The evolution of living organisms in an oxidizing atmosphere has resulted in a complex array of antioxidation mechanisms within cells to protect critical biomolecules from oxidative modifications. Despite this, oxidation of biomolecules as a result of exposure to reactive oxygen species is a constant biochemical battle. Because lipids present themselves often as initial barriers to the free diffusion of reactive oxygen species into the cell, they themselves become targets of oxidation reactions. By and large, the resulting structures are not controlled by enzymatic mechanisms and thus are not programmed by the human genome. Their formation is controlled by chemical reaction mechanisms, and a diverse array of products are made, depending upon the reactive oxygen species as well as the nature of the lipid target. In most cases, these molecules are readily degraded and recycled into the essential building blocks of the cell, but there are some oxidized lipids that escape such protective mechanisms and can exert profound, deleterious effect on cells.
Oxidized lipids can be produced by reactions initiated by non-radical reactive oxygen species such as singlet O2, HOCl, and ozone (O3) or by inorganic free radical species derived from nitric oxide, superoxide, and hydrogen peroxide. Inorganic radical species can react with various lipids, leading to the formation of simple hydroxy fatty acids, oxidized cholesterol species, various isoprostanes, nitro-fatty acids, and lipid aldehydes. The initial reactions of inorganic radicals with lipids involve either hydrogen abstractions of allylic or bisallylic hydrogens or additions to carbon–carbon double bonds. The resulting lipid radicals then can propagate formation of other oxidized lipids in reactions involving isomerization and chain scission. Because inorganic free radical species are formed by cells such as phagocytes during inflammation, oxidized lipids are produced in association with inflammation. However, reactive oxygen species can also come from the environment and the air we breathe, which contains oxides of nitrogen, reactive aldehydes from fires, and even ozone from atmospheric photochemistry.
The first minireview of this series by Freeman, d'Ischia, and co-workers
describes a new group of potential lipid mediators, the nitro-fatty acids
(NO2-FAs).3
Like other oxidized lipids described in this series, this class of modified
fatty acids has been detected in normal mammalian tissues, and the levels of
NO2-FAs increase during inflammation. A nitrogen dioxide
(•NO2) group can add to the cis-double
bonds of common monounsaturated and polyunsaturated fatty acids, ultimately
leading to the formation of NO2-FAs, nitronitrite esters of FAs,
and nitrohydroxy FAs. NO2 is formed from peroxynitrite
(ONOO–) following its protonation or from the peroxynitrite
carbon dioxide adduct 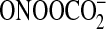 .
Peroxynitrite itself is produced during inflammation from nitric oxide
(•NO) plus superoxide
(
.
Peroxynitrite itself is produced during inflammation from nitric oxide
(•NO) plus superoxide
(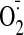 ).
Both free fatty acids and fatty acids esterified to membrane phospholipids can
be nitrated. In the latter case, NO2-FAs can be mobilized by
phospholipase A2. NO2-FAs have the potential to release
•NO, although the biological importance of NO2-FAs
as a reservoir of •NO is not known. NO2-FAs can
also function through •NO (and cGMP)-independent processes to
activate peroxisome proliferator-activated receptor-γ and participate in
the regulation of several enzymes. NO2-FAs appear to function as
anti-inflammatory agents by inhibiting NF-κB activation and inducing
heme oxygenase-1 expression. Finally, NO2-FAs are electrophilic
species that can react with thiols and imidazole groups and thus have the
potential to alter the activity of proteins. An example of this is the
post-translational modification of Keap1 that appears to regulate the function
of the Nrf2 transcription factor involved in antioxidant responses.
).
Both free fatty acids and fatty acids esterified to membrane phospholipids can
be nitrated. In the latter case, NO2-FAs can be mobilized by
phospholipase A2. NO2-FAs have the potential to release
•NO, although the biological importance of NO2-FAs
as a reservoir of •NO is not known. NO2-FAs can
also function through •NO (and cGMP)-independent processes to
activate peroxisome proliferator-activated receptor-γ and participate in
the regulation of several enzymes. NO2-FAs appear to function as
anti-inflammatory agents by inhibiting NF-κB activation and inducing
heme oxygenase-1 expression. Finally, NO2-FAs are electrophilic
species that can react with thiols and imidazole groups and thus have the
potential to alter the activity of proteins. An example of this is the
post-translational modification of Keap1 that appears to regulate the function
of the Nrf2 transcription factor involved in antioxidant responses.
The second minireview in this series by Murphy and Johnson discusses non-enzymatic mechanisms for the formation of oxidized cholesterol by both radical and non-radical pathways. Cholesterol is highly enriched in the plasma membrane compared with internal membranes of cells, and readers will be surprised to learn that in the structured environment of biological membranes, cholesterol is oxidized in preference to polyunsaturated fatty acids. Radical pathways of cholesterol oxidation are most commonly initiated by removal of the allylic hydrogen from C-7 of cholesterol and addition of O2 to form either the 7α- or 7β-hydroperoxyl cholesterol radical. The authors discuss the mechanisms of formation of these cholesterol radicals and the major downstream products, which are 7α,β-hydroxy-, 7-oxo-, and 5,6-epoxycholesterol; 5,6-epoxycholesterol is the major non-enzymatic cholesterol oxidation product of macrophages. Cholesterol can also be oxidized by non-radical pathways involving singlet O2 produced by photosensitization. The major initial product is 5α-hydroperoxycholesterol. In other non-radical pathways, inhaled O3 can add across the 5,6-double bond to form a 1,2,3-trioxolane, which then undergoes decomposition to numerous reactive products. Finally, HOCl formed by neutrophils and HOBr formed by eosinophils during phagocytosis can add to the 5,6-double bond of cholesterol chlorohydrins or bromohydrins, respectively. It is well established that oxidized cholesterol species such as 7α,β-hydroxy-, 7-oxo-, and 5,6-epoxycholesterol are cytotoxic. These compounds, which are enriched in fatty streaks and oxidized low density lipoprotein, appear to function by inducing apoptosis. Although it is unclear whether oxidized cholesterol species formed non-enzymatically affect cholesterol biosynthesis in vivo, some of these compounds can interact in vitro with proteins such as Insig and Scap that are involved in the regulation of 3-hydroxy-3-methylglutaryl-CoA reductase.
