Abstract
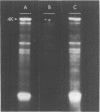
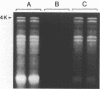
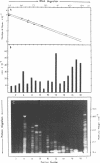
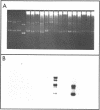
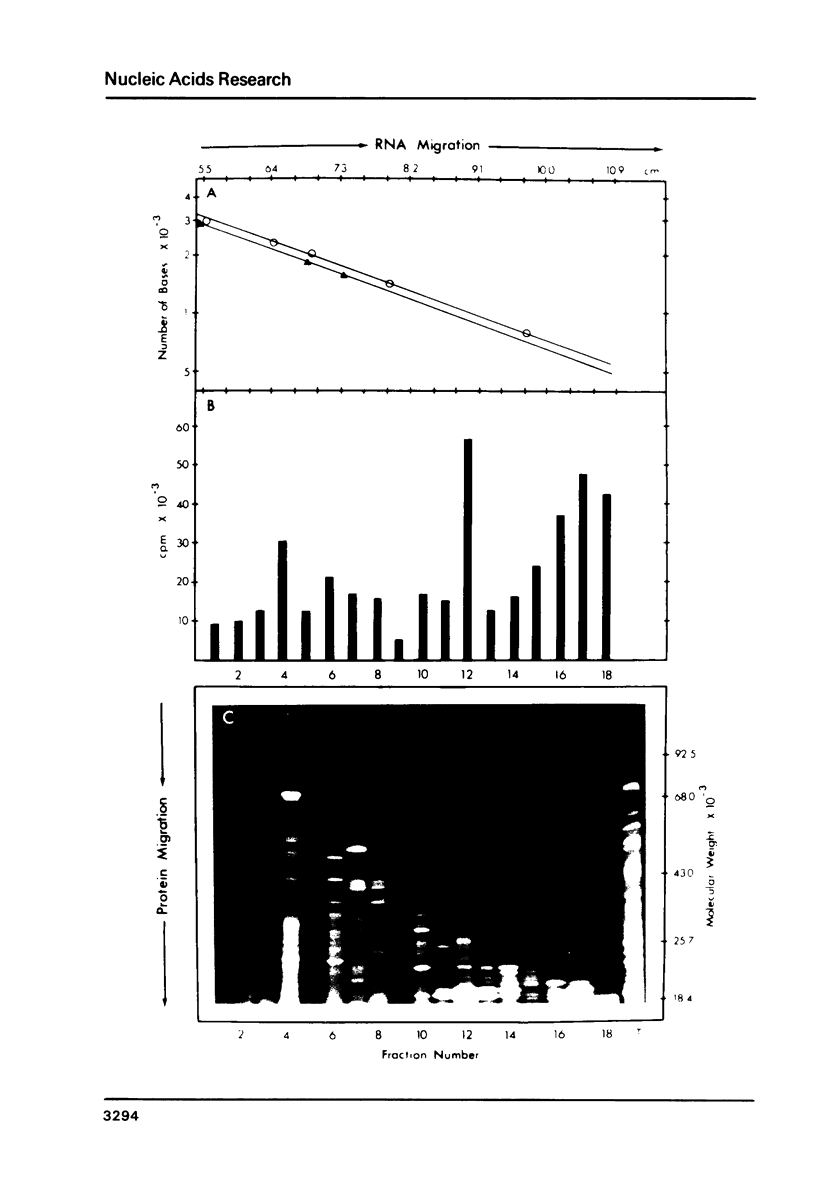
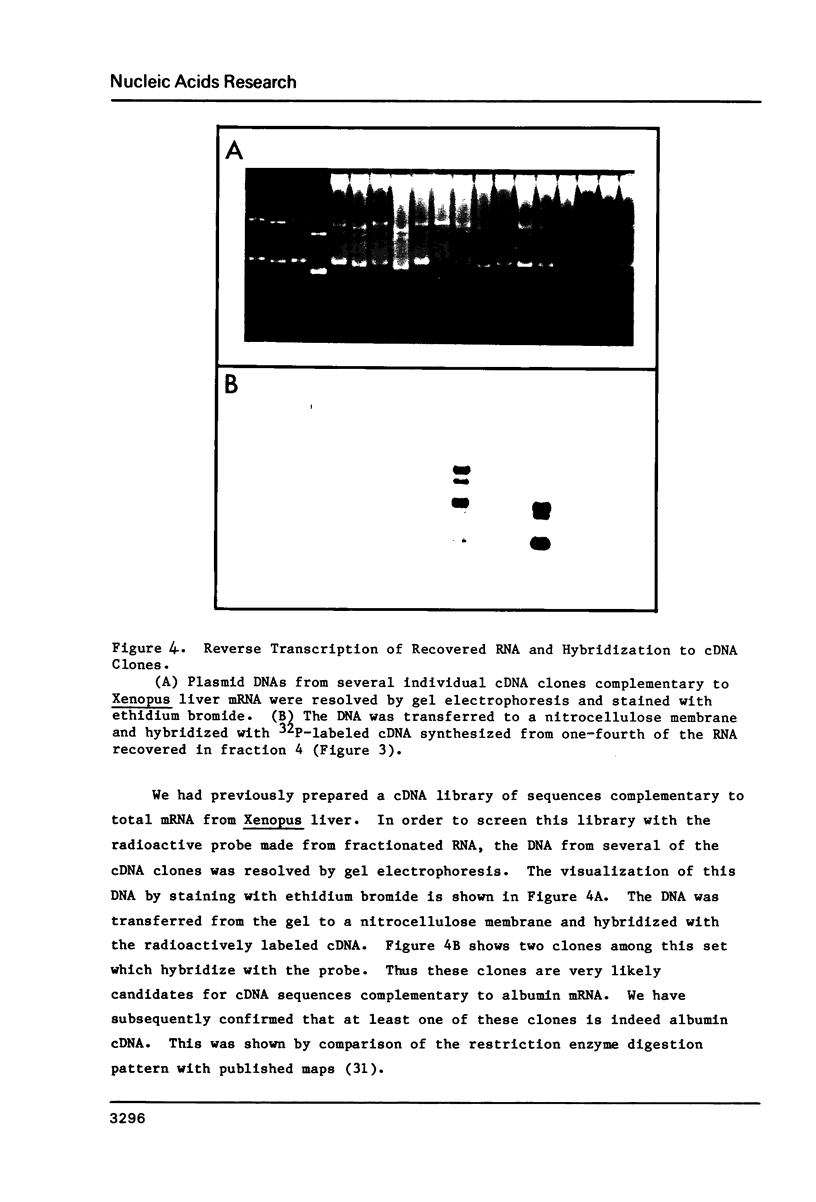
A simple method is described for the efficient recovery of intact mRNA from high resolution agarose gels. Fractionation of RNA is accomplished by gel electrophoresis under denaturing conditions using methylmercuric hydroxide. The RNA in the gel is then transferred electrophoretically to a diethylaminoethyl (DEAE)-membrane. After reversing the methylmercuric modification of the RNA, the membrane is sliced into narrow sections and the RNA is eluted at 65 degrees with a high ionic strength buffer containing 6M guanidine hydrochloride. RNA isolated by this procedure is suitable for subsequent enzymatic reactions, including in vitro translation and reverse transcription. The major advantages offered by this procedure are: 1) The membrane-bound RNA is a replica of the high resolution fractionation pattern achieved in the gel. 2) The immobilization and concentration of RNA and the removal of gel matrix contaminants are all accomplished in one step. 3) Small quantities of RNA are efficiently recovered and are suitable for subsequent biochemical manipulations. The method is of general utility for any biological system. We have applied its use to the fractionation, recovery, and analysis of mRNA from Xenopus liver and have identified cDNA clones complementary to albumin mRNA.
