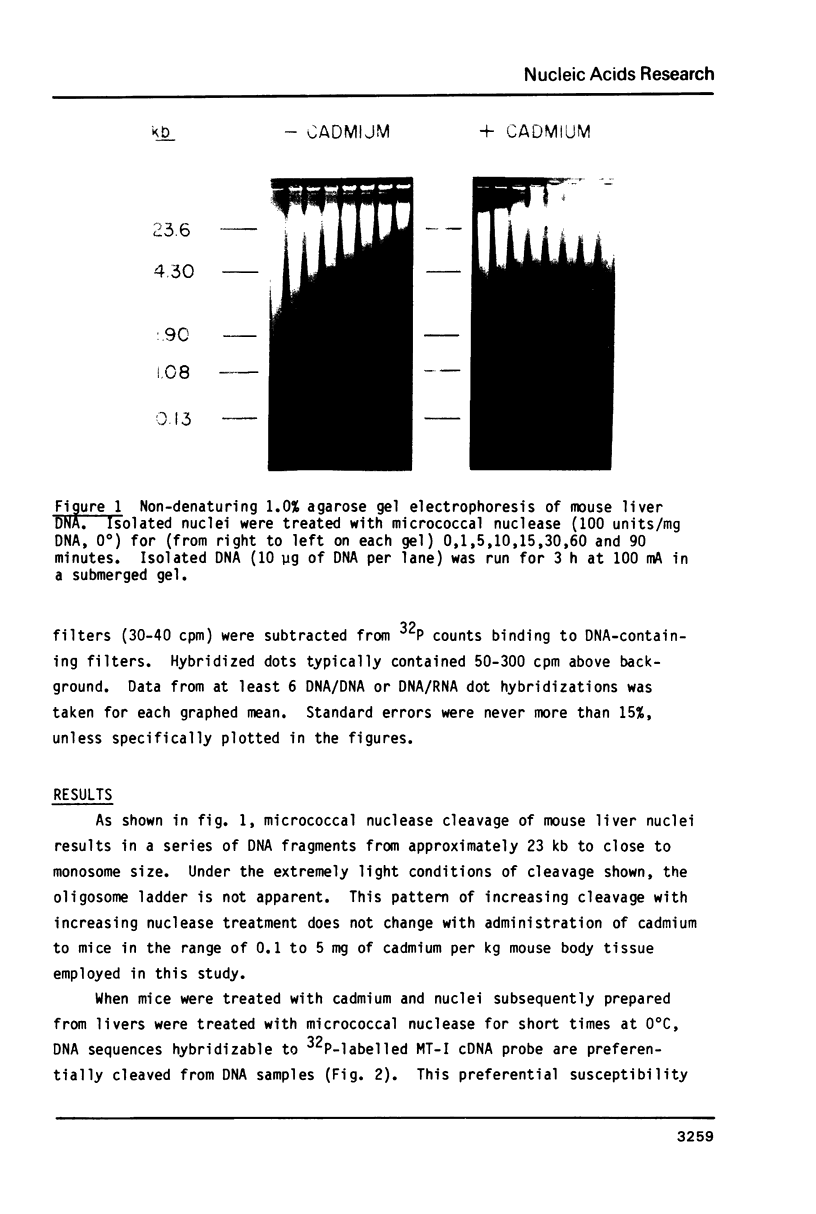
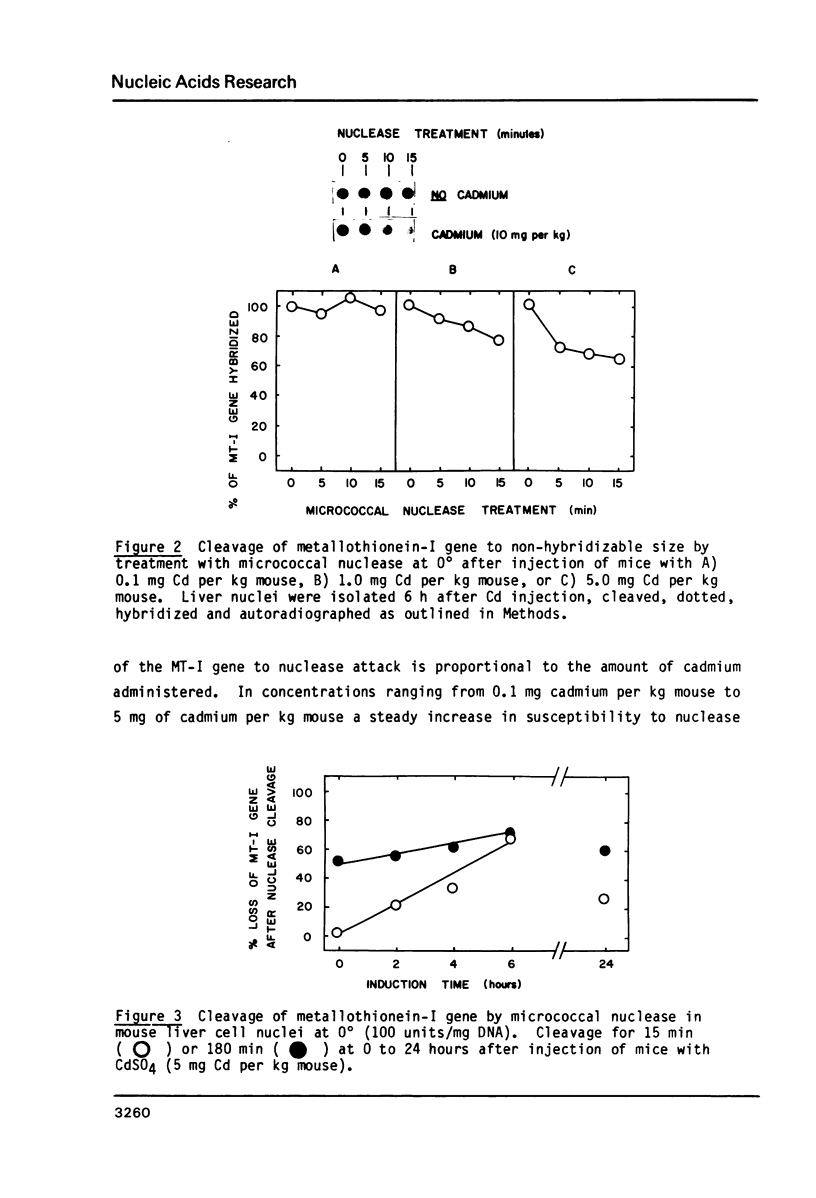
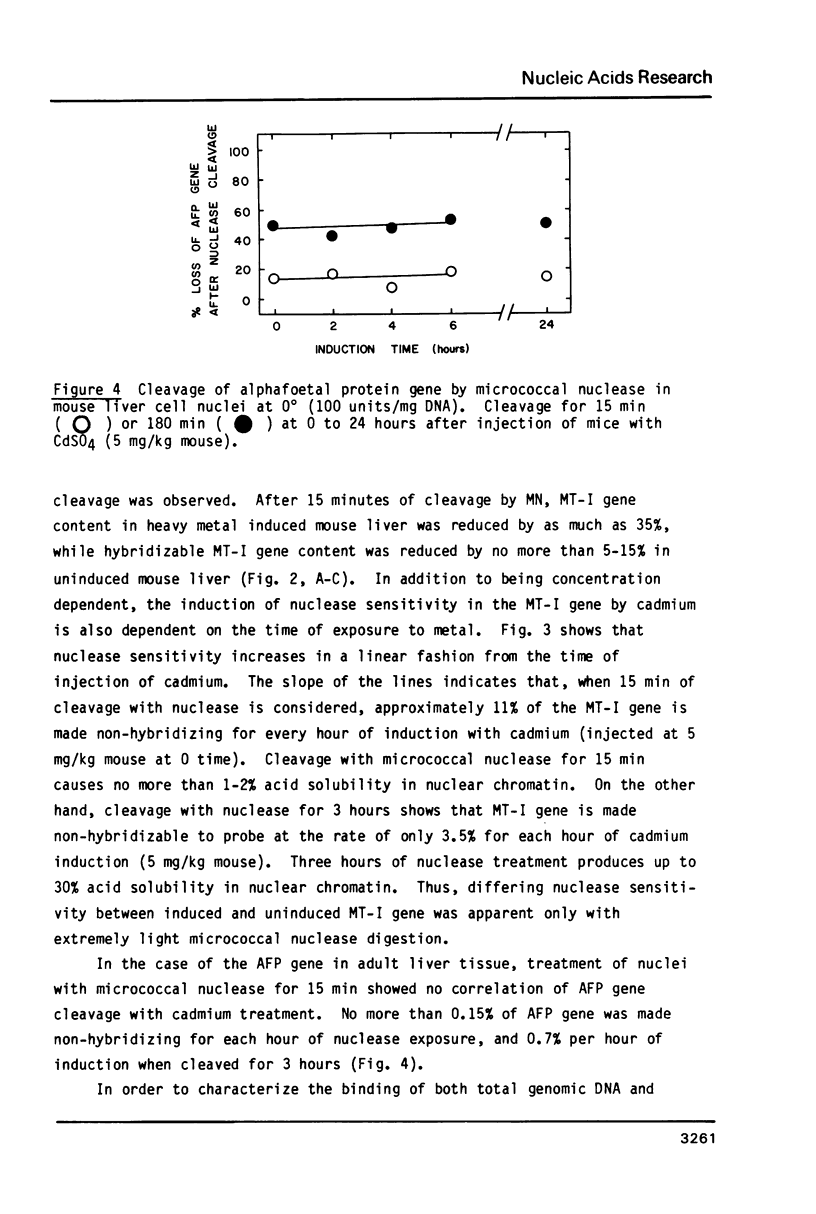
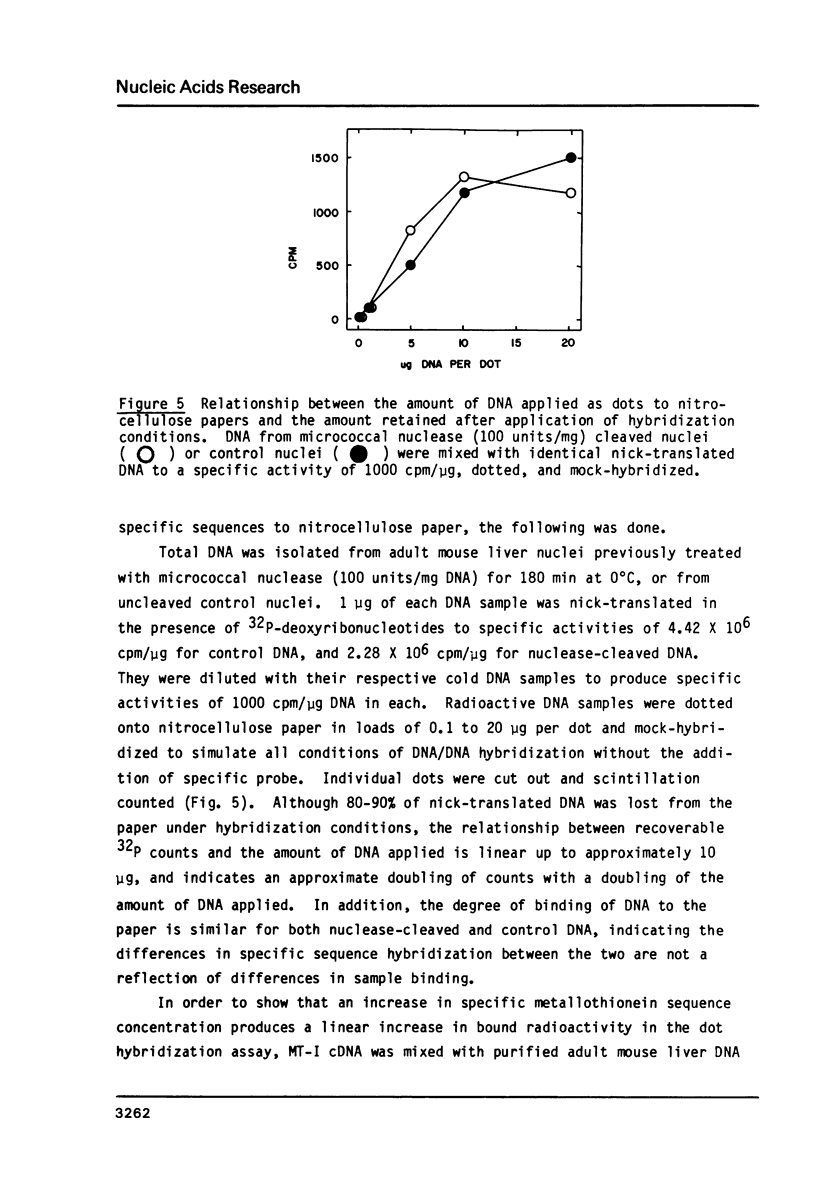
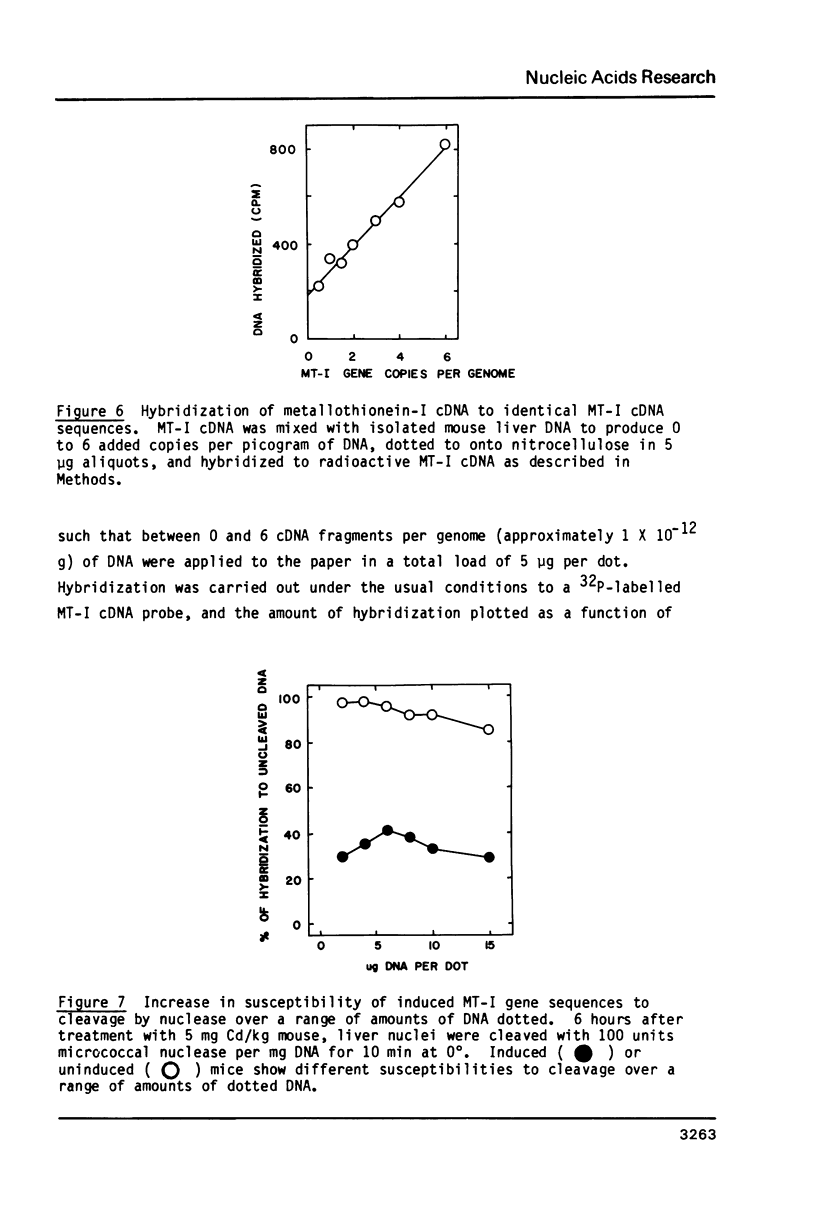
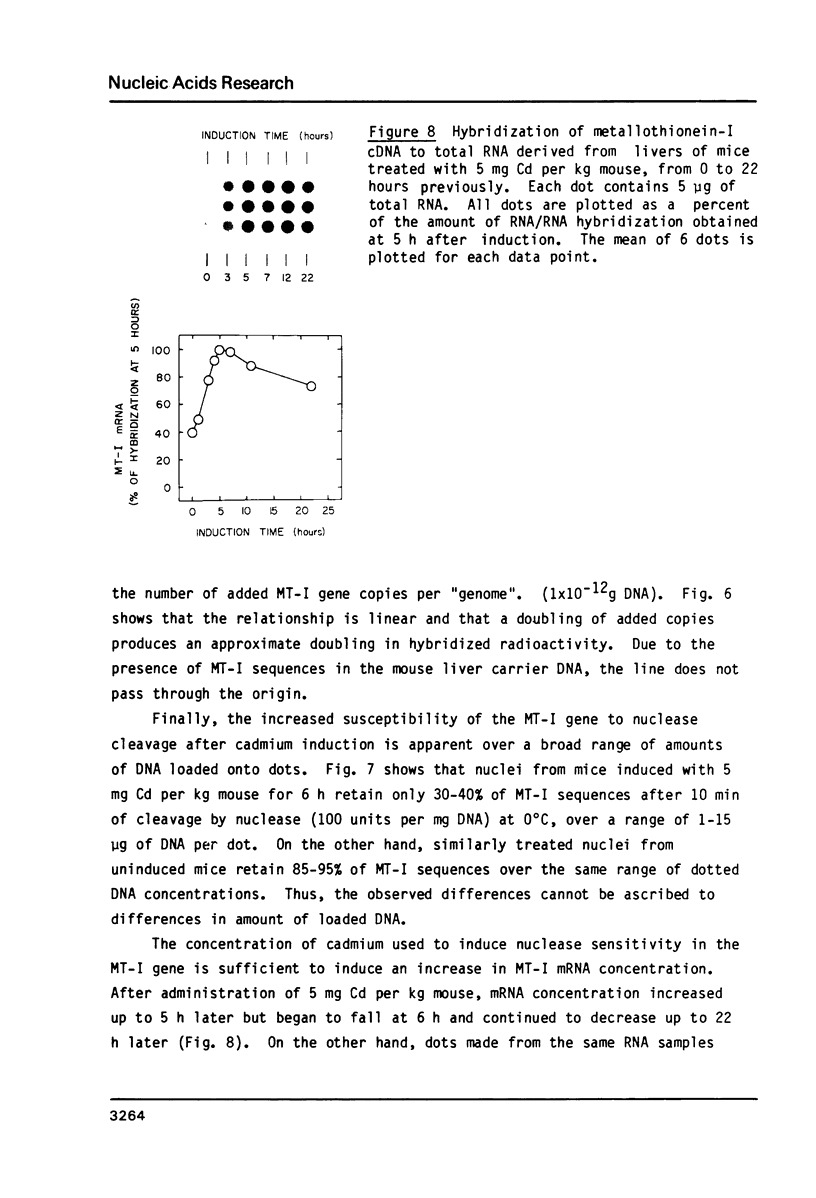
Micrococcal nuclease has been shown to preferentially cleave chromatin in the region of genes actively engaged in transcription. We have used this preferential cleavage to show that the metallothionein (MT) gene in adult mouse liver, when induced to produce mRNA by injection of cadmium, becomes more susceptible to nuclease cleavage. However, the MT gene in uninduced liver, and the alphafoetal protein (AFP) gene in both induced and uninduced liver, remain relatively resistant to nuclease cleavage. The AFP gene is not normally expressed in cadmium induced or uninduced liver. Thus, susceptibility of genes to nuclease cleavage appears to rise with increasing transcription of the gene.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abelev G. I. Alpha-fetoprotein in ontogenesis and its association with malignant tumors. Adv Cancer Res. 1971;14:295–358. doi: 10.1016/s0065-230x(08)60523-0. [DOI] [PubMed] [Google Scholar]
