Abstract
Background
Asthma is a chronic inflammatory disorder, hypothetically caused by autoreactive Th2 cells, whereas Th1 and regulatory T cells may confer protection. The development of Th subpopulations is dependent on the expression of lineage-specific transcription factors.
Purpose
This study aimed to assess the balance of CD4+ T cell populations in asthmatic children.
Methods
Peripheral blood mononuclear cells (PBMC) mRNA expression was assessed in 30 asthmatic children (18 patients with mild asthma and 12 with moderate asthma). Real-time polymerase chain reaction (RT-PCR) quantified TBX21, GATA-3, RORC, FOXP3, and EBI3 mRNA expression. Intracellular cytokine expression of IL-2, IL-4, IL-10, and IFN-γ in CD4+ T cells in asthmatic children was measured by flow cytometry. IL-6 and IL-17 cytokines were assessed in serum by enzyme-linked immunosorbent assay (ELISA).
Results
A significant increase was found in the percentage of CD4+ and CD8+ T cell-producing IL-4, IL-6, and IL-17. A decreased percentage of CD4+ producing IFN-γ in asthmatic children was found. Expression of GATA-3 (Th2), retinoid-related orphan receptor C (RORC) (Th17), and EBI3 were increased in asthmatic patients compared to healthy controls. Expression of FOXP3 (Treg) and TBX21 (Th1) were decreased (P < 0.0001 and P < 0.0001) in asthmatic children. Analysis of transcription factor ratios revealed an increase in the RORC/FOXP3 (P = 0.0001), and a significant decrease of TBX21/GATA-3 (P = 0.0001) ratios in patients with asthma.
Conclusion
Young asthmatics were characterized by increased IL-4 production and low IFN-γ synthesis. The increased serum IL-17 and IL-6 levels sustained an inflammatory environment in young asthmatics. The results indicate that FOXP3 and RORC mRNA expression could be associated with the sustained inflammatory process, transduced by low immune tolerance by Treg cells. The TBX21/GATA-3 and RORC/FOXP3 ratios dysregulation in asthmatics is consistent with the plasticity existing between Th1, Th17, and Treg cells during inflammation.
Keywords: RORC, FOXP3, asthma, transcription factors
Introduction
Asthma is a chronic inflammatory disorder of the airway.1 Its symptoms are highly variable, and the causes of asthma and their interactions remain largely uncertain.2 The heterogeneity of asthma suggests that it is influenced by a multitude of factors, including genetics, family history, age, sex, socioeconomic status, race and/or ethnicity, and a host of recognized environmental factors. The role of specific T cells and their cytokines in the pathogenesis of allergic asthma is now well recognized.3 The infiltration and accumulation of polarized CD4+ T helper (Th)2 cells, degranulated mast cells, and eosinophils in the bronchial mucosa are the pathological features of allergic asthma. Allergic asthma starts with an influx of naive CD4+ T cells and eosinophils into the bronchial mucosa. The priming of the naïve CD4+ T cells to differentiate into proinflammatory Th2 cells, instead of the infection-fighting Th1 cells in the T-cell repertoire, by allergen- activated dendritic cells (DCs) is an important proposed mechanism.4 The progressive increase in the commitment of CD4+ T cells toward a Th2 phenotype is accompanied by an upregulation of the Th2 inflammatory cytokines, such as IL-4, IL-5, IL-9, and IL-13, and an increased expression of the transcriptional factor GATA-3.3 In parallel, the Th2 cells shut off the expression of interferon-γ (IFN-γ) and other Th1 cytokines, such as IL-2. The recent discovery of Th17 in the mediation of corticosteroid-resistant asthma sheds new light on neutrophilic asthma.5 A skewed programming of CD4+ T cells toward Th2 or Th17 phenotype seems to be a primary cause of asthma and other immune dysfunctions of the airway.
Th1-type cells, defined by secretion of IFN-γ, are primarily involved in host defense against intracellular bacteria. Th2-type cells, involved in allergic reactions and defense against parasites, are phenotypically recognized by IL-4, IL-5, and IL-13 production.6,7 Th17 cells, characterized by their secretion of IL-17 A and IL-17F, fill a gap between Th1 and Th2, contributing to immunity against certain extracellular bacteria and fungi, and are typically linked to the defense of mucosal surfaces.8 The Th profile-associated cytokines produced are regulated by the expression of subset-specific transcription factors: TBX21 directs the Th1 cellular program;9 while GATA-3 is specific for Th2 cells.10 Recently, the human RORC (retinoid-related orphan receptor C) mRNA transcript variant 2 was identified, and appears to be responsible for Th17 differentiation.11 The phenotype of CD4+CD25hi regulatory T cells (Tregs) is directed by the transcription factor FOXP3, also frequently used as a specific marker of these cells.12,13 Tregs have been implicated as playing an important role in peripheral tolerance through their ability to suppress effector CD4+ T cells. Recently, the cytokine IL-35, consisting of EBI3 (Epstein–Barr virus-induced gene 3) and p35 (IL-12a), was implicated as a candidate for mediation of suppression and a downstream target of FOXP3.14
Asthma has classically been viewed as Th2-type mediated, but recent studies have shifted attention toward Th17 cells as responsible for the pathological processes.15,16 In order to evaluate the balance between CD4+ populations in asthmatics, the study examined the expression of the CD4+ T cell-related transcription factors in blood from children with mild and moderate asthma. In addition, the study assessed cytokines’ profile in peripheral CD4+ T cells between asthmatic children and healthy controls.
