Abstract
The pro-inflammatory cytokine interleukin (IL)-17 (also known as IL-17A) has been associated with induction of tissue inflammation. Obese individuals exhibit many symptoms of chronic low-grade inflammation, suggesting that IL-17A may impact adipose tissue. However, the role of IL-17A in obesity is largely unexplored. Emerging studies indicate that obesity selectively promotes expansion of the Th17 T-cell lineage, exacerbating disease in murine models of autoimmunity such as EAE and colitis. Human studies support this concept, as new clinical studies suggest that IL-17A is expressed at elevated levels in obese individuals. Conversely, however, an anti-adipogenic role for IL-17A is becoming evident, and therefore the interconnections between IL-17A and fat metabolism may be quite complex. Here, we consolidate the potential implications of IL-17 in relation to obesity and describe the emerging data regarding the role of IL-17A in adipose tissue.
Introduction
The recently discovered T helper 17 (Th17) cells represent a novel subset of CD4 effector T cells. The hallmark cytokine of Th17 cells, Interleukin (IL)-17A, is the first member of a family of six pro-inflammatory cytokines: IL-17A through IL-17F (1). IL-17A signals through a receptor composed of IL-17RA and IL-17RC. Since receptors for IL-17A are distributed ubiquitously, Th17 cells (and other IL-17A-producing cells (2) have been implicated in interactions between the immune system and somatic tissues (3, 4). IL-17A is involved in tissue inflammation by release of other pro-inflammatory cytokines and inducing neutrophil chemotaxis (5). New evidence indicates that the biological roles of IL-17A include several non-immune functions, which have yet to be thoroughly explored. Hence, we review the effects of IL-17A on adipose tissue and the implications for obesity and inflammation.
Connections between obesity and inflammation
Adipose tissue, apart from being a storage depot for lipids, plays a significant role in the integration of endocrine, metabolic, and inflammatory signals (6, 7). Adipocytes form the functional units of adipose tissue and produce various pro-inflammatory cytokines including IL-6, IL-8, TNF-α, IL-10 and IL-18 (6, 8, 9), potentially linking fat and inflammation. Adipocytes and their precursors interact with the immune system, as revealed from numerous studies on inflammation in obesity and other metabolic diseases (10, 11). By virtue of its production by adaptive and innate cells and its actions on somatic tissues, IL-17A may serve as a significant link between adipose tissue and inflammatory immune responses.
Obesity in many senses is considered to be an inflammatory predisposition. For example, low levels of chronic inflammation and macrophage infiltration into adipose tissue are associated with obese conditions (12). Obesity is also noted to predispose to autoimmune disorders, including inflammatory bowel disease (IBD) and psoriasis (13, 14). The Th17 lineage and IL-17A are now recognized to play detrimental roles in a wide variety of autoimmune and inflammatory conditions, such as IBD, psoriasis, systemic lupus erythematosus (SLE) and rheumatoid arthritis (15-18). Therefore it is compelling to identify and understand the interface between the pro-inflammatory cytokine such as IL-17A and obesity.
Thus far only a few reports explore the role of IL-17A in conjunction with adipogenesis or obesity, but the ubiquitous nature of IL-17A in inflammation suggests this is only the tip of the iceberg. This review discusses current findings in relation to the potential association between obesity and Th17 expansion and the effects stimulated by IL-17A on adipogenesis in various model systems (Table 1).
Table.1.
