Abstract
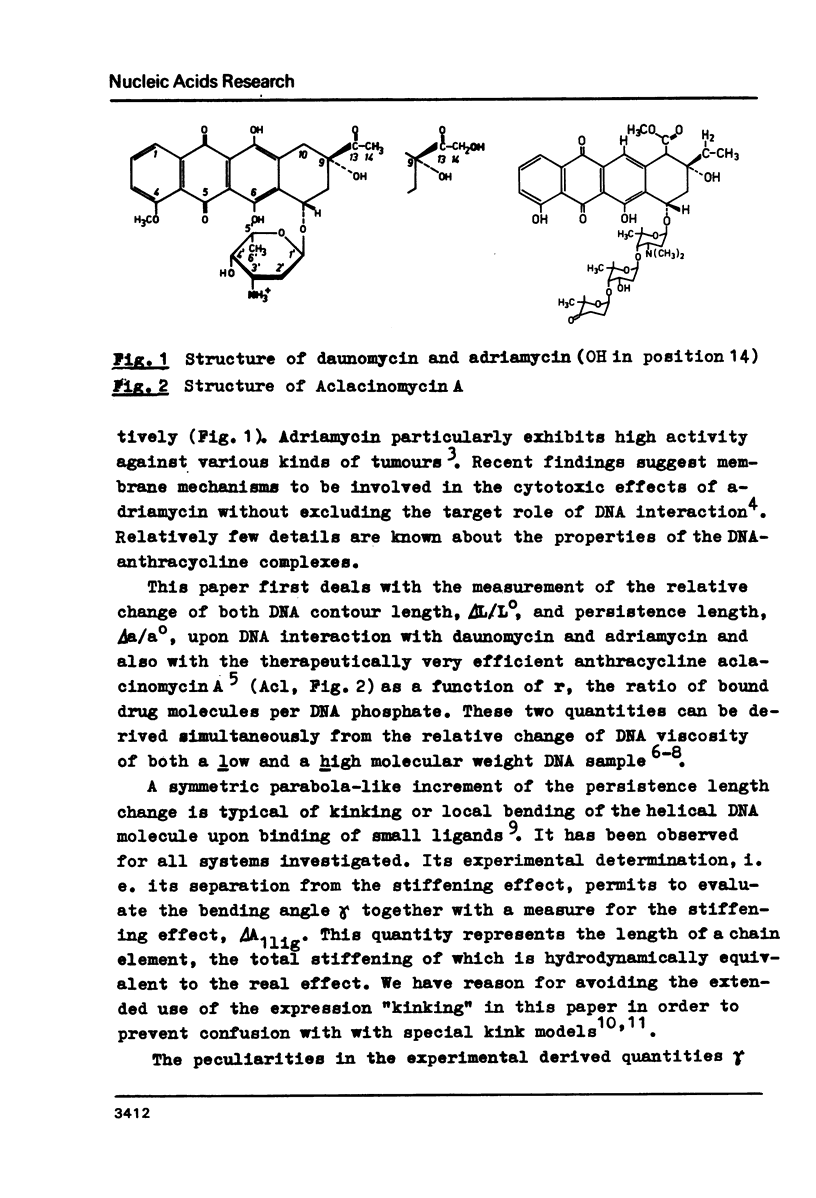
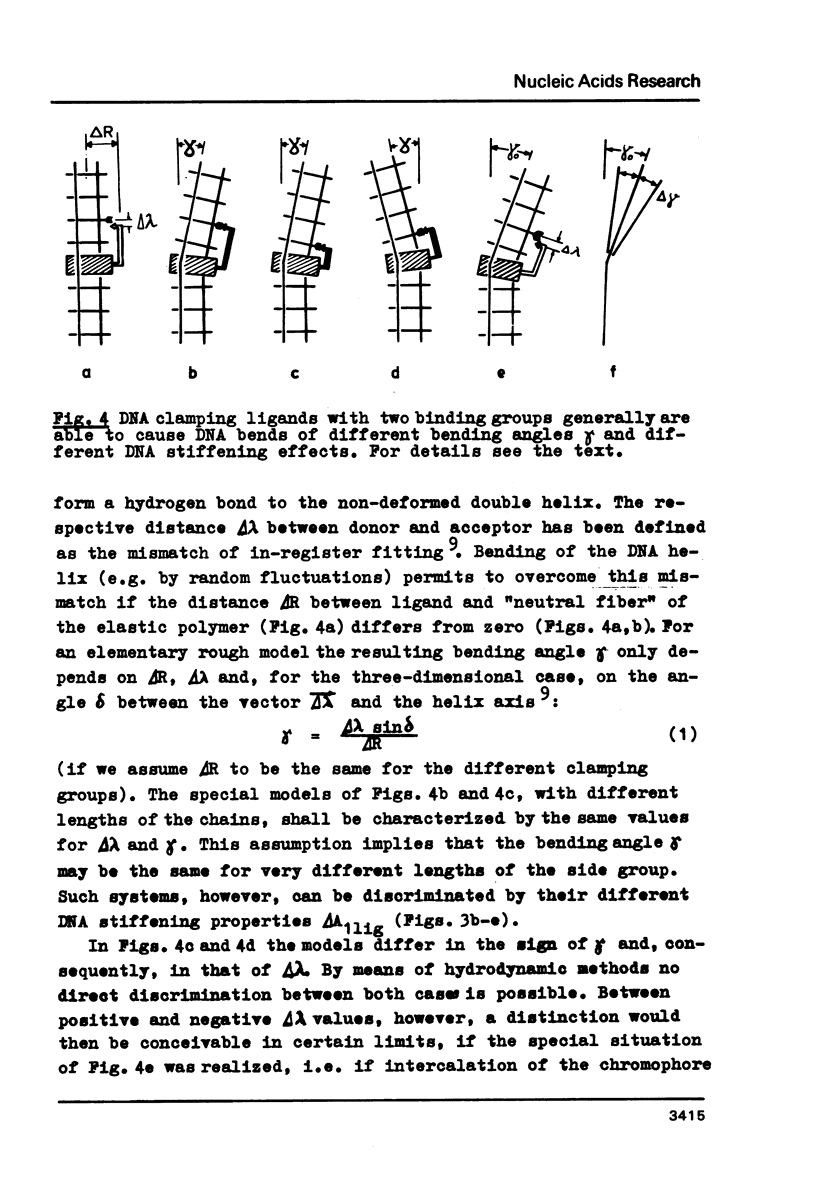
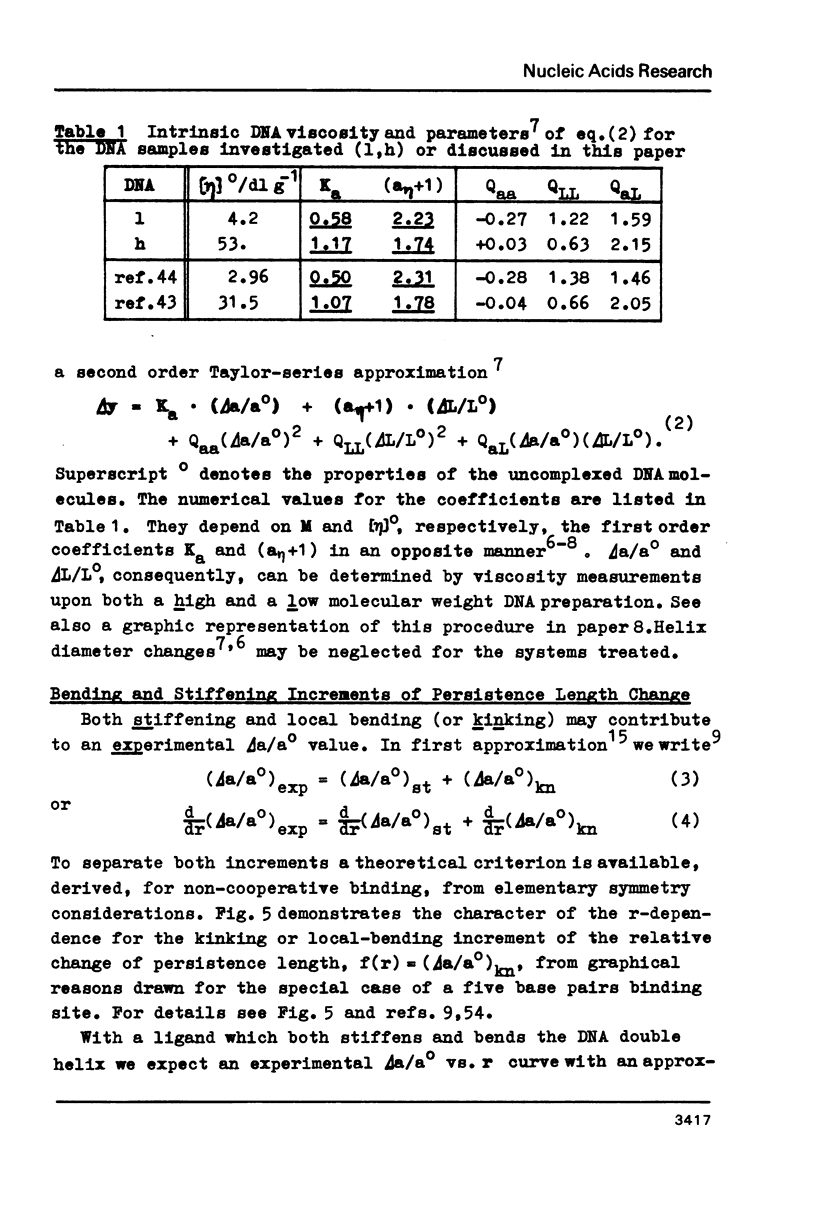
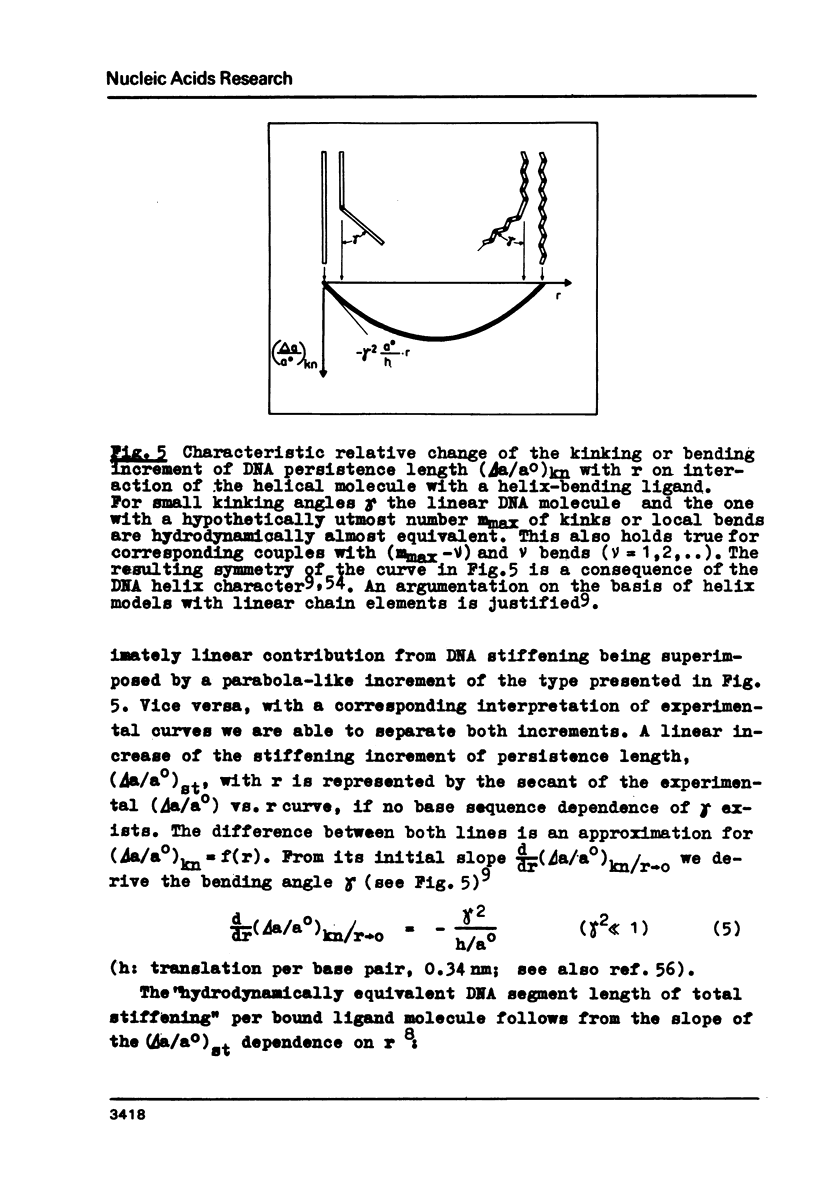
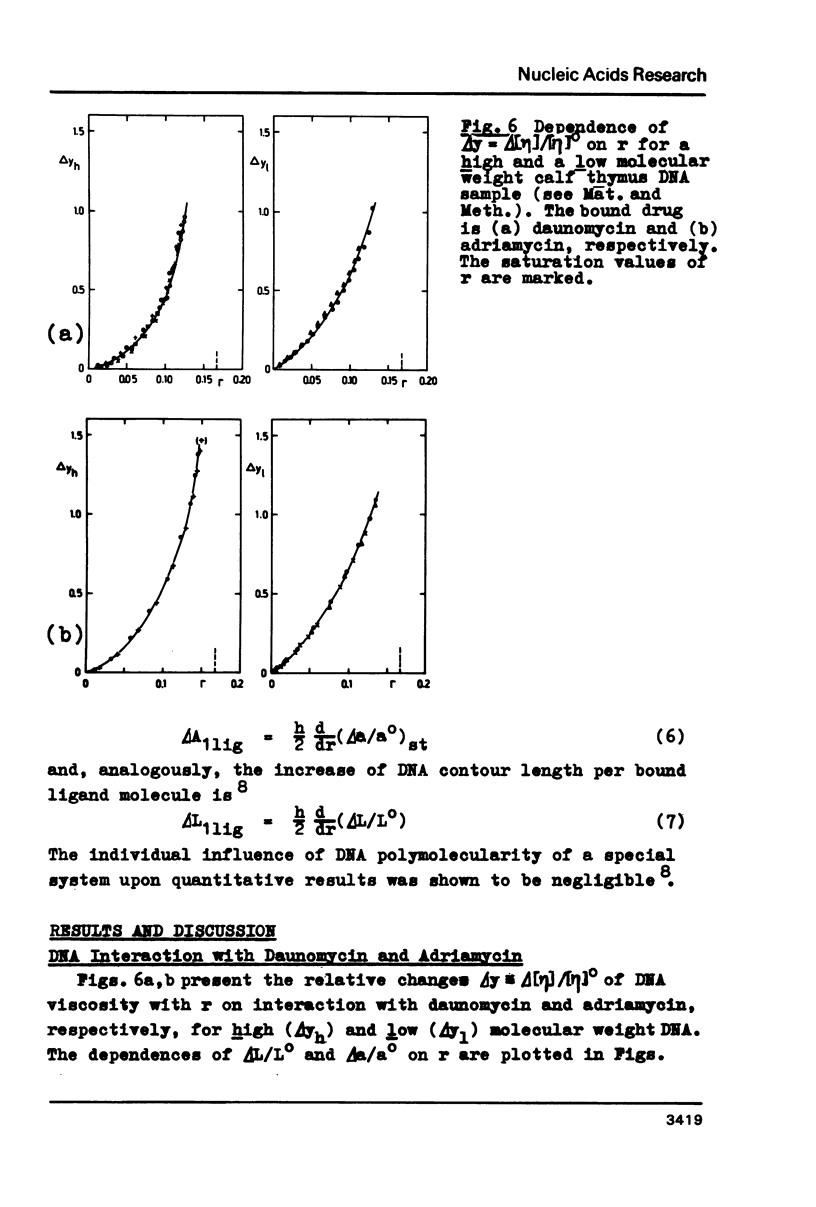
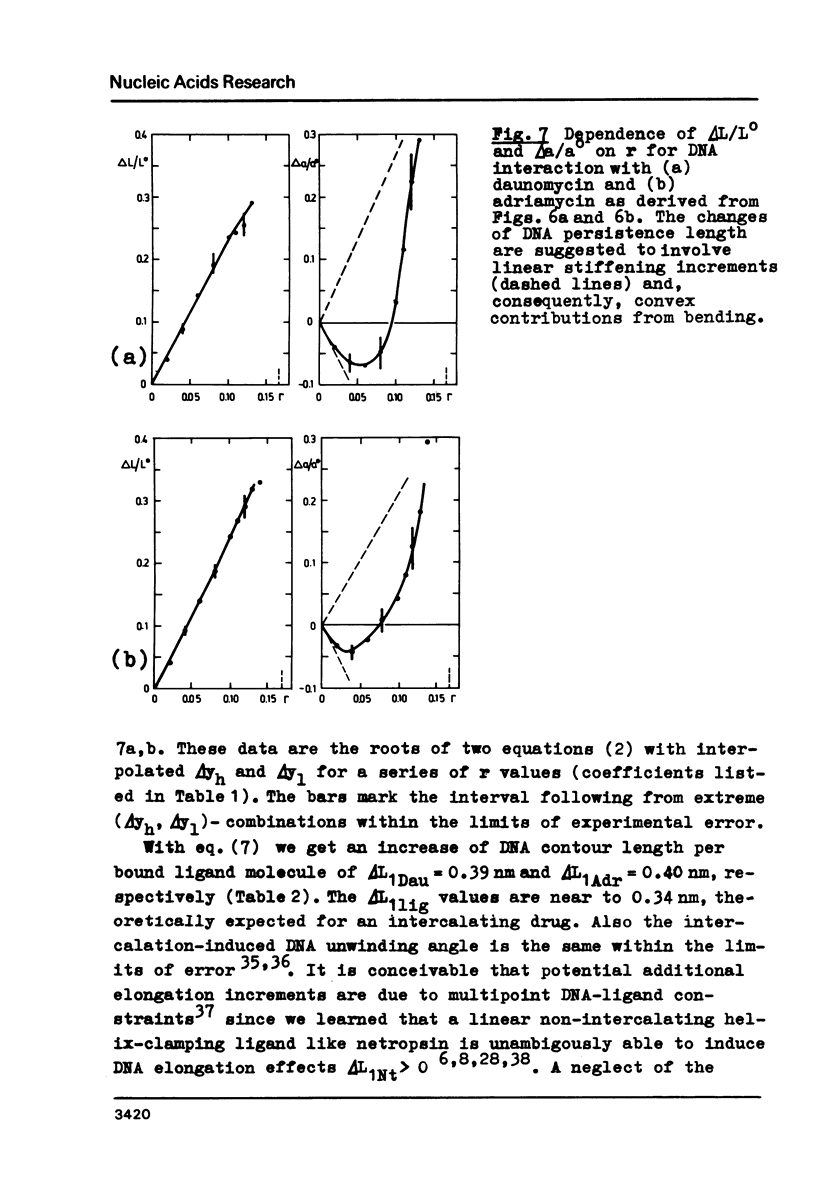
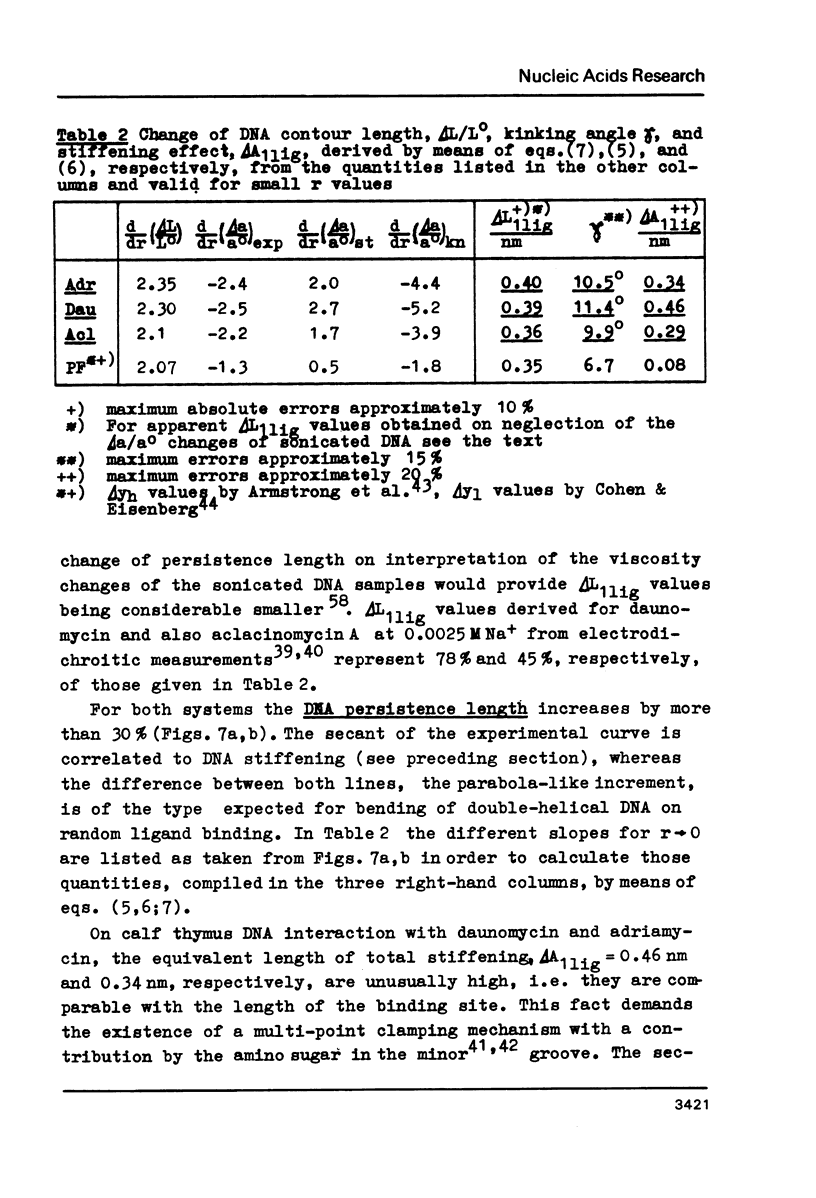
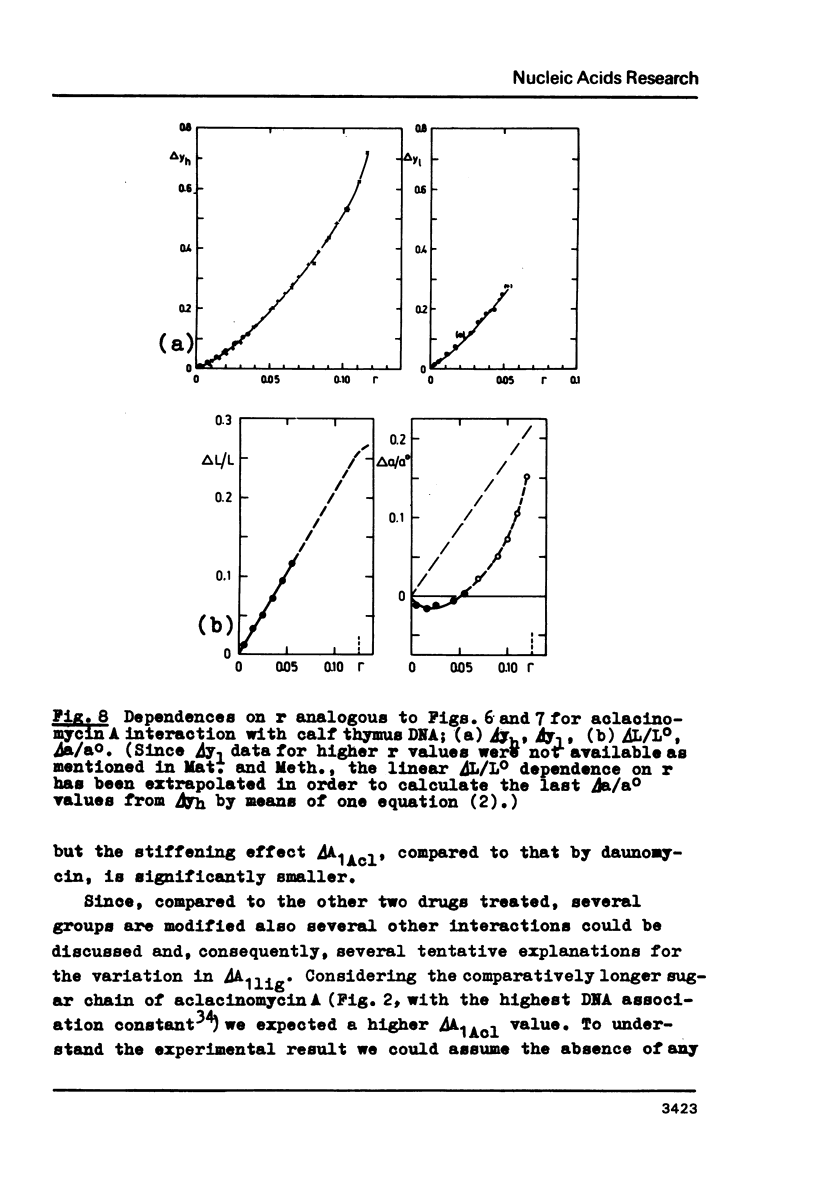
Upon interaction of the three anthracycline antibiotics daunomycin, adriamycin, and aclacinomycin A with calf thymus DNA the relative changes of both DNA contour length, delta L/Lo, and persistence length, delta a/ao, have been determined as a function of r, the ratio of bound ligand molecules per DNA mononucleotide. From the r dependence of delta a/ao a measure for the stiffening effect and also the angle gamma of ligand-induced DNA bending could be derived. Experimental basis are titration viscometric measurements upon both low and high molecular weight DNA. It was found that the DNA