Materials and methods
Study subjects
Thirty children with asthma, diagnosed according to the Global Initiative for Asthma guidelines,17 were enrolled in the study and seen consecutively at the Department of Pediatrics and Respiratory Disease, Homeostasis and Cell Dysfunction Unit Research 99/UR/08-40, Abderrahman Mami Hospital (Ariana, Tunisia). Thirty children without asthma served as healthy controls. The Abderrahman Mami Hospital Ethics Committee approved the project, and the parents and children gave their informed consent to be enrolled in the study. Children with mild and moderate asthma were treated with regular inhaled glucocorticoids (ICS), but variable daily doses were required to control the symptoms (at the time of evaluation daily ICS dose ranged 100–1000 mg/day). Nine children with exacerbation were excluded from the study. Table 1 describes the characteristics of the asthmatic patients in the study: 60.5% had mild asthma and 39.5% had moderate asthma. The study analyzed TBX21, GATA-3, RORC, FOXP3, and EBI3 mRNA transcript in peripheral blood mononuclear cells (PBMC) from young asthmatic patients compared to PBMC from healthy children (mean age ± SD: 14 ± 3.8 years; range: 4–15 years).
Table 1.
Patients’ characteristics
| Characteristics | Data |
|---|---|
| Number of subjects | 30 |
| Gender: female/male | 18/12 |
| Age | 9.8 (4–16) |
| Positive skin test results | 68.4% |
| FVC (%) | 88.38 (58–112) |
| FEV1 (%) | 92.74 (53–120) |
| FEV1/FVC | 0.9 (0.79–1) |
| Passive smoking | 47.3% |
| Severity | |
| Mild | 60% |
| Moderate | 40% |
| CD4/CD8 ratio | 1.08 v. (HC )1.1† |
Notes: P = 0.068.
Abbreviations: FEV1, forced expiratory volume in 1 second; FVC, forced vital capacity.
Cytokines levels in sera
The serum levels of the proinflammatory cytokines IL-6 (pg/mL) and IL-17 (pg/mL) were assessed using a solid phase enzyme-linked immunosorbent assay (ELISA), as recently reported.18 Commercial ELISA kits were provided from R&D Systems® (Minneapolis, MN), Genzyme ( Cambridge, MA), and BioSource™ (Camarillo, CA). Tests were performed according to the manufacturer’s instructions for each product (detection limits of IL-6: 0.094 pg/mL, of IL-17: 4–15 pg/mL).
Real-time polymerase chain reaction (RT-PCR)
RT-PCR was performed on PBMCs. The expression of mRNA was quantified using the Applied Biosystems™ 7500 Fast Real-Time PCR System (Carlsbad, CA), as recently reported.19 Amplification of complementary DNA (cDNA) was performed with the TaqMan® Universal PCR Master Mix (2×), No AmpErase® UNG (Applied Biosystems). A reaction volume of 25 μL (1.0 μL cDNA) was amplified for 40 cycles of 10 seconds at 95°C, and 1 minute at 60°C. All samples were analyzed in duplicate, and transcription expression was calculated as a mean and standard deviation (SD). For quantification of cDNA, a five-point serially four-fold diluted standard curve was developed from cell cultures stimulated with phytohemagglutinin. The mRNA expression of the T cell transcription factors was standardized to 18S (human rRNA) and all results expressed as a ratio. A coefficient of variance <15% was accepted as maximum variation among duplicates. The intra-assay variance for 18S was 4.9%; FOXP3 6.5%; GATA-3 6.1%; RORC 7.2%; TBX21 7.2%; and EBI3 6.2%. Samples revealing an undetectable expression in both duplicates in three subsequent analyses were assigned an expression quantity of zero. Primers and probes for TBX21, RORC, and EBI3 expression were analyzed with TaqMan Gene Expression assays (Applied Biosystems), while GATA-3, FOXP3, and 18S, using 30 FAM/50 TAMRA™-labeled probes were constructed in-house using Primer Express® 3.0 (Applied Biosystems). The following sequences were used: GATA-3 forward CAAAATGAACG GACAG AACCG, reverse GCTCTCCTGGCTGCAGACA, probe CCCCTCATTAAGCCCAAGCGAAGG; FOXP3 forward GTGGCCCGGATGTG AGAA, reverse GCTGCTC CAGAGACTGTACCATCT, probe CCTCAAGCACTG CCAGGCGGAC; 18S forward CGGCTACCACATCCA AGGAA, reverse GCTGGAA TTACCGCGGCT, probe GAGGGCA AGTCTGGTGCCAGCA. High-performance liquid chromatography-purified oligonucleotide primers and probes were bought from MedProbe® (Oslo, Norway). All in-house-designed mRNA amplicons included at least one exon–exon boundary to ensure specificity (marked in bold in the sequences above), and reaction concentration was optimized prior to analysis of samples.