Full text
PDF















Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alwine J. C., Kemp D. J., Stark G. R. Method for detection of specific RNAs in agarose gels by transfer to diazobenzyloxymethyl-paper and hybridization with DNA probes. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5350–5354. doi: 10.1073/pnas.74.12.5350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey J. M., Davidson N. Methylmercury as a reversible denaturing agent for agarose gel electrophoresis. Anal Biochem. 1976 Jan;70(1):75–85. doi: 10.1016/s0003-2697(76)80049-8. [DOI] [PubMed] [Google Scholar]
- Bittner M., Kupferer P., Morris C. F. Electrophoretic transfer of proteins and nucleic acids from slab gels to diazobenzyloxymethyl cellulose or nitrocellulose sheets. Anal Biochem. 1980 Mar 1;102(2):459–471. doi: 10.1016/0003-2697(80)90182-7. [DOI] [PubMed] [Google Scholar]
- Brosius J., Dull T. J., Noller H. F. Complete nucleotide sequence of a 23S ribosomal RNA gene from Escherichia coli. Proc Natl Acad Sci U S A. 1980 Jan;77(1):201–204. doi: 10.1073/pnas.77.1.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brosius J., Palmer M. L., Kennedy P. J., Noller H. F. Complete nucleotide sequence of a 16S ribosomal RNA gene from Escherichia coli. Proc Natl Acad Sci U S A. 1978 Oct;75(10):4801–4805. doi: 10.1073/pnas.75.10.4801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buell G. N., Wickens M. P., Payvar F., Schimke R. T. Synthesis of full length cDNAs from four partially purified oviduct mRNAs. J Biol Chem. 1978 Apr 10;253(7):2471–2482. [PubMed] [Google Scholar]
- Danner D. B. Recovery of DNA fragments from gels by transfer to DEAE-paper in an electrophoresis chamber. Anal Biochem. 1982 Sep 1;125(1):139–142. doi: 10.1016/0003-2697(82)90394-3. [DOI] [PubMed] [Google Scholar]
- Deeley R. G., Gordon J. I., Burns A. T., Mullinix K. P., Binastein M., Goldberg R. F. Primary activation of the vitellogenin gene in the rooster. J Biol Chem. 1977 Nov 25;252(22):8310–8319. [PubMed] [Google Scholar]
- Denhardt D. T. A membrane-filter technique for the detection of complementary DNA. Biochem Biophys Res Commun. 1966 Jun 13;23(5):641–646. doi: 10.1016/0006-291x(66)90447-5. [DOI] [PubMed] [Google Scholar]
- Dunn A. R., Hassell J. A. A novel method to map transcripts: evidence for homology between an adenovirus mRNA and discrete multiple regions of the viral genome. Cell. 1977 Sep;12(1):23–36. doi: 10.1016/0092-8674(77)90182-9. [DOI] [PubMed] [Google Scholar]
- Goldberg D. A. Isolation and partial characterization of the Drosophila alcohol dehydrogenase gene. Proc Natl Acad Sci U S A. 1980 Oct;77(10):5794–5798. doi: 10.1073/pnas.77.10.5794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatfield D., Portugal F. H. Seryl-tRNA in mammalian tissues: chromatographic differences in brain and liver and a specific response to the codon, UGA. Proc Natl Acad Sci U S A. 1970 Nov;67(3):1200–1206. doi: 10.1073/pnas.67.3.1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson V. Studies on histone organization in the nucleosome using formaldehyde as a reversible cross-linking agent. Cell. 1978 Nov;15(3):945–954. doi: 10.1016/0092-8674(78)90278-7. [DOI] [PubMed] [Google Scholar]
- Kutateladze T. V., Axelrod V. D., Gorbulev V. G., Belzhelarskaya S. N., Vartikyan R. M. New procedure of high-voltage electrophoresis in polyacrylamide gel and its application to the sequencing of nucleic acids. Anal Biochem. 1979 Nov 15;100(1):129–135. doi: 10.1016/0003-2697(79)90121-0. [DOI] [PubMed] [Google Scholar]