contour length increases linearly with r by approximately 0.34 nm per bound drug molecule. The comparatively very high DNA stiffening effect measured in solution is understandable as a result of helix clamping by at least two anthracycline groups of sufficient long distance. The variation of gamma on DNA interaction with different anthracycline derivatives find their explanation in terms of different values of the mismatch to in-register binding prior to complex formation. From an analogous interpretation of viscosity measurements by Arcamone and coworkers upon high molecular weight DNA with many anthracycline derivatives it can be concluded that DNA interaction by both amino sugar and 9-acetyl group are responsible for the generation of strong anthracycline binding mediated DNA stiffening effects in solution. (A combined analysis of the viscosity measurements by Cohen & Eisenberg and Armstrong et al. upon DNA interaction with proflavine indicates a very small DNA stiffening effect, gamma = 6.7 sigma and a helix elongation by 0.35 nm per bound ligand molecule.)
Full text
PDF










Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arcamone F. New antitumor anthracyclines. Lloydia. 1977 Jan-Feb;40(1):45–66. [PubMed] [Google Scholar]
- Arlandini E., Vigevani A., Arcamone F. Interaction of new derivatives of daunorubicin and adriamycin with DNA. Farmaco Sci. 1977 May;32(5):315–323. [PubMed] [Google Scholar]
- Berg H., Eckardt K. Zur Interaktion von Anthracyclinen und Anthracyclinonen mit DN. Z Naturforsch B. 1970 Apr;25(4):362–367. [PubMed] [Google Scholar]
- Crick F. H., Klug A. Kinky helix. Nature. 1975 Jun 12;255(5509):530–533. doi: 10.1038/255530a0. [DOI] [PubMed] [Google Scholar]
- Fritzsche H., Triebel H., Chaires J. B., Dattagupta N., Crothers D. M. Studies on interaction of anthracycline antibiotics and deoxyribonucleic acid: geometry of intercalation of iremycin and daunomycin. Biochemistry. 