Abstract
Objective
There is a commonly held belief that randomized, placebo-controlled trials in pediatric critical care should incorporate “rescue” therapy (open-label administration of active drug) when a child’s condition is deteriorating. The ethical, conceptual and analytic challenges related to “rescue” therapy in randomized trials can be misrepresented.
Design
Narrative review.
Methods
The ethical basis of “rescue” therapy, the equipoise concept, and intention-to-treat analysis are examined in the setting of a hypothetical randomized trial comparing corticosteroids versus placebo in pediatric septic shock.
Findings
The perceived need for “rescue” therapy may be partly motivated by the moral imperative to save a child’s life. However, allowing “rescue” therapy in a trial is misconceived and inconsistent with equipoise regarding the efficacy of the study drug. If “rescue” therapy is permitted, intention-to-treat analysis can only compare immediate versus delayed use of the study drug. When “rescue” therapy is beneficial, the observed treatment effect is substantially diminished from true effect of the study drug, leading to increased sample size and thereby placing more children at risk (18 “excess” placebo-arm deaths occur in our hypothetical example). Analysis of a trial incorporating “rescue” therapy cannot definitively assess overall efficacy of the agent, or distinguish beneficial or harmful treatment effects related to timing of drug use.
Conclusions
While a “rescue” therapy component in a randomized trial may be perceived as ethically desirable, inconsistency of “rescue” therapy with full equipoise may itself raise significant ethical concerns. Increased sample sizes expose more children to the risks of study participation, including death. Researchers should be aware that clinical trials designed with “rescue” therapy cannot definitively determine the beneficial or harmful effects of a treatment per se, and can only assess the effects of delayed versus immediate provision of the treatment.
Keywords: clinical trials, randomized; critical care; corticosterone; equipoise; ethics; placebos; shock; septic; rescue therapy
There is a commonly held belief that randomized, placebo-controlled trials in pediatric critical care should routinely incorporate use of “rescue” therapy (open-label administration of active drug) when a child appears to be deteriorating (1,2). In this report, we briefly discuss perceived ethical issues that often motivate the use of “rescue” therapy. We subsequently address the concept of equipoise from ethical, clinical, and study design viewpoints. We describe the effects of a “rescue” therapy component in a randomized trial on statistical power and on interpretation of the study results.
To facilitate our discussion, we consider a hypothetical randomized controlled trial (RCT) of corticosteroids in pediatric septic shock. The trial is based on acknowledged lack of evidence supporting the use of corticosteroids unless a child is known to have true adrenal insufficiency, has received acute or chronic corticosteroid therapy (3), or has received recent dosing of etomidate or ketoconazole (4). The largest, most recent adult clinical trial of adjunctive corticosteroids in severe sepsis concluded that although such intervention hastens the resolution of shock for patients who resolve their shock, it has no beneficial effect in terms of reducing mortality (5). The same trial reported that corticosteroid administration was associated with increased nosocomial infections. Results of small pediatric trials addressing this subject have been conflicting (6,7,8). The most recent evidence-based medicine review on the topic concluded “There is some, albeit limited, evidence for the benefit of low-dose corticosteroids in adults with sepsis. No supporting data are available for the pediatric population; therefore, a randomized controlled trial in septic children is needed.” (9)
MATERIALS AND METHODS
Study design
This report is a narrative review of ethical and biostatistical issues inherent to placebo-controlled randomized trials with a “rescue” therapy component (defined below). Literature relevant to the equipoise concept as applied in the pediatric critical care setting was reviewed. A hypothetical clinical trial example is used to motivate ethical arguments, and to present biostatistical consequences of allowing “rescue” therapy under various conditions.
Key definitions
“Rescue” therapy is defined here as open-label administration, in a deteriorating child, of the same active agent that is being compared to placebo in the randomized trial.
Individual equipoise, as originated by Fried (10), refers to an individual physician’s genuine uncertainty as to the best treatment for a condition.
Collective equipoise, as proposed by Freedman (11), refers to a state of honest, professional disagreement in the overall community of physicians as to best treatment for a condition.
Intention-to-treat (ITT) analysis is the biostatistical approach wherein all subjects randomly assigned to a treatment arm are analyzed in that arm, regardless of adherence to protocol including possible crossover to the other study arm (12).
RESULTS
Ethical issues associated with “rescue” therapy
Pediatric intensivists have an enhanced responsibility to protect the lives and rights of children, a vulnerable population. This responsibility becomes of paramount importance in children at high acute risk of mortality, who often require rapid changes in therapy. The American Academy of Pediatrics (AAP) guidelines note that drug research in children at high risk of mortality is appropriate when standard therapy is ineffective, and there is reasonable expectation that the new therapy may be beneficial (13).
When faced with a dying child, study investigators may perceive a misconceived obligation to prescribe “rescue” therapy for the child, even in the absence of evidence that “rescue” is efficacious (14). In some instances, uncontrolled anecdotes about successful “rescue” may be powerful behavior modification stimuli. Additional pressure to provide “rescue” therapy may come from parents or clinicians who believe that everything must be tried for a dying child. Moreover, published guidelines for patient management (which are based on committee and consensus, and sometimes disseminated in absence of definitive evidence) may be viewed as advocating or even mandating the use of “rescue” therapy in a deteriorating patient.
