Highlights
* Examines the neural correlates of moral judgment in adolescents and adults. * Examines correlation between age and brain activity while rating the severity of moral violations. * Age was positively correlated with temporo-parietal junction and posterior cingulate activity.
Keywords: Adolescent, Adult, Moral, fMRI
Abstract
The neural mechanisms underlying moral judgment have been extensively studied in healthy adults. How these mechanisms evolve from adolescence to adulthood has received less attention. Brain regions that have been consistently implicated in moral judgment in adults, including the superior temporal cortex and prefrontal cortex, undergo extensive developmental changes from adolescence to adulthood. Thus, their role in moral judgment may also change over time. In the present study, 51 healthy male participants age 13–53 were scanned with functional magnetic resonance imaging (fMRI) while they viewed pictures that did or did not depict situations considered by most individuals to represent moral violations, and rated their degree of moral violation severity. Consistent with predictions, a regression analysis revealed a positive correlation between age and hemodynamic activity in the temporo-parietal junction when participants made decisions regarding moral severity. This region is known to contribute to mentalizing processes during moral judgment in adults and suggests that adolescents use these types of inferences less during moral judgment than do adults. A positive correlation with age was also present in the posterior cingulate. Overall, the results suggest that the brain regions utilized in moral judgment change over development.
Functional neuroimaging studies of moral judgment in adults have consistently demonstrated the critical role of several brain regions related to social and affective processing. These regions include the medial prefrontal cortex, posterior temporal cortex including the superior temporal sulcus and temporo-parietal junction, precuneus, posterior cingulate, and the anterior temporal cortex including the temporal poles and amygdala (Greene and Haidt, 2002, Moll et al., 2005). While the involvement of these regions has been demonstrated in many studies of adult moral judgment, their involvement in moral judgment during adolescence, and potential changes from adolescence to adulthood, has been less studied. This represents a significant gap in the literature, given the substantial development that occurs during adolescence in brain structure and function (Toga et al., 2006, Blakemore and Choudhury, 2006, Blakemore, 2008), and moral sensitivity and judgment (Kohlberg, 1969, Murphy and Gillligan, 1980). The goal of this study was to investigate whether the role of brain regions implicated in moral judgment in adults changes between adolescence and adulthood.
Despite the consistency of the neural circuitry underlying moral judgment in adults, the specific contributions of different regions to moral judgment is not fully understood. One region where notable progress has been made is the temporo-parietal junction/TPJ. Studies by Young and colleagues have provided strong evidence for the role of this region in mentalizing, defined as the attribution of mental states such as beliefs and intentions to others, or theory of mind (Frith and Frith, 2003), during moral judgment. Young et al. (2007) reported increased TPJ activity associated with beliefs that an individual intended to harm another, but not when harm was judged to be accidental. They also demonstrated that temporary disruption to TPJ function via transcranial magnetic stimulation led participants to judge attempted harms as less morally wrong and more morally permissible (Young et al., 2010).
Mentalizing (inferring beliefs and/or intentions in others) can be considered a specific type of perspective taking, the latter referring to the general apprehension of another's internal states. Perspective taking skills are generally considered critical in moral development (Kohlberg, 1969, Eisenberg et al., 1983, Eisenberg, 1986). Theories of moral development typically describe early stages as being characterized by hedonistic perspectives (focus on the needs of the self, e.g. avoiding punishment), then progressively integrating perspectives beyond the self (understanding and concerns for the needs and welfare of others). Studies have shown that the more one is able (and inclined) to consider the perspectives of others, the more likely they are to engage in prosocial behavior (e.g. helping others), and the less likely they are to engage in antisocial behavior (e.g. harming others)—in other words, the more likely they are to ‘act morally’ (Roberts and Strayer, 1996, Cohen and Strayer, 1996). Regarding mentalizing in particular, higher levels of performance on mentalizing tasks (false-belief) in early childhood have been shown to predict more sophisticated moral reasoning skills (more frequent references to other-oriented vs. hedonistic needs when making decisions about moral dilemmas) at a later age (Lane et al., 2010).
Mentalizing skills begin to develop in childhood, and continue to do so well into adolescence and adulthood (Dumontheil et al., 2010). This development is accompanied by extensive changes in the structure of brain regions involved in mentalizing, including the superior temporal cortex and the prefrontal cortex (Giedd et al., 1999, Gogtay et al., 2004, Sowell et al., 1999; for reviews see Toga et al., 2006, Blakemore and Choudhury, 2006, Blakemore, 2008). The developmental trajectory differs depending on the specific region; for example, gray matter density in the lateral and superior prefrontal cortex increases until the onset of puberty, followed by a decline throughout adolescence and early adulthood. The superior temporal cortex also shows an increase in gray matter density until puberty followed by a decline, but the decline is substantially more protracted, continuing well into adulthood. The posterior superior temporal cortex, in particular, matures at a relatively later age (Gogtay et al., 2004). Functional neuroimaging studies of mentalizing have also reported age-related changes in activity within these regions. Blakemore et al. (2007) found that adults and adolescents both showed increased activity in the posterior superior temporal sulcus/pSTS and adjacent temporo-parietal junction/TPJ when making attributions about intentional vs. physical causality. Relative to adolescents, adults showed increased activity in the STS. Burnett et al. (2009) found that adults showed increased activity relative to adolescents in the left temporal pole when thinking about social emotions that involved mentalizing, relative to emotions that did not.
Given that mentalizing and its underlying neural substrates undergo extensive developmental changes from adolescence to adulthood, and the demonstrated role of these brain regions in moral judgment, we hypothesized that their involvement in moral judgment would change over time. To investigate this hypothesis, we used functional magnetic resonance imaging (fMRI) to scan 51 healthy adolescent and adult males, age 13–53, as they completed a task in which they viewed three types of pictures: ‘moral’ pictures were unpleasant pictures that depicted situations considered by most people to represent moral violations (e.g. a hand breaking into a house), ‘non-moral’ pictures were unpleasant pictures that did not depict moral violations (e.g. a mutilated hand), and ‘neutral’ pictures were neither unpleasant nor pleasant and did not depict moral violations (e.g. a hand being fingerprinted) and rated the degree of moral violation severity in each picture on a scale from 1 (none) to 5 (severe). We predicted that viewing and making severity decisions about pictures depicting moral violations, relative to non-moral and neutral pictures that did not depict moral violations, would activate brain regions involved in moral judgment including the medial PFC, STS/TPJ, posterior cingulate/PCC, precuneus, and anterior temporal cortex including the amygdala and temporal poles, as we have found in our previous studies in adults (Harenski et al., 2008, Harenski et al., 2010). We further predicted that the engagement of the STS/TPJ and temporal poles in response to moral pictures would be positively correlated with age. These predictions were based on previous findings that the involvement of these regions in mentalizing changes from adolescence to adulthood (Blakemore et al., 2007, Burnett et al., 2009, Güroğlu et al., 2011, Decety et al., 2011), and our expectation that the involvement of mentalizing in moral judgment would also change from adolescence to adulthood. Although the non-moral and neutral pictures depicted similar social situations as the moral pictures and may also engage mentalizing, we did not predict significant age correlations in these conditions with for two reasons: first, prior studies have shown that STS/TPJ and temporal pole activity is stronger in response to moral relative to non-moral or neutral stimuli (Greene and Haidt, 2002, Moll et al., 2005). Second, only our moral pictures depicted intentional harm caused to others. The TPJ has been particularly implicated in making harm intent attributions (Young et al., 2007, Young et al., 2010). Thus we expected that overall STS/TPJ/temporal pole activation and age correlations would be stronger in the moral condition. Previous studies have reported positive correlations between age and activity in the anterior medial prefrontal cortex/aMPFC during mentalizing tasks (Blakemore, 2008). The task used in the present study has been shown to activate the ventromedial prefrontal cortex, but not the more dorsal aMPFC (Harenski et al., 2008, Harenski et al., 2010), thus we did not predict correlations with age in this region. Whether ventromedial prefrontal activity would be correlated with age was an open question. A recent study found no significant correlations between age and prefrontal activity during a moral judgment task; however, only an adolescent sample was examined (Eslinger et al., 2009).