Roles of Th17 cells and IL-17 in relation to obesity
| System | Model | Finding | Reference |
|---|---|---|---|
| Obesit | |||
| Diet Induced Obese Mice DIO |
TNBS colitis and EAE |
DIO predisposes to IL-6-dependent Th17 expansion associated with more severe disease conditions |
Winer et al, 2009 |
| DIO and leptin- deficient Ob/Ob Mice |
Zymosan- induced peritonitis |
IL-17A derived from neutrophils increased in obese mice in response to ZY, and coincided with increased IL-6 levels |
Fantuzzi & Pini, 2010 |
| Obese and lean women | - | Increased levels of IL-17 in addition to IL-23 in blood were detected in obese women than in lean subjects. |
Sumarac-Dumanovic et al 2009 |
| IL-17 mediated inhibition of adiopogenesis | |||
| IL-17RA-/- (KO) mice, 3T3-L1 cells |
Ovariectomy- induced - osteoporosis (OVX) |
IL-17RA-/- mice showed elevated levels of leptin after OVX than controls, leading to increased bone loss. IL-17A and IL-17F treatments of 3T3- L1 preadipocytes cells inhibited adipogenesis. |
Goswami et al, 2009 |
| Human bone marrow- derived mesenchymal stem cells |
- | IL-17A inhibited adipocyte differentiation of hBM-MSCs via COX-2 induction. |
Shin et al, 2009 |
Obesity promotes expansion of Th17 cells and subsequent IL-17 production
Obesity is associated with a state of chronic low-grade inflammation, as illustrated by increased levels of acute-phase proteins and pro-inflammatory mediators in serum of obese individuals compared with lean subjects (6). Under obese conditions, adipose tissue contributes a significant proportion of circulating serum IL-6 (19). IL-6 expression is associated with diet-induced obese mice (DIO) as well as in obese humans (20, 21). Furthermore, IL-6 is required for the differentiation of naïve CD4 T cells into the Th17 lineage (22, 23), and is a major downstream gene target of IL-17A (24, 25).
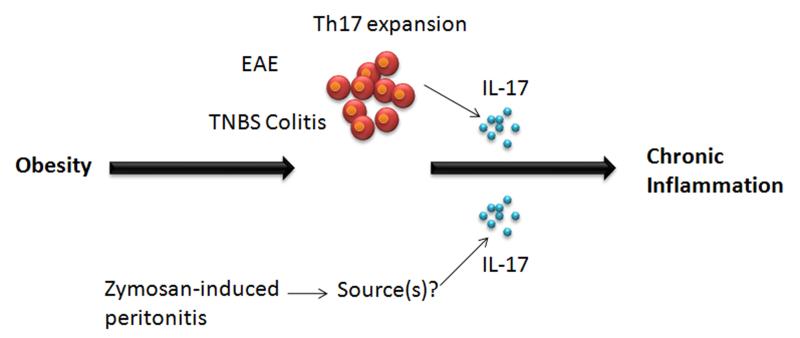
Accordingly, the association of IL-6 with obesity has prompted recent studies to evaluate a connection between obesity and IL-17A-mediated autoimmunity, in particular trinitrobenzene sulfonic acid (TNBS)-induced colitis (a model of IBD) and experimental autoimmune encephalomyelitis (EAE, a model of multiple sclerosis) (Fig. 1) (26). In DIO, mice express higher numbers of Th17 cells, while the sizes of CD4+, IFNγ+ (Th1), Foxp3+ regulatory T cells (Tregs) and GATA+ Th2 cell pools were unaffected, indicating that obesity specifically predisposes to induction of Th17 cells. Following immunization with myelin oligodendrocyte glycoprotein 35-55 (MOG35-55), a well-characterized target antigen used in EAE, DIO also increased Th17 numbers dramatically. However, IL-6-deficient DIO mice did not have enhanced Th17 responses, indicating that IL-6 is crucial for polarizing Th17 responses in this model. Similarly, in TNBS colitis (27), enhanced Th17 responses that were associated with more severe disease outcomes (Table 1, Fig. 1) (26). These intriguing data suggest that obesity predisposes to the generation of Th17 responses at least in part via IL-6, which may in turn exacerbate autoinflammatory diseases such as MS and colitis.
Figure 1. Effects of IL-17A during acute inflammation associated with obesity.
(A) T-cells from diet-induced obese (DIO) mice expand Th17 cell pools and produce progressively more IL-17 than lean littermates, in what has been shown to be an IL-6-dependent process. The increased Th17 bias is associated with more pronounced autoimmune disease during EAE and trinitrobenzene sulfonic acid colitis. In both models DIO mice developed more severe early autoimmune disease, with increased pools of IL-17+ T-cells (26). (B) Levels of IL-17A in peritoneal fluid in response to ZY are elevated in obese leptin-deficient ob/ob mice compared with lean animals. IL-17A is increased in obese mice during acute inflammation and contributes to exacerbation of inflammatory responses, but the cellular sources of IL-17A remain controversial (34).