- Adinolfi M. Human alphafetoprotein 1956-1978. Adv Hum Genet. 1979;9:165–228. [PubMed] [Google Scholar]
- Andersen R. D., Weser U. Partial purification, characterization and translation in vitro of rat liver metallothionein messenger ribonucleic acid. Biochem J. 1978 Dec 1;175(3):841–852. doi: 10.1042/bj1750841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bélanger L., Frain M., Baril P., Gingras M. C., Bartkowiak J., Sala-Trepat J. M. Glucocorticosteroid suppression of alpha1-fetoprotein synthesis in developing rat liver. Evidence for selective gene repression at the transcriptional level. Biochemistry. 1981 Nov 10;20(23):6665–6672. doi: 10.1021/bi00526a022. [DOI] [PubMed] [Google Scholar]
- Chiu J. F., Gabryelak T., Commers P., Massari R. The elevation of alpha-fetoprotein messenger RNA in regenerating rat liver. Biochem Biophys Res Commun. 1981 Jan 15;98(1):250–254. doi: 10.1016/0006-291x(81)91895-7. [DOI] [PubMed] [Google Scholar]
- Chiu J. F., Massari R. J., Schwartz C. E., Meisler N. T., Thanassi J. W. Hormonal modulation of alpha-fetoprotein gene expression in newborn rat livers. Nucleic Acids Res. 1981 Dec 21;9(24):6917–6933. doi: 10.1093/nar/9.24.6917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denhardt D. T. A membrane-filter technique for the detection of complementary DNA. Biochem Biophys Res Commun. 1966 Jun 13;23(5):641–646. doi: 10.1016/0006-291x(66)90447-5. [DOI] [PubMed] [Google Scholar]
- Durnam D. M., Palmiter R. D. Transcriptional regulation of the mouse metallothionein-I gene by heavy metals. J Biol Chem. 1981 Jun 10;256(11):5712–5716. [PubMed] [Google Scholar]
- Durnam D. M., Perrin F., Gannon F., Palmiter R. D. Isolation and characterization of the mouse metallothionein-I gene. Proc Natl Acad Sci U S A. 1980 Nov;77(11):6511–6515. doi: 10.1073/pnas.77.11.6511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garel A., Axel R. Selective digestion of transcriptionally active ovalbumin genes from oviduct nuclei. Proc Natl Acad Sci U S A. 1976 Nov;73(11):3966–3970. doi: 10.1073/pnas.73.11.3966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garel A., Zolan M., Axel R. Genes transcribed at diverse rates have a similar conformation in chromatin. Proc Natl