Because the primary study hypothesis was that age would be positively correlated with activity in brain regions involved in mentalizing during moral judgment, it was important to ensure that the moral judgment task that we used did engage mentalizing processes. Since the task did not employ an overt manipulation of mentalizing processes, or specific instructions to consider the beliefs or intentions of individuals in the pictures, we evaluated this by conducting a pilot study with a separate group of adult participants who completed the same task outside the MRI scanner. After the task, all participants rated the extent to which they utilized a variety of cognitive and affective processes, including mentalizing, in their moral judgments. We predicted that mentalizing would be rated highly among the concepts, indicating that mentalizing contributed significantly to moral judgments. We also conducted the same pilot study with a separate group of male adolescents, to investigate whether adolescents also reported using mentalizing during moral judgment, and whether their use differed significantly from adults.
We included only male participants in the present study, for several reasons. First, studies have shown sex differences in brain development (Giedd et al., 1999, De Bellis et al., 2001), and it was not an aim of our study to compare sex differences in moral brain development. Second, we have previously reported sex differences in hemodynamic activity associated with the moral judgment task used in this study (Harenski et al., 2008). Finally, our choice of male participants was intended to facilitate comparisons to our studies in antisocial/incarcerated populations, in which the participants are mostly male.
1. Methods
1.1. Participants
Eighty-four adult and adolescent males were recruited from community advertisements. Twenty-one of these individuals were not enrolled in the study because they met one or more study exclusion criteria. These included: age greater than 55 years, less than a fourth grade reading level; IQ score less than 80; history of head injury with loss of consciousness for more than 30 min; history of major medical or neurological illness; history of seizures, current or lifetime psychotic disorder, current DSM Axis I or Axis II diagnosis,1 history of psychosis in a first degree relative; history of alcohol or drug dependence, current drug abuse. Twelve additional participants were excluded due to discomfort or claustrophobia in the MRI scanner, excessive head motion during scanning (>5 mm; five adolescents and two adults), or poor task performance (e.g. missing several ratings during the task). Analyses are presented on the remaining 51 participants which included 36 adults (age range 19–53 years, M = 27.3, SD = 8.37) and 15 adolescents (age range 13–18 years, M = 16.5, SD = 1.89). The 36 adults were further subdivided into an ‘older adult’ group (age range 27–53, M = 34.1, SD = 8.05) and a ‘younger adult’ group (age range 19–25, M = 21.7, SD = 1.91). All except one adolescent participant were right handed. All participants provided written informed consent, and the study was conducted in accordance with institutional ethical standards.
To ensure no significant differences in intelligence across age, all participants 16 years of age and older completed the Vocabulary and Matrix Reasoning subtests of the Wechsler Adult Intelligence Scale (WAIS, 1997) which was used to estimate IQ (Ryan et al., 1999). Participants 15 years of age and younger completed the same age-equivalent subtests from the Wechsler Intelligence Scale for Children (WISC, 2003). Mean (±SD) IQ scores were 119.7 (±15.6) for the adult participants and 116.0 (±15.0) for the adolescent participants. The correlation between age and IQ was not significant (r(48) = 0.07, p = 0.63). Two adolescent participants did not complete the IQ test, but had education levels that were comparable to or higher than the majority of the other adolescent participants (12th grade for both participants).
1.2. Stimuli and task
Three picture sets (25 moral, 25 non-moral, 25 neutral) were selected primarily from the International Affective Picture System (IAPS; Lang et al., 1995), and supplemented with pictures from media sources. All moral pictures depicted unpleasant social scenes indicating a moral violation (e.g. a person attacking another person, a drunk driver). Non-moral pictures depicted unpleasant social scenes without moral content (e.g. two individuals arguing, an angry driver). Neutral pictures depicted affectively neutral social scenes without moral content (e.g. two individuals having a conversation, a normal driver). Moral and non-moral pictures were a subset of those used in Harenski and Hamann (2006), and were matched on emotional arousal (based on emotional arousal ratings of pictures from three separate groups of participants: Harenski and Hamann, 2006, Harenski et al., 2008; Harenski, unpublished data). Moral, non-moral, and neutral pictures were also matched for social content by using pictures that depicted similar numbers of individuals and types of social situation (e.g. a set of moral, non-moral, and neutral pictures that involved one male interacting with one female). In addition, the participants in our previous studies who rated pictures on emotional arousal (Harenski and Hamann, 2006, Harenski et al., 2008) also rated them on the degree of social complexity that they perceived to be present in each picture. There were no significant differences in social complexity ratings across the three picture types. Matching on social content also helped ensure that there were similar numbers of faces and bodies in the different conditions, which have been shown to differentially engage the TPJ (Kret et al., 2011). The pictures can be viewed at www.mrn.org/mrt_stimuli.
Participants were informed that they would see a series of pictures depicting people and events. For each picture, they were instructed to rate the moral violation severity on a 1–5 scale, with 5 representing the highest violation severity. If no moral violation was present in the picture, the participant was instructed to give a rating of 1. Emphasis was placed on asking the participants to make ratings based on their own moral values, not what others or society would think was a moral violation. During fMRI scanning, participants completed five practice trials to ensure they understood how to perform the task. In each trial, a picture was displayed for six seconds, while the participant determined whether it represented a moral violation. Next, a rating scale was shown. The rating scale was displayed in continuous presentation format, such that a red bar began at ‘1’ (none) and progressed to ‘5’ (severe) over a period of 4 s. The participant pressed a button to stop the bar when it reached the rating they wished to give.2 This rating format was chosen for simplicity (needing to press only one button rather than several different buttons). Next, a 4-s rest period occurred during which a black screen with a white fixation cross was displayed. Moral, non-moral, and neutral picture trials were presented in a randomized order, and interspersed with ‘null’ fixation trials of the same duration as picture trials. The randomization of the null trials created variable rest periods (14, 24, or 34 s when a picture trial was followed by 1, 2, or 3 null trials, respectively). The 100 total trials (25 moral, 25 non-moral, 25 neutral, and 25 null) were presented across two separate runs. Images were rear-projected into the scanner using an LCD projector, controlled by a PC computer. Tasks were designed and presented and responses were recorded using Presentation (version 10.78, http://nbs.neuro-bs.com).
To determine the extent to which participants use mentalizing to guide moral judgments in the present task, we collected pilot data from an independent sample of 12 adult male participants (mean age = 27.5, range = 22–38) and 10 male adolescent participants (mean age = 16.7, range = 13–18) on the same task outside the MRI scanner.3 In this version of the task, the response format was changed to a Likert scale so that reaction times could be recorded. Following the task, participants were asked to rate the extent to which their moral judgments were based on six different categories: (1) Emotion (emotional responses to pictures), (2) Intentions (beliefs that individuals in the pictures were acting intentionally), (3) Law (whether the picture represented a violation of the law), (4) Empathy (feeling similar emotions to those depicted by individuals in the pictures), (5) Sympathy (feelings of concern/compassion for individuals in the pictures), and (6) Memory (being reminded of one's own personal experiences by the pictures), on a scale from 1 (not at all) to 5 (very much). A group × condition repeated measures ANOVA was conducted to determine whether any of these processes contributed significantly more to moral judgments than the others. A main effect of condition revealed a significant difference across the six rating categories (emotion, intentions, law, empathy, sympathy, memory; F(5, 95) = 12.58, p < 0.0001). Intentionality was rated the highest of all categories, and was rated significantly higher than all other categories (vs. Emotion: t(20) = 4.05, p < 0.001; vs. Law: t(20) = 4.11, p < 0.001; vs. Empathy: t(20) = 5.98, p < 0.0001; vs. Sympathy: t(20) = 3.18, p < 0.005; vs. Memory: t(20) = 8.25, p < 0.0001). Thus, the results indicated that certain types of mentalizing, particularly intentionality attributions, did guide moral judgments in the present task. Sympathy was rated second-highest, and was rated significantly higher than Empathy (t(20) = 4.56, p < 0.001) and Memory (t(20) = 4.79, p < 0.001). The latter two categories were rated lowest. There was no main effect of group nor group × condition interaction (ps > 0.25) (Table 1).