Leptin is an IL-6-family cytokine secreted by adipocytes. Leptin plays a key role in fat turnover, weight control and satiety and also acts as an immunostimulatory molecule with both pro-and anti-inflammatory properties (28, 29). In Th17-mediated inflammation models such as EAE, collagen induced arthritis (CIA), and autoimmune hepatitis, leptin is pro-inflammatory (30, 31). However in inflammatory models that are independent of adaptive immune responses, leptin shows anti-inflammatory properties (32). Murine zymosan-induced (ZY) peritonitis is a well studied model of acute inflammation (33). Leptin-deficient mice (ob/ob) have an exacerbated and prolonged inflammatory response to ZY-induced peritoneal inflammation, associated with high levels of IL-6 (29). Hence, leptin appears to play a protective role exhibiting anti-inflammatory properties during ZY-induced peritonitis in mice. Using the ZY-induced peritoneal inflammation model of acute inflammation, a recent study further probed whether the increase in IL-6 levels in leptin-deficient ob/ob and DIO mice was Th17-dependent (Table 1). Pini and Fantuzzi (34) found that IL-17A levels were increased in obese mice in response to ZY, which coincided with increased IL-6 levels. Neutralization of IL-17A in ZY-treated obese mice also resulted in decreased IL-6. However, neutralization of IL-6 surprisingly did not reduce the levels of IL-17 in the ob/ob mice, thus de-linking IL-6 in this setting.
Although IL-17 is traditionally thought to be expressed by CD4+ Th17 lymphocytes, the cellular source of IL-17A in this acute inflammatory model was reported to be neutrophils, based on intracellular flow cytometry. This issue remains controversial, as most other groups fail to find that neutrophils are a bona fide source of IL-17A. Indeed, this observation contrasts with other studies where IL-17A production is enhanced in CD4+ T cells during DIO (26). Regardless of its source, neutralization of IL-17A in ZY-treated obese mice resulted in decreased neutrophilic recruitment, correlating to decreased levels of neutrophil-recruiting chemokines such as CXCL1 and CXCL2. IL-17A is a strong inducer of CXC chemokines and subsequent neutrophil infiltration, and IL-17-deficiency is associated with marked neutrophil defects in vivo (35). New clinical studies also suggest a potential role for IL-17A in obesity (36). Data in this report show that obese women exhibit increased levels of circulating IL-23 and IL-17, which is statistically independent of leptin or other inflammatory factors such as macrophage migration inhibitory factor (MIF) (36). Interestingly, under inflammatory conditions polarized human Th17 cells have been shown to adhere to mesenchymal stem cells, which leads to their conversion to a Treg-like phenotype (37). Thus in the context of an inflammatory environment, differentiated Th17 cells may exhibit plasticity. However, this is at odds with the idea that obesity induces a pro-inflammatory state, and therefore there seems to be a more complex relationship that warrants much more investigation.
IL-17A inhibits adipogenesis
Unlike classic proinflammatory mediators such as TNF-α and IL-6, T-cell-derived cytokines such as IL-17A and interferon-γ (IFN-γ) have not been investigated extensively in the context of obesity (10, 38). Data from two recent studies indicate that IL-17A inhibits adipogenesis, suggesting an unexpected suppressive role for this cytokine in fat development (Table 1, Fig. 2). IL-17RA-/- mice are known anecdotally to be overweight. While investigating the role of IL-17RA in osteoporosis, our group discovered a surprising anti-adipogenic effect of IL-17A (39). Specifically, a mouse model of osteoporosis was employed to evaluate the role of IL-17A signaling in bone loss caused by estrogen deficiency (induced by ovariectomy, OVX). Since IL-17A drives bone loss in CIA and also induces expression of cytokines known to promote bone loss in osteoporosis models, it has long been presumed in the field that IL-17A would likely promote systemic bone loss following OVX. However, unexpectedly IL-17RA-/- mice showed accelerated systemic and spinal bone loss, which was concomitant with significant weight gain. Phenotyping analysis of serum revealed elevated leptin levels in IL-17RA-/- mice subjected to OVX (39). Thus, the role of IL-17A in regulating bone is more complex than might be inferred from its activities in RA models, and these results hinted at a connection to fat metabolism.