Acad Sci U S A. 1977 Nov;74(11):4867–4871. doi: 10.1073/pnas.74.11.4867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gitlin D., Boesman M. Sites of serum alpha-fetoprotein synthesis in the human and in the rat. J Clin Invest. 1967 Jun;46(6):1010–1016. doi: 10.1172/JCI105590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorin M. B., Tilghman S. M. Structure of the alpha-fetoprotein gene in the mouse. Proc Natl Acad Sci U S A. 1980 Mar;77(3):1351–1355. doi: 10.1073/pnas.77.3.1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keene M. A., Corces V., Lowenhaupt K., Elgin S. C. DNase I hypersensitive sites in Drosophila chromatin occur at the 5' ends of regions of transcription. Proc Natl Acad Sci U S A. 1981 Jan;78(1):143–146. doi: 10.1073/pnas.78.1.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Law S., Tamoaki T., Kreuzaler F., Dugaiczyk A. Molecular cloning of DNA complementary to a mouse alpha-fetoprotein mRNA sequence. Gene. 1980 Jun;10(1):53–61. doi: 10.1016/0378-1119(80)90143-2. [DOI] [PubMed] [Google Scholar]
- Levy-Wilson B., Dixon G. H. Limited action of micrococcal nuclease on trout testis nuclei generates two mononucleosome subsets enriched in transcribed DNA sequences. Proc Natl Acad Sci U S A. 1979 Apr;76(4):1682–1686. doi: 10.1073/pnas.76.4.1682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathis D., Oudet P., Chambon P. Structure of transcribing chromatin. Prog Nucleic Acid Res Mol Biol. 1980;24:1–55. doi: 10.1016/s0079-6603(08)60670-4. [DOI] [PubMed] [Google Scholar]
- Miller D. M., Turner P., Nienhuis A. W., Axelrod D. E., Gopalakrishnan T. V. Active conformation of the globin genes in uninduced and induced mouse erythroleukemia cells. Cell. 1978 Jul;14(3):511–521. doi: 10.1016/0092-8674(78)90237-4. [DOI] [PubMed] [Google Scholar]
- Probst G. S., Bousquet W. F., Miya T. S. Kinetics of cadmium-induced hepatic and renal metallothionein synthesis in the mouse. Toxicol Appl Pharmacol. 1977 Jan;39(1):51–60. doi: 10.1016/0041-008x(77)90176-4. [DOI] [PubMed] [Google Scholar]
- Reeves R. Analysis and reconstruction of Xenopus ribosomal chromatin nucleosomes. Eur J Biochem. 1977 May 16;75(2):545–560. doi: 10.1111/j.1432-1033.1977.tb11555.x. [DOI] [PubMed] [Google Scholar]