Intracellular cytokine staining
Intracellular cytokine staining before and after polyclonal stimulation was assessed, as recently reported.18 Heparinized whole blood was diluted with Gibco® RPMI (Invitrogen™, Carlsbad, CA) and 2% calf serum, and after the addition of 25 μL (25 ng/mL) phorbol 12-myristate 13-acetate (PMA; Sigma®, St Louis, MO), 20 μL (1μg/mL) ionomycin (Sigma), and 10 μL (2.5 μM) monensin (Sigma) incubated in a water bath for 4 hours at 37°C. Stimulated whole blood was distributed in 100 μL aliquots into tubes containing 20 μL anti-human CD3 FITC and CD4 PerCp (Becton Dickinson [BD], Franklin Lakes, NJ), vortexed, and incubated for 20 minutes at room temperature in the dark. To fix cells, 100 μL of IntraPrep Reagent 1 (DAKO, Glostrup, Denmark) was added to the samples followed by vortexing and incubation for 15 minutes at room temperature in the dark, and washing with 2 mL of cell-wash solution. Simultaneous permeabilization of white cells and lysis of red cells was then carried out by adding 100 μL IntraPrep Reagent 2 (DAKO) and incubation. Intracellular staining procedure included addition of 20 μL of anti-IL-2, IL-4, IL-10, and IFN-γ monoclonal antibodies or isotype control mouse antibodies conjugated with phycoerythrin (DAKO). The flow cytometric assessment with FACScan flow cytometry (EPICS XL; Coulter Electronics, Hialeah, FL) was performed on the same day. Acquisition was gated on CD3+CD4+ and CD3+CD8+ cells to define the CD4/CD8 ratio. The percentage of CD3+CD4+ cell-producing IL-2, IL-4, IL-10, and IFN-γ, was determined on dot plots. The acquisition was performed both on samples stimulated with PMA and ionomycin, and on nonstimulated samples, in order to estimate the residual intracellular expression of the cytokines. Activation of the cells was confirmed by estimation of the CD69 expression, which was >90% in all samples.
Statistical analysis
Data were analyzed with IBM® SPSS® (v 15.0.0; SPSS Inc, Chicago, IL). The Mann–Whitney U test was used to analyze independent samples for differences in transcription factor expression. When assessing flow cytometric data, Student’s t-test was used. P values < 0.05 were considered statistically significant.
Results
Expression of FOXP3, GA TA-3, TBX21, and RORC in asthmatic children
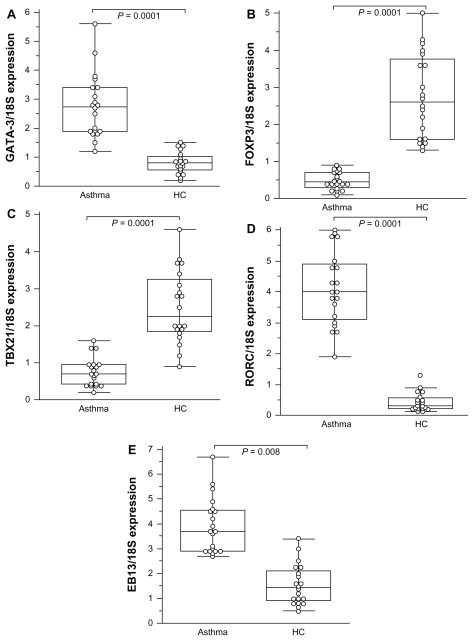
The study detected the expression of mRNA for GATA-3, EBI3, and TBX21, and for FOXP3 in mild asthmatics and moderate asthmatics. The results were expressed as (mean ± SD). Patients with asthma expressed higher Th2-inducing GATA-3 mRNA levels (2.61 ± 1.11; range: 0.97–5.6) than healthy controls (0.82 ± 0.37; range: 1.2–1.5; P = 0.0001) (Figure 1A). No significant difference was observed between mild and moderate asthmatics (2.30 ± 0.97 and 2.08 ± 1.4; P = 0.058; P = 0.92) in the Th2-inducing GATA-3 mRNA level. In contrast, the FOXP3 was significantly decreased in asthmatics (0.51 ± 0.31; range: 0.1–1.72) compared to controls (2.75 ± 1.14; range: 1.3–5; P = 0.0001) (Figure 1B). The levels of FOXP3 mRNA tended to be lower in moderate (0.39 ± 0.21) than mild asthmatic (0.52 ± 0.29) patients without any significance (P = 0.068).