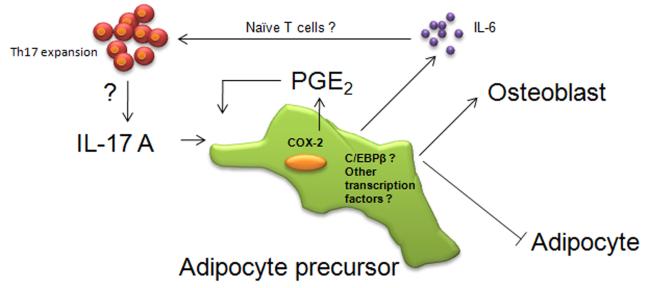
Figure 2. Model for inhibition differentiation of adipocytes by IL-17A.
IL-17A promotes mesenchymal stem cells to undergo osteoblastogenesis and inhibit adipogenesis (39, 43). IL-17A exhibits the inhibitory effect on adipogenesis in part by the secretion of PGE2 via COX-2 induction in differentiated adipocytes (42). During the inhibition of adipogenesis it is not clear whether IL-17RA signaling involves C/EBPβ or other transcription factors. It is also unclear whether the IL-17A-induced expression of IL-6 from adipose tissue under conditions of obesity affects differentiation of naïve T lymphocytes into Th17 cells.
Although leptin promotes satiety, obesity is often associated with leptin resistance and elevated leptin levels (40). Hence the high basal levels of leptin IL-17RA-/- mice may reflect an altered metabolism that predisposes to obesity. Leptin has been shown to cause bone loss through a central pathway in the hypothalamus (40). Conversely, leptin can be bone-protective through a peripheral pathway (40, 41). Since the accentuated bone loss in the IL-17RA-/- mice correlated with elevated leptin, one possible mechanism is that leptin acts via the central pathway to trigger increased bone loss. According to this model, IL-17A would be expected to inhibit leptin expression. Indeed, IL-17A (and the closely related cytokine IL-17F) both downregulated adipogenesis and subsequent leptin production in the 3T3-L1 adipocyte-differentiation system (39). Consistently, IL-17A impaired adipocyte differentiation in human mesenchymal stem cells (MSC) (42). Intriguingly, IL-17A was found to accelerate differentiation of human MSC into mature osteoblasts (43). Together, these observations suggest that IL-17A can skew MSC development. Hence, the balance of MSC differentiation in IL-17RA-/- mice appears to be tipped in favor of adipogenesis rather than osteoblastogenesis during bone remodeling, ultimately favoring accelerated bone destruction in the context of OVX (Fig 2).
Mechanisms for inhibition of adipocyte differentiation by IL-17A
a. C/EBPβ
IL-17A has been implicated as a possible player in the crosstalk between adipose tissue and Th17 associated immune responses, but the detailed mechanisms remains poorly defined. Crucial transcription factors that promote adipocyte differentiation include CCAAT/Enhancer Binding Protein (C/EBP)β, C/EBPδ and peroxisome proliferator-activated receptor γ (PPARγ), among others (44, 45). During adipogenesis, C/EBPβ undergoes sequential phosphorylation at three sites in its internal regulatory domain (Thr188, Ser184 and Thr179) (46). Despite its inhibitory role in adipogenesis, IL-17A causes increased abundance of both C/EBPβ and C/EBPδ in osteogenic and adipogenic cell lines (24, 25, 47). Moreover, IL-17A induces phosphorylation of C/EBPβ on Thr188 and Thr179 in the adipogenic ST-2 cell line (48). Thus, it is unclear how IL-17A acts to inhibit adipogensis while paradoxically activating the adipogenic potential of C/EBPβ. Alternate transcription factors may be implicated downstream of IL-17A signaling leading to the inhibition of differentiating adipocytes, but this has not yet been explored in any detail.
b. COX-2
In studies of adipogenic differentiation of human MSCs, induction of cyclooxygenase 2 (COX-2) was proposed to be at least partially responsible for the inhibitory effect of IL-17A on differentiation. In this study, IL-17A treatment of differentiated adipocytes led to increased COX-2 mRNA levels and subsequently elevated prostaglandin E2 (PGE2). Moreover, COX-2 inhibition reduced the inhibitory effects of IL-17A in this setting (42). PGE2 has been shown to inhibit adipocyte differentiation in mammalian adipocytes (49). Since human adipocytes express PGE2 receptors (50), PGE2 could potentially regulate adipocyte differentiation in either an autocrine or paracrine manner.