Table 1.
Usage of moral-relevant categories during moral judgments.
| Group | Emotion | Intention | Law | Empathy | Sympathy | Memory |
|---|---|---|---|---|---|---|
| Adult | 2.73 (1.10) | 4.45 (0.93) | 2.82 (1.25) | 2.91 (1.04) | 3.55 (1.13) | 2.09 (1.14) |
| Adolescent | 3.40 (1.26) | 4.20 (0.63) | 3.70 (0.95) | 2.70 (1.34) | 3.50 (1.27) | 1.80 (1.03) |
We also compared reaction times of adults and adolescents. A main effect of condition (F(2, 38) = 11.03, p < 0.0001) was found, representing longer reaction times to both moral and non-moral pictures relative to neutral pictures (t(20) = 3.17, p < 0.006; t(20) = 6.63, p < 0.0001, respectively). Reaction times did not differ significantly between moral and non-moral pictures (p = 0.88). There was a marginal group × condition interaction (F(2, 38) = 3.07, p < 0.06). Adolescents showed longer reaction times (M = 1485 ms) relative to adults (M = 1144 ms) in response to neutral pictures (t(19) = 2.10, p < 0.05). Adolescents and adults did not differ in reaction times to moral pictures (M = 1545 ms and 1578 ms, respectively; p = 0.85) or non-moral pictures (M = 1468 ms and 1685 ms, respectively; p = 0.25). The main effect of group was not significant (p = 0.21).
1.3. MRI data acquisition and analysis
MR images were collected with a mobile Siemens 1.5T Avanto with advanced SQ gradients (max slew rate 200 T/m/s; 346 T/m/s vector summation, rise time 200 μs) equipped with a 12 element head coil. The EPI gradient-echo pulse sequence (TR/TE 2000/39 ms, flip angle 90°, FOV 24 cm × 24 cm, 64 × 64 matrix, 3.4 by 3.4 mm in plane resolution, 5 mm slice thickness, 30 slices) effectively covers the entire brain (150 mm) in 2.0 s. Head motion was limited using padding and restraint. Any participant with head motion greater than 5 mm was excluded from analysis.
Functional images were analyzed using Statistical Parametric Mapping software (SPM5). Images were realigned using INRIAlign—a motion correction algorithm unbiased by local signal changes (Freire and Mangin, 2001, Freire et al., 2002). For each participant, the realignment parameters (3 translation; 3 rotations) were entered as covariates of no interest in the statistical model to regress variance due to movement. Functional images were spatially normalized to the MNI template via a 9-parameter affine transformation followed by smoothing with basis functions to account for nonlinear differences (Ashburner and Friston, 1999), and smoothed (8 mm FWHM). High frequency noise was removed using a low pass filter (cutoff – 128 s). Picture presentations (moral, non-moral, and neutral) and the rating period were modeled as separate events. The primary event of interest, picture presentation, was modeled with the standard hemodynamic response function with a 6 s duration. The rating period was modeled as one regressor for all picture ratings with a 4 s hemodynamic response function.
Functional images were computed for each participant that represented hemodynamic responses associated with viewing moral, non-moral, or neutral pictures. Linear contrasts were used to compare across conditions. Although we present results for three separate contrasts (moral > non-moral, moral > neutral, and non-moral > neutral) for completeness, the primary contrast of interest was the correlation between age and hemodynamic activity in the moral > non-moral contrast, since the moral and non-moral conditions were matched on both emotional arousal and social content. Effects that are related to processing moral stimuli should be present in both moral > non-moral and moral > neutral contrasts (meaning they are present for moral stimuli regardless of the comparison condition, which reduces the possibility that findings could be influenced by stimulus qualities that may be more salient in one of the comparison conditions). Moral related processing should be limited in the non-moral > neutral contrast, which represents primarily emotion processing. These analyses were first conducted in the adult and adolescent groups separately. Age-related activity was then examined in the entire sample by regressing participant age in the general linear model for each condition of interest.
Hemodynamic responses associated with individual ‘severity of moral violation’ ratings were also analyzed, using a parametric modulation analysis in which the participant's ratings associated with each picture were entered as covariates. This analysis determined, for each participant, whether increased activity in any brain regions during picture viewing predicted subsequent higher (or lower) violation severity ratings. This analysis was conducted in all participants, then in adolescent and adult groups separately.
Relative to the adolescent participants, the adult participants were double in number and included a wider age range. To more closely examine the nature of age-related changes during moral processing in regions of interest over time, we also examined differences between the older adult, younger adults, and adolescents using a 3 (older adult/younger adult/adolescent) × 3 (moral/non-moral/neutral) ANOVA in SPM5. Post hoc analyses were performed to identify effects specific to each group (e.g. a 2 (adolescent) − 1 (younger adult) − 1 (older adult) contrast in the moral condition).
For all analyses, small volume corrections were applied to regions of interest by creating 10-mm spheres with center coordinates derived from a prior study in an independent adult sample (Harenski et al., 2008), and thresholding the results at 0.05, FWE corrected, in SPM5. ROIs included the ventromedial PFC, bilateral posterior STS/TPJ, PCC, precuneus, bilateral anterior temporal cortex and amygdala.4
Post hoc whole-brain analyses were also conducted for each group and condition of interest. These latter results were thresholded at p < 0.001 uncorrected with an extent threshold of 37 contiguous voxels. The threshold was determined based on Monte Carlo simulation using the AlphaSim program written by D. Ward in AFNI software (http://afni.nimh.nih.gov/).
SPMs were overlaid on a representative high-resolution structural T1-weighted image from a single subject from the SPM5 canonical image set, coregistered to Montreal Neurological Institute (MNI) space. All coordinates are reported in MNI space.
2. Results
2.1. Severity of moral violation ratings
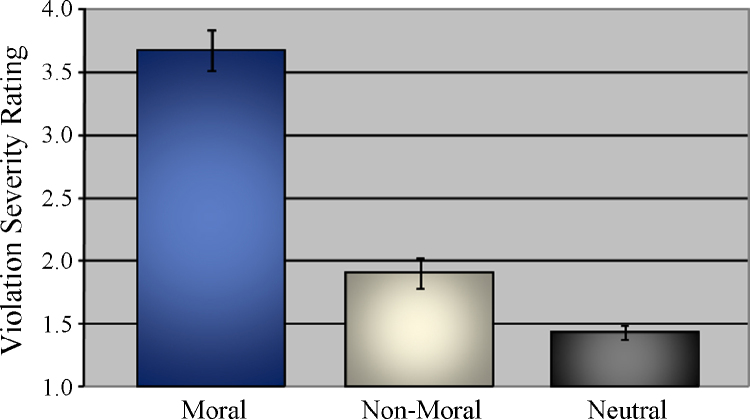
Prior to investigating the brain areas engaged during moral judgment across age, it was important to evaluate the behavioral ratings of pictures made by adolescent and adult participants. This ensures that any associations between brain activity and age are not due to differences in explicit judgments regarding the severity of moral violation in each picture condition. Adult and adolescent participants rated moral pictures (M = 3.68, SD = 0.60) higher on violation severity than non-moral (M = 1.90, SD = 0.48; F(1, 50) = 608.31, p < 0.0001) and neutral (M = 1.43, SD = 0.22; F(1, 50) = 721.19, p < 0.0001) pictures (Fig. 1). Non-moral pictures were also rated higher than neutral pictures (F(1, 50) = 80.46, p < 0.0001). This may reflect the fact that participants occasionally over-interpret what is represented by the non-moral pictures (e.g. if someone is in distress, another person must have caused it). There were no significant correlations between age and ratings in any condition (all ps > 0.30), nor between age and difference ratings between conditions (moral–non-moral, moral–neutral, non-moral–neutral; all ps > 0.15) indicating that adults and adolescents were similarly able to identify moral violations and rate their severity.
Fig. 1.
Moral violation severity ratings by condition.