Figure 1.
Comparison of transcription factor expression fold change in patients with asthma compared to healthy controls (HC ).
Notes: All values represent quotes between transcription factor and 18S expression. In the figure, the horizontal bars within boxes correspond to the median; box limits correspond to 25th and 75th percentiles; and vertical lines indicate the range. The mean values were compared and the P values are indicated on the figure. A significant difference was observed in EBI3 value between asthmatic patients (3.19 ± 1.48) and healthy controls (1.55 ± 0.81; P = 0.008).
The expression of Th1-type TBX21 was significantly decreased in mild and moderate asthmatics (0.75 ± 0.42; range: 0.13–1.9) compared to healthy controls (2.51 ± 0.98; range: 0.9–4.6; P < 0.0001; Figure 1C). No difference was found in the Th1-inducing TBX21 mRNA expression between mild and moderate asthmatics. By contrast, Th17- associated RORC was significantly increased in asthmatics (3.5 ± 1.19; range: 0.97–6) compared to healthy controls (0.44 ± 0.29; range: 0.13–1.8; P < 0.0001; Figure 1D). Levels of Th17-associated RORC were significantly different between mild (4.75 ± 1.19; range: 1.19–4.73) and moderate (5.78 ± 0.97; range: 2.6–6) asthmatic patients (P = 0.0032). The expression of EBI3 mRNA was significantly increased both in mild and moderate asthmatics (3.19 ± 1.48) as compared to controls (1.55 ± 0.81; P = 0.008; Figure 1E).
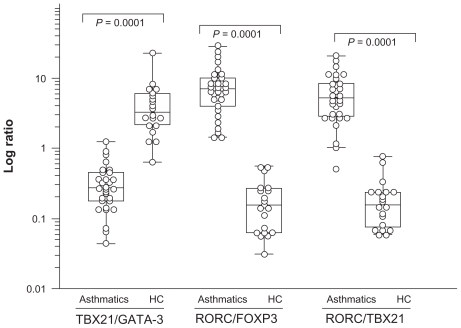
TBX21 and GATA-3 are regarded as reciprocally inhibiting each other.9,20 Similarly, RORC was antagonistically related to FOXP3.21 Therefore, the study calculated ratios of TBX21/GATA-3 and RORC/FOXP3 in order to assess the relative expression of transcription factors (Figure 2). The RORC/FOXP3 ratio exhibited a drastic increase in asthmatic patients (8.55 ± 6.62) both in mild and moderate asthmatics compared to controls (0.198 ± 0.16; P < 0.0001). A significant difference was also observed between asthmatic patients (0.34 ± 0.26) and controls (4.76 ± 4.81; P = 0.0001) regarding TBX21/GATA-3 ratio. As seen in Figure 2, the RORC/TBX21 ratio exhibited a drastic increase in asthmatic patients (6.60 ± 4.96) compared to controls (0.20 ± 0.18; P < 0.0001). The balance between Treg and Th effector cells regulates the degree of inflammation. The increased RORC/TBX21 ratio (Th17/Th1) was used to mirror the level of the inflammation. Comparison of RORC/FOXP3 and RORC/TBX21 revealed an imbalance in the subpopulation of CD4+ T cells among young asthmatics, with a low expression of Treg-associated transcription factors.
Figure 2.
Transcription factor TBX21/GA TA-3, RORC/TBX21, and FOXP3/RORC ratios in asthmatics compared to healthy controls (HC ).
Notes: Ratios are expressed as log transcription of TBX21/GA TA-3 and FOXP3/RORC. TBX21 directs the Th1 cellular program. GA TA-3 is specific for Th2 cells. RORC mRNA transcript variant 2 was identified, and appears to be responsible for Th17 differentiation. The phenotype of CD4+CD25hi regulatory T cells (Tregs) is directed by the transcription factor FOXP3. The P values are indicated on the figure.
Abbreviation: RORC, retinoid-related orphan receptor C.
Serum IL-17 and IL-6 expression in asthmatic children
Cytokine levels in sera of IL-6 and IL-17 were expressed as mean ± standard error (SE). Values of IL-6 and IL-17 were significantly higher in all asthmatic patients compared to healthy children (IL-6: 12.9 ± 4.8 pg/mL vs 4.9 ± 2.7 pg/mL [P = 0. 0012]; IL-17: 17.6 ± 8.5 pg/mL vs 9.4 ± 3.6 pg/mL [P = 0. 0026]). No significant differences were observed in asthmatic groups between mild and moderate asthmatics (IL-6: 11.7 ± 5.2 pg/mL vs 12.3 ± 1.9 pg/mL [P > 0. 05]; IL-17: 16.4 ± 4.3 pg/mL vs 17.2 ± 3.2 pg/mL [P > 0. 05]).
