SUMMARY
Muscle stem cells and their progeny play a fundamental role in the regeneration of adult skeletal muscle. We have previously shown that activation of the canonical Wnt/β-catenin signaling pathway in adult myogenic progenitors is required for their transition from rapidly dividing transient amplifying cells to more differentiated progenitors. Whereas Wnt signaling in Drosophila is dependent on the presence of the co-regulator Legless, previous studies of the mammalian ortholog of Legless, BCL9 (and its homolog, BCL9-2) have not revealed an essential role of these proteins in Wnt signaling in specific tissues and developmental stages. Using Cre-lox technology to delete BCL9 and BCL9-2 in the myogenic lineage in vivo and RNAi technology to knockdown the protein levels in vitro, we show that BCL9 is required for activation of the Wnt/β-catenin cascade in adult mammalian myogenic progenitors. We observed that the nuclear localization of β-catenin and downstream TCF/LEF-mediated transcription, which are normally observed in myogenic progenitors upon addition of exogenous Wnt and during muscle regeneration, were abrogated when BCL9/9-2 levels were reduced. Furthermore, reductions of BCL9/9-2 inhibited the promotion of myogenic differentiation by Wnt and the normal regenerative response of skeletal muscle. These results suggest a critical role of BCL9/9-2 in the Wnt-mediated regulation of adult, as opposed to embryonic, myogenic progenitors.
INTRODUCTION
Myogenic stem cells (satellite cells) are the source myogenic progenitors that are responsible for the successful regeneration of adult muscle tissue after injury. Upon muscle injury, the satellite cells activate, proliferate and differentiate to form nascent muscle fibers. Understanding the coordinated lineage progression and molecular signals that regulate this complex process is of fundamental importance in understanding tissue regeneration. Wnt signaling plays an important role in many stem and progenitor cell functions in various models of tissue and limb regeneration (Kim et al., 2007; Osakada et al., 2007). Recently, we demonstrated that Wnt/β-catenin signaling increases in myogenic progenitors during lineage progression and promotes differentiation of myogenic progenitors in regenerating adult muscle (Brack et al., 2008). Extracellular Wnt inhibitors, DKK1 and sFRP3, inhibited lineage progression and differentiation, demonstrating a requirement of Wnt signaling for effective adult muscle regeneration (Brack et al., 2008). During muscle development, various Wnts have also been shown to mediate myogenic commitment and differentiation (Tajbakhsh et al., 1998; Cossu and Borello, 1999; Anakwe et al., 2003).
The Wnt signaling cascade can be activated through various ligands, but the hallmark of active canonical Wnt signaling is nuclear localized β-catenin (Logan and Nusse, 2004). In the nucleus, β-catenin binds to the TCF/LEF transcription factors to activate downstream target genes. Loss-of-function studies have demonstrated a requirement for β-catenin in satellite cell and myogenic progenitor fate during development and in the adult (Borello et al., 2006; Brunelli et al., 2007; Perez-Ruiz et al., 2008).
In Drosophila, transcriptional activation in response to Wnt involves a nuclear protein complex containing β-catenin and the co-activators Legless and Pygopus (Kramps et al., 2002; Parker et al., 2002; Thompson et al., 2002; Belenkaya et al., 2002). Mutants of legless and pygopus have phenotypes that are very similar to that of the wingless mutant (Kramps et al., 2002; Parker et al., 2002; Thompson et al., 2002; Belenkaya et al., 2002), demonstrating the essential roles of these proteins in the Wnt signaling pathway. Legless and Pygopus act as transcriptional co-activators, promoting Wnt/β-catenin signaling by multiple mechanisms (Townsley et al., 2004; Hoffmans et al., 2005; de la Roche and Bienz, 2007; Carrera et al., 2008). In mammals, there are two orthologs of Legless (termed BCL9 and BCL9-2) and two orthologs of Pygopus (termed Pygopus1 and Pygopus2). The roles of these proteins in the canonical Wnt/β-catenin signaling pathway in mammals are less well characterized. In vitro studies suggest that BCL9 and Pygopus may be essential for Wnt/β-catenin signaling only in very specific cellular contexts (Thompson et al., 2002; Sustmann et al., 2008). Mice that are null for Pygopus1 are viable and fertile with no obvious defects. Mice that are null for Pygopus2 or null for both Pygopus 1 and 2 die shortly after birth and exhibit reduced TCF-mediated signaling but have relatively mild developmental phenotypes (Li et al., 2007; Song et al., 2007; Schwab et al., 2007). Therefore, unlike in Drosophila, the absence of Pygopus does not phenocopy the developmental consequences of disrupted Wnt/β-catenin signaling (Grigoryan et al., 2008). Likewise, mice that are null for both BCL9 and BCL9-2, although embryonic lethal, also do not phenocopy mice in which Wnt/β-catenin signaling has been disrupted (Murphy-Seiler et al., 2008). It should be noted that although divergent roles for BCL9 and BCL9-2 have been reported (Brembeck et al., 2004), there is considerable functional redundancy (Brembeck et al., 2004; Hoffmans and Basler, 2007). Despite the requirement of Legless and Pygopus for canonical Wnt signaling in Drosophila, the roles of BCL9 and Pygopus proteins in Wnt signaling in mammals, either during development or in adult tissue regeneration remains to be determined.
The study of adult stem cells in tissue homeostasis and repair have revealed the fact that, although there are many aspects of development that are recapitulated when stem cells activate to form new tissue, there are also features of adult tissue formation following injury that are not shared by developmental counterparts. For example, muscle development and maturation is normal in FGF6 null mice, whereas muscle regenerative capacity is severely compromised in the adult (Floss et al., 1997). The divergence in regulatory control between embryonic and adult progenitors is only accentuated in the setting of tissue injury in the adult. In that case, stem cells and progenitors function in an environment that is influenced by products of injured, dying, and dead cells, by infiltrating inflammatory cells and factors they secrete, by highly dynamic and perhaps even aberrant extracellular matrix deposition and remodeling, and a host of other features not present in developing tissue. The study of the role of evolutionarily conserved signaling pathways, such as the Wnt pathway, in adult stem cells responding to tissue damage is likely to reveal the full repertoire of regulatory components of the pathway that rarely or never come into play during development. Hence it is essential not only to characterize the role that such signaling pathways play in the biology of adult stem cells, but alto to determine the molecular basis that explains how modulations of the pathway may yield divergent phenotypes during development and during adult tissue repair.
In the current study, we explore the role of BCL9 in adult muscle stem cells participating in tissue repair. Using a tissue-specific knockout approach, we did not observe any obvious muscle phenotype in BCL9-null muscle, despite the importance of Wnt signaling in muscle development (Cossu and Borello, 1999). To our surprise, however, initial studies revealed a dramatic defect in adult muscle regeneration. Further analysis demonstrated that BCL9 is essential for regulating Wnt signaling during specific stages of adult muscle stem cell activation. Knocking down BCL9 in vitro and genetically disrupting BCL9 in vivo both abrogated the Wnt-mediated promotion of differentiation of adult myogenic progenitors. We hypothesize that the co-regulators of the Wnt pathway may be specifically recruited as part of an amplification program that requires rapid and coordinated stem cell activation, proliferative expansion, and differentiation during tissue repair in response to acute injury. These findings may serve as a general model for the understanding defects in adult stem cell functionality associated with genetic alterations that yield no significant developmental phenotypes.
MATERIALS AND METHODS
Animals
Mutant mice were generated in which BCL9 and BCL9-2 were simultaneously inactivated by conditional targeting using the Cre-lox recombination system (Murphy-Seiler et al., 2008). Briefly, a pair of loxP sites was introduced into intronic sequences flanking exon V of BCL9 and exons VI and VII of BCL9-2, respectively, leading to loss of the coding sequences for the β-catenin binding site in BCL9 and the β-catenin and Pygopus binding sites in BCL9-2. The targeting vectors were introduced into embryonic stem cells by electroporation, and single colonies were analyzed for homologous recombination of the BCL9 and BCL9-2 targeting vector. Correctly targeted clones were used to generate chimeric founder mice by blastocyst injection. The founder mice were crossed with FLPeR mice expressing the flp recombinase gene in the ROSA26 locus to delete the FRT flanked PGKneo cassette in germ cells. Mice with two copies of BCL9loxP and BCL9-2loxP were crossed with heterozygous Myf5-Cre mice (Haldar et al., 2007) to generate mice that were null for BCL9/9-2 in muscle and control littermates. To monitor efficiency of Cre-mediated recombination, ROSA26-βgal mice (Soriano, 1999) were crossed with Myf5-Cre mice. Animals were housed and handled in accordance with the guidelines of Veterinary Medical Unit of the VA Palo Alto Health Care System and the Administrative Panel on Laboratory Animal Care of Stanford University.
Reagents
Antibodies to the following proteins used were: Pax7, eMyHC and MyHC; (A4.1025) (DSHB; Iowa City, IA); β-catenin (Pharmingen/Beckton-Dickinson, San Jose, CA); MyoD and Myogenin (Santa Cruz, Santa Cruz, CA); BCL9 (mouse monoclonal; Novus Biologicals, Littleton, CO). The chicken polyclonal Syndecan-4 antibody was generously provided by Bradley Olwin (University of Colorado). Fluorophore secondary conjugates used for immunofluorescence detection were donkey-anti-chicken Alexa488, goat-anti-mouse Alexa488, goat-anti-mouse Alexa546, goat-anti-rabbit Alexa488, and donkey-anti-rat Alexa488 (Invitrogen, Carlsbad, CA). Recombinant Wnt3A was from R&D Systems (Minneapolis, MN).
Single fiber cultures, cell cultures, and satellite cell isolation
Single fiber cultures and satellite cell isolations were performed as described previously (Brack et al., 2007). To measure Wnt-dependent TCF/LEF luciferase reporter activity, LSL cells were used as described previously (Blitzer and Nusse, 2006; Brack et al., 2007). Luciferase activity was normalized to a stably expressed LacZ plasmid.