Because of these issues, many pediatric intensivists perceive an ethical (and possibly even a legal) conflict regarding use of “rescue” therapy when faced with a study participant whose condition is deteriorating. If the trial design does not include a “bailout” clause allowing “rescue” therapy, the obligation to protect the best interests of an individual patient may seem to conflict with the need to preserve the scientific integrity of the trial. However, ethical misconceptions cannot form the basis for requirements for “rescue”, especially when the clinical community does not know if “rescue” actually provides benefit. Evaluation of an agent within controlled trials is ethically preferable to innovative or compassionate use of the agent (15), as occurs in the “rescue” setting.
“Rescue” therapy and equipoise
Randomized trials are not undertaken unless there is some evidence that the therapy may benefit the patient, although evidence of efficacy may be available solely from nonrandomized and/or animal studies. A necessary ingredient for carrying out an RCT design in critical care is equipoise related to a particular therapy (16). While individual equipoise (10) and clinical equipoise (11) are separate justifications for the conduct of RCTs, the former relates to the physician-patient relationship in the conduct of research, whereas the latter constitutes a necessary (though not sufficient) condition for the approval of research protocols by the medical community in general, and the members of institutional review boards in particular.
Shapiro and Glass (17) assert that clinical equipoise must “trump” individual physician uncertainty about the merits of a treatment, and indeed that an individual physician’s equipoise does not affect the moral basis of a trial. As discussed above, it is indeed an ethical misconception to use “rescue” therapy when evidence of a benefit is absent. Nevertheless, when an individual (bedside) physician is faced with an unstable, perhaps moribund child, the individual physician’s uncertainty may take precedence over community perceptions. As noted by Schwab (18), the bioethical question of whether clinical equipoise should constrain legitimate individual equipoise may not have a perfect solution. In the ideal situation, both the medical community and the individual physician would be in equipoise. Both concepts of equipoise address the complementary ethical bases for conducting clinical research, regardless of the perceived distinction between clinical research ethics and the ethics of therapeutic medicine (19).
Actually, a higher standard exists for research subjects than for patients exposed to innovative or compassionate use therapies. Typically, research protocols are reviewed by funding agencies, investigator credentials are scrutinized, a systematic literature review commonly precedes the clinical trial, Institutional Review Board oversight is mandatory, Data Safety Monitoring Boards (DSMBs) review adverse events, clinical research is conducted within an academic environment, and protocolized care is provided for both the placebo and study intervention arms, frequently resulting in improved care for both groups. By embracing the acquisition of sound scientific conclusions, the researcher will ultimately benefit future patients. In contrast, the physician who exposes individual patients to compassionate use therapies is performing an experiment on a single subject, without oversight, and with no prospect of deriving sound scientific conclusions.
Since lack of equipoise before an RCT often leads to the failure of otherwise well-designed trials (20), efforts to establish or document clinical equipoise are often worthwhile (21). In the absence of well-defined clinical practice (wide variation that is largely unexplained, as is the case for corticosteroid prescription for septic shock), it is reasonable to randomize patients to reasonable yet competing beneficial treatment strategies that lie within general boundaries of competent or good care (22). However, physicians enrolling their patients in RCTs (23) and patients who participate in RCTs (24) may not understand the concept of equipoise or its importance. Opinions about clinical utility of corticosteroid therapy in septic shock, for example, demonstrate reluctance of many pediatric intensivists to allocate critically ill children randomly to a placebo-control group in the absence of a “bailout” clause in the trial.
When a “rescue” therapy component is felt to be necessary, it may be argued that there is clinical equipoise regarding use versus non-use of corticosteroids in the initial randomization setting of septic shock, but insufficient equipoise regarding use of corticosteroids if the child’s condition substantially deteriorates. One option would then be to declare insufficient overall equipoise regarding efficacy of corticosteroids to justify a randomized trial.
An alternative argument would be that the perceived necessity for “rescue” therapy reflects equipoise regarding the immediate use of corticosteroids in the setting of septic shock, versus delaying corticosteroid use until such time (if any) when the child’s condition deteriorates sufficiently. Equipoise in this context neither corresponds to, nor follows directly from, equipoise regarding efficacy of corticosteroid use, but rather reflects uncertainty regarding the optimal timing of use (i.e., early versus later use of corticosteroids).
“Rescue” therapy and intention-to-treat analysis
Biostatisticians recommend intention-to-treat (ITT) analysis, wherein all events are assigned to the initially randomized treatment arm, as the primary analysis strategy for RCTs designed to evaluate the superiority of one treatment to another. The ITT approach does have analytic limitations, including sensitivity to missing outcome data (25), and other secondary analysis approaches may be very informative (26). The fundamental motivation for ITT as the primary analysis approach is that comparability of subjects between assigned treatment arms, provided by the randomization process, no longer applies when subjects are grouped according to protocol adherence.
If a significant number of subjects receive “rescue” therapy in a trial, ITT analysis may be viewed as comparing two initial treatment strategies rather than the actual treatments. This comparison of strategies is often cited as an advantage of ITT, since findings can be interpreted as mimicking “what would occur in the real world”. For example, substantial noncompliance to a powerful new drug due to strong side effects would reduce the overall ultimate benefit of this drug in the children initially treated with it. ITT analysis captures the effect of such nonadherence to a protocol, because dropouts from the new drug regimen are retained in the new drug arm.