1982 Aug 17;21(17):3940–3946. doi: 10.1021/bi00260a006. [DOI] [PubMed] [Google Scholar]
- Geller K., Reinert K. E. Evidence for an increase of DNA contour length at low ionic strength. Nucleic Acids Res. 1980 Jun 25;8(12):2807–2822. doi: 10.1093/nar/8.12.2807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogan M., Dattagupta N., Crothers D. M. Transient electric dichroism of rod-like DNA molecules. Proc Natl Acad Sci U S A. 1978 Jan;75(1):195–199. doi: 10.1073/pnas.75.1.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogan M., Dattagupta N., Crothers D. M. Transient electric dichroism studies of the structure of the DNA complex with intercalated drugs. Biochemistry. 1979 Jan 23;18(2):280–288. doi: 10.1021/bi00569a007. [DOI] [PubMed] [Google Scholar]
- Mandelkern M., Elias J. G., Eden D., Crothers D. M. The dimensions of DNA in solution. J Mol Biol. 1981 Oct 15;152(1):153–161. doi: 10.1016/0022-2836(81)90099-1. [DOI] [PubMed] [Google Scholar]
- Manfait M., Alix A. J., Jeannesson P., Jardillier J. C., Theophanides T. Interaction of adriamycin with DNA as studied by resonance Raman spectroscopy. Nucleic Acids Res. 1982 Jun 25;10(12):3803–3816. doi: 10.1093/nar/10.12.3803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuzawa Y., Oki T., Takeuchi T., Umezawa H. Structure-activity relationships of anthracyclines relative to cytotoxicity and effects on macromolecular synthesis in L1210 leukemia cells. J Antibiot (Tokyo) 1981 Dec;34(12):1596–1607. doi: 10.7164/antibiotics.34.1596. [DOI] [PubMed] [Google Scholar]
- Müller W., Crothers D. M. Studies of the binding of actinomycin and related compounds to DNA. J Mol Biol. 1968 Jul 28;35(2):251–290. doi: 10.1016/s0022-2836(68)80024-5. [DOI] [PubMed] [Google Scholar]