2.2. Brain activity during moral picture viewing
In our prior studies with adult male participants (Harenski et al., 2008, Harenski et al., 2010), we compared violation severity judgments of moral and non-moral pictures and found that brain areas associated with social and affective processing, including the medial prefrontal cortex, bilateral pSTS/TPJ, PCC, precuneus, anterior temporal cortex and amygdala, showed greater activity during judgments of moral pictures. The present results, which showed increased activity in nearly all of these regions in adult and adolescent participants (Table 2), are consistent with these findings. We also compared brain activity during violation severity judgments of moral relative to neutral pictures. In adults, greater activity to morally salient pictures was found in the bilateral pSTS/TPJ, MPFC, PCC, precuneus, and inferior frontal gyrus/IFG. Adolescents also showed increased activity in the IFG, precuneus, and MPFC. Other regions showing increased activity during moral relative to non-moral and neutral picture viewing across groups are listed in Table 2.
Table 2.
Brain regions showing increased activity during moral picture viewing.
| Contrast | Region (BA) | MNI coordinates |
FWE | Cluster size | |||
|---|---|---|---|---|---|---|---|
| x | y | z | t | ||||
| Moral > non-moral | Adult (N = 36) | ||||||
| *L. PCC/precuneus (31) | −3 | −54 | 30 | 10.18 | <0.001 | 171 | |
| *L. STS/TPJ (39) | −48 | −66 | 24 | 9.72 | <0.001 | 144 | |
| *L. Ventral MPFC (10) | −3 | 54 | 6 | 8.68 | <0.001 | 171 | |
| *L. PCC (31) | −12 | −54 | 18 | 7.83 | <0.001 | 165 | |
| *R. TPJ (39) | 48 | −66 | 30 | 7.65 | <0.001 | 101 | |
| *L. Amygdala/parahippocampal gyrus | −18 | −9 | −21 | 3.97 | 0.015 | 65 | |
| L. Superior PFC (8) | −21 | 30 | 51 | 7.98 | 171 | ||
| R. Occipital cortex (19) | 48 | −72 | 3 | 7.85 | 1316 | ||
| R. Cerebellum | 9 | −54 | −45 | 5.87 | 49 | ||
| R. Middle frontal gyrus (8) | 27 | 33 | 51 | 4.67 | 62 | ||
| R. Middle temporal gyrus (21) | 63 | −24 | −15 | 4.48 | 66 | ||
| Adolescent (N = 15) | |||||||
| *L. PCC/parahippocampal gyrus (30) | −15 | −48 | 6 | 7.83 | <0.001 | 89 | |
| *L. Ventral MPFC (10) | −9 | 48 | 0 | 7.07 | <0.001 | 158 | |
| *R. TPJ (39) | 48 | −66 | 27 | 6.64 | <0.001 | 94 | |
| *L. STS/TPJ (39) | −57 | −54 | 21 | 6.58 | <0.001 | 145 | |
| *R. Precuneus (7) | 3 | −60 | 33 | 6.08 | 0.002 | 137 | |
| L. Occipital cortex (19) | −48 | −81 | 3 | 6.30 | 44 | ||
| Moral > neutral | Adult (N = 36) | ||||||
| *L. PCC/precuneus (31) | −3 | −57 | 30 | 8.51 | <0.001 | 171 | |
| *L. MPFC (10) | −3 | 57 | 15 | 8.47 | <0.001 | 171 | |
| *L. STS/TPJ (39) | −48 | −66 | 24 | 6.14 | <0.001 | 136 | |
| *L. PCC (29) | −9 | −48 | 15 | 6.08 | <0.001 | 82 | |
| *R. TPJ (39) | 45 | −66 | 33 | 5.77 | <0.001 | 96 | |
| *R. Amygdala/parahippocampal gyrus | 27 | 6 | −21 | 3.87 | 0.018 | 27 | |
| *L. Amygdala | −18 | −6 | −18 | 3.77 | 0.023 | 29 | |
| R. Occipital cortex (19)/cerebellum | 45 | −72 | 0 | 11.56 | 1279 | ||
| R. Cerebellum | 6 | −54 | −45 | 5.23 | 74 | ||
| R. Inferior frontal gyrus (47) | 51 | 33 | 3 | 5.18 | 48 | ||
| R. Superior frontal gyrus (6) | 24 | 0 | 54 | 5.11 | 112 | ||
| L. Precuneus (7) | 15 | −60 | 63 | 4.84 | 85 | ||
| L. Cerebellum | −45 | −72 | −27 | 4.79 | 45 | ||
| L. Cerebellum | −12 | −90 | −30 | 4.52 | 47 | ||
| Adolescent (N = 15) | |||||||
| L. Superior frontal gyrus (6) | −3 | 18 | 63 | 8.37 | 52 | ||
| R. Middle temporal gyrus (37) | 51 | −66 | 3 | 7.46 | 98 | ||
| L. Occipital cortex (19) | −48 | −84 | 3 | 6.06 | 53 | ||
| L. Inferior frontal gyrus (47) | −33 | 18 | −15 | 5.35 | 43 | ||
| L. Precuneus (7) | −18 | −57 | 66 | 5.09 | 62 | ||
| L. Medial frontal gyrus (9) | −3 | 54 | 33 | 4.77 | 56 | ||
| Non-moral > neutral | Adult (N = 36) | ||||||
| *R. Amygdala | 24 | −3 | −21 | 3.80 | 0.022 | 58 | |
| R. Inferior frontal gyrus (45) | 48 | 36 | 3 | 6.05 | 141 | ||
| L. Inferior frontal gyrus (46) | −45 | 39 | 3 | 5.73 | 361 | ||
| R. Lingual gyrus (18) | 18 | −87 | −3 | 5.51 | 185 | ||
| R. Caudate | 12 | 12 | 0 | 4.91 | 62 | ||
| Adolescent (N = 15) | |||||||
| *R. Amygdala/parahippocampal gyrus | 36 | −6 | −18 | 4.42 | 0.037 | 65 | |
| L. Inferior frontal gyrus (46) | −48 | 33 | 9 | 8.60 | 44 | ||
| R. Inferior frontal gyrus (47) | 48 | 45 | 9 | 6.22 | 43 | ||
| R. Middle temporal gyrus (37) | 48 | −63 | −3 | 5.12 | 38 | ||
BA: Brodmann area. FWE: small volume corrected values listed for regions of interest (denoted with a *). Other regions listed are significant at a whole brain threshold of p < 0.001, uncorrected, extent threshold 37 contiguous voxels.
2.3. Correlation with age
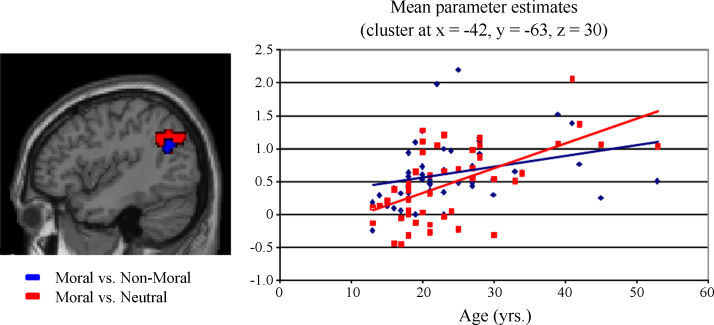
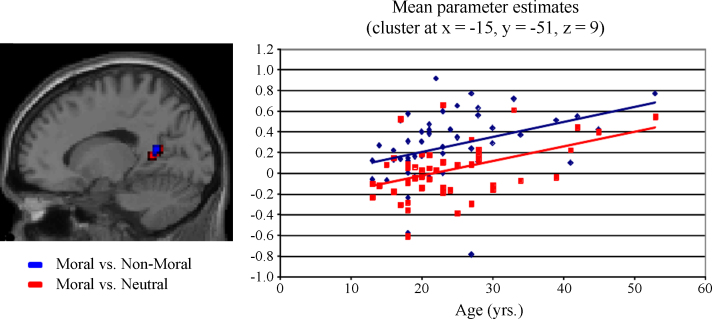
To investigate whether the engagement of brain regions associated with moral judgment changes across age, participant age was entered as a regressor for the contrasts described above. As predicted, a significant positive correlation with age was present in the left pSTS/TPJ during moral relative to non-moral and neutral picture viewing (p < 0.05, corrected, Table 3, Fig. 2). A marginal positive correlation was also present in the right TPJ during moral relative to neutral picture viewing (p < 0.07, corrected). A positive correlation with left pSTS/TPJ was also present during non-moral relative to neutral picture viewing, but at a reduced statistical threshold (p < 0.005, uncorrected). The PCC also showed a positive correlation between activity and age in the moral relative to non-moral and neutral contrasts (p < 0.05, corrected; Table 3, Fig. 3). No significant negative correlations with age were present.