In our hypothetical study, all children assigned to corticosteroid therapy would remain on that therapy, while some initially assigned to placebo would cross over to corticosteroid therapy (“rescue”). Thus, ITT analysis could arguably be viewed as a comparison of initiating corticosteroid therapy immediately at the time a child presents with septic shock, versus delaying corticosteroid therapy until such time as the child’s condition deteriorates. This comparison, while quite possibly of substantial clinical importance, does not directly evaluate the benefit of corticosteroid therapy per se in the setting of septic shock. Nevertheless, investigators considering inclusion of “rescue” therapy in the trial should consider if a strategy of delaying corticosteroid therapy mimics an actual (or, at the least, a possible) real-life approach to treatment of septic shock. If this is indeed the case, a compelling argument for permitting “rescue” therapy would be to compare the effectiveness of two realistic treatment strategies.
Investigators planning this hypothetical study should also consider if there is equipoise regarding delayed versus immediate delivery of corticosteroid therapy. As noted above, this is a somewhat different issue to contend with than efficacy of the drug itself. Ineffectiveness of the therapy implies ineffectiveness of immediate versus delayed delivery, but not vice versa: a trial that conclusively answers the delay issue may not provide an incontrovertible conclusion regarding ultimate efficacy. Moreover, it is biologically less plausible that delayed treatment will be effective if the drug lacks efficacy per se, or that delayed treatment with an effective drug will be superior to immediate treatment. We believe that the timing of therapy (immediate vs. delayed) should not be tested unless the drug’s efficacy has been determined definitively. Coming full circle, such an efficacy determination can be done in a randomized fashion only if rescue therapy is not allowed.
Design of a randomized trial with “rescue” therapy: Case example
We assume investigators have decided to conduct a RCT that permits “rescue” therapy for children with sepsis who are deteriorating after initial randomization. In this hypothetical study, subjects are randomized in a 1:1 ratio to corticosteroid therapy (cortisol 100 mg/m2 load, followed by 30 mg/m2 every 6 hrs for 5 days, and weaned over 2 days) or placebo. Seven-day mortality, analyzed as a binary variable, is the primary study outcome. True 7-day mortality in the target population (in the absence of a treatment effect) is assumed to be 25%. Without crossover to “rescue” therapy, this study would require a total sample size of approximately 700 subjects (350 per arm) to detect a 10% absolute reduction in mortality in the corticosteroid arm at a statistical power of 90%, using a two-sided chi-squared test with a Type I error of 0.05.
We further assume that “rescue” corticosteroid therapy is administered to some subjects (for example, 20%) after substantial clinical deterioration. For the time being, we ignore the issues of whether this “rescue” therapy would be given to subjects assigned to the active arm as well as the placebo arm, and whether unblinding would occur at the time of decision to implement “rescue” therapy.
Effects on the study when “rescue” therapy is effective
In this scenario, we assume that the following hold true in our hypothetical trial:
There is a substantial benefit of immediate corticosteroid therapy: true 7-day mortality is 15% when immediate corticosteroid treatment is initiated, versus 25% mortality if corticosteroids are not administered.
There is a benefit of corticosteroids delivered as “rescue” therapy. This may be the same relative benefit as for immediate corticosteroid therapy, in which case the “rescue” therapy reduces risk of mortality by 40% (the proportion of lives saved with immediate therapy when mortality is reduced from 25% to 15%). Alternatively, any benefit of “rescue” therapy may be weaker than if corticosteroids were administered earlier.
We assume no added benefit or harm of “rescue” therapy among subjects already assigned to corticosteroids
In this hypothetical trial, we first assume that 70 (20%) placebo subjects receive corticosteroid “rescue” therapy at a time when their risk of death (if left untreated) is 50%. The risk of death for the other 280 (80%) placebo subjects is then 18.8% (this keeps overall placebo arm mortality at 25%). If corticosteroid “rescue” therapy has the same relative benefit as when given initially, these 70 high-risk crossovers will have expected mortality reduced from 50% to 30%. Risk of death for the remaining 80% of placebo subjects remains at 18.8%, and thus the total expected mortality for the 350 children initially assigned to placebo is 21%, versus 15% expected mortality in the active arm. Expected overall treatment benefit has diminished from 10% to 6%, due to beneficial “rescue” therapy received by some initially placebo-assigned subjects. Consequently, the study becomes underpowered to detect a true benefit of corticosteroids.
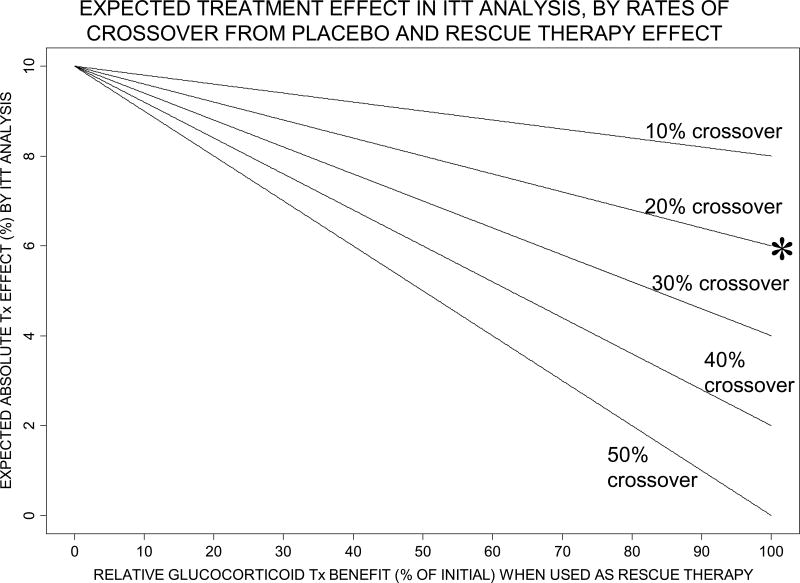
Figure 1 shows how the expected treatment benefit in our hypothetical trial (analyzed by ITT) changes as the relative “rescue” therapy benefit of corticosteroids varies from 0% (no effect) to 100% of the benefit when given initially, and as crossover rates in the placebo arm range from 10% to 50%. The diminishing of expected overall treatment benefit becomes more pronounced as “rescue” therapy benefit increases, and as the proportion of children crossing over to corticosteroid “rescue” therapy increases.