- Patel D. J., Kozlowski S. A., Rice J. A. Hydrogen bonding, overlap geometry, and sequence specificity in anthracycline antitumor antibiotic.DNA complexes in solution. Proc Natl Acad Sci U S A. 1981 Jun;78(6):3333–3337. doi: 10.1073/pnas.78.6.3333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pullman B., Lavery R., Pullman A. Two aspects of DNA polymorphism and microheterogeneity: molecular electrostatic potential and steric accessibility. Eur J Biochem. 1982 May 17;124(2):229–238. doi: 10.1111/j.1432-1033.1982.tb06582.x. [DOI] [PubMed] [Google Scholar]
- Quigley G. J., Wang A. H., Ughetto G., van der Marel G., van Boom J. H., Rich A. Molecular structure of an anticancer drug-DNA complex: daunomycin plus d(CpGpTpApCpG). Proc Natl Acad Sci U S A. 1980 Dec;77(12):7204–7208. doi: 10.1073/pnas.77.12.7204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinert K. E., Geller D., Stutter E. Temperature mediated variation of DNA secondary structure in (A.T) clusters; evidence by use of the oligopeptide netropsin as a structural probe. Nucleic Acids Res. 1981 May 25;9(10):2335–2349. doi: 10.1093/nar/9.10.2335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinert K. E., Stutter E., Schweiss H. Aspects of specific protein-DNA interaction; multi-mode binding of the oligopeptide antibiotic netropsin to (A.T)-rich DNA segments. Nucleic Acids Res. 1979 Nov 10;7(5):1375–1392. doi: 10.1093/nar/7.5.1375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinert K. E., Thrum H., Sarfert E. Counterion dependent variation of DNA secondary structure in (A . T) clusters: evidence by use of netropsin as a structural probe. Nucleic Acids Res. 1980 Nov 25;8(22):5519–5531. doi: 10.1093/nar/8.22.5519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobell H. M., Tsai C. C., Jain S. C., Gilbert S. G. Visualization of drug-nucleic acid interactions at atomic resolution. III. Unifying structural concepts in understanding drug-DNA interactions and their broader implications in understanding protein-DNA interactions. J Mol Biol. 1977 Aug 15;114(3):333–365. doi: 10.1016/0022-2836(77)90254-6. [DOI] [PubMed] [Google Scholar]
- Triton T. R., Yee G. The anticancer agent adriamycin can be actively cytotoxic without entering cells. Science. 1982 Jul 16;217(4556):248–250. doi: 10.1126/science.7089561. [DOI] [PubMed] [Google Scholar]
- Wilson W. D., Keel R. A., Jones R. L., Mosher C. W. Viscometric analysis of the interaction of bisphenanthridinium compounds with closed circular supercoiled and linear DNA. Nucleic Acids Res. 1982 Jul 10;10(13):4093–4106. doi: 10.1093/nar/10.13.4093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zunino F. Studies on the mode of interaction of daunomycin with DNA. FEBS Lett. 1971 Nov 1;18(2):249–253. doi: 10.1016/0014-5793(71)80456-8. [DOI] [PubMed] [Google Scholar]