Table 3.
Brain regions correlated with age during moral picture viewing.
| Contrast | Region (BA) | MNI coordinates |
FWE | Cluster size | |||
|---|---|---|---|---|---|---|---|
| x | y | z | t | ||||
| Moral > non-moral | Positive | ||||||
| *L. PCC (30) | −15 | −51 | 12 | 3.45 | 0.035 | 59 | |
| *L. STS/TPJ (39) | −45 | −60 | 24 | 3.30 | 0.050 | 15 | |
| Negative | |||||||
| — | |||||||
| Moral > neutral | Positive | ||||||
| *L. STS/TPJ (39) | −45 | −63 | 27 | 4.49 | 0.002 | 129 | |
| *L. PCC (30) | −15 | −51 | 9 | 3.58 | 0.025 | 102 | |
| *R. TPJ (39) | 45 | −63 | 36 | 3.14 | 0.066 | 66 | |
| Negative | |||||||
| — | |||||||
| Non-moral > neutral | Positive | ||||||
| — | |||||||
| Negative | |||||||
| — | |||||||
BA: Brodmann area. FWE: small volume corrected values listed for regions of interest (denoted with a *).
Fig. 2.
Correlation between age and pSTS/TPJ activation.
Fig. 3.
Correlation between age and PCC activation.
2.4. Comparisons across age groups
The results of the ANOVA comparing older adults, younger adults, and adolescents revealed a group × condition interaction in right TPJ and left PCC. Post hoc analyses showed that bilateral TPJ activity was significantly greater in older adults compared to younger adults and adolescents during moral (but not non-moral or neutral) picture viewing. Activity in the PCC was significantly greater in older and younger adults compared to adolescents during moral (but not non-moral or neutral) picture viewing. For all brain regions showing differential activity across the three groups, see Table 4.
Table 4.
Brain regions showing increased activity by age group.
| Region (BA) | MNI coordinates |
FWE | Cluster size | ||||
|---|---|---|---|---|---|---|---|
| x | y | z | F | ||||
| Main effect of age | R. Middle Temporal Gyrus (39) | 39 | −72 | 12 | 11.95 | 46 | |
| R. Lingual Gyrus (17) | 15 | −90 | −6 | 10.85 | 40 | ||
| R. Precuneus (7) | 21 | −60 | 54 | 9.21 | 68 | ||
| Age group × condition interaction | *L. PCC (29) | −6 | −45 | 12 | 3.65 | 0.005 | 31 |
| *R. TPJ (39) | 45 | −63 | 36 | 5.02 | 0.058 | 31 | |
| Region (BA) | MNI coordinates |
FWE | Cluster size | ||||
|---|---|---|---|---|---|---|---|
| x | y | z | t | ||||
| Older adult vs. younger adult + adolescent | Moral | ||||||
| *R. TPJ (39) | 45 | −63 | 36 | 3.08 | 0.057 | 92 | |
| *R. ATC (21) | 57 | 0 | −18 | 2.97 | 0.073 | 58 | |
| *L. STS/TPJ (39) | −45 | −72 | 21 | 2.96 | 0.075 | 53 | |
| *L. PCC (29) | −3 | −60 | 15 | 2.95 | 0.076 | 125 | |
| R. PCC (31) | 3 | −42 | 33 | 4.73 | 186 | ||
| R. Precuneus (7) | 18 | −57 | 48 | 4.21 | 318 | ||
| 27 | −63 | 27 | 4.14 | 57 | |||
| L. Middle frontal gyrus (8) | −33 | 30 | 45 | 4.19 | 50 | ||
| L. Superior parietal cortex (7) | −27 | −66 | 57 | 3.98 | 46 | ||
| Non-moral | |||||||
| R. Medial frontal gyrus (6) | 18 | 3 | 63 | 3.92 | 40 | ||
| R. Precuneus (7) | 18 | −60 | 51 | 3.55 | 59 | ||
| Neutral | |||||||
| R. Precuneus | 21 | −60 | 51 | 4.36 | 196 | ||
| 27 | −66 | 27 | 4.36 | 50 | |||
| L. Precentral gyrus (6) | −48 | −3 | 48 | 4.22 | 49 | ||
| R. Middle frontal gyrus (6) | 21 | 6 | 66 | 4.04 | 46 | ||
| L. Superior parietal cortex (7) | −30 | −63 | 60 | 3.87 | 164 | ||
| L. Paracentral lobule (5) | −3 | −39 | 54 | 3.64 | 42 | ||
| Younger adult + adolescent vs. older adult | No significant group differences across conditions | ||||||
| Older adult + younger adult vs. adolescent | Moral | ||||||
| *L STS/TPJ (39) | −51 | −69 | 18 | 3.78 | 0.009 | 85 | |
| *L. PCC (29) | 3 | −57 | 9 | 3.41 | 0.025 | 143 | |
| *R. ATC (21) | 60 | −3 | −12 | 3.43 | 0.023 | 44 | |
| R. Middle temporal gyrus (39) | 45 | −60 | 6 | 4.73 | 81 | ||
| R. PCC (23) | 6 | −45 | 24 | 4.61 | 245 | ||
| R. Superior temporal gyrus (41) | 45 | −33 | 0 | 4.58 | 127 | ||
| L. Anterior cingulate (32) | −6 | 15 | 45 | 4.15 | 138 | ||
| L. Middle temporal gyrus (39) | −48 | −72 | 9 | 4.09 | 54 | ||
| R. Occipital cortex (19) | 36 | −69 | −12 | 3.99 | 130 | ||
| L. Putamen | −24 | 9 | 3 | 3.89 | 37 | ||
| L. Lingual gyrus (18) | −9 | −78 | −12 | 3.88 | 43 | ||
| R. Superior parietal cortex | 36 | −60 | 51 | 3.77 | 47 | ||
| Non-moral | |||||||
| *L STS/TPJ (39) | −51 | −69 | 18 | 3.09 | 0.054 | 22 | |
| R. Middle temporal gyrus (39) | 42 | −57 | 6 | 4.59 | 61 | ||
| R. Superior temporal gyrus (22) | 48 | −36 | 3 | 4.00 | 60 | ||
| Neutral | |||||||
| *L STS/TPJ (39) | −51 | −69 | 18 | 2.84 | 0.096 | 21 | |
| R. Middle temporal gyrus (39) | 39 | −72 | 15 | 4.41 | 51 | ||
| Adolescent vs. younger adult + older adult | No significant group differences across conditions | ||||||
| Older adult + adolescent vs. younger adult | No significant group differences across conditions | ||||||
| Younger adult vs. older adult + adolescent | Moral | ||||||
| L. Parahippocampal gyrus (19) | −33 | −54 | −9 | 5.55 | 477 | ||
| R. Superior temporal gyrus (41) | 48 | −39 | 9 | 4.11 | 38 | ||
| Non-moral | |||||||
| L. Fusiform gyrus (19) | −36 | −72 | −12 | 4.62 | 60 | ||
| R. Lingual gyrus (17) | 15 | −90 | −6 | 4.32 | 184 | ||
| R. Superior temporal gyrus (41) | 48 | −39 | 6 | 4.13 | 41 | ||
| Neutral | |||||||
| R. Lingual gyrus (17) | 15 | −90 | −6 | 4.26 | 85 | ||
BA: Brodmann area. FWE: small volume corrected values listed for regions of interest (denoted with a *). Other regions listed are significant at a whole brain threshold of p < 0.001, uncorrected, extent threshold 37 contiguous voxels.