- Reeves R. Ribosomal genes of Xenopus laevis: evidence of nucleosomes in transcriptionally active chromatin. Science. 1976 Oct 29;194(4264):529–532. doi: 10.1126/science.973136. [DOI] [PubMed] [Google Scholar]
- Ruoslahti E., Seppälä M. alpha-Fetoprotein in cancer and fetal development. Adv Cancer Res. 1979;29:275–346. doi: 10.1016/s0065-230x(08)60849-0. [DOI] [PubMed] [Google Scholar]
- Shapiro S. G., Squibb K. S., Markowitz L. A., Cousins R. J. Cell-free synthesis of metallothionein directed by rat liver polyadenylated messenger ribonucleic acid. Biochem J. 1978 Dec 1;175(3):833–840. doi: 10.1042/bj1750833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shepherd J. H., Mulvihill E. R., Thomas P. S., Palmiter R. D. Commitment of chick oviduct tubular gland cells to produce ovalbumin mRNA during hormonal withdrawal and restimulation. J Cell Biol. 1980 Oct;87(1):142–151. doi: 10.1083/jcb.87.1.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Squibb K. S., Cousins R. J. Synthesis of metallothionein in a polysomal cell-free system. Biochem Biophys Res Commun. 1977 Apr 11;75(3):806–812. doi: 10.1016/0006-291x(77)91544-3. [DOI] [PubMed] [Google Scholar]