Frequencies of T cell-producing IL-2, IL-4, IL-10, and IFN-γ spontaneously and after stimulation in asthma patients and healthy controls
Mean values of frequencies of CD4+ T cell-producing IL-2, IL-4, IL-10, and IFN-γ, before and after stimulation are shown in Table 2. Unstimulated CD4+ T cells in whole blood staining positively for IL-2, IL-4, and IL-10 were similar in children with asthma compared to healthy controls. Frequencies of CD4+ T cell-producing IFN-γ were significantly reduced in asthmatic children (P = 0.0001) compared to healthy subjects.
Table 2.
Percentage of CD4+ T cells producing IL-2, IL-4, IL-10, and IFN-γ spontaneously and after in vitro stimulation (data are shown as mean ± SE )
| IL-2 | IL-4 | IL-10 | IFN -γ | ||
|---|---|---|---|---|---|
| Spontaneously | Asthmatics | 3.24% ± 0.17% | 3.22% ± 0.36% | 3.52% ± 0.51% | 1.86% ± 0.43%* |
| HC | 3.92% ± 0.28% | 3.19% ± 0.22% | 4.06% ± 0.46% | 3.52% ± 0.92% | |
| After stimulation | Asthmatics | 6.09% ± 0.45% | 9.58% ± 0.43%* | 7.92% ± 0.5% | 6.72% ± 0.39%* |
| HC | 6.21% ± 0.33% | 5.60% ± 0.39% | 8.58% ± 0.47% | 12.5% ± 1.92% |
Notes: P < 0.05; no differences were observed in IL-2 and IL-10 synthesis between healthy controls and asthmatics, spontaneously and after stimulation.
Abbreviation: HC, healthy controls.
After in vitro stimulation, frequencies of CD4+ T cells staining positively for IL-2 and IL-10 were similar in asthmatics and healthy controls (Table 2). The frequencies of CD4+ T cell-producing IL-4 were significantly higher in asthma patients than in the healthy control group after in vitro stimulation; in contrast, a significantly low level of IFN-γ was observed in asthmatic children (P = 0.0001).
Discussion
Asthma arises from multiple interactions of infiltrating and structural cells in the context of chronic airway inflammation that is orchestrated by Th1, Th2, Th17, and Treg cells.20 Recent data indicates that a deficient control of presumed pathogenic Th2- and Treg-mediated mechanisms may be involved in asthma.22 This study assessed the expression of CD4-related transcription factors on PBMC to evaluate the balance between circulating Th subpopulations in young asthmatics. PBMC cells from asthmatic patients were associated with a low expression of FOXP3, a marker of Treg cells. In addition, the study found a high expression of the regulatory factor EBI3 in asthmatics compared with control groups. The expression of GATA-3 and RORC, the Th2 and Th17-associated transcription factors themselves, exhibited a higher increase in asthmatic patients. Using stimulated CD4+ T cell-producing cytokines, the study found significantly higher frequencies of CD4+ T cells expressing IL-4 compared to healthy children. Similarly to other reports, stimulated T cells were accompanied by significantly decreased IFN-γ production in patients with asthma, as compared to age-matched healthy controls.
The number of circulating Tregs in peripheral blood was significantly altered in asthmatic patients, as recently reported.23 In the current study, the data reported decreased expression of FOXP3 in asthmatic children. The stability of FOXP3 in Treg cells could be diminished under strong inflammatory conditions.24,25 Provoost et al reported a deficiency in FOXP3 protein expression within CD4+CD25+ T cells of asthmatic patients.26 Recent studies have elegantly demonstrated that FOXP3+ T cells expressing T-bet, IRF4, or STAT3 specifically suppress the Th1, Th2, or Th17 response in vivo, respectively.26
This study found high levels of EBI3 expression in asthmatic patients. EBI3 was found to constitute a part of IL-35, a cytokine associated with immunosuppressive effects of Tregs in mice.4,27 EBI3 receptor signaling influences a variety of immune cell types and can inhibit both Th1 and Th2 responses. Recently Dokmeci et al reported that EBI3 might play an inhibitory role in allergic asthma development.28 EBI3 in human immune regulation is not confirmed and more data are needed. A straight interaction exists between Th1, Th2, Th17, and Treg cells under the influence of many factors (TGF-β, retinoic acid, IL-6, IL-17, IL-10, etc)29 and a conversion from one kind to another population is possible. Treg cells can become IL-17-producing cells upon stimulation with IL-6 and low concentration of TGF-β. Recently it was reported that naturally regulatory T cells (nTreg) and FOXP3 were discovered as immune suppressors critical for self-tolerance and immune homeostasis. These nTreg cells were shown to convert into proinflammatory cells.30
Observations of increased expression of IL-17 in blood and in inflammatory sites support a Th17-mediated pathogenesis.31,32 The Th17/Treg ratio was found to be significantly higher in asthmatics, and this finding is compatible with a role for Th17 as an inflammatory factor in asthma. The young patients were characterized in their sera by a significant increase of IL-6 and IL-17 cytokines having a prominent role in inflammation in childhood asthma. Analysis of the distribution of Th17 cells revealed the presence of inflammation occurring when the number of Th17 cells exceeds the number of Th1 cells.31 Such a situation was observed in the asthmatic patients group where Th17 cells exceed Th1 cells. A wide range of Th17/Th1 ratios permitted exacerbation of the disease. In asthma patients, Th17 differentiated cells are able to release IL-17. Th17 cells also induced IFN-γ (Th1) production.33,34 In the patients, the high Th17/Th1 ratio in asthmatics indicated low IFN-γ–CD4+ T cells production spontaneously and after in vitro stimulation. The role of IFN-γ in the pathogenesis of asthma is more complex. Regarding the wide range production of proinflammatory cytokines by Th17 cells (IL-17), it is expected that Th17 is a potent pathogenic factor in disease immunopathophysiology, in particular, in asthma.