Silencing of BCL9 and BCL9-2 expression
Two pairs of siRNA oligonucleotides targeting the BCL9 and BCL9-2 transcripts as well as negative control siRNA oligonucleotides (from Invitrogen, Carlsbad, CA) were used in these studies. One pair was selected due to a more consistent and higher level of gene silencing. The sequences were CCTTCCTGGGTTTGCAGGAATGATA and CCCGGATTTGGAGGTATGCAGAGTA for BCL9 and BCL9-2, respectively. Myoblasts (isolated and purified according to standard procedures (Brack et al., 2008) or LSL cells (Blitzer and Nusse, 2006) were plated in 6-well plates at either 50% or 90% confluency, respectively, and transfected 24 hours later with siRNA using Lipofectamine 2000 (Invitrogen) in Optimem (Gibco BRL, Rockville, MD). Cells were incubated in proliferation medium (20% fetal bovine serum (FBS; Mediatech, Hendon, VA) in Hams F-10 (Gibco BRL)) for 24 hours and then analyzed either immediately or after treatment with Wnt3A for 18 hours.
Modulation of Wnt signaling in vitro
Single fiber cultures were incubated in plating medium (10% horse serum (HS; Gibco BRL) and 0.5% chick embryo extract (CEE; US Biological, Swampscott, MA) in Dulbecco’s modified Eagle’s medium (DMEM; Gibco BRL)) for 3 days and then treated with Wnt3A for 8 hours and analyzed for β-catenin localization in growth medium (10% HS/DMEM). Myoblasts or LSL cells treated with control or BCL9/9-2 siRNA as described above were switched to growth medium for 8 hours with or without Wnt3A and analyzed for β-catenin, or for 18 hours and analyzed for luciferase activity as described previously (Brack et al., 2007).
Muscle injury and modulation of Wnt signaling in regenerating muscle
Injury to whole muscle was made by injection of barium chloride (50 µl, 1.2%) into 30 sites in the lower limb. Focal injuries to TA muscles were made by applying a metal probe, 4 mm in diameter that had been cooled on dry ice directly to the exposed muscle surface for 10 sec. To modulate Wnt levels, 10 µl of Wnt3A or control solution was introduced into the muscle surrounding the injury site by direct intramuscular injection.
Histology and immunofluorescence
Muscles were dissected and embedded for cryostat sectioning. Immunofluorescence was performed on 12 µm thick fixed sections (4% PFA, 10 min) and incubated in MOM blocking solution (Vector Labs, Burlingame, CA) according to the manufacturer’s guidelines. Sections were incubated in eMyHC primary antibody (at 1/5 dilution), washed and blocked in 5% goat serum (GS) in 0.2% Triton X-100 in PBS (PBT) and then incubated with fluorophore-conjugated antibody (Alexa goat anti-mouse546). Immunofluorescence was performed on fixed cells after permeabilization with 0.2% PBT for 10 min and blocked with 5% GS in PBT. Cells were incubated in primary antibodies overnight at 4°C at the following dilutions: Pax7 (1/200), Myogenin (1/300), MyoD (1/150), β-catenin (1/100), BCL9 (1/150). Cells were washed and blocked in 5% GS/PBT and then incubated with fluorophore-conjugated secondary antibody (Alexa goat anti-mouse546, Alexa goat anti-mouse488, goat anti-rabbit488, or goat anti-rabbit546 at 1/1500) and DAPI to visualize nuclei for 1 hr at room temperature. β-galactosidase was determined by X-gal staining (Invitrogen) as described previously (Brack et al., 2007).
Quantification of lineage progression and differentiation in vitro
Myogenic cells from single fiber cultures were analyzed for the percentage of cells that expressed Myogenin or MyHC. To measure morphological differentiation in myogenic cells in fusion medium (3% HS/DMEM), the Fusion Index was calculated as the ratio of the number of nuclei present in MyHC+ myotubes (3 or more nuclei) normalized to the total number of nuclei × 100.
Analysis of fiber size and fiber number in injured area
Images of immunostained serial sections of regenerating muscle were collected using a 20X objective. The number and cross-sectional area of every regenerating muscle fiber (denoted by central nucleation and expression of eMyHC) was quantified in the mid-region of the injury using Axiovision AC software (Zeiss) and normalized to cross-sectional areas of adjacent non-injured muscle fibers in stained sections. Every eMyHC+ fiber within the regenerating area was counted to reduce selection bias. Three sections were counted per muscle to minimize sampling errors and any variations in fiber size along the length of fiber. A minimum of 3 mice per time-point within each genotype was analyzed.
Fluorescent activated cell sorting
To enrich for myogenic progenitors, cells were isolated from myofibers as described above and then purified by sorting for cells that express Syndecan-4 (Cornelison et al., 2001). The Syndecan-4 antibody was directly conjugated to IgG-APC using Alexa Fluor647 Monoclonal Antibody Labeling Kit (Invitrogen) according to the manufacturer’s recommendations. Cells were incubated in sorting medium (10% HS in Hams F-10) for 5 min then incubated in anti-Syn-4-APC (1/100), anti-CD31-FITC (1/200), and anti-CD45-FITC (1/200) for 1 hour on ice. Cells were washed, centrifuged and re-suspended in sorting medium. Cells were analyzed using FACS Vantage SE (BD Biosciences). Cells were sorted with a gating hierarchy of forward and side scatter and gated to include cells with fluorescence staining above isotype control for anti-Syn-4-APC and negative for anti-CD31-FITC and anti-CD45-FITC. A minimum of 20,000 cells were collected per sample. After collection, the cells were washed, spun and prepared for further analysis.
Real time RT-PCR
RNA was isolated from muscle using TRIZOL reagent (Invitrogen) and cDNA was synthesized using Superscript First Strand Synthesis System for RT-PCR (Invitrogen) according to the manufacturer’s instructions. Quantitation by real-time PCR was carried out on a MyiQ real time PCR (Biorad) using probes against BCL9, BCL9-2, Axin2 and GAPDH (Applied Biosystems). The quantitation of gene expression was normalized to GAPDH.
Statistical Analysis
A minimum of 3 replicates was done for experiments presented. Data are presented as means and standard errors of the mean. Comparisons between groups were done using Kruskall Wallis comparison and a Dunn’s multiple comparison post hoc test. Differences were considered statistically significant at the p < 0.05 level.
RESULTS
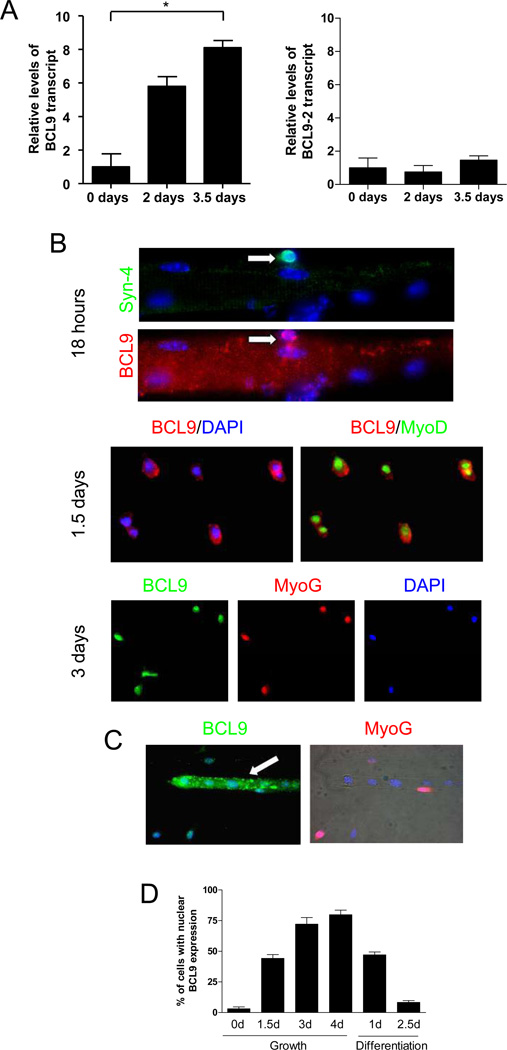
To investigate if BCL9 and BCL9-2 are present in satellite cells and their progeny, tibialis anterior (TA) muscles were either harvested or injured and left to heal for either 2 or 3.5 days and then harvested. Myogenic progenitors were isolated and purified by flow cytometry, yielding a highly-enriched (97%) population of myogenic cells (SOM Fig. 1). We observed that the BCL9 transcript was low in satellite cells and increased during myogenic lineage progression (Fig. 1A). Conversely, BCL9-2 transcript did not change during myogenic lineage progression in vivo. The expression of BCL9 was also tested at the protein level using immunohistochemical analysis of satellite cells and their progeny in single fiber cultures (Fig. 1B). Cells were stained for Syndecan-4 (a satellite cell marker), MyoD (a marker of proliferating myogenic progenitors), and Myogenin (a marker of differentiation commitment). During satellite cell activation (18 hours-1.5 days after isolation), BCL9 protein was detected in the cytoplasm of Syndecan-4+ satellite cells.
Figure 1. BCL9 and BCL9-2 expression in myogenic progenitors during lineage progression.
(A) Transcript levels of BCL9 (left panel) and BCL9-2 (right panel) assessed by real-time qRT-PCR analysis of satellite cells (0 days after injury) and their progeny (2 and 3.5 days after muscle injury) obtained by FACS sorting. Transcript levels were normalized to GAPDH and expressed relative to day 0. (* p < 0.05) (B) Representative images of single muscle fibers incubated for different times after isolation. Various antibodies (to Syndecan-4 (Syn-4), MyoD, and Myogenin (Myog)) were used in combination with an antibody against BCL9. Nuclei were visualized with DAPI. (C) Cells incubated in growth medium for 3 days were subsequently switched to differentiation medium for 2.5 days to facilitate myotube formation and analyzed for BCL9 and Myogenin expression. (D) Quantitative analysis of BCL9 immunohistochemistry as shown in panel B and C. Histogram shows the percentage of myogenic cells with BCL9 expression detectable in the nucleus after different times in growth or differentiation conditions.