Figure 1.
Expected treatment (Tx) effect (difference between corticosteroid arm and placebo arm 7-day mortality) by intention-to-treat (ITT) analysis, according to relative corticosteroid benefit when used as “rescue” therapy (horizontal axis) and to proportion of placebo arm subjects crossing over to “rescue” therapy (10% to 50%, as labeled on each curve). The risk of mortality among crossovers from placebo, if “rescue” therapy not given, is assumed to be 50%. The specific example discussed in the text, where 20% crossover to rescue therapy equally (100%) as effective as initial therapy reduces expected Tx effect from 10% to 6%, is labeled by * in this Figure.
As the expected treatment benefit in the ITT analysis diminishes, sample size required for acceptable power increases. Figure 2 shows necessary sample sizes to achieve 90% power under the Figure 1 scenarios. In our specific example discussed above, the sample size required to detect an absolute 10% treatment benefit increases from 700 subjects to a possibly unattainable 1780 when “rescue” therapy is permitted, because expected treatment benefit diminishes to 6%. Figure 3 shows statistical power that the original sample size of 700 subjects achieves under varying amounts of crossover and “rescue” therapy effect. Power drops to unacceptably low levels under very realistic combinations of moderate crossover rates and moderate relative benefit of corticosteroid “rescue” therapy. In our above example, the statistical power with the original sample size of 700 decreases to approximately 50% after accounting for beneficial “rescue” therapy.
Figure 2.
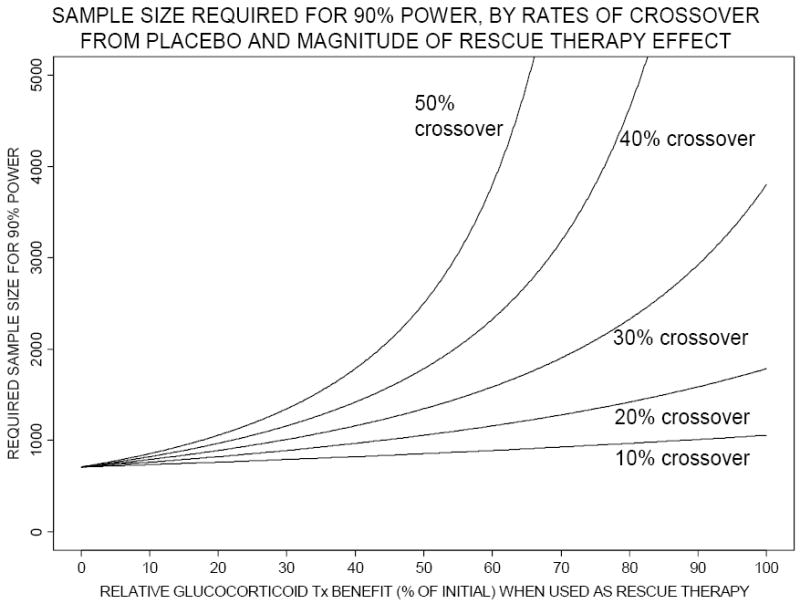
Total study sample size required for 90% power (using a two-sided chi-squared test with Type I error=0.05) using intention-to-treat analysis, according to relative corticosteroid benefit when used as “rescue” therapy (horizontal axis) and to proportion of placebo arm subjects crossing over to “rescue” therapy (10% to 50%, as labeled on each curve). The risk of mortality among crossovers from placebo, if “rescue” therapy not given, is assumed to be 50%. Tx denotes treatment.
Figure 3.
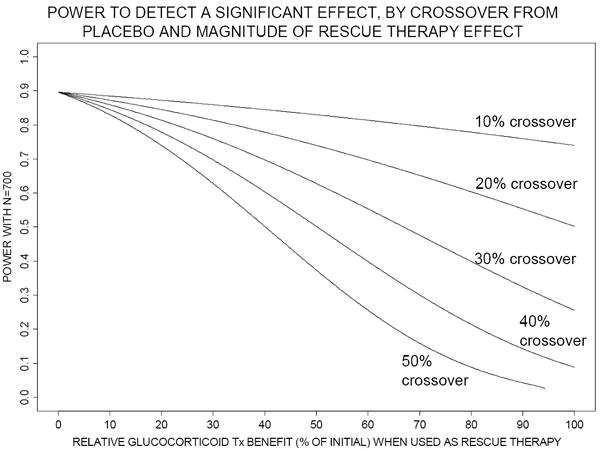
Statistical power achieved with 700 total subjects (using a two-sided chi-squared test with Type I error=0.05) using intention-to-treat analysis, according to relative corticosteroid benefit when used as “rescue” therapy (horizontal axis) and to proportion of placebo arm subjects crossing over to “rescue” therapy (10% to 50%, as labeled on each curve). The risk of mortality among crossovers from placebo, if “rescue” therapy not given, is assumed to be 50%. Tx denotes treatment.