Interplay between Treg and Th17 probably exist in asthma. The overexpression of RORC2 in naïve T cells reduces levels of FOXP3; small interfering RNA- mediated knockdown of RORC2 enhances its expression. RORC2 mediates this inhibition at least partially by binding to two out of four ROR-responsive elements on the FOXP3 promoter.35 RORC promotes high FOXP3 levels and decreased expression of proinflammatory cytokines. Burgler et al identified RORC2 as a polarizing factor in transcriptional cross-regulation and provides novel viewpoints on the control of immune tolerance versus effector immune responses.35 Unexpectedly, as reported recently by Ayyoub et al, a significant proportion of circulating memory FOXP3+ Tregs secrete IL-17 and express high levels of RORγt ex vivo.36 These findings shed light on the close relationship between human Tregs and Th17 cells and suggest that, in addition to their well-recognized immune suppressive functions, memory FOXP3+ Treg cells likely play additional, yet to be described, proinflammatory functions.37 Future studies addressing these issues will undoubtedly contribute to further elucidation of the mechanisms regulating the balance between inflammation and homeostasis in asthma.
These data are consistent with a recent report demonstrating that TBX21 T-1993C polymorphism represses TBX21 expression and Th1 cytokine production through control of YY1, which might result in the imbalance between Th1 and Th2 immune responses in autoimmune or allergic diseases.37,38
The results show the plasticity existing between Th1, Th17, and Treg cells during young asthma. The study did not find significant differences of CD4+-IL-10 production after in vitro stimulation. This cytokine was reported to inhibit the production of IFN-γ of Th1 CD4+ cell clones via its ability to abrogate the production of monocyte/macrophage cytokines such as IL-12. 39,40 It is well recognized that IL-10 is produced by Th1, Th2, Th17, and particularly by Treg cells (CD4+CD25+FOXP3+), and that the transcription factors TBX21, GATA-3, RORC, and FOXP3 can be co-expressed in some Treg cells and exist in vivo.33
A critical role for T cells, and especially Th17 and Treg cells, in young asthmatics is now widely accepted. The precise effects of TBX21 and GATA-3 in the pathogenesis of asthma are still not fully understood and the finding of overexpression of RORC and underexpression of FOXP3 in asthma suggests that these proteins have a significant role in this process.
Acknowledgments
Study design, data interpretation, and manuscript preparation: Agnes Hamzaoui, Kamel Hamzaoui. Data collection: Haïfa Maalmi, Anissa Berraïes. Statistical analysis: Agnes Hamzaoui, Haïfa Maalmi, Anissa Berraïes, Kamel Hamzaoui. Literature search and funds collection: Agnes Hamzaoui, Haïfa Maalmi, Anissa Berraïes, Hanadi Abid, Jamel Ammar, Kamel Hamzaoui. All authors read and approved the final manuscript.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Kumar RK, Hitchins MP, Foster PS. Epigenetic changes in childhood asthma. Dis Model Mech. 2009;2(11–12):549–553. doi: 10.1242/dmm.001719. [DOI] [PubMed] [Google Scholar]
- 2.Lemanske RF, Jr, Brusse WW. Asthma: clinical expression and molecular mechanisms. J Allergy Clin Immunol. 2010;125(2 Suppl 2):S95–102. doi: 10.1016/j.jaci.2009.10.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ray A, Khare A, Krishnamoorthy N, et al. Regulatory T cells in many flavors control asthma. Mucosal Immunol. 2010;3(3):216–229. doi: 10.1038/mi.2010.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hammad H, Lambrecht BN. J Allergy Clin Immunol. 2. Vol. 118. Recent: progress; 2006. in the biology of airway dendritic cells and implications for understanding the regulation of asthmatic inflammation; pp. 331–336. [DOI] [PubMed] [Google Scholar]
- 5.Barnes PJ. New molecular targets for the treatment of neutrophilic diseases. J Allergy Clin Immunol. 2007;119(5):1055–1062. doi: 10.1016/j.jaci.2007.01.015. [DOI] [PubMed] [Google Scholar]
- 6.Mosmann TR, Cherwinski H, Bond MW, et al. Two types of murine helper T cell clone. I. Definition according to profiles of lymphokine activities and secreted proteins. J Immunol. 1986;136(7):2348–2357. [PubMed] [Google Scholar]