After 3 days in growth medium (when a proportion of cells are committing to differentiation), BCL9 was detected in the nuclei of Myogenin+ progenitors. By contrast, BCL9 was not present in the nuclei of multi-nucleated myotubes and instead was localized to the cytoplasm with a punctuate pattern (Fig. 1C). These data suggest that BCL9 protein localization changes during myogenic lineage progression, initially cytoplasmic in proliferating progenitors, shifting to the nuclei of differentiating progenitors, and then reverting back to a cytoplasmic localization in differentiated post-mitotic myotubes (Fig. 1D). The localization of BCL9 in the nuclei of myogenic progenitors occurs in the same phase as the peak of Wnt signaling, during the differentiation of proliferating progentiors (Brack et al., 2008). Therefore, the nuclear localization of BCL parallels that of β-catenin. We were not able to demonstrate the same shift in localization for β-catenin immunohistochemically as we could for BCL9, simply because the cytoplasmic levels of β-catenin were so high that the increased nuclear localization of β-catenin that is responsible for the increased readout of Wnt signaling in the cells is just not discernible.
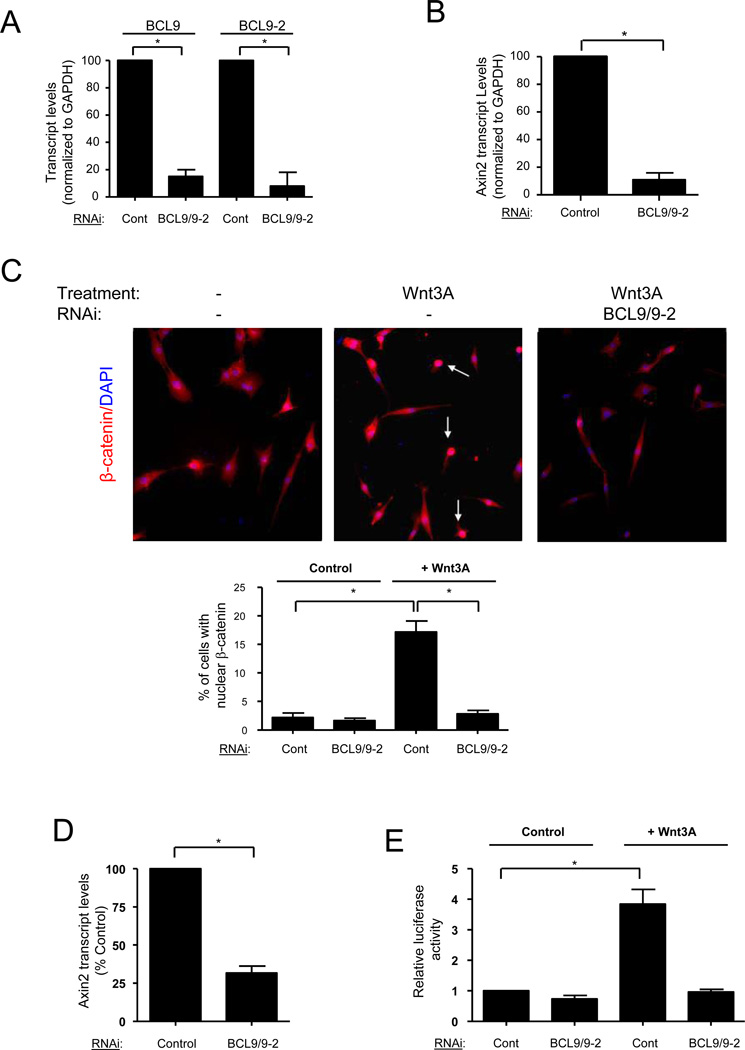
We next tested whether BCL9 and BCL9-2 mediate Wnt/β-catenin signaling in myogenic progenitors. Primary myoblasts were treated with control siRNA or siRNA against BCL9 and BCL9-2 in combination. Absolute and relative levels of BCL9 and BCL9-2 transcripts were determined by quantitative real-time RT-PCR. BCL9 and BCL9-2 transcripts were reduced to <15% of control levels by treatment with targeting siRNAs (Fig. 2A). We analyzed two readouts of Wnt/β-catenin signaling in siRNA-treated myoblasts: the abundance of Axin2 transcript, a downstream Wnt target (Jho et al., 2002), and nuclear localization of β-catenin. In myoblasts treated with BCL9/9-2 siRNA, Axin2 levels were dramatically reduced compared to those in controls (Fig. 2B). In proliferating myoblasts, β-catenin was rarely detected in nuclei, indicative of low endogenous Wnt signaling in the population as reported previously (Brack et al., 2007). In the presence of Wnt3A, an increased fraction of cells had detectable nuclear β-catenin staining compared to untreated cells (Fig. 2C), indicating an activation of the canonical Wnt signaling cascade. In cells treated with BCL9/9-2 siRNA, exogenous Wnt3A did not induce nuclear translocation of β-catenin (Fig. 2C). Furthermore, Axin2 induction by exogenous Wnt3A was reduced in BCL9/9-2 siRNA treated cells compared to control cells (Fig. 2D). Together these data indicate BCL9/9-2 is an essential mediator of canonical Wnt/β-catenin signaling in adult myogenic progenitors.
Figure 2. BCL9/9-2 mediates canonical Wnt signaling in myogenic progenitors.
(A) Transcript levels of BCL9 and BCL9-2 were assessed by real-time qRT-PCR analysis of primary myoblasts after treatment with control siRNA or with siRNA against BCL9 and BCL9-2 in combination. (Average Ct values for Controls: GAPDH - 16.7; BCL9 - 29.2; BCL9-2 - 26.6; average Ct values for BCL9/9-2 RNAi-treated: GAPDH - 16.6; BCL9 - 32.4; BCL9-2 - 29.1). Transcript levels were normalized to GAPDH and expressed relative to control siRNA treated myoblasts. (* p < 0.01) (B) Transcript levels of Axin2 in primary myoblasts treated as in Panel A. Transcript levels were normalized to GAPDH and expressed relative to control siRNA treated myoblasts. (** p < 0.01) (C) Representative images are shown of immunohistochemistry of β-catenin in primary myoblasts. Primary myoblasts were treated with BCL9/9-2 siRNA or control siRNA and subsequently with Wnt3A (40 ng/ml) or control (0.2% BSA) solution for 8 hours in growth medium (arrows indicate β-catenin+ nuclei). Quantitative analysis of the percentage of cells with nuclear localized β-catenin is shown below. (* p < 0.05) (D) Transcript levels of Axin2 in primary myoblasts treated with Wnt3A after addition of either BCL9/9-2 or control siRNA. Transcript levels were normalized to GAPDH and expressed relative to control siRNA treated myoblasts. (* p < 0.05) (E) Wnt reporter activity in LSL cells transfected with control or BCL9/9-2 siRNA subsequently treated with Wnt3A (20 ng/ml) or control solution for 18 hours. Data are expressed as relative luciferase activity (* p < 0.05)
To investigate a more general role of BCL9/9-2 in TCF/LEF-mediated transcription, we used the LSL cell line, a mammalian fibroblast line harboring a stable reporter that provides a readout of Wnt-mediated TCF/LEF-dependent transcription (Blitzer and Nusse, 2006; Brack et al., 2007). LSL cells were transfected with either control or BCL9/9-2 siRNA and then exposed to control medium or medium containing Wnt3A. In BCL9/9-2 siRNA treated cells, the Wnt3A-mediated increase in reporter activity was blocked (Fig. 2E). These data demonstrate that the essential role of BCL9/9-2 in the Wnt/β-catenin signaling pathway is not limited to myogenic progenitors but may include cells derived from other adult tissues. The effect of the inhibition of Wnt signaling on myogenic differentiation is considered below.
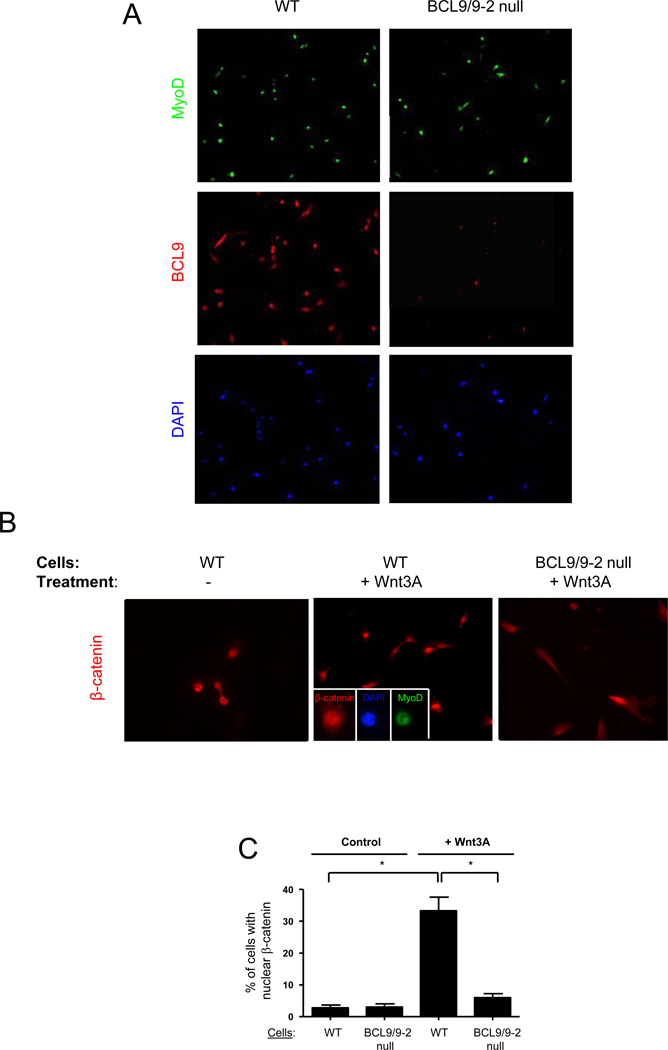
As an alternative approach to investigate whether BCL9/9-2 is essential for Wnt/β-catenin signaling, we generated myogenic progenitors in which the BCL9 and BCL9-2 genes were deleted. Mice in which BCL9 and BCL9-2 were flanked by loxP sites (Murphy-Seiler et al., 2008) were crossed with Myf5-Cre mice (Haldar et al., 2007) to delete BCL9 and BCL9-2 specifically in Myf5-expressing muscle cells. To assess Cre-dependent recombination in muscle cell populations, Myf5-Cre mice were crossed with ROSA26-βgal reporter mice (Soriano, 1999), thereby providing an estimate the percentage of satellite cells that expressed Myf5 at some point in their history by the expression of β-gal. We observed that approximately 96% of satellite cells and their progeny from adult skeletal muscle had robust expression of β-gal (SOM Fig. 2). Any inefficiency of Cre expression from the Myf5 locus or any inefficiency of recombination in Cre-expressing cells would render that number an underestimate. Nevertheless, these data demonstrate that the Myf5-Cre mouse is suitable for loss-of-function studies in postnatal muscle stem cells and their progeny.