IL-17A promotes IL-6 production in differentiated adipocytes
IL-17A influences production of pro-inflammatory cytokines from adipose tissue as well as other mesenchymal cell types (5, 51). Despite the fact that IL-17A stimulation of IL-6 is a prominent response in most somatic cells, the role of IL-17A on regulation of IL-6, if any, during adipogenesis inhibition remains unclear (19). Results from Shin et al, (42) showed significantly lower levels of IL-6 in differentiated adipocytes compared to undifferentiated MSCs (Table 1, Fig 2). This dampening of IL-6 expression could be due to phosphorylation of C/EBPβ during differentiation. Phosphorylation of C/EBPβ is required for adipocyte differentiation, but the modification also downregulates C/EBPβ-responsive genes such as il6 (46, 48). However, in human MSC cultures, IL-17A triggers a significant rise in the levels of IL-6 in differentiated adipocytes (42). This increased IL-6 may favor Th17 cell differentiation, which may in turn lead to IL-17A-induced IL-6 upregulation. Although IL-17A induces IL-6 expression in many somatic tissue cells, it remains unclear whether IL-17A can modulate obesity and metabolic status, or whether the increase in IL-6 levels in the adipose tissue under conditions of obesity affects differentiation of naïve T lymphocytes.
Summary and Future Perspectives
Given the close proximity of the adipose tissue and lymphatic tissue, adipocytes as well as their precursors interact intimately with the immune system. Adipocytes not only produce adopogenic cytokines, but can participate in the regulation of the immune system via secretion of cytokines such as IL-6. In turn, inflammatory cytokines such as IL-6 and IL-17A regulate the differentiation of adipocytes and their capacity to secrete adipokines and chemokines. Emerging studies reveal a role for IL-17A in obesity, and the observations made in rodents are probably relevant models of human disease. Furthermore, IL-17A inhibits adipocyte development in both murine and human systems. These findings in concert indicate that IL-17A mediates many important interactions between adipose tissue and the immune system.
Understanding the role of IL-17A in metabolic processes has direct clinical implications. In particular, clinical trials using anti-IL-17 therapies are underway for treating rheumatoid arthritis, psoriasis and other autoimmune conditions (52). Although this strategy shows promise for alleviating disease, reducing IL-17A may also cause complications with respect not only to opportunistic infections but also obesity.
Acknowledgments
SLG was supported by NIH grants DE019424 and AR054389.
Abbreviations
- C/EBP
CCAAT/Enhancer Binding Protein
- CIA
collagen-induced arthritis
- DIO
diet-induced obesity
- EAE
experimental autoimmune encephalomyelitis
- IBD
inflammatory bowel disease
- MSC
mesenchymal stem cell
- OVX
ovariectomy
- PPARγ
peroxisome proliferators-activated receptor γ
References
- 1.Gaffen SL. Structure and signalling in the IL-17 receptor family. Nat Rev Immunol. 2009;9:556–567. doi: 10.1038/nri2586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cua DJ, Tato CM. Innate IL-17-producing cells: the sentinels of the immune system. Nat Rev Immunol. 2010;10:479–489. doi: 10.1038/nri2800. [DOI] [PubMed] [Google Scholar]
- 3.Yu J, Gaffen SL. Interleukin-17: A novel inflammatory cytokine that bridges innate and adaptive immunity. Front. Biosci. 2008;13:170–177. doi: 10.2741/2667. [DOI] [PubMed] [Google Scholar]