- Stalder J., Seebeck T., Braun R. Accessibility of the ribosomal genes to micrococcal nuclease in Physarum polycephalum. Biochim Biophys Acta. 1979 Feb 27;561(2):452–463. doi: 10.1016/0005-2787(79)90153-9. [DOI] [PubMed] [Google Scholar]
- Stalder J., Seebeck T., Braun R. Degradation of the ribosomal genes by DNAse I in Physarum polycephalum. Eur J Biochem. 1978 Oct;90(2):391–395. doi: 10.1111/j.1432-1033.1978.tb12616.x. [DOI] [PubMed] [Google Scholar]
- Thomashow M. F., Nutter R., Montoya A. L., Gordon M. P., Nester E. W. Integration and organization of Ti plasmid sequences in crown gall tumors. Cell. 1980 Mar;19(3):729–739. doi: 10.1016/s0092-8674(80)80049-3. [DOI] [PubMed] [Google Scholar]
- Weintraub H., Groudine M. Chromosomal subunits in active genes have an altered conformation. Science. 1976 Sep 3;193(4256):848–856. doi: 10.1126/science.948749. [DOI] [PubMed] [Google Scholar]
- Wu C., Wong Y. C., Elgin S. C. The chromatin structure of specific genes: II. Disruption of chromatin structure during gene activity. Cell. 1979 Apr;16(4):807–814. doi: 10.1016/0092-8674(79)90096-5. [DOI] [PubMed] [Google Scholar]