We observed that only 8% of myogenic cells obtained from Myf5-Cre,BCL9lox/lox,BCL9-2lox/lox mice and purified by flow cytometry were positive for BCL9 expression compared with more than 90% from control mice (Fig. 3A). To assess the effect of BCL9/9-2 deletion on Wnt/β-catenin signaling, we purified myogenic cells by flow cytometry, maintained them for 2 days in culture, and then incubated them in growth medium in the presence or absence of Wnt3A for 8 hours. In the control myogenic cells, β-catenin was detected in the nucleus of 33% of cells (Fig. 3B, C). However, in the “BCL9/9-2 null” myogenic cells, nuclear β-catenin was detected in only 6% of cells, comparable to that observed in control cells in the absence of Wnt3A. Therefore, loss of BCL9/9-2 prevents the nuclear localization of β-catenin in myogenic cells normally induced by Wnt3A.
Figure 3. Loss of BCL9/9-2 function in muscle cells in vivo alters canonical Wnt signaling in myogenic progenitors.
(A) Purified satellite cells were obtained from control and BCL9/9-2 null muscle after FACS sorting and maintained in culture for 18 hours. Cells were stained for MyoD and BCL9. (B) Representative images showing β-catenin in activated myogenic progenitors FACS-purified from control and BCL9/9-2 null muscle, maintained in culture for 18 hrs, and then maintained in the presence or absence of Wnt3A for 8 hrs. Inset in central panel shows β-catenin co-localization with MyoD and DAPI in the nucleus. (C) Quantitative analysis of the percentage of activated myogenic progenitors with nuclear localized β-catenin from studies as in panel B. (** p < 0.01)
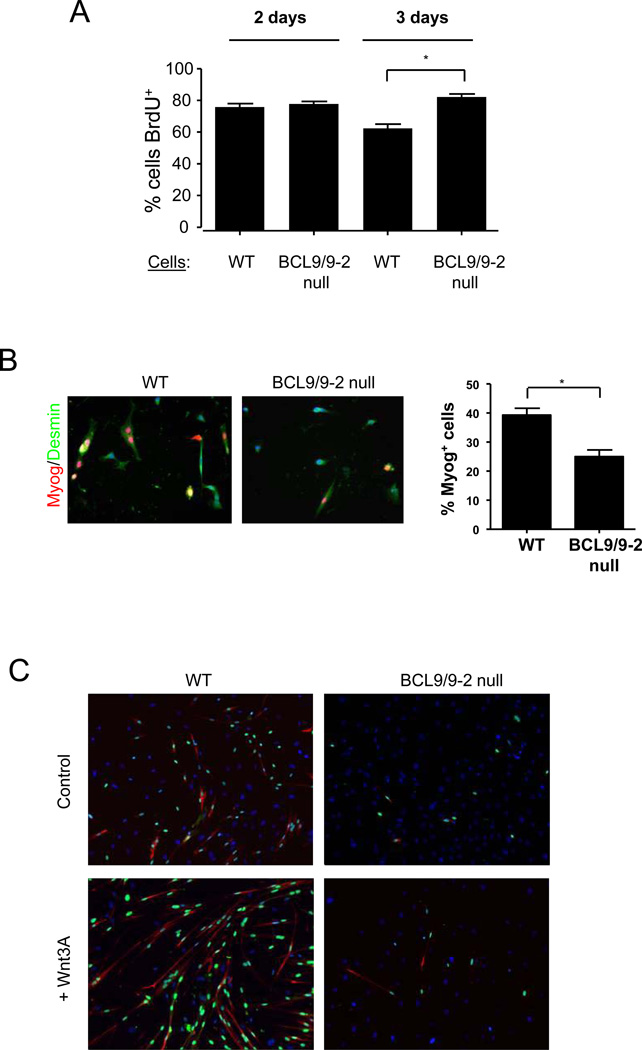
We previously demonstrated that Wnt3A promotes lineage progression and differentiation of myogenic progenitors (Brack et al., 2008). The inability to activate Wnt/β-catenin signaling in BCL9/9-2 null myogenic progenitors led us to investigate the role of BCL9/9-2 in myogenic lineage progression. We first analyzed proliferation of myogenic progenitors from single muscle fiber cultures using BrdU incorporation. Cultures treated with the Wnt inhibitor DKK1 showed a dose-dependent increase in BrdU incorporation, consistent with the role of Wnt inhibiting proliferation and promoting differentiation (SOM Fig. 3). The percentage of myogenic cells that incorporated BrdU was similar in BCL9/9-2 null and control cultures at 2 days (Fig. 4A). Between 2 and 3 days in culture when cells begin to commit to differentiation (Zammit et al., 2004), we observed a decline in cells incorporating BrdU in control cultures. However, in BCL9/9-2 null myogenic progenitors, there was no decline in BrdU incorporation at this time.
Figure 4. Loss of BCL9/9-2 impairs the commitment to myogenic differentiation in vitro.
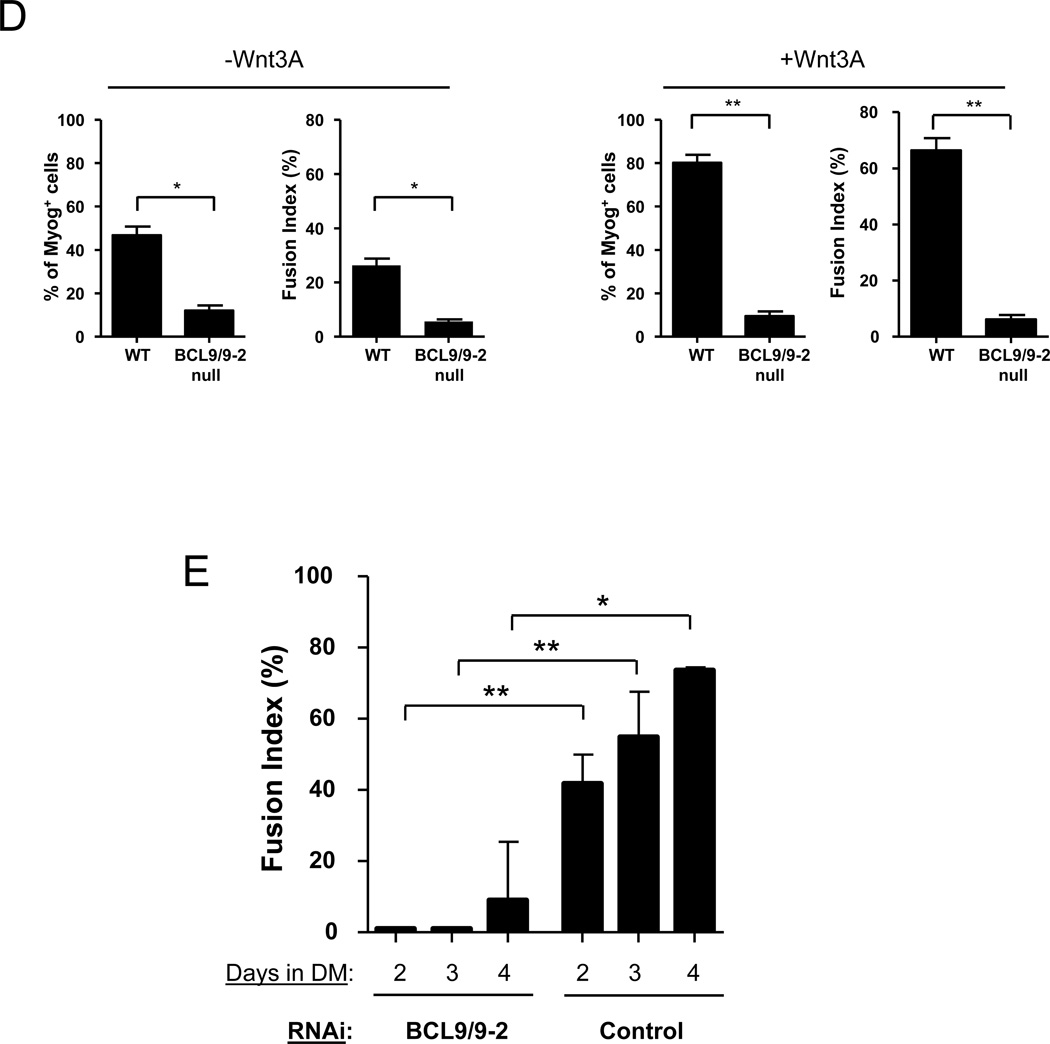
(A) Single fibers were isolated from BCL9/9-2 null and control muscles and cultured for 2 or 3 days in plating medium. BrdU was added for the final 7 hours and cells were immunostained with anti-BrdU and anti-Desmin antibodies. Quantitative analysis is shown as the percentage of BrdU+/Desmin+ cells. (* p < 0.05) (B) Myogenic progenitors from single fiber cultures were incubated in plating medium for 3 days, switched to differentiation medium for 8 hours, and immunostained for Desmin and Myogenin (Myog). Quantitative analysis is shown as the percentage of Desmin+ cells expressing Myogenin in control and BCL9/9-2 null progenitors. (* p < 0.05) (C) Myogenic progenitors from single fiber cultures were switched to differentiation medium for 2 days in the absence (upper panels) or presence (lower panels) of Wnt3A (40 ng/ml). Cultures were immunostained for Myogenin (green) and MyHC (red). DAPI (blue) stains all nuclei. (D) Quantitative analysis of cells from (C). Histograms represent the percentage of Myogenin+ cells and the Fusion Index during differentiation in the absence (left panels) and presence (right panels) of Wnt3A. (** p < 0.01; * p < 0.05) (E) At different times in differentiation medium, the Fusion Index was determined in primary myoblast cultures after treatment with either a control or BCL9/9-2 siRNA in combination. A minimum of 500 cells counted per condition. (** p < 0.01; * p < 0.05)
To address whether BCL9/9-2 plays an important role in Wnt-mediated myogenic differentiation, we analyzed the differentiation of myogenic progenitors in which BCL9/-2 had been knocked down by RNAi or knocked out by Cre-mediated recombination. We first analyzed the expression of Myogenin in BCL9/9-2 null and control progenitors incubated in differentiation medium. In control cultures, 40% of cells expressed Myogenin after 8 hrs of differentiation, whereas only 24% of BCL9/9-2 null myogenic cells expressed Myogenin (Fig. 4B), a difference that persisted when cells were exposed to differentiation medium for up to 2 days (Fig. 4C). To assess whether BCL9/9-2 null myogenic cells were altered in their ability to terminally differentiate, cultures maintained in differentiation medium for 2 days were analyzed for the expression of Myosin Heavy Chain (MyHC) and for fusion. In BCL9/9-2 null cultures, fewer cells expressed MyHC and fewer cells underwent fusion compared to control cultures (Fig. 4C, D). Furthermore, the promotion of terminal differentiation by Wnt3A was nearly completely abrogated in BCL9/9-2 null cultures (Fig. 4C, D). Likewise, the fusion of myogenic progenitors in which BCL9/9-2 had been knocked down by RNAi showed an even greater inhibition compared to control cells (Fig. 4E). Therefore either reducing levels of BCL9/9-2 by RNAi or genetically deleting BCL9/9-2 expression inhibits active Wnt/β-catenin signaling and impairs the ability of myogenic cells to commit to differentiation in vitro.