- 7.de Waal Malefyt R, Figdor CG, Huijbens R, et al. Effects of IL-13 on phenotype, cytokine production, and cytotoxic function of human monocytes. Comparison with IL-4 and modulation by IFN-gamma or IL-10. J Immunol. 1993;151(11):6370–6381. [PubMed] [Google Scholar]
- 8.Curtis MM, Way SS. Interleukin-17 in host defence against bacterial, mycobacterial and fungal pathogens. Immunology. 2009;126(2):177–185. doi: 10.1111/j.1365-2567.2008.03017.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Szabo SJ, Kim ST, Costa GL, et al. A novel transcription factor, T-bet, directs Th1 lineage commitment. Cell. 2000;100(6):655–669. doi: 10.1016/s0092-8674(00)80702-3. [DOI] [PubMed] [Google Scholar]
- 10.Zheng W, Flavell RA. The transcription factor GATA-3 is necessary and sufficient for Th2 cytokine gene expression in CD4 T cells. Cell. 1997;89(4):587–596. doi: 10.1016/s0092-8674(00)80240-8. [DOI] [PubMed] [Google Scholar]
- 11.Ivanov II, McKenzie BS, Zhou L, et al. The orphan nuclear receptor RORgammat directs the differentiation program of proinflammatory IL-17+ T helper cells. Cell. 2006;126(6):1121–1133. doi: 10.1016/j.cell.2006.07.035. [DOI] [PubMed] [Google Scholar]
- 12.Sakaguchi S, Sakaguchi N, Asano M, et al. Immunologic self-tolerance maintained by activated T cells expressing IL-2 receptor alpha-chains (CD25). Breakdown of a single mechanism of self-tolerance causes various autoimmune diseases. J Immunol. 1995;155(3):1151–1164. [PubMed] [Google Scholar]
- 13.Fontenot JD, Gavin MA, Rudensky AY. Foxp3 programs the development and function of CD4+CD25+ regulatory T cells. Nat immunol. 2003;4(4):330–336. doi: 10.1038/ni904. [DOI] [PubMed] [Google Scholar]
- 14.Collison LW, Pillai MR, Chaturvedi V, et al. Regulatory T cell suppression is potentiated by target T cells in a cell contact, IL-35- and IL-10-dependent manner. J Immunol. 2009;182(10):6121–6128. doi: 10.4049/jimmunol.0803646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Souwer Y, Szegedi K, Kapsenberg ML, et al. IL-17 and IL-22 in atopic allergic disease. Curr Opin Immunol. 2010;22(6):821–826. doi: 10.1016/j.coi.2010.10.013. [DOI] [PubMed] [Google Scholar]
- 16.Kim YS, Choi SJ, Tae YM, et al. Distinct roles of vascular endothelial growth factor receptor-1- and receptor-2-mediated signaling in T cell priming and Th17 polarization to lipopolysaccharide-containing allergens in the lung. J Immunol. 2010;185(9):5648–5655. doi: 10.4049/jimmunol.1001713. [DOI] [PubMed] [Google Scholar]
- 17.van Weel C, Bateman ED, Bousquet J, et al. Asthma management pocket reference 2008. Allergy. 2008;63(8):997–1004. doi: 10.1111/j.1398-9995.2008.01643.x. [DOI] [PubMed] [Google Scholar]
- 18.Hamzaoui K, Hamzaoui A, Guemira F, et al. Cytokine profile in Behçet’s disease patients. Relationship with disease activity. Scand J Rheumatol. 2002;31(4):205–210. doi: 10.1080/030097402320318387. [DOI] [PubMed] [Google Scholar]
- 19.Hamzaoui K, Borhani Haghighi A, Ghorbel IB, et al. RORC and Foxp3 axis in cerebrospinal fluid of patients with neuro-Behçet’s disease. J Neuroimmunol. 2011;233(1–2):249–253. doi: 10.1016/j.jneuroim.2011.01.012. [DOI] [PubMed] [Google Scholar]
- 20.Ouyang W, Ranganath SH, Weindel K, et al. Inhibition of Th1 development mediated by GATA-3 through an IL-4-independent mechanism. Immunity. 1998;9(5):745–755. doi: 10.1016/s1074-7613(00)80671-8. [DOI] [PubMed] [Google Scholar]
- 21.Zhou L, Lopes JE, Chong MM, et al. TGF-beta-induced Foxp3 inhibits T(H)17 cell differentiation by antagonizing RORgammat function. Nature. 2008;453(7192):236–240. doi: 10.1038/nature06878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Koyasu S, Moro K. Innate Th2-type immune responses and the natural helper cell, a newly identified lymphocyte population. Curr Opin Allergy Clin Immunol. 2011;11(2):109–114. doi: 10.1097/ACI.0b013e3283448808. [DOI] [PubMed] [Google Scholar]