2.5. Brain activity associated with moral severity ratings
The results of the parametric modulation analysis, which evaluated associations between individual moral severity ratings and brain activity, did not reveal any significant differences in moral severity rating-brain activity associations in regions of interest between adults and adolescents.
3. Discussion
The present study tested the hypothesis that brain regions implicated in mentalizing would show increased engagement during moral judgment from adolescence to adulthood. Previous studies have shown that brain regions associated with mentalizing and moral judgment, particularly the anterior and posterior temporal cortex, show less engagement during mentalizing tasks in adolescents relative to adults. Here we directly investigated the engagement of these brain regions in adolescents and adults during a moral judgment task. In accordance with hypotheses, age was positively correlated with hemodynamic activity in the pSTS/TPJ while participants made severity decisions about pictures depicting moral violations. A similar pattern was found in the PCC. These results suggest that the involvement of specific brain regions and their associated functions in moral judgment changes from adolescence to adulthood.
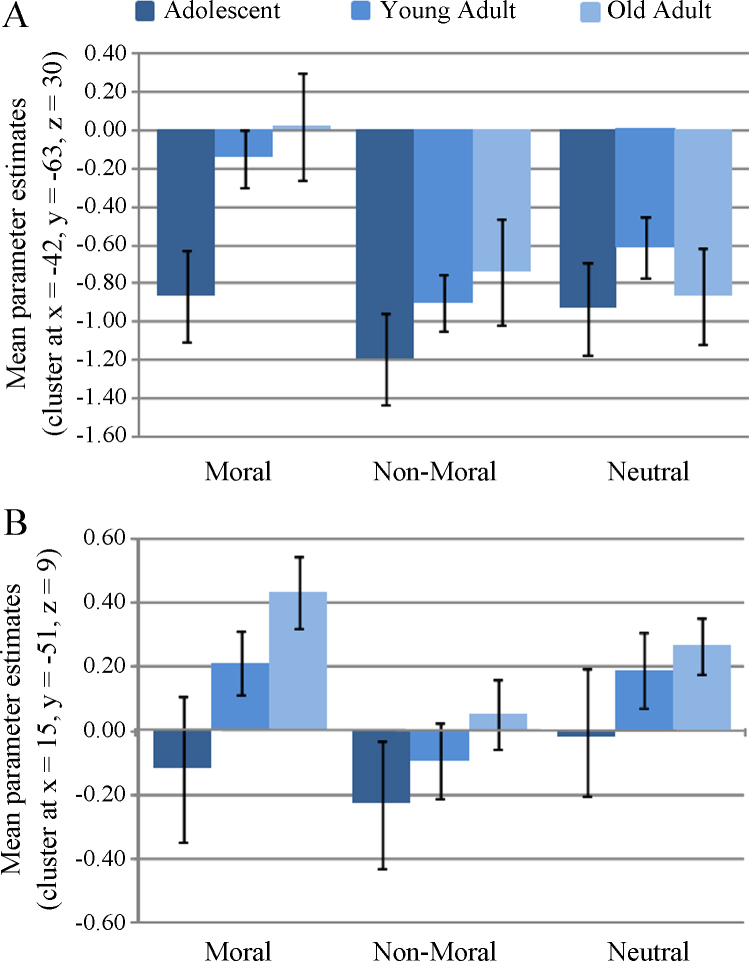
Making decisions about the violation severity of pictures depicting moral violations was associated with increased pSTS/TPJ activity in adolescents and adults; and the magnitude of activity was positively correlated with age. This region has been implicated in belief and intentionality attributions related to moral judgment in adults (Young et al., 2007, Young et al., 2010). The present results may indicate that adolescents, relative to adults, use this type of mentalizing less during moral judgment. Beliefs and intentions may be progressively integrated into moral judgments over time. Although the role of the pSTS/TPJ in making intentionality attributions during moral judgment has been well demonstrated, studies have also highlighted other functions of this region. For example, the right TPJ has been implicated in empathy, sense of agency and self-other discrimination, as well in redirecting attention to task-relevant stimuli (Decety and Lamm, 2007, Mitchell, 2008). Decety and Lamm (2007) proposed that rTPJ is associated with a variety of lower-level processes (e.g. redirection of attention) that contribute to higher level functions such as mentalizing or empathy. Regarding empathy, the results of our pilot study showed that empathy was ranked quite low compared to intentionality in its importance in making moral judgments, suggesting it was less (consciously) utilized by both adults and adolescents. Attentional redirection may be related to TPJ function in the context of moral judgment, though this has not specifically studied to our knowledge, nor have studies shown that these processes are critically related to moral development. Self-other discriminations are highly related to mentalizing, and our TPJ findings might relate more generally to adolescent vs. adults’ tendency to distinguish between self and others during moral judgment. Overall, given the demonstrated association between mentalizing and moral development, adult moral judgment, and TPJ function, we consider mentalizing, intentionality attributions in particular, to be the most parsimonious interpretation of the correlation between age and pSTS/TPJ activity during moral judgment (Fig. 4).
Fig. 4.
pSTS/TPJ (A) and PCC (B) activation by condition and age group.
We also found a positive correlation between age and activity in the PCC during moral picture processing. Unlike the finding in the pSTS/TPJ, this region has not typically been implicated in previous studies of mentalizing. The role of this region in moral judgment is also not well understood. It is possible that adolescents are less sensitive to certain aspects of moral content that engage the PCC. The PCC has been linked to emotional and self-reflective processing, which may contribute to moral judgments (Fink et al., 1996, Maddock, 1999, Damasio et al., 2000, Vogt and Laureys, 2005, Johnson et al., 2006). The PCC result may appear at first to be at odds with findings from a study which found that adolescents showed increased activity in this region during the evaluation of moral dilemmas (Pujol et al., 2008). However, this study included only adolescent participants and did not compare adolescents to adults. The adolescents in our study did activate this region in response to moral pictures, but the degree of activity significantly increased with age.
Although both TPJ and PCC activity increased with age during moral picture processing, a closer examination of age groups revealed different patterns of increased activity within each region. Whereas right TPJ activity was greater in older adults relative to younger adults and adolescents, PCC activity was greater in both older adults and younger adults relative to adolescents. This suggests different developmental trajectories related to moral processing in each region. PCC activity appears to increase during moral processing by young adulthood, whereas increased right TPJ engagement during moral processing appears to occur later in adulthood.
Our study hypotheses were based on previous functional imaging studies of mentalizing in adolescents relative to adults. Although our finding of a positive correlation between age and activity in the STS/TPJ is somewhat consistent with the finding by Blakemore et al. (2007) that adolescents showed less activity than adults in this region during a mentalizing task, the latter result occurred in a region of STS that was well inferior to the one which we observed, which highly overlapped with the TPJ. A possible reason for this difference is the type of mentalizing that is involved in a particular task. For example, the task used in Blakemore et al. (2007) involved making inferences about intention and causality in a variety of situations, such as changing seats in a movie theatre in order to have a better view of the screen. In contrast, the task used in adult moral judgment studies by Young et al., 2007, Young et al., 2010 involved making inferences regarding a person's belief about causing harm to another person. The TPJ region activated in the Young et al. studies more closely resembles the one we observed, likely reflecting more similarity in the types of inferences made in this task (i.e. beliefs about causing harm) and our task. Differences in the type of mentalizing might also explain why we did not observe correlations between age and other brain regions which have previously shown age-related changes in mentalizing, such as the anterior medial prefrontal cortex/aMPFC (Blakemore et al., 2007, van den Bos et al., 2011). This region is involved in self-referential processing related to mentalizing (e.g. imagining what other people think of oneself; Amodio and Frith, 2006, Frith and Frith, 2008). Thus, the involvement of these processes in moral judgment may change less between adolescence and adulthood. It is also possible our moral task does not engage the specific self-referential processes related to the aMPFC (the present study and prior studies using this task typically find more ventral mPFC activity), which is why no significant correlation was present.