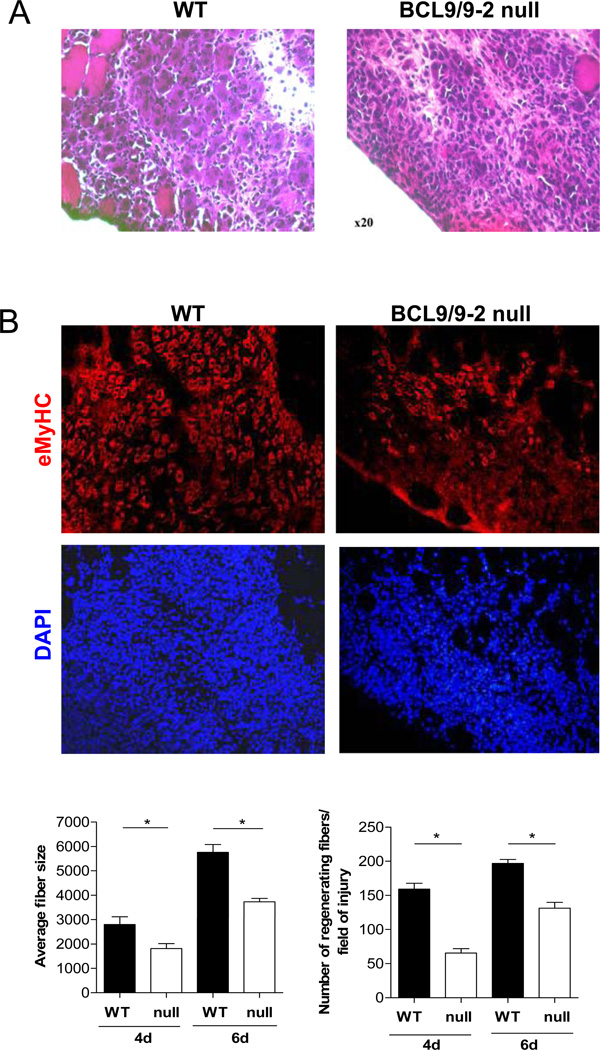
To confirm that these results in vitro reflected the behavior of myogenic cells in vivo, we injured control and BCL9/9-2 null muscles and allowed the muscles recover for either 4 or 6 days. In BCL9/9-2 null muscles, there appeared fewer and smaller caliber multinucleated myofibers based on histological analysis (Figs. 5A, B). We observed a markedly reduced number of nascent fibers (identified by the expression of embryonic Myosin Heavy Chain (eMyHC), a myosin gene expressed in newly form myofibers but not in mature fibers) in BCL9/9-2 null muscles compared to control muscles, and the fibers were of smaller caliber at both 4 and 6 days of regeneration (Fig. 5B). These in vivo data support the in vitro data and are consistent with the requirement for BCL9/9-2 in myogenic progenitors for their commitment to differentiation during muscle regeneration.
Figure 5. Loss of BCL9/9-2 impairs muscle regeneration in vivo.
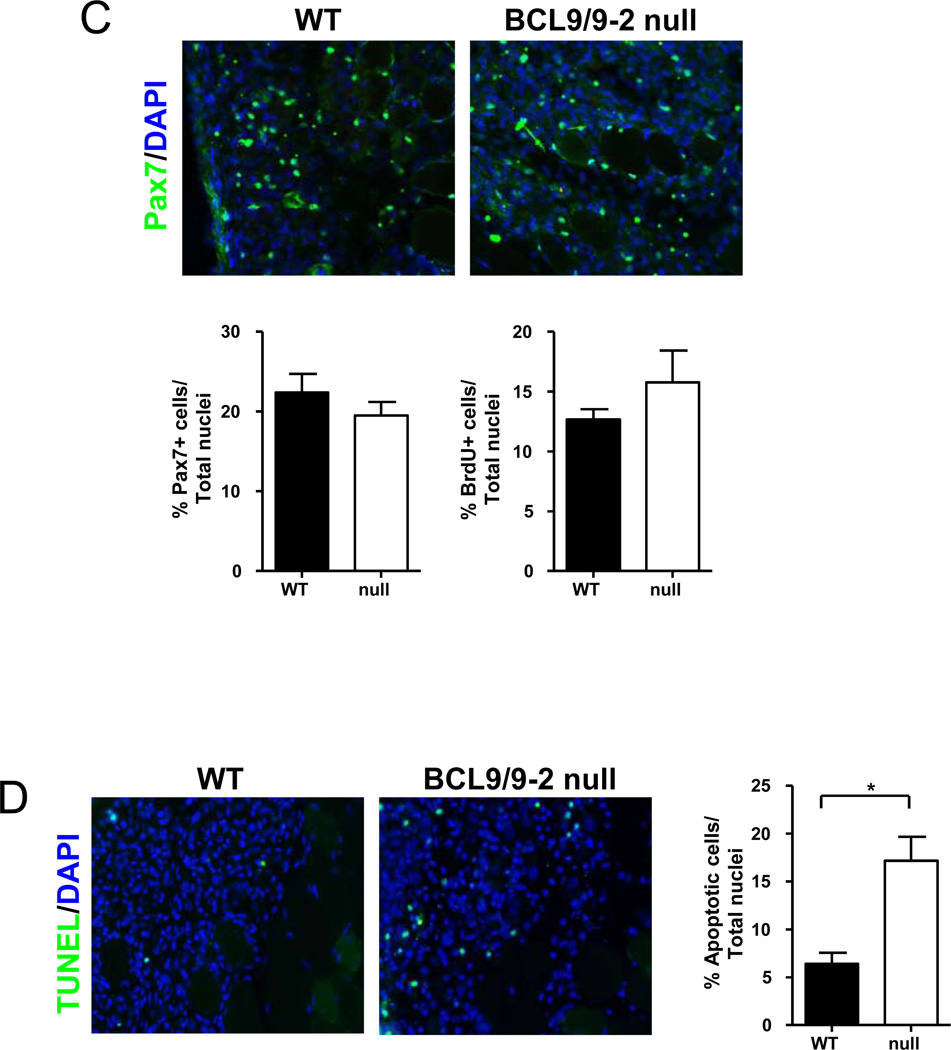
Cross sections of regenerating TA muscle 4 days after freeze injury in control (WT) (left panels) and BCL9/9-2 null muscle (null) (right panels). (A) Sections were stained with H&E for histological analysis. (B) Sections were stained with eMHC to identify newly formed myotubes. Quantitative analyses of the number of regenerating fibers normalized to cross-sectional area (left histogram) and the average size of nascent muscle fibers in regenerating tissue (right histogram) in control and BCL9/9-2 null muscles after 4 and 6 days of regeneration. (C) Sections stained with Pax7 to identify satellite cell progeny. Quantitative analysis of the number of Pax7+ cells normalized to total (DAPI+) number of nuclei in the regenerating area of muscle in WT and null muscles after 4 days of regeneration (left histogram). Quantitative analysis of the number of BrdU+ cells normalized to total (DAPI+) number of nuclei in the regenerating area of muscle in WT and null muscles after 4 days of regeneration (right histogram). (D) TUNEL staining of muscle cross sections 4 days after injury. Quantitative analysis of the number of TUNEL+ cells normalized to total (DAPI+) number of nuclei in the regenerating area of muscle in WT and null muscles after 4 days of regeneration represented in histogram. (n = 3 for the quantitative analyses; * p < 0.05)
To determine whether the regeneration defects in the absence of BCL9/9-2 are associated with a reduction in the proliferation of myogenic progenitors, we injected mice with BrdU following injury and subsequently stained sections for BrdU and Pax7. The number of Pax7+ and BrdU+ cells in regenerating muscle was not significantly affected by disruption of BCL9/9-2 function (Fig. 5C). We also assessed the survival of myogenic progenitors in the absence of BCL9/9-2 by TUNEL staining of sections of regenerating muscle. There was a significant increase in the number of TUNEL+ cells in BCL9/9-2 null muscles compared to control muscles (Fig. 5D). The data suggest that disrupting BCL9/9-2 function does not limit differentiation by inhibiting proliferation but rather by promoting apoptosis of myogenic progenitors.