- 4.Ouyang W, Kolls JK, Zheng Y. The biological functions of T helper 17 cell effector cytokines in inflammation. Immunity. 2008;28:454–467. doi: 10.1016/j.immuni.2008.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Onishi RM, Gaffen SL. Interleukin-17 and its target genes: mechanisms of interleukin-17 function in disease. Immunology. 2010;129:311–321. doi: 10.1111/j.1365-2567.2009.03240.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fantuzzi G. Adipose tissue, adipokines, and inflammation. J Allergy Clin Immunol. 2005;115:911–919. doi: 10.1016/j.jaci.2005.02.023. quiz 920. [DOI] [PubMed] [Google Scholar]
- 7.Rajala MW, Scherer PE. Minireview: The adipocyte--at the crossroads of energy homeostasis, inflammation, and atherosclerosis. Endocrinology. 2003;144:3765–3773. doi: 10.1210/en.2003-0580. [DOI] [PubMed] [Google Scholar]
- 8.Patton JS, Shepard HM, Wilking H, et al. Interferons and tumor necrosis factors have similar catabolic effects on 3T3 L1 cells. Proc Natl Acad Sci U S A. 1986;83:8313–8317. doi: 10.1073/pnas.83.21.8313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bruun JM, Pedersen SB, Richelsen B. Regulation of interleukin 8 production and gene expression in human adipose tissue in vitro. J Clin Endocrinol Metab. 2001;86:1267–1273. doi: 10.1210/jcem.86.3.7264. [DOI] [PubMed] [Google Scholar]
- 10.Dandona P, Aljada A, Bandyopadhyay A. Inflammation: the link between insulin resistance, obesity and diabetes. Trends Immunol. 2004;25:4–7. doi: 10.1016/j.it.2003.10.013. [DOI] [PubMed] [Google Scholar]
- 11.Guilherme A, Virbasius JV, Puri V, Czech MP. Adipocyte dysfunctions linking obesity to insulin resistance and type 2 diabetes. Nat Rev Mol Cell Biol. 2008;9:367–377. doi: 10.1038/nrm2391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dandona P, Aljada A, Ghanim H, et al. Increased plasma concentration of macrophage migration inhibitory factor (MIF) and MIF mRNA in mononuclear cells in the obese and the suppressive action of metformin. J Clin Endocrinol Metab. 2004;89:5043–5047. doi: 10.1210/jc.2004-0436. [DOI] [PubMed] [Google Scholar]
- 13.Setty AR, Curhan G, Choi HK. Obesity, waist circumference, weight change, and the risk of psoriasis in women: Nurses’ Health Study II. Arch Intern Med. 2007;167:1670–1675. doi: 10.1001/archinte.167.15.1670. [DOI] [PubMed] [Google Scholar]
- 14.Hass DJ, Brensinger CM, Lewis JD, Lichtenstein GR. The impact of increased body mass index on the clinical course of Crohn’s disease. Clin Gastroenterol Hepatol. 2006;4:482–488. doi: 10.1016/j.cgh.2005.12.015. [DOI] [PubMed] [Google Scholar]
- 15.Langowski JL, Zhang X, Wu L, et al. IL-23 promotes tumour incidence and growth. Nature. 2006;442:461–465. doi: 10.1038/nature04808. [DOI] [PubMed] [Google Scholar]
- 16.Crispin JC, Tsokos GC. IL-17 in systemic lupus erythematosus. J Biomed Biotechnol. 2010;2010:943254. doi: 10.1155/2010/943254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lubberts E. IL-17/Th17 targeting: on the road to prevent chronic destructive arthritis? Cytokine. 2008;41:84–91. doi: 10.1016/j.cyto.2007.09.014. [DOI] [PubMed] [Google Scholar]
- 18.Garrett-Sinha L, John S, Gaffen S. IL-17 and the Th17 lineage in systemic lupus erythematosus. Curr Op. Rheum. 2008;20:519–525. doi: 10.1097/BOR.0b013e328304b6b5. [DOI] [PubMed] [Google Scholar]
- 19.Naugler WE, Karin M. The wolf in sheep’s clothing: the role of interleukin-6 in immunity, inflammation and cancer. Trends Mol Med. 2008;14:109–119. doi: 10.1016/j.molmed.2007.12.007. [DOI] [PubMed] [Google Scholar]
- 20.Febbraio MA. gp130 receptor ligands as potential therapeutic targets for obesity. J Clin Invest. 2007;117:841–849. doi: 10.1172/JCI30453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bastard JP, Jardel C, Bruckert E, et al. Elevated levels of interleukin 6 are reduced in serum and subcutaneous adipose tissue of obese women after weight loss. J Clin Endocrinol Metab. 2000;85:3338–3342. doi: 10.1210/jcem.85.9.6839. [DOI] [PubMed] [Google Scholar]