- Laskey R. A., Mills A. D. Quantitative film detection of 3H and 14C in polyacrylamide gels by fluorography. Eur J Biochem. 1975 Aug 15;56(2):335–341. doi: 10.1111/j.1432-1033.1975.tb02238.x. [DOI] [PubMed] [Google Scholar]
- Lehrach H., Diamond D., Wozney J. M., Boedtker H. RNA molecular weight determinations by gel electrophoresis under denaturing conditions, a critical reexamination. Biochemistry. 1977 Oct 18;16(21):4743–4751. doi: 10.1021/bi00640a033. [DOI] [PubMed] [Google Scholar]
- Loening U. E. The fractionation of high-molecular-weight ribonucleic acid by polyacrylamide-gel electrophoresis. Biochem J. 1967 Jan;102(1):251–257. doi: 10.1042/bj1020251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- McMaster G. K., Carmichael G. G. Analysis of single- and double-stranded nucleic acids on polyacrylamide and agarose gels by using glyoxal and acridine orange. Proc Natl Acad Sci U S A. 1977 Nov;74(11):4835–4838. doi: 10.1073/pnas.74.11.4835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelham H. R., Jackson R. J. An efficient mRNA-dependent translation system from reticulocyte lysates. Eur J Biochem. 1976 Aug 1;67(1):247–256. doi: 10.1111/j.1432-1033.1976.tb10656.x. [DOI] [PubMed] [Google Scholar]
- Roberts B. E., Paterson B. M. Efficient translation of tobacco mosaic virus RNA and rabbit globin 9S RNA in a cell-free system from commercial wheat germ. Proc Natl Acad Sci U S A. 1973 Aug;70(8):2330–2334. doi: 10.1073/pnas.70.8.2330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salim M., Maden B. E. Nucleotide sequence of Xenopus laevis 18S ribosomal RNA inferred from gene sequence. Nature. 1981 May 21;291(5812):205–208. doi: 10.1038/291205a0. [DOI] [PubMed] [Google Scholar]
- Saris C. J., Franssen H. J., Heuyerjans J. H., van Eenbergen J., Bloemers H. P. Blotting of RNA onto ion exchange paper allowing subsequent characterization by in situ translation in addition to blot hybridization. Nucleic Acids Res. 1982 Aug 25;10(16):4831–4843. doi: 10.1093/nar/10.16.4831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith H. O. Recovery of DNA from gels. Methods Enzymol. 1980;65(1):371–380. doi: 10.1016/s0076-6879(80)65048-4. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Stellwag E. J., Dahlberg A. E. Electrophoretic transfer of DNA, RNA and protein onto diazobenzyloxymethyl (DBM) - paper. Nucleic Acids Res. 1980 Jan 25;8(2):299–317. doi: 10.1093/nar/8.2.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahl G. M., Stern M., Stark G. R. Efficient transfer of large DNA fragments from agarose gels to diazobenzyloxymethyl-paper and rapid hybridization by using dextran sulfate. Proc Natl Acad Sci U S A. 1979 Aug;76(8):3683–3687. doi: 10.1073/pnas.76.8.3683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westley B., Wyler T., Ryffel G., Weber R. Xenopus laevis serum albumins are encoded in two closely related genes. Nucleic Acids Res. 1981 Aug 11;9(15):3557–3574. doi: 10.1093/nar/9.15.3557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winberg G., Hammarskjöld M. L. Isolation of DNA from agarose gels using DEAE-paper. Application to restriction site mapping of adenovirus type 16 DNA. Nucleic Acids Res. 1980 Jan 25;8(2):253–264. doi: 10.1093/nar/8.2.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zain S., Sambrook J., Roberts R. J., Keller W., Fried M., Dunn A. R. Nucleotide sequence analysis of the leader segments in a cloned copy of adenovirus 2 fiber mRNA. Cell. 1979 Apr;16(4):851–861. doi: 10.1016/0092-8674(79)90100-4. [DOI] [PubMed] [Google Scholar]