Effects on the study when “rescue” therapy is ineffective or harmful
A trial that is adequately powered to find a treatment effect assuming beneficial “rescue” therapy will also be adequately powered when there is truly no beneficial effect of “rescue” therapy. Returning again to our specific example, suppose that we enroll 1780 subjects, to achieve desired power assuming “rescue” therapy effect of corticosteroids is equal to the initial beneficial effect. If there is in fact no “rescue” therapy effect, the expected treatment effect by ITT analysis is 10%, and power to detect a difference between treatment arms is over 99.9%.
What if “rescue” therapy is in fact harmful to some extent? Postulating the effects on trial results in this setting is challenging. If corticosteroids are harmful as “rescue” therapy, they may also be less beneficial than expected when given as initial therapy. Moreover, corticosteroids delivered as “rescue” therapy in a blinded trial might cause harm in the active arm as well as the placebo arm.
For facilitating discussion, assume that prognosis in the active treatment arm is unaffected by “rescue” therapy. This could occur if additional corticosteroids do not affect prognosis of a child already receiving this treatment, or in a “blinded crossover” design (ethically debatable) where subjects failing on the assigned treatment arm are given the “opposite” treatment as “rescue” therapy. In this case, the expected event rate by ITT analysis in our example would be 15% in the active arm, and some rate over 25% in the placebo subjects (who fail to receive the initial benefit of steroid therapy, and are in addition harmed by the “rescue” therapy). The power of ITT analysis to detect an overall treatment difference is then even higher than when “rescue” therapy has no effect.
ITT analysis cannot distinguish between primary therapy and “rescue” therapy effects
Scenarios can be constructed where expected mortality rates are close or far apart between treatment arms, depending on the relative use and harm/benefit of “rescue” therapy in the placebo arm (and possibly in the active arm as well). In all instances, ITT analysis will appropriately compare the overall assigned treatment strategies. However, the ITT analysis by itself cannot evaluate whether the “rescue” therapy component is beneficial, irrelevant, or harmful. ITT analysis cannot definitively discern whether any observed difference between treatment arms (or lack thereof) is due to initial steroid effect versus “rescue” therapy effect. This is a critical limitation if appreciable numbers of subjects receive “rescue” therapy in one or both treatment arms.
Alternative analysis strategies, such as comparisons of adherers to each assigned treatment arm or comparisons by final treatment received (26), can provide important evidence regarding harm versus benefit of “rescue” therapy. However, moving subjects between treatment arms, or removing selected patients entirely from an analysis, may create highly unbalanced subgroups to be compared. Therefore, non-ITT analyses must attempt to adjust for the higher risk profile, at baseline and at time of deterioration, of the patients receiving “rescue” therapy. Despite careful efforts at risk adjustment, such comparisons no longer attain the balance between study groups effectively guaranteed by randomization, and must be viewed as secondary analyses. Without direct randomization to “rescue” therapy, the efficacy of “rescue” therapy cannot be reliably evaluated in a trial with a “rescue” therapy component.
Ethics revisited: Considering sample size issues
Clearly, if a beneficial effect of corticosteroids as “rescue” therapy is expected, then a substantially increased number of children are required in many scenarios (Figure 2). As sample size requirements increase, more children will be subjected to whichever treatment is inferior, as well as to other inherent study risks.
Assume now that in our hypothetical trial incorporating “rescue” therapy, the investigators heed biostatistical advice and enroll 1780 children, 890 per treatment arm. If there is no interim data analysis, and if the 15% corticosteroid arm and 21% post-”rescue”-therapy placebo arm mortality rates hold exactly, then 53 more children are expected to die in the placebo arm (in which 187 deaths are expected) than in the corticosteroid arm (with approximately 134 expected deaths). This difference of 53 deaths between treatment arms is higher, by 18 deaths, than the corresponding difference of 35 expected in a study with no “rescue” therapy effect that enrolls 350 children per arm. Thus, although effective “rescue” therapy reduces risk of mortality for each individual placebo-assigned child, an “excess” of 18 additional children in the placebo arm are expected to die in the larger “rescue” therapy trial. In general, as the beneficial effect of “rescue” therapy increases, the number of “excess” deaths in the placebo arm increases. Figure 4 shows this expected number of “excess” placebo arm deaths, relative to the trial without rescue therapy, for various scenarios; this number is most dramatic under high crossover rates when large numbers of subjects are required for acceptable study power (as was shown in Figure 2). We believe that these “excess” placebo arm deaths, due to incorporating “rescue” therapy into the trial, are a particularly important ethical issue to consider.
Figure 4.