- 22.Harrington LE, Hatton RD, Mangan PR, et al. Interleukin 17-producing CD4+ effector T cells develop via a lineage distinct from the T helper type 1 and 2 lineages. Nat Immunol. 2005;6:1123–1132. doi: 10.1038/ni1254. [DOI] [PubMed] [Google Scholar]
- 23.Park H, Li Z, Yang XO, et al. A distinct lineage of CD4 T cells regulates tissue inflammation by producing interleukin 17. Nat Immunol. 2005;6:1133–1141. doi: 10.1038/ni1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ruddy MJ, Wong GC, Liu XK, et al. Functional cooperation between interleukin-17 and tumor necrosis factor-alpha is mediated by CCAAT/enhancer-binding protein family members. J Biol Chem. 2004;279:2559–2567. doi: 10.1074/jbc.M308809200. [DOI] [PubMed] [Google Scholar]
- 25.Shen F, Ruddy MJ, Plamondon P, Gaffen SL. Cytokines link osteoblasts and inflammation: microarray analysis of interleukin-17- and TNF-alpha-induced genes in bone cells. J Leukoc Biol. 2005;77:388–399. doi: 10.1189/jlb.0904490. [DOI] [PubMed] [Google Scholar]
- 26.Winer S, Paltser G, Chan Y, et al. Obesity predisposes to Th17 bias. Eur J Immunol. 2009;39:2629–2635. doi: 10.1002/eji.200838893. [DOI] [PubMed] [Google Scholar]
- 27.Kastelein RA, Hunter CA, Cua DJ. Discovery and biology of IL-23 and IL-27: related but functionally distinct regulators of inflammation. Annu Rev Immunol. 2007;25:221–242. doi: 10.1146/annurev.immunol.22.012703.104758. [DOI] [PubMed] [Google Scholar]
- 28.Bluher S, Mantzoros CS. Leptin in humans: lessons from translational research. Am J Clin Nutr. 2009;89:991S–997S. doi: 10.3945/ajcn.2008.26788E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pini M, Gove ME, Sennello JA, van Baal JW, Chan L, Fantuzzi G. Role and regulation of adipokines during zymosan-induced peritoneal inflammation in mice. Endocrinology. 2008;149:4080–4085. doi: 10.1210/en.2008-0327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Faggioni R, Jones-Carson J, Reed DA, et al. Leptin-deficient (ob/ob) mice are protected from T cell-mediated hepatotoxicity: role of tumor necrosis factor alpha and IL-18. Proc Natl Acad Sci U S A. 2000;97:2367–2372. doi: 10.1073/pnas.040561297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Siegmund B, Lear-Kaul KC, Faggioni R, Fantuzzi G. Leptin deficiency, not obesity, protects mice from Con A-induced hepatitis. Eur J Immunol. 2002;32:552–560. doi: 10.1002/1521-4141(200202)32:2<552::AID-IMMU552>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 32.Bernotiene E, Palmer G, Talabot-Ayer D, Szalay-Quinodoz I, Aubert ML, Gabay C. Delayed resolution of acute inflammation during zymosan-induced arthritis in leptin-deficient mice. Arthritis Res Ther. 2004;6:R256–263. doi: 10.1186/ar1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Leypoldt JK, Kamerath CD, Gilson JF. Acute peritonitis in a C57BL/6 mouse model of peritoneal dialysis. Adv Perit Dial. 2007;23:66–70. [PubMed] [Google Scholar]
- 34.Pini M, Fantuzzi G. Enhanced production of IL-17A during zymosan-induced peritonitis in obese mice. J Leukoc Biol. 2010;87:51–58. doi: 10.1189/jlb.0309188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kolls JK, Linden A. Interleukin-17 family members and inflammation. Immunity. 2004;21:467–476. doi: 10.1016/j.immuni.2004.08.018. [DOI] [PubMed] [Google Scholar]
- 36.Sumarac-Dumanovic M, Stevanovic D, Ljubic A, et al. Increased activity of interleukin-23/interleukin-17 proinflammatory axis in obese women. Int J Obes (Lond) 2009;33:151–156. doi: 10.1038/ijo.2008.216. [DOI] [PubMed] [Google Scholar]
- 37.Ghannam S, Pene J, Torcy-Moquet G, Jorgensen C, Yssel H. Mesenchymal stem cells inhibit human Th17 cell differentiation and function and induce a T regulatory cell phenotype. J Immunol. 2010;185:302–312. doi: 10.4049/jimmunol.0902007. [DOI] [PubMed] [Google Scholar]