- 23.Hamzaoui A, Ammar J, Hamzaoui K. Regulatory T cells in induced sputum of asthmatic children: association with inflammatory cytokines. Multidiscip Respir Med. 2010;5(1):22–30. doi: 10.1186/2049-6958-5-1-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Oldenhove G, Bouladoux N, Wohlfert EA, et al. Decrease of Foxp3+ Treg cell number and acquisition of effector cell phenotype during lethal infection. Immunity. 2009;31(5):772–786. doi: 10.1016/j.immuni.2009.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Koch MA, Tucker-Heard G, Perdue NR, et al. The transcription factor T-bet controls regulatory T cell homeostasis and function during type 1 inflammation. Nat Immunol. 2009;10(6):595–602. doi: 10.1038/ni.1731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Provoost S, Maes T, van Durme YM, et al. Decreased FOXP3 protein expression in patients with asthma. Allergy. 2009;64(10):1539–1546. doi: 10.1111/j.1398-9995.2009.02056.x. [DOI] [PubMed] [Google Scholar]
- 27.Williams LM, Rudensky AY. Maintenance of the Foxp3-dependent developmental program in mature regulatory T cells requires continued expression of Foxp3. Nat Immunol. 2007;8(3):277–284. doi: 10.1038/ni1437. [DOI] [PubMed] [Google Scholar]
- 28.Dokmeci E, Xu L, Robinson E, et al. EBI3 deficiency leads to diminished T helper type 1 and increased T helper type 2 mediated airway inflammation. Immunology. 2011;132(4):559–566. doi: 10.1111/j.1365-2567.2010.03401.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhou L, Chong MM, Littman DR. Plasticity of CD4+ T cell lineage differentiation. Immunity. 2009;30(5):646–655. doi: 10.1016/j.immuni.2009.05.001. [DOI] [PubMed] [Google Scholar]
- 30.Wang Y, Souabni A, Flavell RA, et al. An intrinsic mechanism predisposes Foxp3-expressing regulatory T cells to Th2 conversion in vivo. J Immunol. 2010;185(10):5983–5992. doi: 10.4049/jimmunol.1001255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Xing J, Wu Y, Ni B. Th9: a new player in asthma pathogenesis? J Asthma. 2011;48(2):115–125. doi: 10.3109/02770903.2011.554944. [DOI] [PubMed] [Google Scholar]
- 32.Stromnes IM, Cerretti LM, Liggitt D, et al. Differential regulation of central nervous system autoimmunity by T(H)1 and T(H)17 cells. Nat Med. 2008;14(3):337–342. doi: 10.1038/nm1715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhu J, Yamane H, Paul WE. Differentiation of effector CD4 T cell populations. Annu Rev Immunol. 2010;28:445–489. doi: 10.1146/annurev-immunol-030409-101212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Deng Y, Chen W, Zang N, et al. The antiasthma effect of neonatal BCG vaccination does not depend on the Th17/Th1 but IL-17/IFN-γ balance in a BALB/c mouse asthma model. J Clin Immunol. 2011;31(3):419–429. doi: 10.1007/s10875-010-9503-5. [DOI] [PubMed] [Google Scholar]
- 35.Burgler S, Mantel PY, Bassin C, et al. RORC2 is involved in T cell polarization through interaction with the FOXP3 promoter. J Immunol. 2010;184(11):6161–6169. doi: 10.4049/jimmunol.0903243. [DOI] [PubMed] [Google Scholar]
- 36.Ayyoub M, Deknuydt F, Raimbaud I, et al. Human memory FOXP3+ Tregs secrete IL-17 ex vivo and constitutively express the T(H)17 lineage-specific transcription factor RORgamma t. Proc Natl Acad Sci U S A. 2009;106(21):8635–8640. doi: 10.1073/pnas.0900621106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Li JR, Li JG, Deng GH, et al. A common promoter variant of TBX21 is associated with allele specific binding to Yin-Yang 1 and reduced gene expression. Scand J Immunol. 2011;73(5):449–458. doi: 10.1111/j.1365-3083.2011.02520.x. [DOI] [PubMed] [Google Scholar]
- 38.Wang YH, Voo KS, Liu B, et al. A novel subset of CD4(+) T(H)2 memory/effector cells that produce inflammatory IL-17 cytokine and promote the exacerbation of chronic allergic asthma. J Exp Med. 2010;207(11):2479–2491. doi: 10.1084/jem.20101376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Moore KW, O’Garra A, de Waal Malefyt R, et al. Interleukin-10. Annu Rev Immunol. 1993;11:165–190. doi: 10.1146/annurev.iy.11.040193.001121. [DOI] [PubMed] [Google Scholar]
- 40.Rennick D, Berg D, Holland G. Interleukin-10: an overview. Prog Growth Factor Res. 1992;4(3):207–227. doi: 10.1016/0955-2235(92)90020-i. [DOI] [PubMed] [Google Scholar]