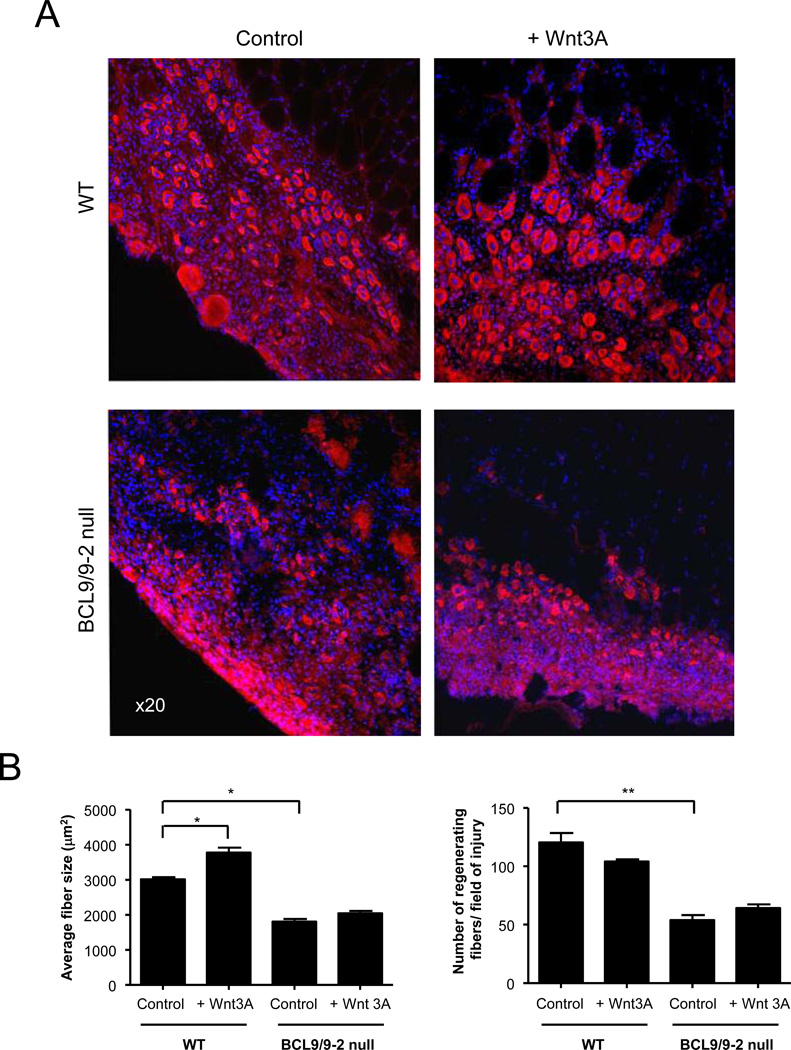
The promotion of myogenic differentiation by Wnt signaling in vivo (Brack et al., 2008) was also markedly suppressed by the absence of BCL9/9-2. Wnt3A or vehicle was injected intramuscularly 3 days after the injury to control and BCL9/9-2 null muscles, and muscles were analyzed 1 day later. In the absence of exogenous Wnt3A, the regenerating BCL9/9-2 null muscle had fewer myotubes and those myotubes were smaller in size compared to littermate controls (Fig. 6A, B). Control muscles injected with Wnt3A showed an accelerated differentiation and fusion resulting in larger myotubes, consistent with our previous findings (Brack et al., 2008). However, in BCL9/9-2 null muscles injected with Wnt3A, the myotube number and size was not significantly different from vehicle-injected muscles (Fig. 6B). Thus, the absence of BCL9/9-2 inhibits myogenic differentiation mediated by exogenous Wnt.
Figure 6. Wnt3A requires BCL9/9-2 in myogenic progenitors to accelerate myogenic differentiation in vivo.
(A) Muscles from adult (4 month) control and BCL9/9-2 null muscle were treated with either Wnt3A (10 µl of 60 ng/ml) or control solution (10 µl of 0.1% BSA) 3 days after injury and analyzed one day later. Sections were stained for eMyHC. (B) Quantitative analyses of average size of nascent muscle fibers in regenerating tissue 4 days after injury (left panel) and number of regenerating fibers normalized to cross-sectional area (right panel) in regenerating tissue in control and BCL9/9-2 null muscles treated in presence or absence of Wnt3A. (n = 3 for the quantitative analyses; * p < 0.05)
DISCUSSION
In the present study, we have used loss-of-function approaches to analyze the effects of reductions in the mammalian orthologs of Legless (BCL9 and its homolog BCL9-2) in postnatal myogenesis. Reconstitution of mammalian BCL9 and BCL9-2 into Drosophila legless mutants showed a functional redundancy between the two homologs (Hoffmans and Basler, 2007). However in the context of zebrafish, BCL9 could not compensate for the loss of BCL9-2 function, suggesting functional differences between the two BCL9 homologs (Brembeck et al., 2004). In the present study, to circumvent possible redundancy between BCL9 and BCL9-2, both genes were targeted. Further studies will be required to resolve the specific cellular and developmental contexts that under which the two homologs can substitute for one another or not. We show that BCL9/9-2 is required for the activation of the canonical Wnt pathway in a mammalian stem cell population, revealing an evolutionary conservation of this pathway in this context. Furthermore, we have also demonstrated a critical functional role of BCL9/9-2 within adult myogenic progenitors for their Wnt-mediated commitment to differentiation and effective muscle regeneration.
In Drosophila, legless was identified in a genetic screen based on a phenocopy of the wingless mutant, with epistatic experiments indicating its function was at the level or downstream of β-catenin (Kramps et al., 2002). Wnt signaling appears almost completely dependent on both Legless and Pygopus in Drosophila (Clevers, 2006), where these two proteins bind to TCF and β-catenin in the nucleus to fully activate canonical Wnt signaling (Kramps et al., 2002; Parker et al., 2002; Thompson et al., 2002; Hoffmans and Basler, 2004; Townsley et al., 2004; Hoffmans et al., 2005; Stadeli and Basler, 2005). BCL9/Legless appears to function as an adaptor protein, providing a molecular link between Pygopus and β-catenin (Stadeli and Basler, 2005), but BCL9/Legless and Pygopus may function independently of one another and each has intrinsic transactivation activity (Hoffmans et al., 2005; de la Roche and Bienz, 2007; Sustmann et al., 2008). Recently, it was shown that Pygopus may also promote gene transcription by binding to the Med12 and Med13 subunits of the Drosophila mediator complex (Carrera et al., 2008).
In vertebrates, the roles of BCL9 and Pygopus in the Wnt/β-catenin signaling pathway are less clear. Over-expression studies in mammalian cells have shown that BCL9 and Pygopus are sufficient to elevate Wnt/β-catenin signaling in vitro (Brembeck et al., 2004; Townsley et al., 2004; Sustmann et al., 2008). Knock-down experiments have demonstrated a requirement for BCL9 and Pygopus in the nuclear localization of β-catenin in some mammalian cell lines and a functional requirement of BCL9 in mesoderm patterning (a Wnt8A-mediated process) in zebrafish (Thompson et al., 2002; Brembeck et al., 2004). Deletion of Pygopus2 leads to mild developmental defects in some, but not all, Wnt-dependent tissues, and the defects appear to be independent of the Wnt signaling pathway (Li et al., 2007; Song et al., 2007; Schwab et al., 2007). Deletion of both Pygopus genes in mice leads to defects in kidney development without an abrogation of TCF/LEF-dependent signaling, and no skeletal muscle phenotype was observed despite a reduction of TCF/LEF-dependent signaling in somites (Schwab et al., 2007). The deletion of BCL9 results in embryonic lethality, whereas mice that are null for BCL9-2 die shortly after birth, but neither exhibit marked defects in Wnt-dependent developmental processes (Murphy-Seiler et al., 2008). Mice that are deficient in both genes die even earlier than BCL9 null mice, but again without a clear Wnt phenotype. Therefore Wnt regulation of mammalian development does not appear to be dependent on these co-activators that are so essential for Wnt signaling during Drosophila development.
The finding that BCL9 is an essential co-activator of the Wnt pathway, at least for the promotion of differentiation, in adult myogenic progenitors highlights the point that adult stem cells may depend on similar signaling pathways as their embryonic counterparts but that the regulation of these pathways, as well as their transcriptional outputs, may differ. Given the different environments in which embryonic and adult stem and progenitors reside, it is not surprising that their responses to environmental cues would differ. Adult stem and progenitor cells are involved in tissue homeostasis and repair in the context of a fully developed organism and a completely different systemic milieu than the embryo, perhaps necessitating additional levels of control of cellular response to activating or repressive stimuli.
It will be interested to determine which of the pleiotropic effect of Wnt in adult stem cells, even within cells of a particular lineage (see below), may be due to differential requirements of co-activators. In that sense, the studies of this report analyze Wnt-mediated differentiation of proliferation progenitors in the context of tissue injury. This is a condition of rapid, synchronous activation of stem cells followed by coordinated and synchronous differentiation associated with very high levels of Wnt signaling (Brack et al., 2008). As such, the specific requirement for BCL9/9-2 during repair of acute tissue injury may be as an “amplification factor”, whereas the more gradual cellular responses during development or tissue homeostasis may not have such a requirement. The other data that are consistent with this notion are the in vitro data of BLC9 and Pygopus requirement for Wnt signaling (Thompson et al., 2002; Sustmann et al., 2008). The cellular response to growth conditions in vitro are more likely to reflect the kind of acute stress and activation of tissue injury where the normal tissue environment is disrupted, and this may explain why co-activator dependence of the Wnt pathway is observed in culture but not from the corresponding cells and tissues in vivo during development.