- 38.Shoelson SE, Herrero L, Naaz A. Obesity, inflammation, and insulin resistance. Gastroenterology. 2007;132:2169–2180. doi: 10.1053/j.gastro.2007.03.059. [DOI] [PubMed] [Google Scholar]
- 39.Goswami J, Hernandez-Santos N, Zuniga LA, Gaffen SL. A bone-protective role for IL-17 receptor signaling in ovariectomy-induced bone loss. Eur J Immunol. 2009;39:2831–2839. doi: 10.1002/eji.200939670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lee NJ, Wong IP, Baldock PA, Herzog H. Leptin as an endocrine signal in bone. Curr Osteoporos Rep. 2008;6:62–66. doi: 10.1007/s11914-008-0011-y. [DOI] [PubMed] [Google Scholar]
- 41.Thomas T, Gori F, Khosla S, Jensen MD, Burguera B, Riggs BL. Leptin acts on human marrow stromal cells to enhance differentiation to osteoblasts and to inhibit differentiation to adipocytes. Endocrinology. 1999;140:1630–1638. doi: 10.1210/endo.140.4.6637. [DOI] [PubMed] [Google Scholar]
- 42.Shin JH, Shin DW, Noh M. Interleukin-17A inhibits adipocyte differentiation in human mesenchymal stem cells and regulates pro-inflammatory responses in adipocytes. Biochem Pharmacol. 2009;77:1835–1844. doi: 10.1016/j.bcp.2009.03.008. [DOI] [PubMed] [Google Scholar]
- 43.Huang H, Kim HJ, Chang EJ, et al. IL-17 stimulates the proliferation and differentiation of human mesenchymal stem cells: implications for bone remodeling. Cell Death Differ. 2009;16:1332–1343. doi: 10.1038/cdd.2009.74. [DOI] [PubMed] [Google Scholar]
- 44.Cao Z, Umek RM, McKnight SL. Regulated expression of three C/EBP isoforms during adipose conversion of 3T3-L1 cells. Genes Dev. 1991;5:1538–1552. doi: 10.1101/gad.5.9.1538. [DOI] [PubMed] [Google Scholar]
- 45.Rosen ED, MacDougald OA. Adipocyte differentiation from the inside out. Nat Rev Mol Cell Biol. 2006;7:885–896. doi: 10.1038/nrm2066. [DOI] [PubMed] [Google Scholar]
- 46.Tang QQ, Gronborg M, Huang H, et al. Sequential phosphorylation of CCAAT enhancer-binding protein beta by MAPK and glycogen synthase kinase 3beta is required for adipogenesis. Proc Natl Acad Sci U S A. 2005;102:9766–9771. doi: 10.1073/pnas.0503891102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ding J, Nagai K, Woo JT. Insulin-dependent adipogenesis in stromal ST2 cells derived from murine bone marrow. Biosci Biotechnol Biochem. 2003;67:314–321. doi: 10.1271/bbb.67.314. [DOI] [PubMed] [Google Scholar]
- 48.Shen F, Li N, Gade P, et al. IL-17 receptor signaling inhibits C/EBPbeta by sequential phosphorylation of the regulatory 2 domain. Sci Signal. 2009;2:ra8. doi: 10.1126/scisignal.2000066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Vassaux G, Gaillard D, Darimont C, Ailhaud G, Negrel R. Differential response of preadipocytes and adipocytes to prostacyclin and prostaglandin E2: physiological implications. Endocrinology. 1992;131:2393–2398. doi: 10.1210/endo.131.5.1330499. [DOI] [PubMed] [Google Scholar]
- 50.Subbaramaiah K, Hudis C, Chang SH, Hla T, Dannenberg AJ. EP2 and EP4 receptors regulate aromatase expression in human adipocytes and breast cancer cells. Evidence of a BRCA1 and p300 exchange. J Biol Chem. 2008;283:3433–3444. doi: 10.1074/jbc.M705409200. [DOI] [PubMed] [Google Scholar]
- 51.Moseley TA, Haudenschild DR, Rose L, Reddi AH. Interleukin-17 family and IL-17 receptors. Cytokine Growth Factor Rev. 2003;14:155–174. doi: 10.1016/s1359-6101(03)00002-9. [DOI] [PubMed] [Google Scholar]
- 52.Hueber W, Patel D, Dryja T, et al. Effects of AIN457, a fully human antibody to interleukin-17A, on psoriasis, rheumatoid arthritis, and uveitis. Sci Transl Med. 2010;2:1–9. doi: 10.1126/scitranslmed.3001107. [DOI] [PubMed] [Google Scholar]