The minireview by Hazen focuses on the properties of oxidized fatty acyl chains in phospholipids of the plasma membrane and oxidized low density lipoprotein that permit the oxidized phospholipids to interact with class B scavenger receptors such as CD36 that are found on the surface of cells of the innate immune system. We learn that CD36 interacts with γ-hydroxy(or oxo)-α,β-unsaturated carbonyl moieties located at the terminus of a shortened chain acylated at the sn-2 position of phospholipids. These oxidized groups originate from chain scission reactions that commonly occur after formation of lipid hydroperoxides. Oxidized phospholipids bearing the γ-hydroxy(or oxo)-α,β-unsaturated carbonyl become oriented in the membrane with the oxidized end of the acyl chain protruding into the interfacial layer of the membrane, where the exposed, oxidized group can serve as a ligand for the scavenger receptor. For example, oxidized phosphatidylserine on apoptotic cells interacts with CD36 on macrophages to initiate phagocytosis. Hazen also describes evidence for the Lipid Whisker Model of cell membranes, in which oxidized portions of fatty acyl chains are found in the more hydrophilic interfacial region of membranes rather than being localized to the hydrophobic core.
In the fourth minireview of this series, Milne, Yin, and Morrow provide an overview of the chemistry and biochemistry of isoprostanes. Isoprostanes are regio- and stereoisomers of prostaglandins and thromboxanes formed non-enzymatically from long chain polyunsaturated fatty acids via free radical-induced peroxidation. Isoprostanes derived from arachidonic acid were first identified almost 20 years ago. They are found in greater abundance even in healthy animals than the corresponding prostaglandin isomers formed through the cyclooxygenase pathway. Isoprostane levels in tissues, plasma, and urine are greatly increased during lipid peroxidation, and F2-isoprostanes are now recognized as the best available biomarker for oxidative stress. More recently, it has become clear that polyunsaturated fatty acids such as eicosapentaenoic acid and docosahexaenoic acid are also precursors of isoprostanes. In all cases, the formation of these compounds begins with free radical-initiated abstraction of an allylic hydrogen and addition of a molecule of oxygen to the acyl chain. This occurs when fatty acids are esterified to phospholipids. Isoprostanes are subsequently mobilized by phospholipase A2. Some isoprostanes have biologically potent effects as mediators of oxidant injury. An example is 8-isoprostaglandin F2α, which, as an agonist of the G-protein-linked thromboxane receptor, can elicit vasoconstriction.
In the fifth minireview in this series, Schneider, Porter, and Brash discuss the possible chemical routes for the formation of 4-hydroxynonenal (HNE). This α,β-unsaturated aldehyde is the one formed in greatest abundance upon free radical-induced peroxidation of linoleic acid (18:2ω6), and its toxicity has been studied in detail over many years. Linoleic acid is the polyunsaturated fatty acid found in the largest amounts in membrane phospholipids. γ-Hydroxy-α,β-unsaturated aldehyde moieties found at the ω terminus of acyl chains esterified to phospholipids are those described by Hazen as ligands for the CD36 scavenger receptor. Examination of the formation of HNE has led Schneider, Porter, and Brash to introduce an alternative paradigm for free radical-initiated lipid peroxidation and the formation of HNE and related aldehydes. It was formerly thought that dimerization and polymerization and other intermolecular reactions of oxidized acyl chains occurred during the termination stage of lipid peroxidation. It now appears that free radical dimers, radicals derived from these dimers, and other products of intermolecular reactions appear earlier in lipid peroxidation at the initiation and propagation stages. It also appears that these intermolecular reactions occur concomitant with the formation of α,β-unsaturated aldehydes. The authors describe a scheme whereby HNE is formed as a product of chain scission of an oxidized dimer of fatty acid chains.
The final minireview by Blair in the Oxidized Lipid Series focuses on one of the potentially toxic events that occurs as a downstream consequence of non-enzymatic lipid peroxidation. This is the formation of DNA adducts from electrophilic γ-substituted α,β-unsaturated aldehydes, including 4-oxononenal and 4-hydroperoxynonenal. Not surprisingly, the reactions involve additions to the adenine, guanine, and cytosine rings to form cyclic etheno analogs. The carbons of the etheno group are the α- and β-carbons of the electrophile. To some extent, DNA repair enzymes can excise these damaged nucleotides, and Blair discusses the analytical tools in development to quantify these etheno products in urine and tissues.
This minireview will be reprinted in the 2008 Minireview Compendium, which will be available in January, 2009. This is the first article of seven in the Oxidized Lipids Minireview Series.
Footnotes
The abbreviations used are: NO2-FAs, nitro-fatty acids; HNE, 4-hydroxynonenal.