In myogenic cells, ligands in the Wnt/β-catenin pathways, such as Wnt1 and Wnt3A, can promote differentiation of proliferating myogenic progenitors in vitro and in vivo (Rochat et al., 2004; Brack et al., 2008). The fact that the ability of Wnt3A to promote myogenic differentiation is abrogated in the absence of BCL9/9-2 in myogenic progenitors suggests that this effect is mediated through the Wnt/β-catenin cascade. Loss of BCL9/-2 leads to an approximately 40% reduction in the number and size of regenerating myofibers in vivo. It is possible that myogenic progenitors are capable of entering the myogenic differentiation program in a Wnt/β-catenin-independent manner, albeit less efficiently. Wnt ligands have been shown to influence numerous signaling pathways besides the Wnt/β-catenin cascade, such as the NFAT, FGF and BMP pathways (Katoh and Katoh, 2007). It is possible that other pathways cooperate with the Wnt cascade to co-ordinate differentiation and when Wnt/β-catenin signaling is compromised, such pathways will initiate myogenic differentiation with reduced efficacy.
We have not examined the responses to other Wnts under the same conditions, either other members of the Wnt family that typically activate the canonical pathway or Wnts that typically activate non-canonical Wnt signaling cascades. However, it remains to be determined whether BCL9/9-2 is required for all of the pleiotropic actions of Wnt on stem/progenitor cells in postnatal myogenesis, even for the canonical Wnt/β-catenin pathway. For example, we have previously described that exposure of resting satellite cells to Wnt can induce a change in fate from a myogenic lineage to a fibrogenic lineage (Brack et al., 2007). Furthermore, in other recent studies, it was reported that activation of the Wnt/β-catenin pathway can, on the one hand, promote self-renewal (Perez-Ruiz et al., 2008) and, on the other hand, promote proliferative expansion (Kitzmann et al., 1998). We did not investigate whether self-renewal was directly affected by BCL9/9-2 in vivo, however when we analyzed regenerating muscle for the satellite cell marker, Pax7, we did not observe any differences in the number of Pax7 cells between control and BCL9/9-2 null muscle. However direct comparisons of the results are difficult because different in vitro and in vivo conditions were used. For example, different approaches to activate the Wnt pathway were used, such as the addition of ligand to the medium and the overexpression of β-catenin. Clearly, these are non-equivalent. Equally importantly, the state of the satellite cells at the time of activation of the Wnt pathway ranged from quiescent to actively proliferating. It is possible that each stage of satellite cell activation represents a specific state during which activation of the Wnt cascade has a unique cellular consequence compared to any other stage. This could be achieved through many differences in cellular context, including the expression of different Wnt receptors in different populations or the involvement of specific co-activators such as BCL9 at different stages of myogenic lineage progression. Such pleiotropic, even opposing, effects of Wnt in cells of a single lineage are not unprecedented. The apparently opposing views as to whether Wnt signaling promotes or inhibits cardiogenesis was reconciled when the answer was found to be both, depending on the time of Wnt signaling during cardiogenesis in zebrafish or during cardiogenic differentiation of ES cells (Ueno et al., 2007). The molecular details of what renders progenitors responsive to Wnts but with such divergent outcomes remains to be determined. In view of recent studies highlighting the role of the BCL9/Pygopus complex in specifying the transcriptional read out of active Wnt signaling (Fiedler et al., 2008), it would be interesting to compare histone modifications of Wnt target genes in myogenic progenitors at different stage along the myogenic lineage. It is possible that the histone code, as read by the BCL9/Pygopus complex, determines the transcriptional read out and divergent phenotypic consequences of activation of the Wnt/β-catenin pathway.
We have used a muscle specific promoter, Myf5, to drive the expression of Cre recombinase and delete BCL9 and BCL9-2 in myogenic progenitors. The Myf5 locus is expressed in myogenic progenitors during development and in quiescent and activated satellite cells in the adult (Cornelison and Wold, 1997; Kassar-Duchossoy et al., 2005; Bajard et al., 2006). Using a ROSA26-βgal reporter mouse to determine Myf5-Cre recombination efficiency, we observed β-gal in 100% of adult muscle fibers and in 96% of satellite cells and activated myogenic progenitors. β-gal in the adult muscle fibers reflects cells that had expressed Myf5 at some time in their developmental history and contributed to the development of the adult muscle fibers, possibly from the Myf5+ myoblasts in the myotome which give rise to the majority of the limb muscles (Kassar-Duchossoy et al., 2005; Bajard et al., 2006; Haldar et al., 2007). Using YFP as a reporter of Myf5-Cre recombination, Kuang et al. reported that 90% of satellite cells have passed through a Myf5+ stage during their development (Kuang et al., 2007). This discrepancy (90% vs 96%) between the YFP and β-gal reporter may be related to issues of sensitivity or to the fact that the two Myf5-Cre strains were independently generated and may have subtle differences in expression patterns. With such high recombination efficiency in satellite cells, their progeny and adult muscle fibers, Myf5-Cre mouse is a useful tool to delete genes in a tissue-specific manner. However, it will be important to ascertain whether cells that are negative for reporter genes in these systems are a result of inefficiencies in all the steps necessary for reporter gene expression (Cre expression, recombination, transcription at the reporter gene locus) or truly reflect a developmental history that does not involve expression of the Myf5 locus.
In summary, the fact that BCL9 is required for activation of the canonical Wnt/β-catenin signaling cascade in Drosophila and in a mammalian system suggests BCL9 maybe a central component of a conserved mechanism for fine tuning the amount and/or duration of Wnt/β-catenin signaling, not only through its presence but also its cellular location, in specific cellular contexts. In turn, this may control the numerous functional properties of stem cells and their progeny during tissue regeneration which depend on the activation canonical Wnt signaling pathway. BCL9 proteins may act as amplifiers of Wnt signaling under such cellular contexts when rapid activation and cessation of the pathway are necessary for temporal control of cell fate decisions.
Supplementary Material
ACKNOWLEDGEMENTS
The authors would like to thank Brad Olwin for the generous provision of the Syndecan-4 antibody, and Anant Vasudevan and Pinky Tripathi for technical assistance. This work was supported by grants from the NIH (NS36409, AG23806, and an NIH Director’s Pioneer Award) and the Department of Veterans Affairs (Merit Review) to TAR.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
AUTHOR CONTRIBUTION
ASB participated in the design, execution and interpretation of the experiments and writing of the manuscript; FS-M and JH participated in the execution of the experiments; JD and SE contributed to the generation of the BCL9loxP, BCL9-2loxP mice; MA generated and provided the BCL9loxP, BCL9-2loxP mice, CK generated and provided the Myf5-Cre mice, and both contributed to the preparation of the manuscript; TAR participated in the design and interpretation of experiments and the writing of the manuscript.
COMPETING FINANCIAL INTERESTS
The authors declare that they have no competing financial interests.
REFERENCES
- Anakwe K, Robson L, Hadley J, Buxton P, Church V, Allen S, et al. Wnt signalling regulates myogenic differentiation in the developing avian wing. Development. 2003;130:3503–3514. doi: 10.1242/dev.00538. [DOI] [PubMed] [Google Scholar]
- Bajard L, Relaix F, Lagha M, Rocancourt D, Daubas P, Buckingham ME. A novel genetic hierarchy functions during hypaxial myogenesis: Pax3 directly activates Myf5 in muscle progenitor cells in the limb. Genes Dev. 2006;20:2450–2464. doi: 10.1101/gad.382806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belenkaya TY, Han C, Standley HJ, Lin X, Houston DW, Heasman J, Lin X. pygopus Encodes a nuclear protein essential for wingless/Wnt signaling. Development. 2002;129:4089–4101. doi: 10.1242/dev.129.17.4089. [DOI] [PubMed] [Google Scholar]
- Blitzer JT, Nusse R. A critical role for endocytosis in Wnt signaling. BMC Cell Biol. 2006;7:28-. doi: 10.1186/1471-2121-7-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borello U, Berarducci B, Murphy P, Bajard L, Buffa V, Piccolo S, et al. The Wnt/beta-catenin pathway regulates Gli-mediated Myf5 expression during somitogenesis. Development. 2006;133:3723–3732. doi: 10.1242/dev.02517. [DOI] [PubMed] [Google Scholar]
- Brack AS, Conboy IM, Conboy MJ, Shen J, Rando TA. A temporal switch from notch to Wnt signaling in muscle stem cells is necessary for normal adult myogenesis. Cell Stem Cell. 2008;2:50–59. doi: 10.1016/j.stem.2007.10.006. [DOI] [PubMed] [Google Scholar]
- Brack AS, Conboy MJ, Roy S, Lee M, Kuo CJ, Keller C, Rando TA. Increased Wnt signaling during aging alters muscle stem cell fate and increases fibrosis. Science. 2007;317:807–810. doi: 10.1126/science.1144090. [DOI] [PubMed] [Google Scholar]