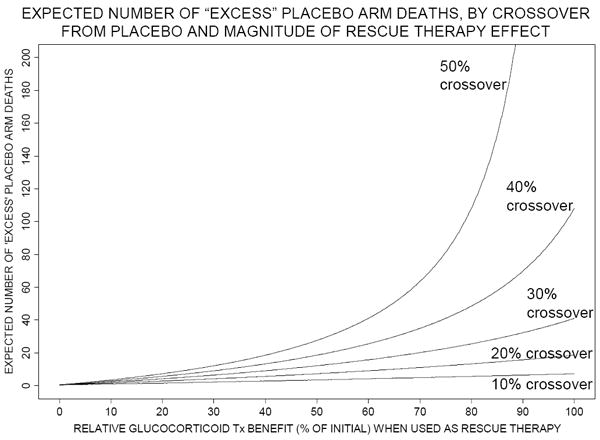
Number of “excess” deaths expected in the placebo arm when the total number of subjects is as required for 90% power, according to relative corticosteroid benefit when used as “rescue” therapy (horizontal axis) and to proportion of placebo arm subjects crossing over to “rescue” therapy (10% to 50%, as labeled on each curve). Expected number of “excess” placebo arm deaths = (expected number of deaths in the placebo arm – expected number of deaths in the active arm) – 35, since (as described in text) in a trial without “rescue” therapy, 35 more deaths are expected in the placebo arm than in the active arm. The risk of mortality among crossovers from placebo, if “rescue” therapy not given, is assumed to be 50%. Tx denotes treatment.
The numbers discussed above apply if “rescue” therapy benefit has been accurately estimated. If sample size has been increased assuming a beneficial “rescue” therapy effect, but corticosteroid “rescue” therapy is truly ineffective or harmful among patients initially receiving placebo, additional deaths will occur. If “rescue” therapy is truly ineffective in our study with 1780 children, we expect 222 placebo arm deaths (25% of 890), 134 more than the 88 deaths expected among 350 placebo arm patients in a trial powered assuming no “rescue” therapeutic effect. This difference increases further if “rescue” therapy is harmful to some extent.
If an unexpected non-effect or harmful effect of “rescue” therapy is restricted to the placebo arm, the corticosteroid arm treatment benefit will be substantially larger than originally estimated. Interim analyses of the study data will then have a good chance of terminating the study early and reducing number of children exposed to study risks.
Assume we modify our hypothetical 1780-subject study to include four equally spaced interim data analyses with conservative monitoring (27). If there is truly no “rescue” therapy effect, there is a 54% chance of stopping the “overpowered” study early, after enrolling the first 700 patients. In fact, even if the “rescue” therapy effect is exactly as expected, four interim analyses would reduce expected number of study deaths by nearly 25%, accounting for possible early stopping (28).
Interim analyses are imperative in a study with a “rescue” therapy component, due to the double uncertainty regarding estimation of “rescue” as well as initial therapeutic effect. However, statistical and ethical issues associated with such interim analyses must be kept in mind. If a trial is stopped early due to treatment superiority, treatment effects are prone to be biased upwards (29,30,31) and, moreover, information provided to the clinical community about primary and secondary outcomes, subgroup effects, and other parameters will be limited. Children may then be subjected to a treatment whose assumed risk-benefit ratio has been inflated due to bias (30), or possibly subjected to risk in future trials carried out due to limited information from a previous terminated trial (32). These issues reinforce use of conservative monitoring boundaries, and the use of such boundaries only as guidelines for DSMBs deliberating continuation of an ongoing study. DSMBs must also evaluate patient enrollment, consistency of primary and secondary endpoint findings, adverse events, and all other relevant information in their recommendations for future study conduct.
If “rescue” therapy is also permitted for children assigned to initial corticosteroid therapy, is harmful, and is frequently used, the power of the trial to find an overall treatment effect will be further diminished from the scenario just discussed. Scenarios can be constructed when “rescue” therapy in both trial arms causes unacceptably low power to find an overall treatment difference. The critical issues are that the harm of “rescue” therapy could be difficult to detect and quantify, and that a harmful “rescue” therapy component could mask a beneficial effect of initial corticosteroid use.
DISCUSSION AND SUMMARY
“Rescue” therapy is inconsistent with the concept of full equipoise regarding efficacy of an agent or therapy. A clinical trial with “rescue” therapy is justifiable, and properly analyzable, only if there is both equipoise and clinical interest in comparing strategies of immediate versus delayed use of the agent. ITT analysis of such a trial cannot determine if immediate administration of the agent is beneficial compared to placebo, if use of the agent in the “rescue” setting is of benefit, or if the agent is efficacious per se. Thus, we believe that a trial with a “rescue” therapy component is not appropriate when efficacy of the agent, rather than optimal timing of treatment with the agent, is in question.
If an agent is beneficial when used as “rescue” therapy, and in fact frequently used as such, the expected treatment overall benefit in the ITT analysis will be substantially diminished compared to the expected efficacy of the agent per se. Not accounting for the placebo-arm benefit of “rescue” therapy in the study design can make the trial fatally underpowered, leading to a final conclusion of “A larger study is necessary” in the ultimate manuscript. Studies properly powered to include “rescue” therapy may not be feasible because of substantially increased sample size requirements. Even if a sufficiently large study can be carried out, additional children will be subjected to study risks. Additional placebo arm deaths may occur in the larger study, although each individual placebo-randomized child’s risk is in fact reduced when “rescue” therapy is effective.