A recent study (Decety et al., 2011) reported increased activity with age in the ventromedial prefrontal cortex/vmPFC in response to pictures showing intentional physical harm caused to a person compared to accidental harm. In the present study we did not find evidence that vmPFC activity in response to moral violations increased with age. This may be explained by several differences between our study and Decety et al. (2011). The participants in Decety et al. (2011) included young children, whereas our participants were adolescents and adults only. It is possible that we would have observed vmPFC differences if young children were included in the study. Also, unlike Decety et al. (2011), our study included male participants only. Perhaps most importantly, the participants in Decety et al. (2011) did not make moral judgments of the pictures during scanning. We have previously shown differential vmPFC activity during implicit (no moral judgment) vs. explicit (moral judgment) moral picture processing (Harenski et al., 2010). It is also worth noting that the pictures used in Decety et al. (2011) involved direct physical harm caused to a person, whereas our pictures varied from direct physical harm (e.g. physical assault) to implied harm or potential for harm (e.g. a woman smoking while pregnant). Their pictures also showed bodily actions but not faces, whereas nearly all of our pictures showed faces and facial expressions. Whether these and/or other factors influence the involvement of the vmPFC, or other regions, in moral judgment over age are important considerations for future research.
A possible alternate explanation for reduced TPJ and PCC activity in adolescent participants is that they found the moral rating task more difficult than the adults. To evaluate the relative task difficulty experienced by adolescents and adults, it could be informative to compare average reaction times across groups. Our task design does not allow for the recording of meaningful reaction time data, because reaction time is determined by the chosen rating. However, as noted earlier, the adults and adolescents in our pilot study did not show significant differences in reaction times to moral (or non-moral) pictures. Thus, it is unlikely that our results reflect greater task difficulty and longer response times for adolescents compared to adults.
Although we ensured that moral pictures did not differ from non-moral pictures on variables such as general emotional arousal and social content, it is possible that moral pictures differed on other types of variables that were not specifically examined. For example, five of the 25 moral pictures represented historical events, such as the 9/11 attacks. Consistent with the group results, the ratings of those individual pictures were not positively correlated with age, indicating that adolescent participants perceived a similar degree of moral salience in those pictures as did the adults. Nonetheless, the age at which these events were encoded differs across participants. While this only impacts a few of the moral pictures used in the present study, the question of how brain activity may vary as a function of the age that morally salient events are encoded is an interesting avenue for future research.
The present study was conducted in males only. As noted earlier, gender differences have been found in brain development (Giedd et al., 1999, De Bellis et al., 2001). For example, frontal and parietal gray matter peak 1 year earlier in females relative to males (Giedd et al., 1999). We have previously reported gender differences in brain activity during moral judgment (Harenski et al., 2008). For example, males show a stronger correlation between TPJ activity and moral severity ratings relative to females. In our pilot study, adult males reported a relatively stronger reliance on intentionality attributions during moral judgments compared to adult females (intentionality being rated significantly higher than all five categories in males, compared to three out of five categories in females). Thus, correlations between age and TPJ activity during moral judgment might not be as strong in females relative to males. Overall, it will be important to examine age effects in females, and in females relative to males, in future studies.
In summary, the findings of the present study demonstrate that the engagement of brain regions implicated in adult moral judgment, including the pSTS/TPJ and PCC, increases with age. These findings suggest that from adolescence to adulthood individuals progressively integrate more information about the mental states of others, such as intentionality, into moral judgments. It should be noted that the present results may not necessarily generalize to different moral judgment tasks, such as evaluating complex moral dilemmas. This is a question for future research. Overall, the results indicate that the neural correlates of moral judgment change from adolescence to adulthood.
Acknowledgments
We thank Katherine Tremba and Kristin Macias for assistance with data collection and management, and our anonymous reviewers for helpful suggestions. This work was supported by NIMH grants R01 MH071896-01 and R01 MH070539-01 and NIDAR01 DA026505-01 to K. Kiehl.
Footnotes
If the participant was not taking medication for the disorder, had been symptom free for more than a year, and did not have a recurrent history, they were included in the study.
The continuous presentation format of the rating scale could affect the ratings of individuals who did not fully attend to the stimuli. In other words, a higher rating could be given because the participant was slow to respond rather than because they intended to give a high violation severity rating. To address this issue, responses were not accepted after the bar reached ‘5’. If a participant was indeed not paying attention during the task, they should have many ‘missed’ ratings. Participants who had multiple missed ratings (more than 5 out of the 75 pictures) were excluded from analysis (N = 1).
Data from 12 adult females were also collected; however because only males were included in the current study and in the adolescent pilot study, we report results from the adult males only.
Activations in ATC and right amygdala were not reported in Harenski et al. (2008), but were present at a statistical threshold lower than the one that was utilized (p < 0.01 vs. p < 0.005).
References
- Amodio D.M., Frith C.D. Meeting of minds: the medial frontal cortex and social cognition. Nature Reviews Neuroscience. 2006;4:268–277. doi: 10.1038/nrn1884. [DOI] [PubMed] [Google Scholar]
- Ashburner J., Friston K.J. Nonlinear spatial normalization using basis functions. Human Brain Mapping. 1999;7:254–266. doi: 10.1002/(SICI)1097-0193(1999)7:4<254::AID-HBM4>3.0.CO;2-G. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blakemore S.-J., Ouden H.D., Choudhury S., Frith C. Adolescent development of the neural circuitry for thinking about intentions. Social Cognitive and Affective Neuroscience. 2007;2:130–139. doi: 10.1093/scan/nsm009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blakemore S.-J., Choudhury S. Development of the adolescent brain: implications for executive function and social cognition. Journal of Child Psychology and Psychiatry. 2006;47:296–312. doi: 10.1111/j.1469-7610.2006.01611.x. [DOI] [PubMed] [Google Scholar]
- Blakemore S.-J. The social brain in adolescence. Nature Reviews Neuroscience. 2008;9:267–277. doi: 10.1038/nrn2353. [DOI] [PubMed] [Google Scholar]
- Burnett S.G., Bird G., Moll J., Frith C., Blakemore S.-J. Development during adolescence of the neural processing of social emotion. Journal of Cognitive Neuroscience. 2009;21:1736–1750. doi: 10.1162/jocn.2009.21121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen D., Strayer J. Empathy in conduct-disordered and comparison youth. Developmental Psychology. 1996;32:988–998. [Google Scholar]
- Damasio A.R., Grabowski T.J., Bechara A., Damasio H., Ponto L.L.B., Parvizi J., Hichwa R.D. Subcortical and cortical brain activity during the feeling of self-generated emotions. Nature Neuroscience. 2000;3:1049–1056. doi: 10.1038/79871. [DOI] [PubMed] [Google Scholar]
- De Bellis M.D., Keshavan M.S., Beers S.R. Sex differences in brain maturation during childhood and adolescence. Cerebral Cortex. 2001;11:552–557. doi: 10.1093/cercor/11.6.552. [DOI] [PubMed] [Google Scholar]
- Decety J., Lamm C. The role of the right temporoparietal junction in social interaction: How low-level computational processes contribute to meta-cognition. The Neuroscientist. 2007;13:580–593. doi: 10.1177/1073858407304654. [DOI] [PubMed] [Google Scholar]