- Brembeck FH, Schwarz-Romond T, Bakkers J, Wilhelm S, Hammerschmidt M, Birchmeier W. Essential role of BCL9-2 in the switch between beta-catenin's adhesive and transcriptional functions. Genes Dev. 2004;18:2225–2230. doi: 10.1101/gad.317604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunelli S, Relaix F, Baesso S, Buckingham M, Cossu G. Beta catenin-independent activation of MyoD in presomitic mesoderm requires PKC and depends on Pax3 transcriptional activity. Dev. Biol. 2007;304:604–614. doi: 10.1016/j.ydbio.2007.01.006. [DOI] [PubMed] [Google Scholar]
- Carrera I, Janody F, Leeds N, Duveau F, Treisman JE. Pygopus activates Wingless target gene transcription through the mediator complex subunits Med12 and Med13. Proc. Natl. Acad. Sci. U. S. A. 2008;105:6644–6649. doi: 10.1073/pnas.0709749105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clevers H. Wnt/beta-catenin signaling in development and disease. Cell. 2006;127:469–480. doi: 10.1016/j.cell.2006.10.018. [DOI] [PubMed] [Google Scholar]
- Cornelison DD, Filla MS, Stanley HM, Rapraeger AC, Olwin BB. Syndecan-3 and syndecan-4 specifically mark skeletal muscle satellite cells and are implicated in satellite cell maintenance and muscle regeneration. Dev. Biol. 2001;239:79–94. doi: 10.1006/dbio.2001.0416. [DOI] [PubMed] [Google Scholar]
- Cornelison DD, Wold BJ. Single-cell analysis of regulatory gene expression in quiescent and activated mouse skeletal muscle satellite cells. Dev. Biol. 1997;191:270–283. doi: 10.1006/dbio.1997.8721. [DOI] [PubMed] [Google Scholar]
- Cossu G, Borello U. Wnt signaling and the activation of myogenesis in mammals. EMBO J. 1999;18:6867–6872. doi: 10.1093/emboj/18.24.6867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de la Roche M, Bienz M. Wingless-independent association of Pygopus with dTCF target genes. Curr Biol. 2007;17:556–561. doi: 10.1016/j.cub.2007.01.063. [DOI] [PubMed] [Google Scholar]
- Fiedler M, Sanchez-Barrena MJ, Nekrasov M, Mieszczanek J, Rybin V, Muller J, et al. Decoding of methylated histone H3 tail by the Pygo-BCL9 Wnt signaling complex. Mol. Cell. 2008;30:507–518. doi: 10.1016/j.molcel.2008.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Floss T, Arnold HH, Braun T. A role for FGF-6 in skeletal muscle regeneration. Genes Dev. 1997;11:2040–2051. doi: 10.1101/gad.11.16.2040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grigoryan T, Wend P, Klaus A, Birchmeier W. Deciphering the function of canonical Wnt signals in development and disease: conditional loss- and gain-of-function mutations of beta-catenin in mice. Genes Dev. 2008;22:2308–2341. doi: 10.1101/gad.1686208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haldar M, Hancock JD, Coffin CM, Lessnick SL, Capecchi MR. A conditional mouse model of synovial sarcoma: insights into a myogenic origin. Cancer Cell. 2007;11:375–388. doi: 10.1016/j.ccr.2007.01.016. [DOI] [PubMed] [Google Scholar]
- Hoffmans R, Basler K. Identification and in vivo role of the Armadillo-Legless interaction. Development. 2004;131:4393–4400. doi: 10.1242/dev.01296. [DOI] [PubMed] [Google Scholar]
- Hoffmans R, Basler K. BCL9-2 binds Arm/beta-catenin in a Tyr142-independent manner and requires Pygopus for its function in Wg/Wnt signaling. Mech. Dev. 2007;124:59–67. doi: 10.1016/j.mod.2006.09.006. [DOI] [PubMed] [Google Scholar]
- Hoffmans R, Stadeli R, Basler K. Pygopus and legless provide essential transcriptional coactivator functions to armadillo/beta-catenin. Curr. Biol. 2005;15:1207–1211. doi: 10.1016/j.cub.2005.05.054. [DOI] [PubMed] [Google Scholar]
- Jho EH, Zhang T, Domon C, Joo CK, Freund JN, Costantini F. Wnt/beta-catenin/Tcf signaling induces the transcription of Axin2, a negative regulator of the signaling pathway. Mol. Cell Biol. 2002;22:1172–1183. doi: 10.1128/MCB.22.4.1172-1183.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kassar-Duchossoy L, Giacone E, Gayraud-Morel B, Jory A, Gomes D, Tajbakhsh S. Pax3/Pax7 mark a novel population of primitive myogenic cells during development. Genes Dev. 2005;19:1426–1431. doi: 10.1101/gad.345505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katoh M, Katoh M. WNT signaling pathway and stem cell signaling network. Clin. Cancer Res. 2007;13:4042–4045. doi: 10.1158/1078-0432.CCR-06-2316. [DOI] [PubMed] [Google Scholar]
- Kim JB, Leucht P, Lam K, Luppen C, Ten BD, Nusse R, Helms JA. Bone regeneration is regulated by wnt signaling. J. Bone Miner. Res. 2007;22:1913–1923. doi: 10.1359/jbmr.070802. [DOI] [PubMed] [Google Scholar]
- Kitzmann M, Carnac G, Vandromme M, Primig M, Lamb NJ, Fernandez A. The muscle regulatory factors MyoD and myf-5 undergo distinct cell cycle-specific expression in muscle cells. J. Cell Biol. 1998;142:1447–1459. doi: 10.1083/jcb.142.6.1447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramps T, Peter O, Brunner E, Nellen D, Froesch B, Chatterjee S, et al. Wnt/wingless signaling requires BCL9/legless-mediated recruitment of pygopus to the nuclear beta-catenin-TCF complex. Cell. 2002;109:47–60. doi: 10.1016/s0092-8674(02)00679-7. [DOI] [PubMed] [Google Scholar]
- Kuang S, Kuroda K, Le GF, Rudnicki MA. Asymmetric self-renewal and commitment of satellite stem cells in muscle. Cell. 2007;129:999–1010. doi: 10.1016/j.cell.2007.03.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li B, Rheaume C, Teng A, Bilanchone V, Munguia JE, Hu M, et al. Developmental phenotypes and reduced Wnt signaling in mice deficient for pygopus 2. Genesis. 2007;45:318–325. doi: 10.1002/dvg.20299. [DOI] [PubMed] [Google Scholar]
- Logan CY, Nusse R. The Wnt signaling pathway in development and disease. Annu. Rev. Cell Dev. Biol. 2004;20:781–810. doi: 10.1146/annurev.cellbio.20.010403.113126. [DOI] [PubMed] [Google Scholar]
- Murphy-Seiler F, Deka J, Eyckerman S, Aguet M. The role of BCL9/9-2 in intestinal development, regeneration, and cancer. Cancer Cell. 2008 submitted. [Google Scholar]
- Osakada F, Ooto S, Akagi T, Mandai M, Akaike A, Takahashi M. Wnt signaling promotes regeneration in the retina of adult mammals. J. Neurosci. 2007;27:4210–4219. doi: 10.1523/JNEUROSCI.4193-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker DS, Jemison J, Cadigan KM. Pygopus, a nuclear PHD-finger protein required for Wingless signaling in Drosophila. Development. 2002;129:2565–2576. doi: 10.1242/dev.129.11.2565. [DOI] [PubMed] [Google Scholar]
- Perez-Ruiz A, Ono Y, Gnocchi VF, Zammit PS. beta-Catenin promotes self-renewal of skeletal-muscle satellite cells. J. Cell Sci. 2008;121:1373–1382. doi: 10.1242/jcs.024885. [DOI] [PubMed] [Google Scholar]
- Rochat A, Fernandez A, Vandromme M, Moles JP, Bouschet T, Carnac G, Lamb NJ. Insulin and wnt1 pathways cooperate to induce reserve cell activation in differentiation and myotube hypertrophy. Mol. Biol. Cell. 2004;15:4544–4555. doi: 10.1091/mbc.E03-11-0816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwab KR, Patterson LT, Hartman HA, Song N, Lang RA, Lin X, Potter SS. Pygo1 and Pygo2 roles in Wnt signaling in mammalian kidney development. BMC. Biol. 2007;5:15-. doi: 10.1186/1741-7007-5-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song N, Schwab KR, Patterson LT, Yamaguchi T, Lin X, Potter SS, Lang RA. pygopus 2 has a crucial, Wnt pathway-independent function in lens induction. Development. 2007;134:1873–1885. doi: 10.1242/dev.001495. [DOI] [PubMed] [Google Scholar]
- Soriano P. Generalized lacZ expression with the ROSA26 Cre reporter strain. Nat. Genet. 1999;21:70–71. doi: 10.1038/5007. [DOI] [PubMed] [Google Scholar]
- Stadeli R, Basler K. Dissecting nuclear Wingless signalling: recruitment of the transcriptional co-activator Pygopus by a chain of adaptor proteins. Mech. Dev. 2005;122:1171–1182. doi: 10.1016/j.mod.2005.07.004. [DOI] [PubMed] [Google Scholar]
- Sustmann C, Flach H, Ebert H, Eastman Q, Grosschedl R. Cell-type-specific function of BCL9 involves a transcriptional activation domain that synergizes with beta-catenin. Mol. Cell Biol. 2008;28:3526–3537. doi: 10.1128/MCB.01986-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tajbakhsh S, Borello U, Vivarelli E, Kelly R, Papkoff J, Duprez D, et al. Differential activation of Myf5 and MyoD by different Wnts in explants of mouse paraxial mesoderm and the later activation of myogenesis in the absence of Myf5. Development. 1998;125:4155–4162. doi: 10.1242/dev.125.21.4155. [DOI] [PubMed] [Google Scholar]
- Thompson B, Townsley F, Rosin-Arbesfeld R, Musisi H, Bienz M. A new nuclear component of the Wnt signalling pathway. Nat. Cell Biol. 2002;4:367–373. doi: 10.1038/ncb786. [DOI] [PubMed] [Google Scholar]
- Townsley FM, Cliffe A, Bienz M. Pygopus and Legless target Armadillo/beta-catenin to the nucleus to enable its transcriptional co-activator function. Nat. Cell Biol. 2004;6:626–633. doi: 10.1038/ncb1141. [DOI] [PubMed] [Google Scholar]
- Ueno S, Weidinger G, Osugi T, Kohn AD, Golob JL, Pabon L, et al. Biphasic role for Wnt/beta-catenin signaling in cardiac specification in zebrafish and embryonic stem cells. Proc. Natl. Acad. Sci. U. S. A. 2007;104:9685–9690. doi: 10.1073/pnas.0702859104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zammit PS, Golding JP, Nagata Y, Hudon V, Partridge TA, Beauchamp JR. Muscle satellite cells adopt divergent fates: a mechanism for self-renewal? J. Cell Biol. 2004;166:347–357. doi: 10.1083/jcb.200312007. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.