Planning of a trial with a “rescue” therapy component necessitates assumptions, not necessarily evaluable post-study, regarding both the immediate and the “rescue” benefits of the agent. If “rescue” therapy is truly ineffective or is harmful to patients initially treated with placebo, a study designed assuming “rescue” therapy benefit can have very high power to detect a treatment effect. Interim data monitoring, with high possibility of early study termination in this setting, can limit the number of children exposed to risk in this situation. However, “rescue” therapy use in the active study arm may lead to a variety of scenarios, some unfavorable, regarding overall study power.
Trials in the critical care setting have varied in allowing rescue therapy. A randomized assessment of corticosteroids in adult ARDS patients allowed crossovers, subsequently reporting high crossover rates from placebo (33) that complicated interpretation of results (34). However, in the UK Collaborative ECMO Trial, pediatric critical care study physicians voluntarily and successfully eschewed crossover from conventional therapy to ECMO (35). The hypothetical trial in our report reflects issues that our research network will face in a possible future randomized evaluation of corticosteroids in pediatric septic shock.
The statistical assumptions and simplifications made in this report are intended to emphasize general concepts rather than provide estimates specifically usable for actual clinical settings. Collaboration of clinicians and biostatisticians is required to determine the most appropriate sample size estimates, analytic techniques, and interim monitoring schemes for an actual study design. The fundamental ethical and analytical issues addressed in this report must be taken into account when assessing the appropriateness, feasibility, and utility of including “rescue” therapy in a clinical trial.
Acknowledgments
This work was supported by the following cooperative agreements from the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD), National Institutes of Health (NIH), Department of Health and Human Services (DHHS): U10HD050096, U10HD049981, U10HD500009, U10HD049945, U10HD049983, U10HD050012 and U01HD049934.
Footnotes
The Authors have no potential conflicts of interest to disclose.
References cited
- 1.Morris AD, Zaritsky AL, LeFever G. Evaluation of ethical conflicts associated with randomized, controlled trials in critically ill children. Crit Care Med. 2000;28:1152–1156. doi: 10.1097/00003246-200004000-00039. [DOI] [PubMed] [Google Scholar]
- 2.Czaja A, Zimmerman JJ. Endpoints/treatment algorithms for RCTs of steroids in pediatric sepsis. Abstr 458. Crit Care Med. 2004;32:A127. [Google Scholar]
- 3.Carcillo JA, Fields AI. American College of Critical Care Medicine Task Force Committee Members: Clinical practice parameters for hemodynamic support of pediatric and neonatal patients in septic shock. Crit Care Med. 2002;30:1365–1378. doi: 10.1097/00003246-200206000-00040. [DOI] [PubMed] [Google Scholar]
- 4.Zimmerman JJ. A history of adjunctive glucocorticoid treatment for pediatric sepsis: moving beyond steroid pulp fiction toward evidence-based medicine. Pediatr Crit Care Med. 2007;8:530–9. doi: 10.1097/01.PCC.0000288710.11834.E6. [DOI] [PubMed] [Google Scholar]
- 5.Sprung CL, Annane D, Keh D, et al. Hydrocortisone therapy for patients with septic shock. N Engl J Med. 2008;358:111–124. doi: 10.1056/NEJMoa071366. [DOI] [PubMed] [Google Scholar]
- 6.Sumarmo, Talogo W, Asrin A, et al. Failure of hydrocortisone to affect outcome in dengue shock syndrome. Pediatrics. 1982;69:45–49. [PubMed] [Google Scholar]
- 7.Tassniyom S, Vasanawathana S, Chirawatkul A, et al. Failure of high-dose methylprednisolone in established dengue shock syndrome: a placebo-controlled, double-blind study. Pediatrics. 1993;92:111–115. [PubMed] [Google Scholar]
- 8.Slusher T, Gbadero D, Howard C, et al. Randomized, placebo-controlled, double blinded trial of dexamethasone in African children with sepsis. Pediatr Infect Dis J. 1996;15:579–583. doi: 10.1097/00006454-199607000-00004. [DOI] [PubMed] [Google Scholar]
- 9.van Schaik SM. Do pediatric patients with septic shock benefit from steroid therapy? A critical appraisal of “Low-dose hydrocortisone improves shock reversal and reduces cytokine levels in early hyperdynamic septic shock” by Oppert et al. Pediatr Crit Care Med. 2007;8:174–176. doi: 10.1097/01.PCC.0000257037.10815.C9. Crit Care Med 2005; 33:2457-2464. [DOI] [PubMed] [Google Scholar]
- 10.Fried C. Medical experimentation: personal integrity and social policy. Amsterdam: North-Holland Publishing; 1974. [Google Scholar]
- 11.Freedman B. Equipoise and the ethics of clinical research. N Engl J Med. 1987;317:141–145. doi: 10.1056/NEJM198707163170304. [DOI] [PubMed] [Google Scholar]
- 12.Fisher LD, Dixon DO, Herson J, et al. Intention-to-treat in clinical trials. In: Peace KE, editor. Statistical issues in drug research and development. New York: Marcel Dekker; 1990. pp. 331–350. [Google Scholar]