- Decety J., Michalska K., Kinzler K. The contribution of emotion and cognition to moral sensitivity: a neurodevelopmental study. Cerebral Cortex. 2011 doi: 10.1093/cercor/bhr111. (Epub ahead of print) [DOI] [PubMed] [Google Scholar]
- Dumontheil I., Apperly I.A., Blakemore S.J. Online usage of theory of mind continues to develop in late adolescence. Developmental Science. 2010;13:331–338. doi: 10.1111/j.1467-7687.2009.00888.x. [DOI] [PubMed] [Google Scholar]
- Eisenberg N. Erlbaum; Hillsdale, NJ: 1986. Altruistic Emotion, Cognition, and Behavior. [Google Scholar]
- Eisenberg N., Lennon R., Roth K. Prosocial development: a longitudinal study. Developmental Psychology. 1983;19:846–855. [Google Scholar]
- Eslinger P.J., Robinson-Long M., Realmuto J., Moll J., deOliveira-Souza R., Tovar-Moll F. Developmental frontal lobe imaging in moral judgment: Arthur Benton's enduring influence 60 years later. Journal of Clinical and Experimental Neuropsychology. 2009;31(2):158–169. doi: 10.1080/13803390802298064. [DOI] [PubMed] [Google Scholar]
- Fink G.R., Markowitsch H.J., Reinkemeier M., Bruckbauer T., Kessler J., Heiss W.D. Cerebral representation of one's own past: neural networks involved in autobiographical memory. Journal of Neuroscience. 1996;16:4275–4292. doi: 10.1523/JNEUROSCI.16-13-04275.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freire L., Mangin J.F. Motion correction algorithms may create spurious brain activations in the absence of subject motion. Neuroimage. 2001;14:709–722. doi: 10.1006/nimg.2001.0869. [DOI] [PubMed] [Google Scholar]
- Freire L., Roche A., Mangin J.F. What is the best similarity measure for motion correction in fMRI time series? IEEE Transactions on Medical Imaging. 2002;21:470–484. doi: 10.1109/TMI.2002.1009383. [DOI] [PubMed] [Google Scholar]
- Frith C.D., Frith U. Implicit and explicit processes in social cognition. Neuron. 2008;60:503–510. doi: 10.1016/j.neuron.2008.10.032. [DOI] [PubMed] [Google Scholar]
- Frith U., Frith C.D. Development and neurophysiology of mentalizing. Philosophical Transactions of the Royal Society of London Series B: Biological Sciences. 2003;358:459–473. doi: 10.1098/rstb.2002.1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giedd J.N., Blumenthal J., Jeffries N.O. Brain development during childhood and adolescence: a longitudinal MRI study. Nature Neuroscience. 1999;2:861–863. doi: 10.1038/13158. [DOI] [PubMed] [Google Scholar]
- Gogtay N., Giedd J.N., Lusk L. Dynamic mapping of human cortical development during childhood through early adulthood. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:8174–8179. doi: 10.1073/pnas.0402680101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greene J., Haidt J. How (and where) does moral judgment work? Trends in Cognitive Sciences. 2002;6:517–523. doi: 10.1016/s1364-6613(02)02011-9. [DOI] [PubMed] [Google Scholar]
- Güroğlu, B., van den Bos, W., van Dijk, E., Rombouts, S.A.R.B., Crone, E.A., 2011. Dissociable brain networks involved in development of fairness considerations: understanding intentionality behind unfairness. Neuroimage, doi:10.1016/j.neuroimage.2011.04.032 in press. [DOI] [PubMed]
- Harenski C.L., Antonenko O., Shane M.S., Kiehl K.A. A functional imaging investigation of moral deliberation and moral intuition. Neuroimage. 2010;49:2707–2716. doi: 10.1016/j.neuroimage.2009.10.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harenski C.L., Antonenko O., Shane M., Kiehl K. Gender differences in neural mechanisms underlying moral sensitivity. Social, Cognitive, and Affective Neuroscience. 2008;3(4):313–321. doi: 10.1093/scan/nsn026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harenski C.L., Hamann S. Neural correlates of regulating negative emotions related to moral violations. Neuroimage. 2006;30:313–324. doi: 10.1016/j.neuroimage.2005.09.034. [DOI] [PubMed] [Google Scholar]
- Johnson M.K., Raye C.L., Mitchell K.M., Touryan S.R., Greene E.J., Nolen-Hoeksema S. Dissociating medial frontal and posterior cingulate activity during self reflection. Social, Cognitive, and Affective Neuroscience. 2006;1:56–64. doi: 10.1093/scan/nsl004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohlberg L. Stage and sequence: the cognitive-developmental approach to socialization. In: Goslin D.A., editor. Handbook of Socialization Theory and Research. Academic Press; New York: 1969. pp. 151–235. [Google Scholar]
- Kret M.E., Pichon S., Grèzes J., de Gelder B. Similarities and differences in perceiving threat from dynamic faces and bodies. An fMRI study. Neuroimage. 2011;54:1755–1762. doi: 10.1016/j.neuroimage.2010.08.012. [DOI] [PubMed] [Google Scholar]
- Lane J.D., Wellman H.M., Olson S.L., LaBounty J., Kerr D.C. Theory of mind and emotion understanding predict moral development in early childhood. British Journal of Developmental Psychology. 2010;28:871–889. doi: 10.1348/026151009x483056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang P.J., Bradley M.M., Cuthbert B.N. National Institute of Mental Health Center for the Study of Emotion and Attention; Bethesda, MD: 1995. International Affective Picture System (IAPS) [Google Scholar]
- Maddock R.J. The retrosplenial cortex and emotion: new insights from functional neuroimaging of the human brain. Trends in Neurosciences. 1999;22:310–316. doi: 10.1016/s0166-2236(98)01374-5. [DOI] [PubMed] [Google Scholar]
- Mitchell J. Activity in the right temporo-parietal junction is not selective for theory-of-mind. Cerebral Cortex. 2008;18:262–271. doi: 10.1093/cercor/bhm051. [DOI] [PubMed] [Google Scholar]
- Moll J., Zahn R., de Oliveira-Souza R., Krueger F., Grafman J. The neural basis of human moral cognition. Nature Reviews: Neuroscience. 2005;6:799–809. doi: 10.1038/nrn1768. [DOI] [PubMed] [Google Scholar]
- Murphy J.M., Gillligan C. Moral development in late adolescence and adulthood: a critique and reconstruction of Kohlberg's theory. Human Development. 1980;23:77–104. [Google Scholar]
- Pujol J., Reixach J., Harrison B.J., Timoneda-Gallart C., Vilanova J.C., Perez-Alvarez F. Posterior cingulate activation during moral dilemma in adolescents. Human Brain Mapping. 2008;29:910–921. doi: 10.1002/hbm.20436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts W., Strayer J. Empathy, emotional expressiveness, and prosocial behavior. Child Development. 1996;67:449–470. [Google Scholar]
- Ryan J.J., Lopez S.J., Werth T.R. Development and preliminary validation of a Satz–Mogel short form of the Wais-III in a sample of persons with substance abuse disorders. International Journal of Neuroscience. 1999;98:131–140. doi: 10.3109/00207459908994796. [DOI] [PubMed] [Google Scholar]
- Sowell E.R., Thompson P.M., Holmes C.J., Batth R., Jernigan T.L., Toga A.W. Localizing age-related changes in brain structure between childhood and adolescence using statistical parametric mapping. Neuroimage. 1999;9:587–597. doi: 10.1006/nimg.1999.0436. [DOI] [PubMed] [Google Scholar]
- Toga A.W., Thompson P.M., Sowell E.R. Mapping brain maturation. Trends in Neurosciences. 2006;29:148–159. doi: 10.1016/j.tins.2006.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Bos W., van Dijk E., Westenberg M., Rombouts S.A.R.B., Crone E.A. Changing brains, changing perspectives: the neurocognitive development of reciprocity. Psychological Science. 2011;22:60–70. doi: 10.1177/0956797610391102. [DOI] [PubMed] [Google Scholar]
- Vogt B.A., Laureys S. Posterior cingulate, precuneal and retrosplenial cortices: cytology and components of the neural network correlates of consciousness. Progress in Brain Research. 2005;150:205–217. doi: 10.1016/S0079-6123(05)50015-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler D. Psychological Corporation; New York, NY: 1997. Wechsler Adult Intelligence Scale. [Google Scholar]
- Wechsler D. Psychological Corporation; San Antonio, TX: 2003. Wechsler Intelligence Scale for Children–Fourth Edition. [Google Scholar]
- Young L., Cushman F., Hauser M., Saxe R. The neural basis of the interaction between theory of mind and moral judgment. Proceedings of the National Academy of Sciences. 2007;104:8235–8240. doi: 10.1073/pnas.0701408104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young L., Camprodon J.A., Hauser M., Pascual-Leone A., Saxe R. Disruption of the right temporoparietal junction with transcranial magnetic stimulation reduces the role of beliefs in moral judgment. Proceedings of the National Academy of Sciences. 2010;107:6753–6758. doi: 10.1073/pnas.0914826107. [DOI] [PMC free article] [PubMed] [Google Scholar]