- 13.Committee on Drugs. American Academy of Pediatrics: Guidelines for the ethical conduct of studies to evaluate drugs in pediatric populations. Pediatrics. 1995;95:286–294. [PubMed] [Google Scholar]
- 14.Koski G. Ethics, science, and oversight of critical care research: the Office for Human Research Protections. Am J Respir Crit Care Med. 2004;169:982–986. doi: 10.1164/rccm.2402022. [DOI] [PubMed] [Google Scholar]
- 15.Fost N. Ethical dilemmas in medical innovation and research: distinguishing experimentation from practice. Semin Perinatol. 1998;22:223–232. doi: 10.1016/s0146-0005(98)80038-4. [DOI] [PubMed] [Google Scholar]
- 16.Miller PB, Weijer C. Rehabilitating equipoise. Kennedy Inst Ethics J. 2003;13:93–118. doi: 10.1353/ken.2003.0014. [DOI] [PubMed] [Google Scholar]
- 17.Shapiro SH, Glass KC. Why Sackett’s analysis of randomized controlled trials fails, but needn’t. CMAJ. 2000;163:834–835. [PMC free article] [PubMed] [Google Scholar]
- 18.Schwab AP. Determining research through underdetermined treatment. [8/29/2008];Virtual Mentor. 2004 6 doi: 10.1001/virtualmentor.2004.6.11.jdsc1-0411. http://virtualmentor.ama-assn.org/2004/11/jdsc1-0411.html. [DOI] [PubMed]
- 19.Miller FG, Brody H. Clinical equipoise and the incoherence of research ethics. J Med Philos. 2007;32:151–165. doi: 10.1080/03605310701255750. [DOI] [PubMed] [Google Scholar]
- 20.Ruddell M, Spencer A, Hill K, et al. Fluoxetine vs placebo for depressive symptoms after stroke: failed randomised controlled trial. Int J Geriatr Psychiatry. 2007;22:963–965. doi: 10.1002/gps.1771. [DOI] [PubMed] [Google Scholar]
- 21.Harrison JD, Carter J, Young JM, et al. Difficult clinical decisions in gynecological oncology: identifying priorities for future clinical research. Int J Gynecol Cancer. 2006;16:1–7. doi: 10.1111/j.1525-1438.2006.00424.x. [DOI] [PubMed] [Google Scholar]
- 22.Fost N. Ethical dilemmas in medical innovation and research: distinguishing experimentation from practice. Semin Perinatol. 1998;22:223–232. doi: 10.1016/s0146-0005(98)80038-4. [DOI] [PubMed] [Google Scholar]
- 23.Ziebland S, Featherstone K, Snowdon C, et al. Does it matter if clinicians recruiting for a trial don’t understand what the trial is really about? Qualitative study of surgeons’ experiences of participation in a pragmatic multi-centre RCT. Trials. 2007;8:4. doi: 10.1186/1745-6215-8-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Robinson EJ, Kerr C, Stevens A, et al. Lay conceptions of the ethical and scientific justifications for random allocation in clinical trials. Soc Sci Med. 2004;58:811–824. doi: 10.1016/s0277-9536(03)00255-7. [DOI] [PubMed] [Google Scholar]
- 25.Wright CC, Sim J. Intention-to-treat approach to data from randomized controlled trials: a sensitivity analysis. J Clin Epidemiol. 2003;56:833–842. doi: 10.1016/s0895-4356(03)00155-0. [DOI] [PubMed] [Google Scholar]
- 26.Peduzzi P, Detre K, Wittes J, et al. Intent-to-treat analysis and the problem of crossovers. An example from the Veterans Administration coronary bypass surgery study. J Thorac Cardiovasc Surg. 1991;101:481–487. [PubMed] [Google Scholar]
- 27.O’Brien PC, Fleming TR. A multiple testing procedure for clinical trials. Biometrics. 1979;35:549–556. [PubMed] [Google Scholar]
- 28.Jennison C, Turnbull B. Group sequential methods with applications to clinical trials. Boca Raton: Chapman and Hall; 2000. [Google Scholar]
- 29.Schulz KF, Grimes DA. Multiplicity in randomised trials II: subgroup and interim analyses. Lancet. 2005;365:1657–1661. doi: 10.1016/S0140-6736(05)66516-6. [DOI] [PubMed] [Google Scholar]
- 30.Mueller PS, Montori VM, Bassler D, et al. Ethical issues in stopping randomized trials early because of apparent benefit. Ann Intern Med. 2007;146:878–881. doi: 10.7326/0003-4819-146-12-200706190-00009. [DOI] [PubMed] [Google Scholar]
- 31.Piantadosi S. Clinical trials: a methodologic perspective. New York: John Wiley & Sons; 1997. [Google Scholar]
- 32.Buchanan DR, Miller FG. A public health perspective on research ethics. J Med Ethics. 2006;32:729–733. doi: 10.1136/jme.2006.015891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Meduri GU, Headley AS, Golden E, et al. Effect of prolonged methylprednisolone therapy in unresolving acute respiratory distress syndrome: a randomized controlled trial. JAMA. 1998;280:159–165. doi: 10.1001/jama.280.2.159. [DOI] [PubMed] [Google Scholar]
- 34.Brun-Buisson C, Brochard L. Corticosteroid therapy in acute respiratory distress syndrome: better late than never? JAMA. 1998;280:182–183. doi: 10.1001/jama.280.2.182. [DOI] [PubMed] [Google Scholar]
- 35.UK Collaborative ECMO Group. The collaborative UK ECMO (Extracorporeal Membrane Oxygenation) trial: follow-up to 1 year of age. [8/29/2008];Pediatrics. 1998 101:e1. doi: 10.1542/peds.101.4.e1. http://www.pediatrics.org/cgi/content/full/101/4/e1. [DOI] [PubMed]